Synthesis and Characterization of Chelating Hyperbranched Polyester Nanoparticles for Cd(II) Ion Removal from Water
Abstract
:1. Introduction
2. Results and Discussion
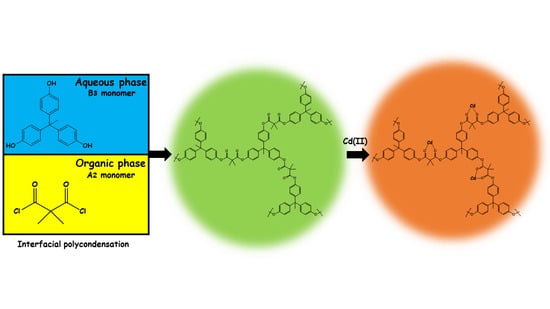
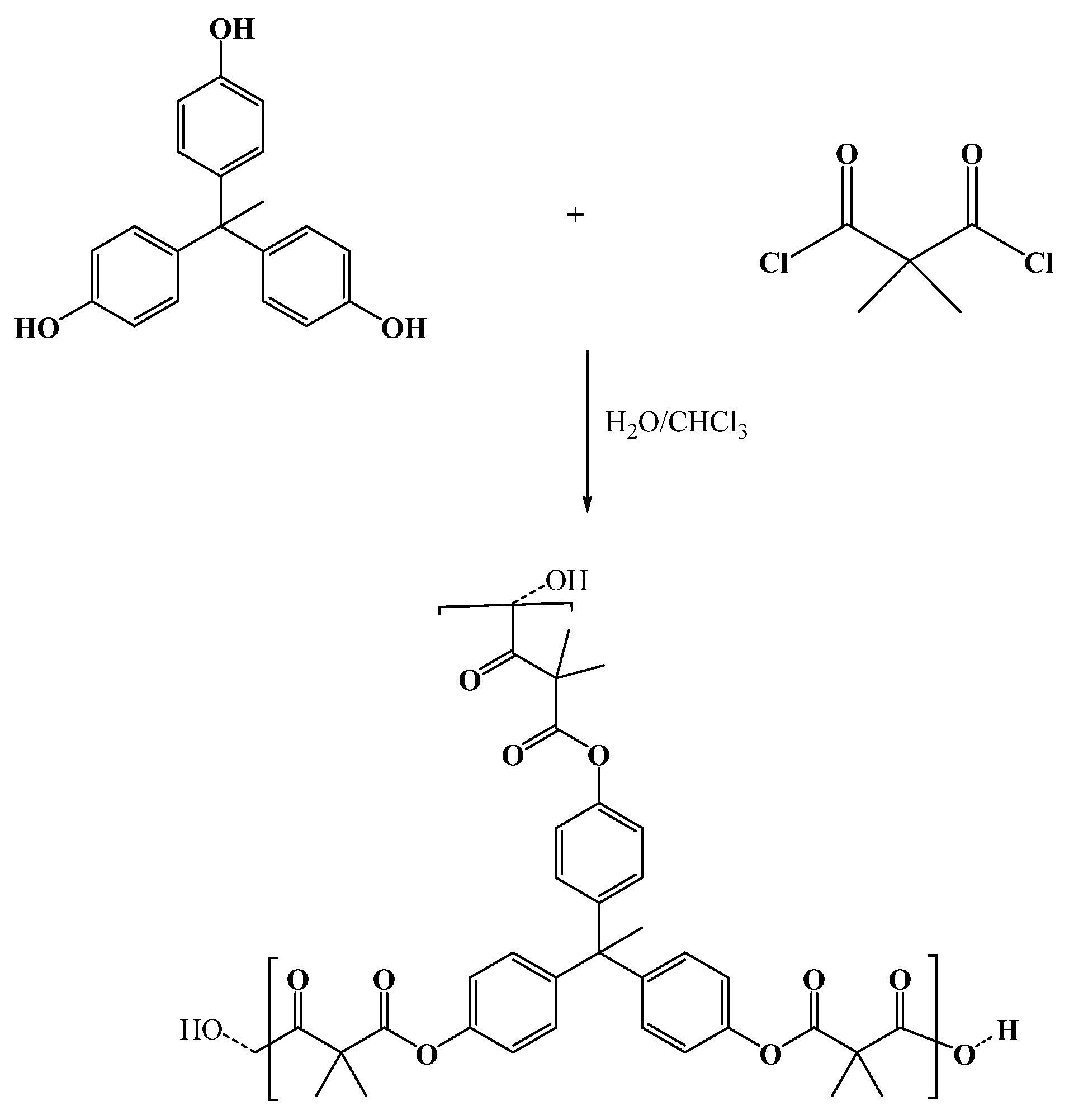
2.1. Synthesis of Chelating Hyperbranched Polyester Based on THPE by Interfacial Polycondensation
2.2. Molecular Weight Measurement
2.3. FTIR Spectra Analysis
2.4. Solid NMR Spectrum Analysis
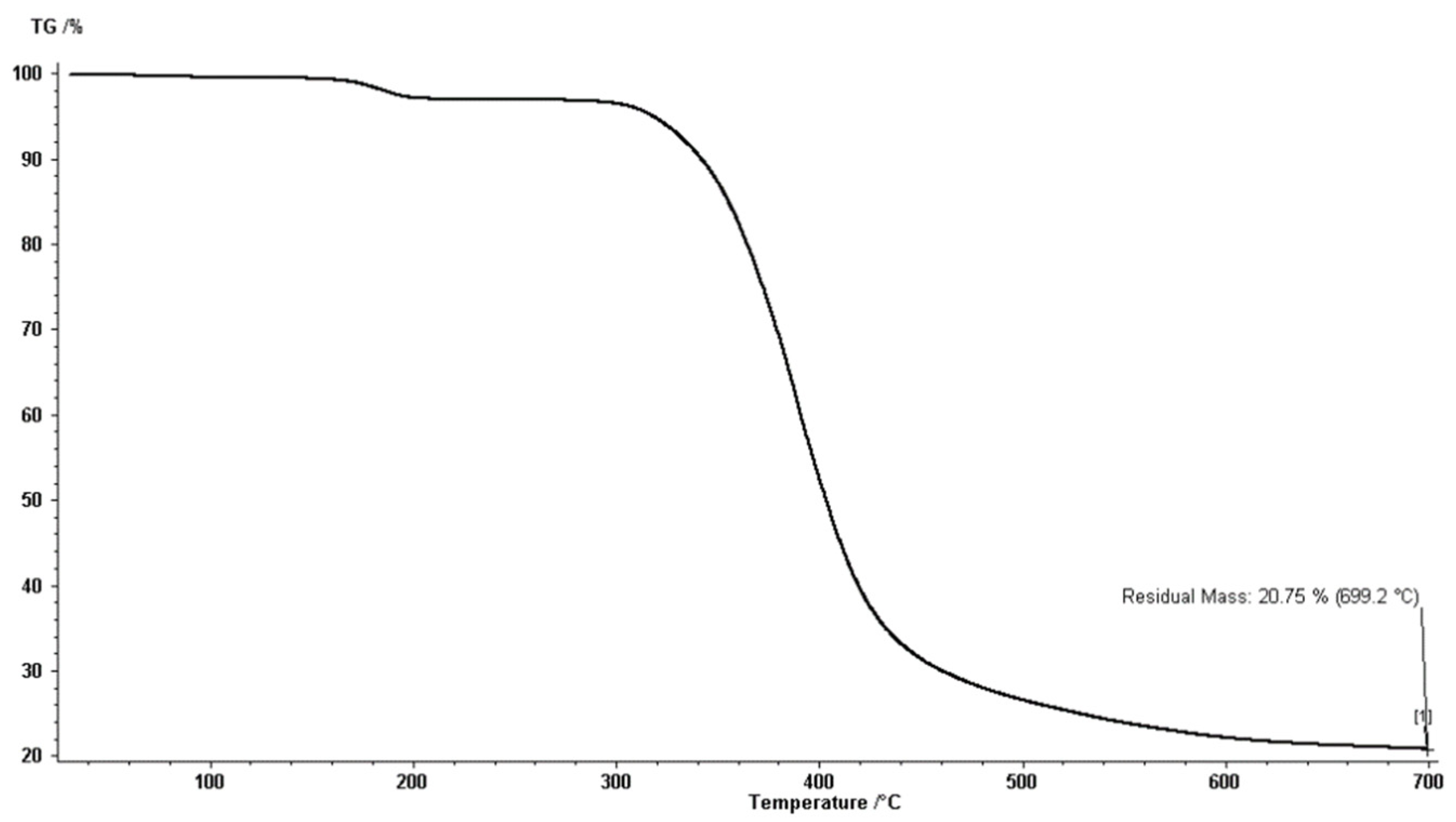
2.5. Thermal Stability
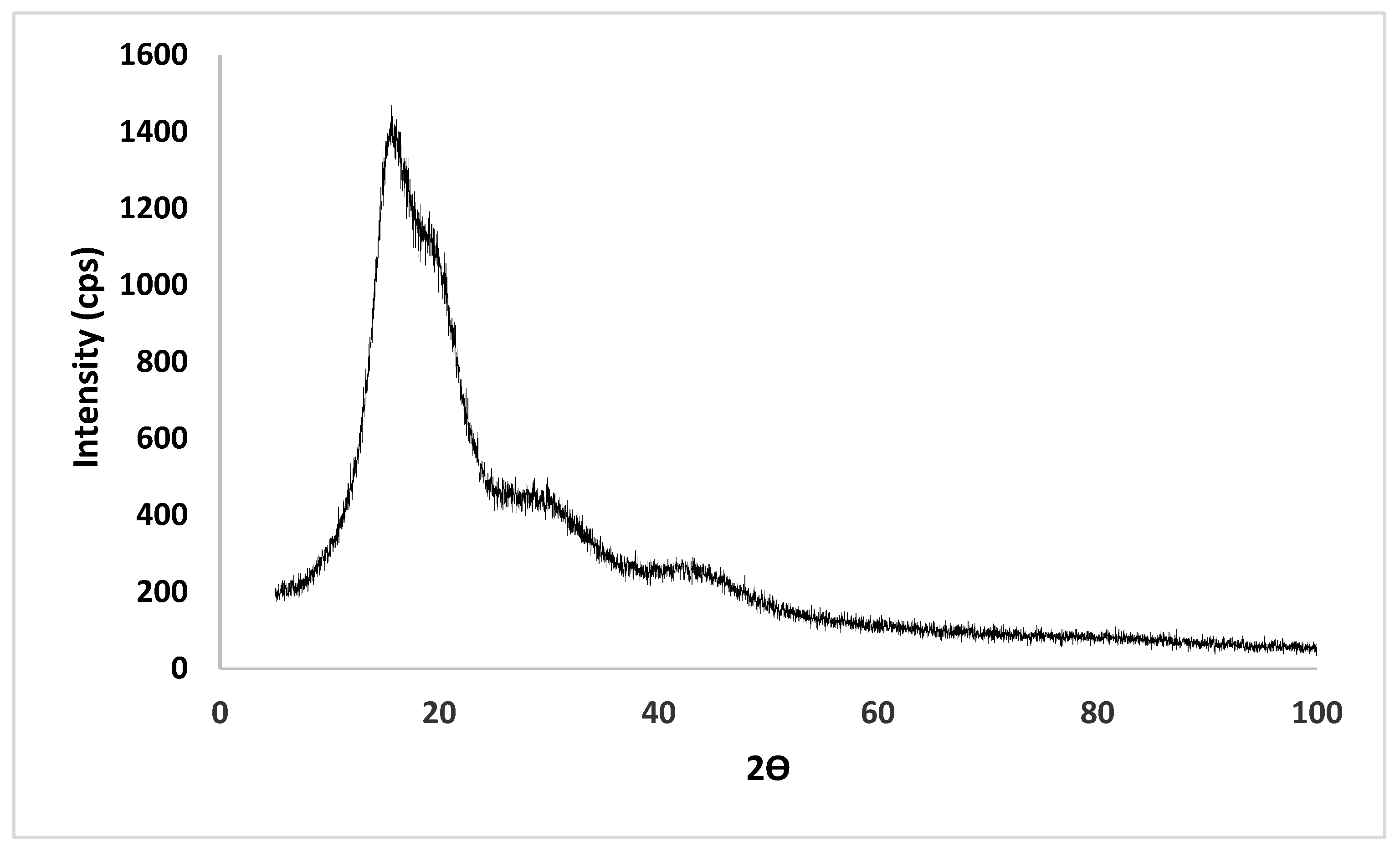
2.6. XRD Analysis
2.7. Particle Size Analysis
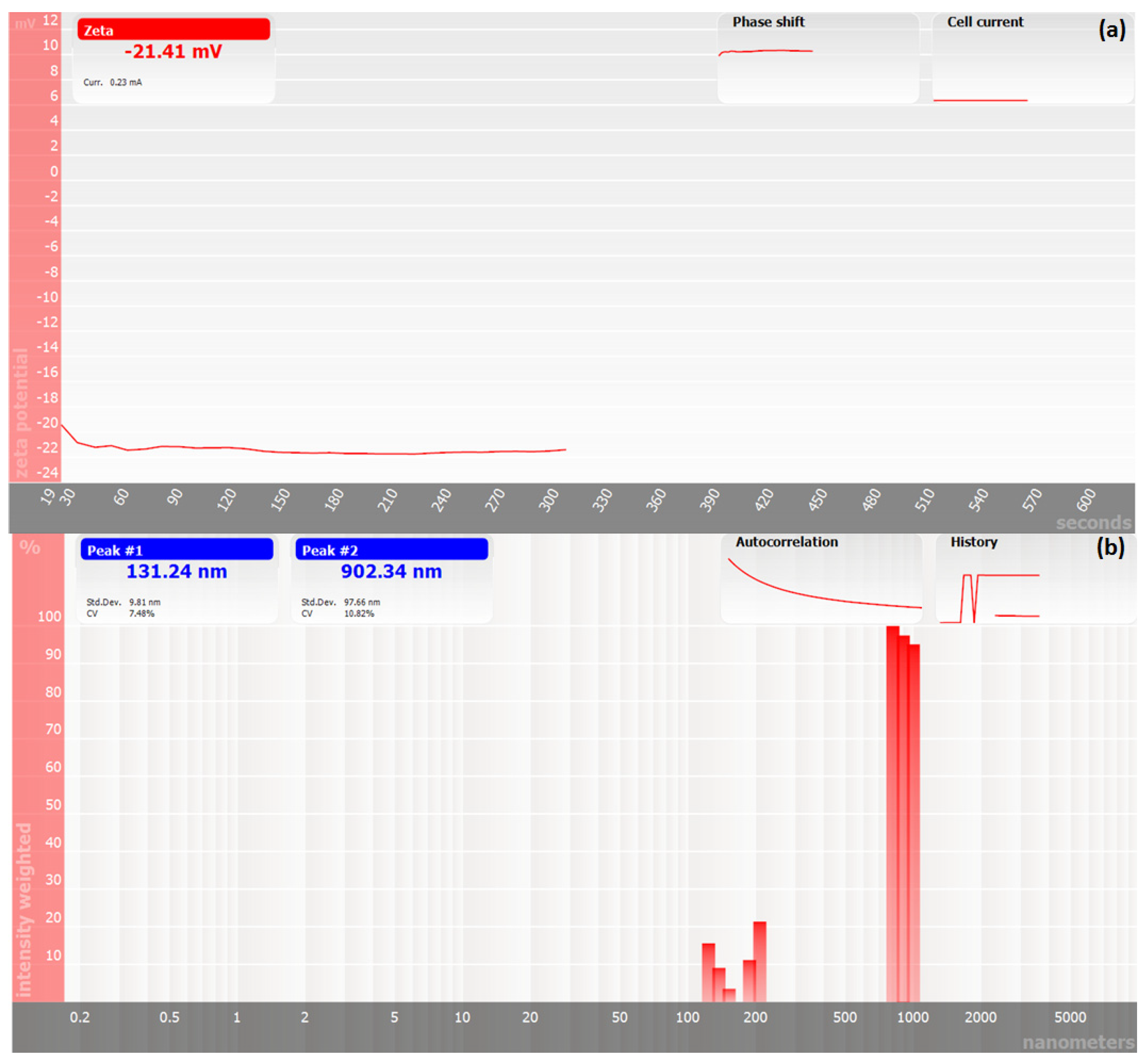
2.7.1. Zeta Potential and Particle Size Analysis
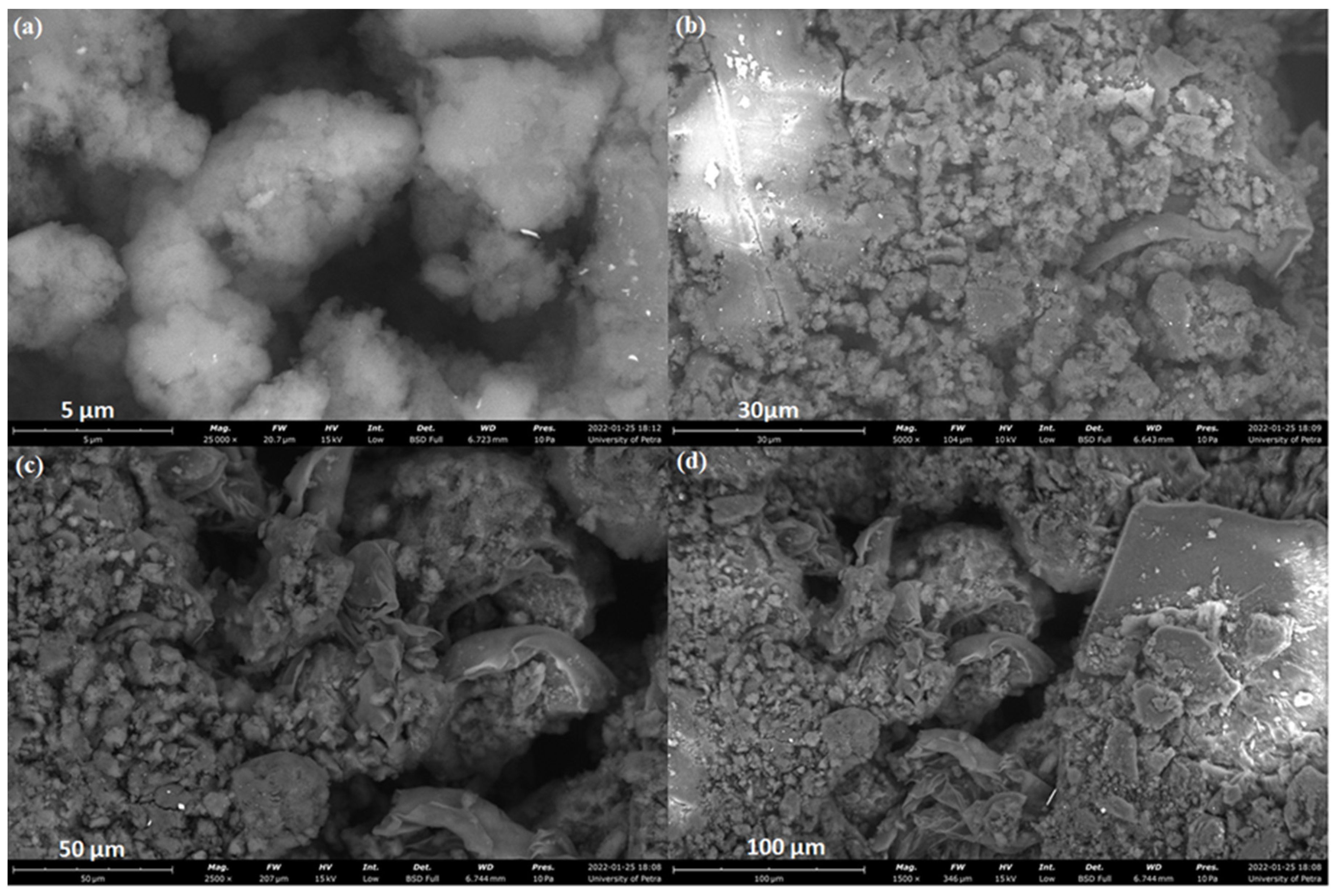
2.7.2. SEM Analysis
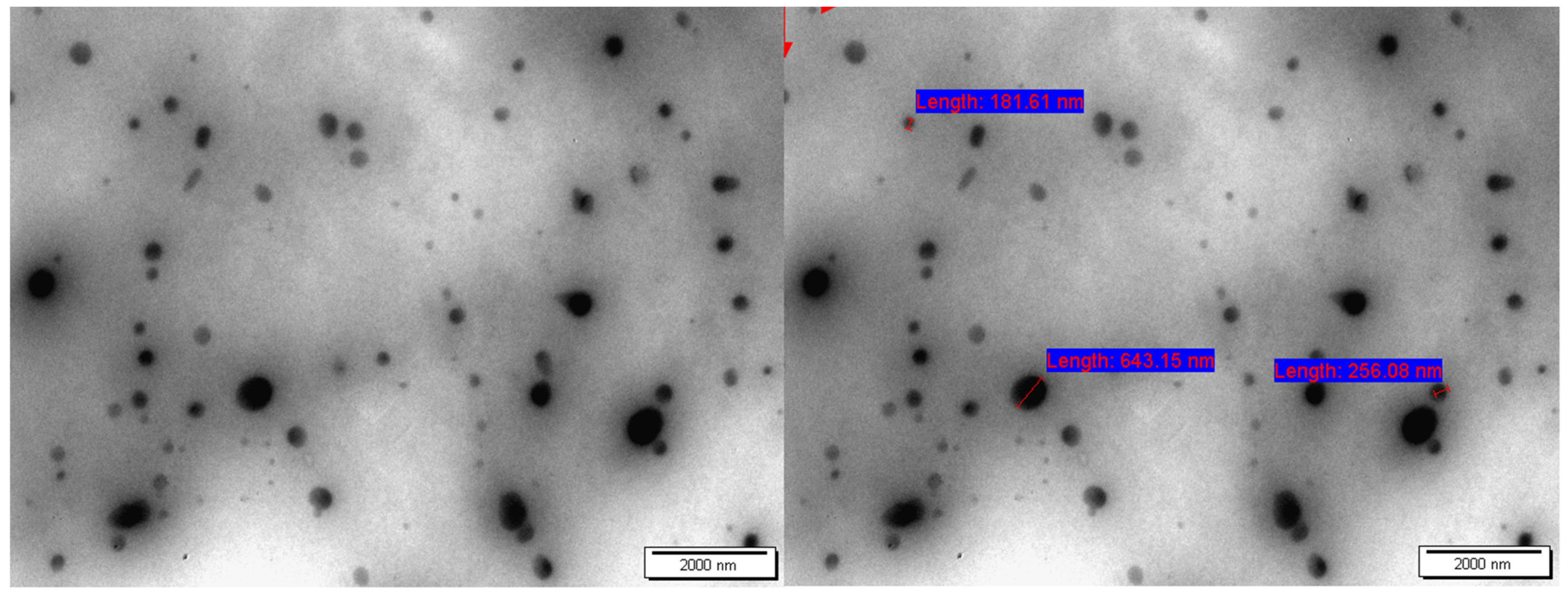
2.7.3. TEM Analysis
2.8. Metal Uptake Behavior of Cd(II) Ions from Water
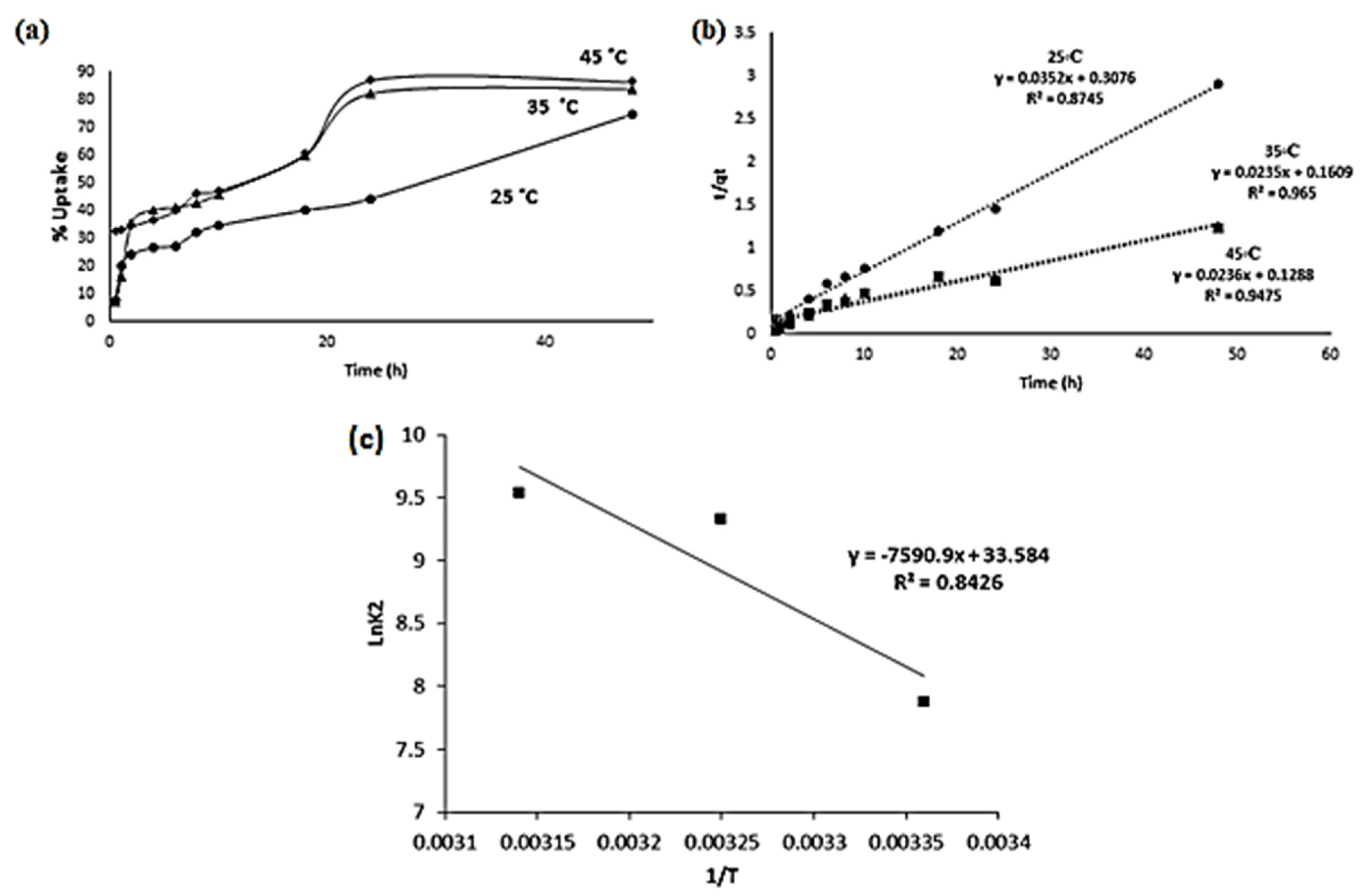
2.8.1. Kinetics Study of Cd(II) Ion Uptake by the Polyester
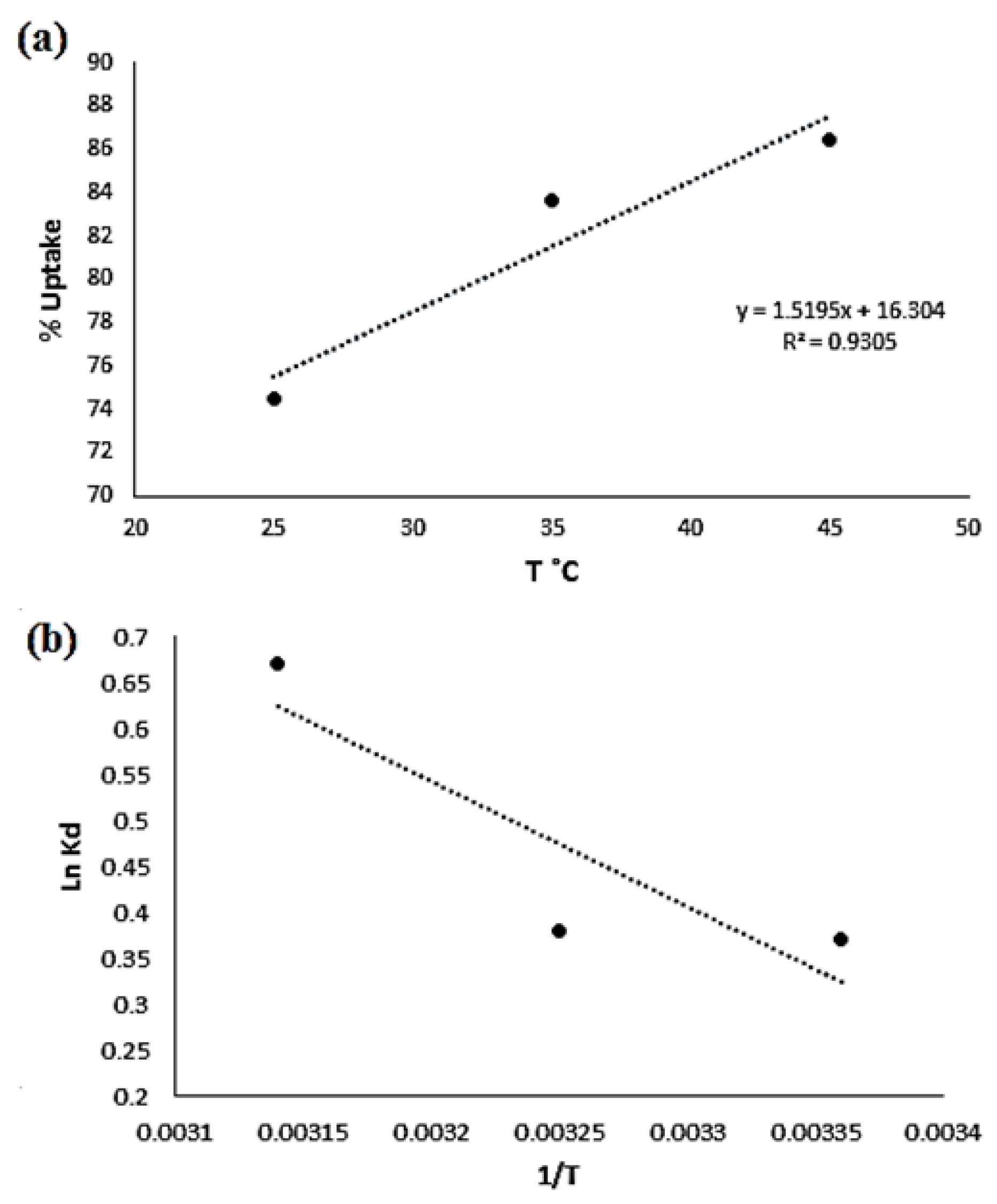
2.8.2. Thermodynamics of Adsorption
2.8.3. Desorption Studies
3. Experimental Section
3.1. Materials
3.2. Synthesis of Hyperbranched Polyester Nanoparticles
3.3. Swelling Capacity
3.4. Characterization
3.4.1. Particle Size, Zeta Potential Analysis, and Molecular Weight Measurement
3.4.2. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
3.4.3. Solid Nuclear Magnetic Resonance (NMR) Spectroscopy
3.4.4. Thermal Gravimetric Analysis (TGA)
3.4.5. X-ray Diffraction (XRD) Analysis
3.4.6. Scanning Electron Micrographs (SEM)
