2.1. Glycopeptides
Glycopeptides are the most successful class of antibiotic CSs in CE. This class includes substances like avoparcin, balhimycin, eremomycin, ristocetin A, teicoplanin, and vancomycin. Glycopeptides are cyclic heptapeptides made up of an aglycon part with aminosaccharide moieties attached to it. Three or four fused macrocyclic rings (composed of linked amino acids and substituted phenols) compose the aglycon, which has a distinctive “basket” shape. The saccharide moieties connected to the aglycon “basket” can spin freely and take on a variety of shapes. They have a high number of stereogenic centers, as well as a number of functional groups that can participate in stereoselective interactions. In acidic solutions, these antibiotics absorb strongly below 250 nm and exhibit a small minimum of around 260 nm (allowing direct UV detection between 250 and 260 nm) [
15,
18,
22].
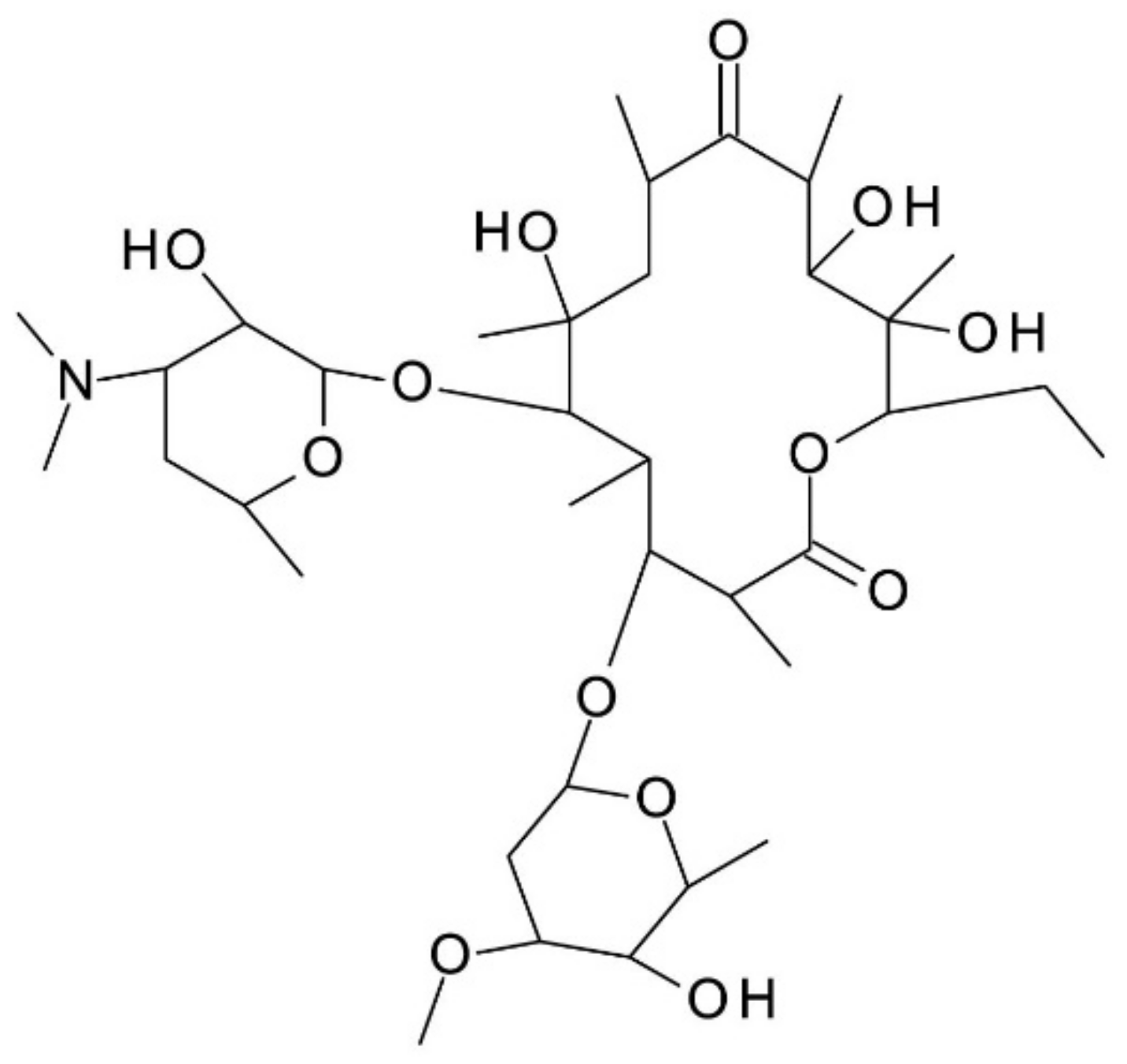
Vancomycin (C
66H
75Cl
2N
9O
24) is a macrocyclic glycopeptide antibiotic produced by the soil actinobacteria
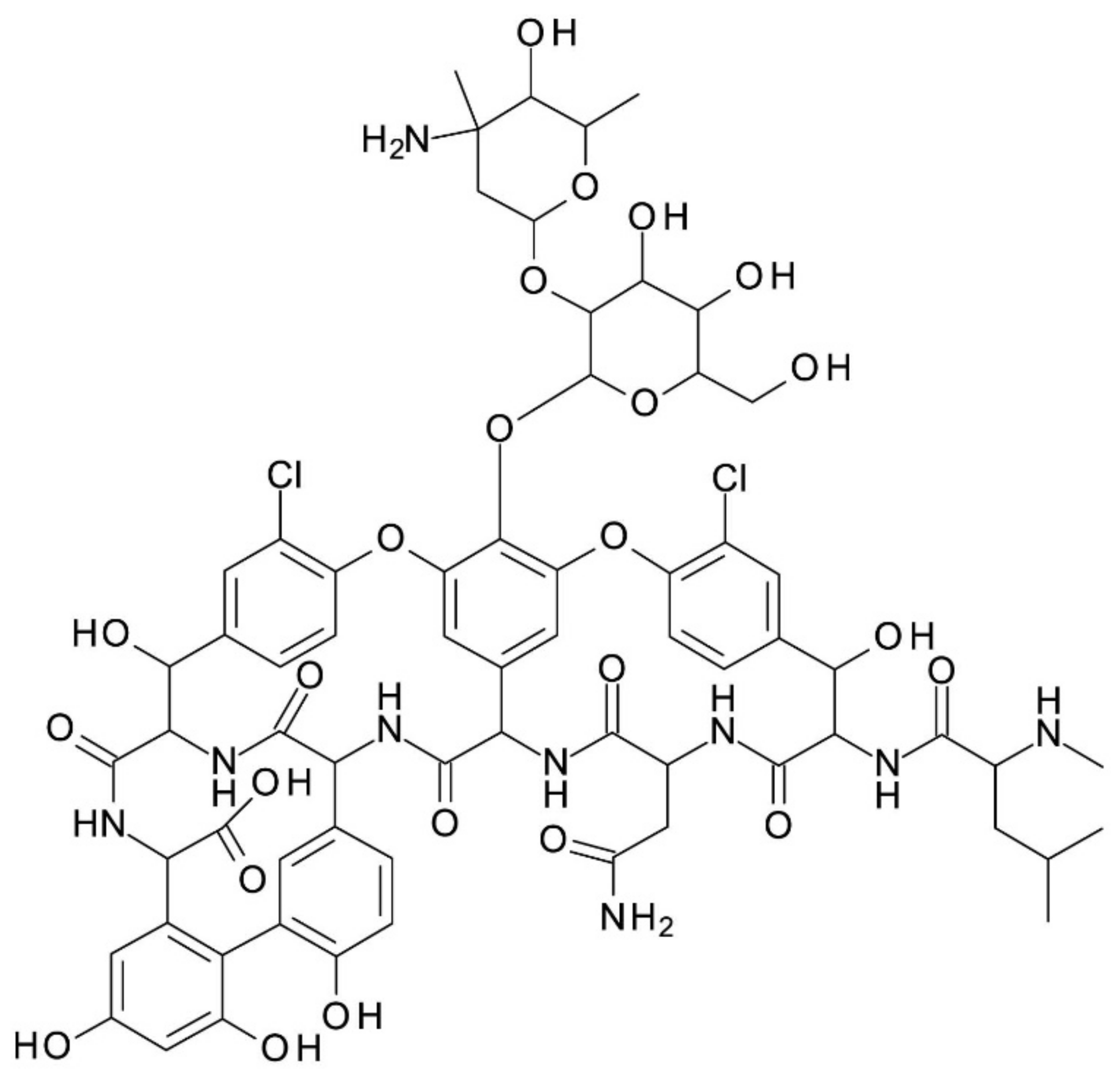
Amycolatopsis orientalis (Nocardia orientalis). It has an amphoteric character, its structure consisting of a seven-membered peptide chain constituted of two chlorinated beta-hydroxytyrosine molecules, three substituted phenyl-glycines,
N-methyl-leucine, and aspartic acid amine; one of the phenyl-glycine has a disaccharide moiety composed of vancosamine and glucose attached to it. It has 18 stereogenic centers, 9 hydroxy groups, 2 amine groups (primary and secondary amine), 7 amido groups, and 2 chloride substituents on two different aromatic rings. It is used in the form of hydrochloride. Vancomycin’s chemical structure is presented in
Figure 1. It has six reported pKa values: 2.9, 7.2, 8.6, 9.6, 10.5, and 11.7. Its isoelectric point (
pI) is 7.2. It is soluble in water and polar aprotic solvents, slightly soluble in methanol and insoluble in higher alcohols. The aqueous solution of vancomycin deteriorates within 2–4 days at room temperature and 6–7 days at 4 °C [
22,
23].
Vancomycin is the most commonly used glycopeptide antibiotic in CE enantio-separations. Vancomycin can be employed as uncharged or charged CS based on BGE pH values, having positive or negative mobility at pH buffer values below and above 7.2, respectively, due to the presence in its structure of both amino and carboxylic groups. Electrostatic interactions are thought to have a substantial part in the mechanism of enantiomer separation, where vancomycin is used as CS, and hydrophobic and hydrogen bonding interactions are the essential secondary interactions that generate the chiral recognition process [
22,
23,
24].
Vancomycin was the first glycopeptide employed as CS in CE; Amstrong et al. published in 1994 a study in which vancomycin was used for the chiral separation of approximately 100 chiral compounds (