3.4.7. Transmission Electron Microscopy (TEM)
3.5. The Removal of Cd(II) Ions from Water
3.5.1. Preparation of Stock Solutions of Metal
3.5.2. Metal Uptake Characteristics of the Polymer by Batch Experiments
3.5.3. Desorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rahimzadeh, M.R.; Kazemi, S.; Moghadamnia, A.A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar]
- Bernhoft, R.A. Cadmium toxicity and treatment. Sci. World J. 2013, 2013, 394652. [Google Scholar] [CrossRef]
- Zhao, G.; Huang, X.; Tang, Z.; Huang, Q.; Niu, F.; Wang, X. Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: A review. Polym. Chem. 2018, 9, 3562–3582. [Google Scholar] [CrossRef]
- Ha, H.T.; Huong, N.T.; Dan, L.L.; Tung, N.D.; Trung, V.B.; Minh, T.D. Removal of Heavy Metal Ion Using Polymer-Functionalized Activated Carbon: Aspects of Environmental Economic and Chemistry Education. J. Anal. Methods Chem. 2020, 2020, 8887488. [Google Scholar] [CrossRef] [PubMed]
- Cyganowski, P.; Dzimitrowicz, A. A Mini-Review on Anion Exchange and Chelating Polymers for Applications in Hydrometallurgy, Environmental Protection, and Biomedicine. Polymers 2020, 12, 784. [Google Scholar] [CrossRef] [Green Version]
- Dzhardimalieva, G.I.; Uflyand, I.E. Design Strategies of Metal Complexes Based on Chelating Polymer Ligands and Their Application in Nanomaterials Science International Commission on Illumination. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1305–1393. [Google Scholar] [CrossRef]
- Haratake, M.; Yasumoto, K.; Ono, M.; Akashi, M.; Nakayama, M. Synthesis of hydrophilic macroporous chelating polymers and their versatility in the preconcentration of metals in seawater samples. Anal. Chim. Acta 2006, 561, 183–190. [Google Scholar] [CrossRef]
- Garg, B.S.; Sharma, R.K.; Bhojak, N.; Mittal, S. Chelating Resins and Their Applications in the Analysis of Trace Metal Ions. Microchem. J. 1999, 114, 94–114. [Google Scholar] [CrossRef]
- Al-blawi, A.N.; Khalili, F.I.; Sweileh, B.A. Synthesis and Characterization of Poly(1,4-benzenedimethylene phthalate) and the Study of its ability to sorb Pb (II), Cd (II), and Zn (II) ions. Arch. Org. Inorg. Chem. Sci. 2018, 1, 1–17. [Google Scholar]
- Fawwaz, K.; Faten, A.; Sweileh, S.B. Removal of Pb (II), Cd (II) and Zn (II) ions using a new poly (1,3-cyclohexylene oxalate). Polymer 2017, 21, 25–36. [Google Scholar]
- Hamidi, S.T.; Sweileh, B.A.; Khalili, F.I. Preparation and characterization of poly (bisphenol A oxalate) and studying its chelating behavior towards some metal ions. Solvent Extr. Ion Exch. 2008, 26, 37–41. [Google Scholar]
- Abu-awwad, I.F.; Khalili, F.I.; Sweileh, B.A. Preparation and Characterization of Poly (2,2-dimethyl-1,3-propylene oxalate) Polymer and the Study of its Metal Uptake Behavior Toward Pb (II), Cd (II), and Hg (II) Ions. Solvent Extr. Ion Exch. 2010, 28, 682–705. [Google Scholar] [CrossRef]
- Qu, R.; Wang, C.; Ji, C.; Sun, C.; Sun, X.; Cheng, G. Preparation, Characterization, and Metal Binding Behavior of Novel Chelating Resins Containing Sulfur and Polyamine. J. Appl. Polym. Sci. 2005, 95, 1558–1565. [Google Scholar] [CrossRef]