N-derivatized amino acids, nonsteroidal anti-inflammatory drugs, antineoplastic drugs, and other carboxylic acid compounds). Low concentrations of vancomycin (2–5 mM) in a 100 mM phosphate BGE over a pH range of 4–7 were used in the determinations; good resolution (in some cases above 10) but also long migration times (in some cases over 30 min) were obtained. It was assumed that the fact that vancomycin occurs as a cationic species in the utilized BGE is primarily responsible for its capacity to resolve the model anionic substances. Separations were optimized by establishing the influence of CS concentration, BGE pH, and organic modifier type and concentration (acetonitrile, methanol, 2-propanol) on the chiral resolution. The tendency of vancomycin to interact with the walls of uncoated fused silica capillaries was found to decrease separation efficiency and increase analysis times [
23].
Fanali and Desiderio used vancomycin as CS for the CE enantio-separation of loxiglumide, a cholecystokinin antagonist used in the treatment of gastrointestinal pathologies. Chiral resolution was achieved in approximately 12 min using a 50 mM phosphate BGE at pH 6.0, and 3 mM vancomycin as CS. The effects of CS concentration and BGE pH on enantiomer resolution were investigated. A partial separation zone approach (polyacrylamide coating of the capillary in conjunction with “counter-flow”, while the capillary was filled with CS-containing BGE at low pressure, keeping the detector cell free of CS) was used to minimize the effect of vancomycin UV absorption, which allowed an excellent limit of detection (LOD) of 0.5 µg/mL for each enantiomer. Using the optimized conditions concentrations of 0.2% (
w/w) distomer (
l-loxiglumide) can be detected. The method was applied for chiral purity control in pharmaceutical preparations [
25].
Vespalec et al. used vancomycin as CS for the chiral separation of amino-quinolylcarbamate derivatized amino acids containing sulfur and selenium. Separation in both uncoated and coated capillaries was investigated. The association constants of vancomycin were found to be in the same order of magnitude, as for β-CD. It was demonstrated that the chemical composition, pH, and concentration of the BGE have a significant impact on the vancomycin-analyte interactions [
26]. The advantages and drawbacks of using vancomycin for the separation of amino acid derivatives were later summarized by the same research group [
24].
Ward et al. used coated capillaries to suppress electroosmotic flow (EOF) and a counter-current CE separation technique to test the efficacy of vancomycin as CS. Nonsteroidal anti-inflammatory drugs and dansyl amino acids were used as model molecules. The utilized “partial separation zone technique” (“partial filling method”) improved analyte detection sensitivity significantly [
27].
In the “counter-current” CE separation technique, the capillary is first filled with vancomycin-containing BGE, then negatively charged analytes are injected at the cathode of the capillary and detected at the anode; this improves detection sensitivity significantly and practically eliminates wall adsorption effects, resulting in higher resolutions and faster analysis times [
28].