- Chen, H.; Jia, J.; Duan, X.; Yang, Z.; Kong, J. Reduction-cleavable hyperbranched polymers with limited intramolecular cyclization, via click chemistry. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2374–2380. [Google Scholar] [CrossRef]
- Gao, C.; Yan, D. Hyperbranched polymers: From synthesis to applications. Prog. Polym. Sci. 2004, 29, 183–275. [Google Scholar] [CrossRef]
- Srinivasa, V.; Rao, A.B.S. Photoactive liquid crystalline polymers: A comprehensive study of linear and hyperbranched polymers synthesized by A2B2, A2B3, A3B2, and A3B3 approaches. Polym. Chem. 2011, 49, 1319–1330. [Google Scholar]
- Zheng, Y.; Li, S.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef]
- Saha, A.; Ramakrishnan, S. Unimolecular micelles and reverse micelles based on hyperbranched polyethers—Comparative study of AB2 + A-R and A2 + B3 + A-R type strategies. Polym. Chem. 2008, 47, 80–91. [Google Scholar] [CrossRef]
- Lee, S.; Eom, Y.; Park, J.; Lee, J.; Kim, S.Y. Micro-hydrogel Particles Consisting of Hyperbranched Polyamidoamine for the Removal of Heavy Metal Ions from Water. Sci. Rep. 2017, 7, 10012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, I.; Noh, H.; Baek, J. Hyperbranched macromolecules: From synthesis to applications. Molecules 2018, 23, 657. [Google Scholar] [CrossRef] [Green Version]
- Asaad, J.N.; Ikladious, N.E.; Awad, F. Evaluation of some new hyperbranched polyesters as binding agents for heavy metals. Can. J. Chem. Eng. 2013, 91, 257–263. [Google Scholar] [CrossRef]
- Santra, S.; Kumar, A. Facile synthesis of aliphatic hyperbranched polyesters based on diethyl malonate and their irreversible molecular encapsulation. Chem. Commun. 2004, 18, 2126–2127. [Google Scholar] [CrossRef]
- Hassan, M.; Afzal, A.; Tariq, M.; Ahmed, S. Synthesis of the hyper—Branched polyamides and their effective utilization in adsorption and equilibrium isothermal study for cadmium ion uptake. J. Polym. Res. 2021, 28, 200. [Google Scholar] [CrossRef]
- Nagavarma, B.V.N.; Yadav, H.K.S.; Ayaz, A.; Vasudha, L.S.; Shivakumar, H.G. Different techniques for preparation of polymeric nanoparticles—A review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- Castro, K.C.; Costa, J.M.; Nogueira, M.G.; Gabriela, M.; Campos, N. Drug-loaded polymeric nanoparticles: A review. Int J. Polym. Mater. Polym. Biomater. 2020, 71, 1–13. [Google Scholar] [CrossRef]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Martino, P. Polymeric nanocapsules as nanotechnological alternative for drug delivery system: Current status, challenges and opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Ali, D.K.; Al-Zuheiri, A.M.; Sweileh, B.A. International Journal of Polymer Analysis and pH and reduction sensitive bio-based polyamides derived from renewable dicarboxylic acid monomers and cystine amino acid. Int. J. Polym. Anal. Charact. 2017, 22, 361–373. [Google Scholar] [CrossRef]
- Marturano, V.; Ambrogi, V.; Cerruti, P.; Giamberini, M.; Tylkowski, B. Photo-triggered release in polyamide nanosized capsules. AIP Conf. Proc. 2014, 1599, 234–237. [Google Scholar]