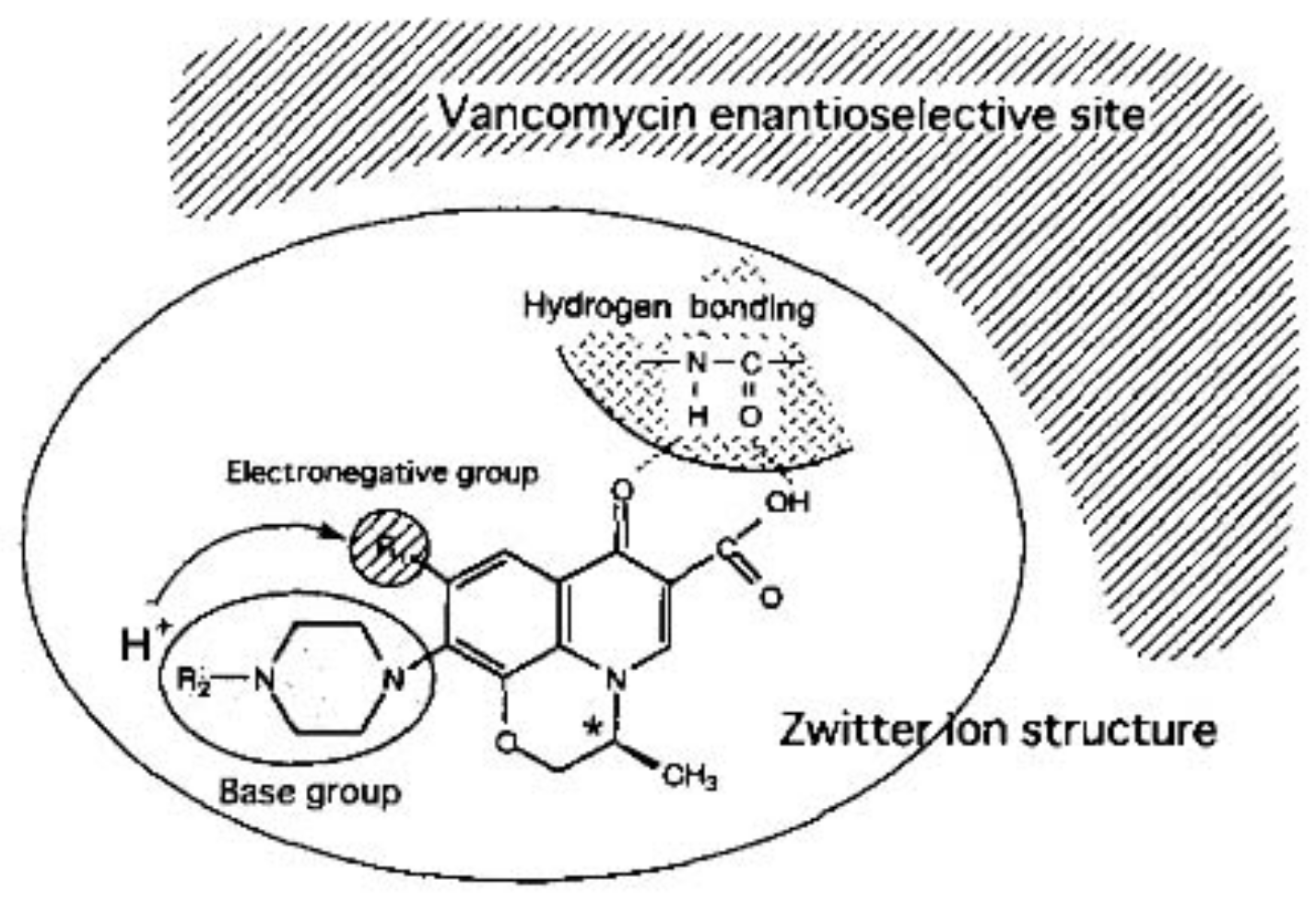
Arai et al. studied the chiral separation of antibacterial quinolone carboxylic acids, including ofloxacin, by CE using vancomycin as CS. The best results were obtained when using 100 mM acetate BGE at pH 4.0 and 5 mM vancomycin as CS. The molecular interaction between the quinolone carboxylic acids and vancomycin, based on structural differences, was explored, as well as the influence of experimental settings on the enantio-separation. The presumed interactions between the analyte and the CS were identified as being hydrogen bonding and amide linkages with piperidinyl, keto, and carboxyl groups; the molecular sizes of the quinolones which have to fit the hydrophobic pockets of the CS, and aromatic interaction with the naphthyl ring of the quinolones (
Figure 2) [
29].
Desiderio et al. used vancomycin as CS for the enantio-separation of herbicides: aryloxypropionic (mecoprop, fenoprop, and dichlorprop),
N-benzoyl-
N-(3-chloro-4-fluorophenyl)-2-aminopropionic acid (flamprop) and aryloxyphenoxypropionic (haloxyfop, fluazifop, diclofop, and fenoxaprop) derivatives. Because of the vancomycin positive charge and the absence/reduction in EOF at the operational pH, the detector path was kept free of absorbing vancomycin during detection by applying a partial filling technique, which produced a remarkable enhancement in detection sensitivity. CS concertation, BGE pH, and capillary temperature were found to strongly influence chiral separation. Baseline resolution was obtained for all analytes in less than 10 min using 75 mM Britton–Robinson BGE at pH 5.0 containing 6 mM vancomycin as CS. The method was used in environmental analysis to detect a soil extract treated with haloxyfop ethoxyethyl ester, which hydrolyzed to the acidic metabolite haloxyfop [
30].
Fanali et al. employed the use of the partial filling technique together with capillary electrophoresis–electrospray ionization-mass spectrometry (CE–ESI-MS), for the enantio-separation of arylpropionic acids anti-inflammatory drugs (carprofen, etodolac, flurbiprofen, ibuprofen, ketoprofen, naproxen) using vancomycin as CS. At acidic pH, vancomycin electrophoretic mobility as a positively charged CS allowed avoiding contaminating the ESI-MS source with the CS. The method was used to determine enantiomers of ibuprofen and its phase I metabolites, as well as etodolac and its metabolites, in urine samples [
31].
Bednar et al. evaluated the use of teicoplanin and vancomycin as CS for the chiral separation of UV nonabsorbing compounds, aspartic and glutamic acid enantiomers. A polyacrylamide-coated capillary using the partial filling-counter current method and an aqueous-organic BGE in the pH range of 4.5–6.5 composed of sorbic acid/histidine was used in the determination. The influence of CS concentration, BGE pH, organic modifier concentration, type, and concentration of absorbing co-ion (indirect UV detection) on the enantio-resolution was studied. The better results were obtained when using vancomycin (10 mM sorbic acid/histidine, pH 5.0, 10 mM vancomycin), which allowed reasonably good chiral resolution of the investigated molecules. The optimized method was applied for the analysis of real-life samples, such as teeth dentine and beer [
32].
Fanali et al. studied the enantio-separation of derivatized
N-acetyl amino acids (
N-acetyl glutamic acid, cystine, proline, serine, tyrosine) using CE, vancomycin as CS and applying a partial-filling countercurrent technique. The effect of CS concentration, BGE pH, capillary temperature, and organic modifier on the chiral resolution was studied. Baseline separation of all five analytes was obtained using a 20 mM ammonium acetate at pH 5.0 and 2.5 mM vancomycin as CS [
33].
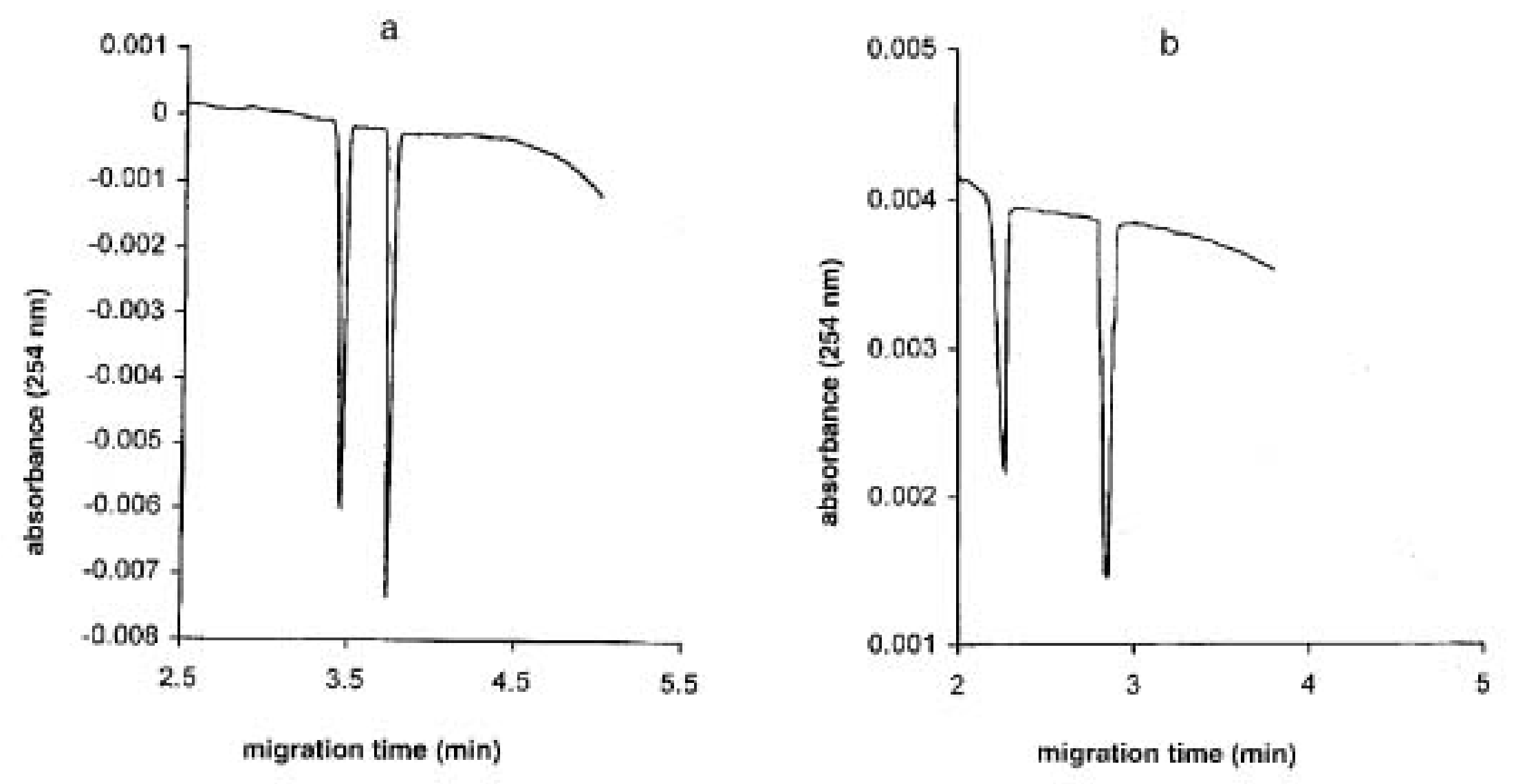
Fanali et al. developed a CE chiral separation method for carboxymethylcysteine and
N-acetamido-carboxymethylcysteine using vancomycin as CS. Separations were carried out in a polyacrylamide-coated capillary filled with a sorbic acid/histidine BGE at a pH range 4.5–6.5 using partial filling-countercurrent technique. In order to boost the sensitivity of the method, indirect UV detection was adopted due to the low absorption of the investigated enantiomers. The influence of CS concentration, BGE pH, organic modifier type, and concentration on enantio-resolution and migration times was investigated.
Figure 3 shows the enantio-separation of the two analytes at the optimum CS concentration [
34].
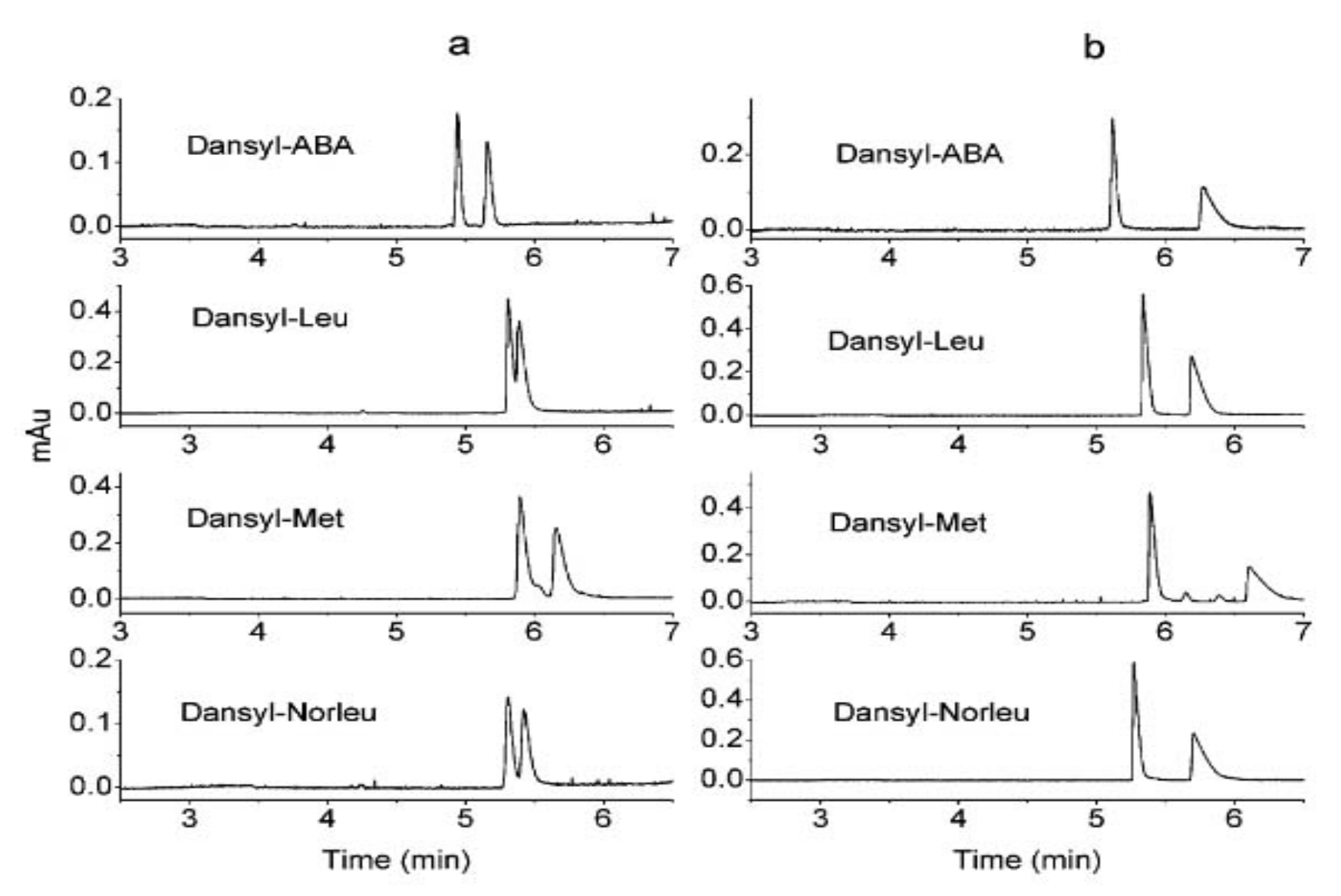
Kang et al. published a mechanistic study of chiral separation with vancomycin and balhimycin as CS. CE was used to study the role of the sugar moiety of the two glycopeptides (vancomycin and balhimycin have the same aglycon and almost similar sugar moieties) in chiral recognition. Balhimycin’s enantio-selectivity for dansylated α-amino acids was found to be 2.6 times higher than that of vancomycin (
Figure 4). When the sugar amino group of balhimycin was blocked by an
N-carbamoylation process, the enantio-selectivity was dramatically reduced compared to vancomycin, which remained almost the same after carbamoylation. Because the dimerization characteristics of glycopeptides are connected to their glycosylation patterns, a dimerization-based mechanism was proposed to explain this phenomenon. A synergistic effect between dimerization and the formation of the glycopeptide-analyte complex can explain the correlation between high dimerization constants and improved enantio-selectivity; consequently, the chiral recognition of glycopeptides with high dimerization constants may be aided by their dimer structure. [
35].
Wang et al. developed a CE method for the determination of stereoisomeric impurity of folinic acid diastereomers with vancomycin as CS. The effects of BGE concentration, BGE pH, CS concentration, organic modifier concentration, capillary temperature, and applied voltage on the enantio-resolution were studied. The best results were obtained when using 100 mM Tris-phosphate buffer at pH 6.0 containing 1 mM vancomycin and 5% acetonitrile on a poly(dimethylacrylamide) dynamically coated capillary. Folinic acid diastereomers were baseline separated in 7.5 min using the optimized conditions [
36].
Zhang et al. used vancomycin as CS for the CE enantio-separation of five nonsteroidal anti-inflammatory arylpropionic derivatives (carprofen, ibuprofen, ketoprofen, naproxen, pranoprofen). A synergistic system using chiral ionic liquids (ILs) was created to decrease adsorption to the capillary wall and UV absorption of the CS, potentially lowering the quantity of vancomycin required for enantio-separation. The synergistic impact of two ILs based on amino acid ester,
l-alanine, and
l-valine tert-butyl ester bis (trifluoromethane) sulfonamide, with the antibiotic CS, was evaluated. The influence of several parameters, such as BGE pH and composition, CS concentration, chiral ILs concentration, organic modifier type, and concentration on the enantio-resolution was evaluated by means of Statistical Product and Service Solutions (IBM, Armonk, NY, USA). For all model analytes, the combination resulted in a considerable improvement in enantio-separation when compared with chiral Ils or vancomycin alone. The introduction of an organic solvent (methanol) helped to increase the enantio-resolution even more. The method was applied to verify the enantiomeric purity of (