- Onseca, A.C.; Coelho, J.F.J.; Valente, J.F.A.; Correia, T.R.; Correia, I.J.; Gil, M.H.; Simões, P.N. Poly(ester amide)s based on (L)-lactic acid oligomers and α-amino acids: Influence of the α-amino acid side chain in the poly(ester amide)s properties. J. Biomater. Sci. Polym. Ed. 2013, 24, 1391–1409. [Google Scholar] [CrossRef] [PubMed]
- Su, J.F.; Wang, L.X.; Ren, L.; Huang, Z.; Meng, X.W. Preparation and characterization of polyurethane microcapsules containing n-octadecane with styrene-maleic anhydride as a surfactant by interfacial polycondensation. J. Appl. Polym. Sci. 2006, 102, 4996–5006. [Google Scholar] [CrossRef]
- Ali, D.K.; Sweileh, B.A. Novel Bio-based Bisfuranic Polyesters by Interchange Reactions Between Bisfuranic Diester Bearing Pendent Carboxylic Acid Group and Bio-based Dihydroxy Compounds. J. Polym. Environ. 2018, 26, 1940–1949. [Google Scholar] [CrossRef]
- Hosseinzadeh, M. Sorption of lead ion from aqueous solution by carboxylic acid groups containing adsorbent polymer. J. Chil. Chem. Soc. 2019, 64, 4466–4470. [Google Scholar] [CrossRef] [Green Version]
- Nishida, M.; Tanaka, T.; Hayakawa, Y.; Nishida, M. Solid-State Nuclear Magnetic Resonance (NMR) and Nuclear Magnetic Relaxation Time Analyses of Molecular Mobility and Compatibility of Plasticized. Polymers 2018, 10, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawelbeit, A.; Yu, M. Structural developments of the reversible confinement of ionic liquids in nylon 6 fibres: Solid state 13C nuclear magnetic resonance. J. Phys. Conf. Ser. 2021, 2021, 012080. [Google Scholar] [CrossRef]
- Aziz, S.; Marf, A.; Dannoun, E.; Brza, M.; Abdullah, R. The Study of the Degree of Crystallinity, Electrical Equivalent Circuit, and Dielectric Properties of Polyvinyl Alcohol (PVA)-Based Biopolymer Electrolytes. Polymers 2020, 12, 2184. [Google Scholar] [CrossRef]
- Souza, T.G.F.; Ciminelli, V.S.T.; Mohallem, N.D.S. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J. Phys. Conf. Ser. 2016, 733, 012039. [Google Scholar] [CrossRef] [Green Version]
- Pabisch, S.; Feichtenschlager, B.; Kickelbick, G.; Peterlik, H. Effect of interparticle interactions on size determination of zirconia and silica based systems—A comparison of SAXS, DLS, BET, XRD and TEM. Chem. Phys. Lett. 2012, 521, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Vicente-Alique, E.; Núñez-Ramírez, R.; Vega, J.F.; Hu, P.; Martínez-Salazar, J. Size and conformational features of ErbB2 and ErbB3 receptors: A TEM and DLS comparative study. Eur. Biophys. J. 2011, 40, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Wang, T. Adsorption properties of hyperbranched aliphatic polyester grafted attapulgite towards heavy metal ions. J. Hazard. Mater. 2007, 149, 75–79. [Google Scholar] [CrossRef] [PubMed]



| T (°C) | Adsorption Rate Constants (k) | Calculated Adsorbed at Equilibrium (qe cal) | Experimental Adsorbed at Equilibrium (qe exp) |
|---|---|---|---|
| 25 | 2.62 × 103 | 28.41 | 27.93 |
| 35 | 1.13 × 104 | 42.55 | 36.89 |
| 45 | 1.39 × 104 | 42.37 | 39.13 |