S)-naproxen in pharmaceutical preparations [
37].
An application of chiral CE-ESI-MS using vancomycin as CS for the enantio-separation of amino acids was published by Sanchez-Hernandez et al. Amino acids were derivatized using 9-fluorenylmethoxycarbonyl chloride to facilitate their interaction with vancomycin and the production of precursor ions with higher m/z, which were used in MS investigations. To avoid ionization suppression and generate a suitable MS signal, the authors employed a positively charged coated capillary (polybrene-coated capillary) in combination with the partial filling technique to avoid the contamination of the ion source by the nonvolatile CS. The simultaneous enantiomeric separation of 17 amino acids (two of which were nonprotein amino acids) took around 20 min under optimal conditions [
38].
Ristocetin A (C
94H
108N
8O
44) is a macrocyclic glycopeptide antibiotic, produced by the bacteria
Amycolatopsis lurida (Nocardia lurida). The aglycon consists of four fused macrocyclic rings (12 membered, 14 membered, and two 16 membered rings), to which several sugars (arabinose, glucose, mannose, rhamnose) are covalently bonded. The four macrocyclic rings form a “basket” shape structure. It has 38 stereogenic centers, 7 aromatic rings, 6 amide groups, 21 hydroxy groups, 2 primary amino groups, and 1 methyl ester. It is soluble in acidic aqueous solutions and less soluble at neutral pH; is soluble in polar organic and insoluble in non-polar organic solvents [
18,
22].
Armstrong et al. were the first to test ristocetin A as CS in CE in 1995 just as in the case of vancomycin a year before. Over 120 chiral analytes were tested, including a variety of
N-blocked amino acids, non-steroidal anti-inflammatory drugs and other compounds containing carboxylic acid groups. The influence of CS concentration, BGE pH, and organic modifier concentration on chiral resolution and migration time was studied. Because of the low amounts of ristocetin A required for enantio-resolution and its low absorbance (by comparison with vancomycin) at wavelengths higher than 250 nm, direct UV detection was possible. Enantioselective association appears to be aided by electrostatic interactions between the chiral analyte and the CS [
39].
Mohr et al. studied the chiral separation of six α-hydroxy acids (mandelic acid, 4-bromomandelic acid, 3-hydroxymandelic acid, 3-hydroxy-4-metoxymandelic acid, 4-hydroxymandelic acid, and 4-methoxymandelic acid,) by capillary electrochromatography (CEC) using ristocein A as CSP (a 25 % particle loaded ristocetin A continuous bed). Negatively charged chemicals moved towards the anode under initial conditions (0.05% triethylamine and 0.1% acetic acid in methanol, normal polarity), and were carried on by the weak cathodic EOF, resulting in long analysis times of over one hour. To reverse the EOF and shorten the analysis time, a cationic surfactant cetyltrimethylammoniumbromide was used as a mobile phase additive. The shortest retention times (less than 20 min) were obtained when a mobile phase with a concentration of 25 mM cetyltrimethylammoniumbromide was used [
40].
Teicoplanin is a mixture of five major macrocyclic glycopeptide antibiotic analogs (Teicoplanin A
2-1—Teicoplanin A
2-5) and four minor ones (Teicoplanin R
S-1—Teicoplanin Rs-4), produced by bacteria
Actinoplanes teichomyceticus. The aglycon consists of four fused macrocyclic rings, to which three carbohydrate moieties consisting of
N-acetylglucosmanine and mannose are attached. The length and shape of a sidechain linked to a third carbohydrate moiety
d-glucosamine, are the sole differences between major and minor components. Teicoplanin is distinguished by the presence of an
N-acyl hydrocarbon chain in one of its glucosamine moieties. Four of the seven aromatic rings are phenolic moieties that can be ionized, and two include chlorine substituents. Teicoplanin is considered to be a lipoglycopeptide [
18,
22].
MDL 63,246 (Hepta-tyr), a semisynthetic glycopeptide antibiotic structurally related to the teicoplanin family, has been tested in CE chiral separations by Fanali et al. Separations were carried out using a polyacrylamide-coated capillary in partial filling-counter current mode and aqueous-organic buffers in the pH range 4–6. The influence of CS concentration, BGE pH, organic modifier concentration, and capillary temperature on the enantio-resolution was studied. The best results were obtained at pH 5.0 using low concentrations of antibiotic (0.55–1.11 mM). The addition of an organic solvent (acetonitrile) to the BGE was necessary due to the limited solubility of Hepta-tyr in aqueous solution. Even in coated capillaries, remarkable adsorption of Hepta-tyr on the capillary wall has been reported [
41].
Avoparcin is a mixture of two macrocyclic glycopeptide antibiotics α-avoparcin (C
89H
102ClN
9O
36) and β-avoparcin (C
89H
101Cl
2N
9O
36), which differ by the presence of an additional chlorine atom in β-avoparcin. It has 32 stereogenic centers, 7 aromatic rings, 6 amide groups, 16 hydroxyl groups, 3 amine groups (2 primary amines, 1 secondary amine), a carboxylic group, and 4 carbohydrate side chains [
42].
Avoparcin capacity as CS in CE was tested by Ekborg-Ott et al. using as chiral model molecules
N-blocked amino acids and aryl propionic anti-inflammatory drugs. A comparative study on the chiral separation efficiency of avoparcin, ristocetin A, teicoplanin, and vancomycin on
N-3,5-dinitrobenzoyl-derivatized amino acids has been made; the use of vancomycin resulted in the longest migration times, ristocetin A the shortest, while the migration times obtained when using avoparcin were intermediate. No connections have been established between the efficiency/inefficiency of a certain CS towards an analyte; however, in all studied situations at least one of the tested CSs offered good enantio-resolution, while the others in the same analytical conditions offered poor separation. A low concentration of CS (0.4 mM) was used to allow direct UV detection at 254 nm. The tendency of avoparcin to bind to the capillary wall was observed [
42]
Balhymicin is a structural analog of vancomycin isolated from the fermentation broth of
Amycolatopsis balhimycina (Amycolatopsis mediterranei). Balhimycin has the same aglycon as vancomycin, made up of a heptapeptide backbone cyclized in the side chains of aromatic amino acids; the type and linking position of the sugar moieties are different. Vancomycin has a disaccharide connected to the aglycon, whereas balhimycin has two sugar moieties, a
d-glucose and an oxo-vancosamine, coupled at two distinct locations to the aglycon. It has 17 stereogenic centers and 3 atropisomeric macrocyclic rings with a dehydrovancosamine sugar attached to it [
43].
Balhimycin and its halogenate analogue bromobalhimycin were evaluated as CSs in CE by Jiang et al. A combined approach of the dynamic surface coating technique, the co-EOF electrophoresis technique, and the partial filling technique was used for the chiral separation of 16 acidic model drugs (dansyl amino acids, aryl propionic anti-inflammatory drugs). A comparative study was made on enantio-recognition capacity of balhimycin, bromobalhimycin, and vancomycin. For most of the tested compounds, balhimycin proved to have the highest enantio-resolution capacity [
44].
The same research group conducted a comparative study evaluating the enantio-recognition capacity of balhimycin, bromobalhimycin, and its dehalogenated analogue dechlorobalhimycin on model dansyl amino acids and aryl propionic anti-inflammatory drugs. The observed enantio-resolution capacity of balhimycin was much higher in all cases, which indicates that the chlorine substituents were important in the enantio-resolution of the model analytes (
Figure 5). To explain this phenomenon, a dimerization-based mechanism was proposed; each monomer’s two chlorine substituents, which mutually penetrate the cavity of the neighboring dimer molecule, are thought to promote dimerization and, as a result, enantio-separation. The cavity generated by the aglycon is thought to be the principal site of interaction between test analytes and antibiotics, and it is this cavity that is responsible for stereoselective inclusion [
45].
Eremomycin (C
73H
89ClN
10O
26) is another structural analog of vancomycin, isolated from the culture filtrate of
Actinomycete numbered INA-238. It has 22 chiral centers, 3 sugar moieties, 5 aromatic rings, a carboxylic group, 9 hydroxyl groups, 7 amido groups, and 3 amino groups [
46].
Prokhorova et al. evaluated the potential of eremomycin as CS in CE using as model substances aryl propionic anti-inflammatory drugs (fenoprofen, flurbiprofen, ibuprofen, indoprofen, ketoprofen). The electrophoretic characteristics of eremomycin, as well as its stability in solution, have been studied. The effect of CS concentration, BGE type, and pH, organic modifier type and concentration on the enantio-resolution was studied. Low concentration of CS was used (2.5 mM), which allowed direct UV detection, eremomycin exhibiting low absorbance between 260 and 350 nm. The migration order of the enantiomers was established. The enantio-separations of profens in CE with eremomycin as CS added in the BGE and in HPLC with eremomycin as CSP showed comparable tendencies [
47].
Prokhorova et al. also studied an interesting combination of coupled chitosan capillary coating and eremomycin CS for the CE enantio-separation of several acids (fenoprofen, flurbiprofen, ibuprofen, indoprofen, ketoprofen, mandelic acid, α-methoxyphenylacetic acid, 3-phenylbutiric acid, 2-phenoxypropionic acid). Eremomycin adsorption on the capillary wall and two types of chitosan-based coatings (chitosan and linked chitosan) were studied. The applicability of chitosan-coated capillary over fused silica capillary has been demonstrated in terms of enantio-resolution and time analysis [
48].
2.3. Macrolides
Macrolides consist of macrocyclic lactone rings containing 14, 15, or 16 atoms which allow the inclusion complexation of chiral analytes. Various deoxy-sugar or amino sugar residues are glycosidically linked to the substituted lactone macrocycle. This class includes substances, such as azithromycin, boromycin, clarithromycin, erythromycin, and gamithromycin. Macrolides interference with UV detection of analytes is lower than in the case of glycopeptides because their structure lacks aromatic rings, resulting in poorer UV absorption [
15,
18].