| Adsorbent | Adsorption Capacities (mg/g) | Reference |
|---|---|---|
| Linear polyester | 3.0 | [10] |
| Aliphatic Hyperbranched polyester | 0.29 | [37] |
| Hyperbranched polyester nanoparticles | 27 | This work |
| Eluting Agent | % Recovery | % Cumulative Recovery | ||||
|---|---|---|---|---|---|---|
| First Fraction | Second Fraction | Third Fraction | Fourth Fraction | Fifth Fraction | ||
| 0.1 M HNO3 | 10.50 | 5.87 | 3.37 | 2.34 | 1.80 | 23.88 |
| 0.1 M EDTA | 4.32 | 4.02 | 4.02 | 4.02 | 3.92 | 20.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alregeb, F.; Khalili, F.; Sweileh, B.; Ali, D.K. Synthesis and Characterization of Chelating Hyperbranched Polyester Nanoparticles for Cd(II) Ion Removal from Water. Molecules 2022, 27, 3656. https://doi.org/10.3390/molecules27123656
Alregeb F, Khalili F, Sweileh B, Ali DK. Synthesis and Characterization of Chelating Hyperbranched Polyester Nanoparticles for Cd(II) Ion Removal from Water. Molecules. 2022; 27(12):3656. https://doi.org/10.3390/molecules27123656
Chicago/Turabian StyleAlregeb, Faten, Fawwaz Khalili, Bassam Sweileh, and Dalia Khalil Ali. 2022. "Synthesis and Characterization of Chelating Hyperbranched Polyester Nanoparticles for Cd(II) Ion Removal from Water" Molecules 27, no. 12: 3656. https://doi.org/10.3390/molecules27123656
APA StyleAlregeb, F., Khalili, F., Sweileh, B., & Ali, D. K. (2022). Synthesis and Characterization of Chelating Hyperbranched Polyester Nanoparticles for Cd(II) Ion Removal from Water. Molecules, 27(12), 3656. https://doi.org/10.3390/molecules27123656