Erythromycin is a macrolide isolated from
Streptomyces erythreus (
Saccharopolyspora erythraea) containing a 14-atom macrocyclic lactone ring, with a characteristic “basket” shape. The lactonic macrocycle of erythromycin is called erythronolide, which is glycosidically bound to a 6-deoxy sugar (
l-cladinose) and an amino sugar (desozamine). It has 10 stereogenic centers, two sugar moieties, three hydroxyl groups, a methoxy group, and a tertiary amine group. Erythromycin’s chemical structure is presented in
Figure 6. It can be positively charged in acidic and neutral BGE because of its dimethyl amino group (pKa 8.8), on the desozamine moiety. However, the relative instability of erythromycin under acidic conditions represents a limiting factor of its applicability [
52,
53].
Ha et al. investigated erythromycin and five related substances (erythromycin
N-oxide, anhydroerythromycin, anhydroerythromycin
N-oxide, erythralosamine, erythralosamine
N-oxide) as potential CS in CE. Phosphate BGEs at two different pH levels (3.0, 7.0) and sodium tetraborate BGE at pH 9.2 were used; different concentrations of CS ranging from 0.1 to 10 mM were added to the BGE. Erythromycins had stronger interactions with acidic compounds than with neutral or weakly basic chemicals, especially in an acidic medium. However, none of the 21 chiral drugs (with different structural characteristics and electrophoretic behavior) showed enantio-separation in the studied conditions; over 3000 runs were made in 70 different experimental conditions. Based on the variation in electrophoretic mobility of the compounds in the presence of different concentrations of CS, the complexation constants for the compounds that showed interaction were calculated. Computational modelling was used to determine the size of erythromycin; the aglycone ring was found to be only half the size of the β-CD cavity, which could explain why the use of erythromycin as CS had such a negative result in the investigation [
53]. The lack of an organic component in the BGE and a low CS concentration may have prevented chiral separation; as the highest concentration of erythromycin in acidic and neutral aqueous medium was 10 mM, while the highest concentration in basic aqueous medium was 5 mM.
The applicability of erythromycin as CS in CE was tested by Huo et al. on four antihepatitis biphenyldimethylester derivatives. The effects of CS concentration, BGE pH, organic modifiers, capillary temperature, and applied voltage on enantio-resolution were studied. The enatio-resolution as well as migration times of the analytes, improved when CS concentration was increased. Additionally, the content of organic solvents in the BGE influenced strongly the enantio-separation. The best results were obtained when using a BGE containing 50 mM phosphate at pH 6.0 and 50% methanol, and 20 mM erythromycin as CS [
54].
Erythromycin lactobionate was applied by Xu et al. as CS for the non-aqueous capillary electrophoresis (NACE) enantio-separation of six model basic chiral drugs (chloroquine,
N,N-dimethyl-3-(2-methoxyphenoxy)-3-propylamine, duloxetine, nefopam, primaquine, propranolol). In this study, erythromycin lactobionate was used which exhibits higher solubilization in aqueous solutions, consequently, CS concentrations up to 12% were used. The influence of CS concentration, BGE pH and concentration, capillary temperature, and applied voltage on enantio-separation was tested; the results showed that CS concentration and BGE pH influenced strongly the enantio-resolution [
55].
Chen et al. also employed erythromycin lactobionate as CS in NACE for the enantio-separation of two extensively used basic chiral drugs a serotonin and norepinephrine inhibitor antidepressant (duloxetine) and a β-blocker (propranolol). The best results were obtained when using a BGE composed of 50 mM Tris, 100 mM boric acid in methanol, and 100 mM erythromycin as CS. The influence of CS concentration, BGE pH and composition, organic solvent type, capillary temperature, and applied voltage on enatio-resolution and migration times was studied [
56].
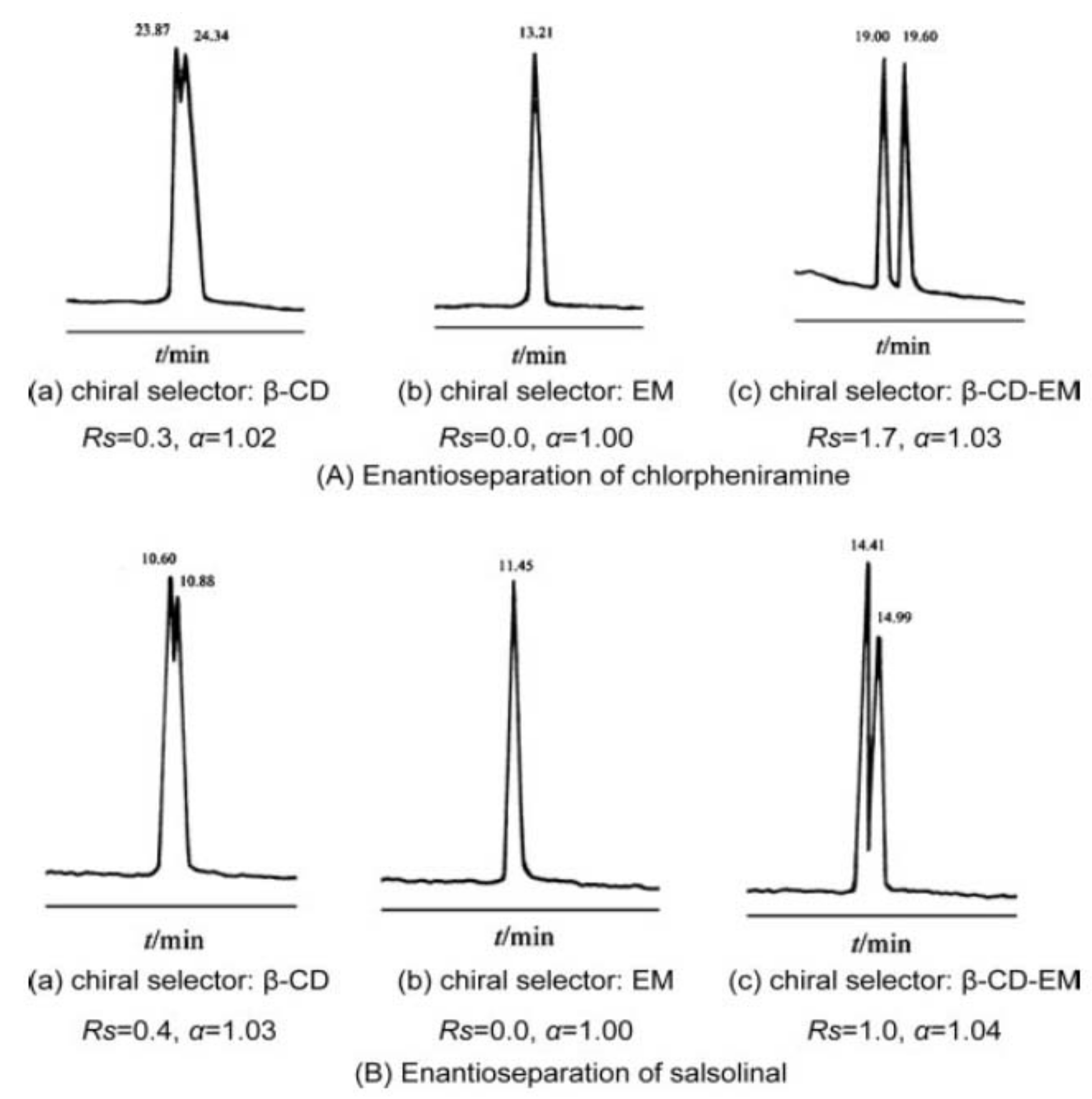
An interesting idea was exploited by Dai et al., who synthetized and tested as CS a water-soluble β-CD-derivatized erythromycin. The goal of substituting 1-oxygen-2,3-epoxypropane at the main hydroxyl site of β-CD is to create a molecule that has both β-CD and erythromycin functionalities. The chemical structure of β-CD-derivatized erythromycin was confirmed using FTIR, 1H NMR, and MALDI-TOF-MS experiments. The influence of CS concentration, BGE pH and concentration, organic modifier, and applied potential, on the enantio-separation was investigated. Β-CD-derivatized erythromycin showed improved enantioselectivities compared with single β-CD and erythromycin as CS, for the enantio-separation of three model drugs (chlorpheniramine, propranolol, salsolinol) (
Figure 7). The best results were obtained when using a BGE containing 20 mM phosphate at pH 3.01 (chlorpheniramine, propranolol) or pH 4.98 (salsolinol) and 20% methanol, and 15–20 mM β-CD-derivatized erythromycin [
57].
Clarithromycin is a semisynthetic macrolide containing a 14-membered macrocyclic lactone ring. It has two sugar moieties, four hydroxyl groups, two methoxy groups, and one tertiary amino group. Clarithromycin has various properties that make it an interesting CS candidate, such as good solubility in methanol (as a salt), low solvent viscosity, and especially low UV absorption [
52].
Clarithromycin lactobionate was used by Yu et al. as CS for the NACE chiral separation of nine model basic molecules (amlodipine, atenolol, bisoprolol, esmolol, labetalol, metoprolol, nefopam, propranolol, ritodrine). The influence of CS concentration, BGE pH and composition, organic modifier type and concentration, and applied voltage on the enantio-resolution and migration times was studied. The best results were obtained when using 12.5 mM sodium borate BGE at pH 7.3–7.5 in a mixture with 50% methanol and 60 mM clarithromycin as CS. Another eight basic chiral drugs (chlorphenamine, chloroquine, citalopram, duloxetine, landiolol, sertraline, sotalol, trimetazidine) were tested but no chiral resolution was obtained. Utilizing Statistical Product and Service Solutions, a comparison of the influences of the analyzed parameters on enantio-separation was investigated using multivariate analysis of variance. Based on statistical results, CS concentration and BGE pH were the most important parameters that influence enantio-separation [
58].
Lebedeva et al. used clarithromycin as CS in NACE for the chiral separation of 11 chiral drugs (amines and amino alcohols: alprenolol, atenolol, clenbuterol, fenoterol, labetalol, methoxyphenamine, metoprolol, pindolol, propranolol, sotalol, and synephrine). To optimize separation the influence of CS concentration, BGE pH, applied voltage, and the capillary temperature were studied. The best results were achieved in a methanolic solution of 100 mM citric acid, 10 mM sodium hydroxide, 240–300 mM boric acid, and 60–75 mM clarithromycin as CS. The method was applied for the quantification of metoprolol and propranolol enantiomers in pharmaceutical preparations [
59].
Yu et al. applied clarithromycin lactobionate in dual CS systems in combination with 4 neutral derivatized CDs (glucose-β-CD, hydroxyethyl-β-CD, hydroxypropyl-β-CD, methyl-β-CD) for the CE enantio-separation of nefopam. Nefopam was a substance that was only partially resolved in their previous study when clarithromycin lactobionate was used as a single CS [
55]. The effects of CS concentration and BGE pH on enantio-resolution were studied. The use of all four dual CS systems resulted in baseline enantio-resolution (
Figure 8). The separation of another six chiral drugs (atenolol, bisoprolol, esmolol, metoprolol, propranolol, ritodrine) was also evaluated with the four CS dual systems; synergistic effects were detected in all four CS systems. [
60].
Azithromycin is a semisynthetic macrolide containing a 15-membered macrocyclic lactone ring; derived from erythromycin, however, it differs from erythromycin as it contains a methyl-substituted nitrogen atom in the lactone ring. It has two sugar moieties, four hydroxyl groups, a methoxy group, and two tertiary amino groups. It is insoluble in water but quite soluble in alcohols, with low viscosity in these solvents and moderate UV absorption due to the lack of aromatic rings in the structure [
18,
52].
Azithromycin was used by Kumar and Park as CS in NACE for the enantio-separation of six chiral substances (carvedilol, cetirizine, citalopram, darifenacin, sertraline, tryptophan). As BGE, a polar organic mixture of acetonitrile, methanol, acetic acid, and triethylamine was used for enantio-separation. The effects of CS concentration, acetonitrile/methanol ratio, applied voltage, and capillary temperature on enantio-separation were examined. The best results were obtained using a BGE composed of acetonitrile/methanol/acetic acid/triethylamine (80:20:0.1:0.1%,
v/v/v/v) and 4–6% (
w/v) azithromycin as CS [
61].
Lebedeva et al. used azithromycin as CS for the NACE enantio-separation of eight model basic derivatives (chlorpheniramine, doxylamine, hydroxyzine, isoproterenol, methoxyphenamine, synephrine, terbutaline, tetrahydrozoline). The effects of BGE composition, CS concentration, organic solvent type, capillary temperature, and applied voltage on the enantio-separation were studied. Enantio-separation was achieved only for two of the studied analytes, methoxyphenamine and tetrahydrozoline. The best results were obtained when using a BGE composed of 30 mM boric acid, 75 mM tributylamine, and 90 mM azithromycin as CS in methanol. LOD values for tetrahydrozoline were 5 µg/mL for both of the enantiomers. The method was applied to determine tetrahydrozoline in eye drops [
62].
Boromycin is a macrolide containing a stereogenic borate moiety. It is a macrodiolide Böeseken complex (J. Böeseken studied boric acid complexes with polyols containing a
d-valine ester). It contains 16 stereogenic centers and has two hydroxyl groups, each attached to a stereogenic center. It is insoluble in water and soluble in polar organic solvents [
63].
Maier et al. tested boromycin as CS in NACE for the chiral separation of six primary amines (2-amino-1-phenylethanol,
p-hydroxynorephedrine, α-methylbenzylamine, norepinephrine, octopamine, and tryptophanol). The influence of CS concentration, BGE composition and concentration, and organic solvents type on enantio-resolution was established. The best results were obtained when using a BGE composed of 75 mM Tris and 50 mM boric acid in methanol, at pH 9.0 and 20 mM boromycin as CS. With the exception of tryptophanol, which was only partially resolved, practically all of the analytes were baseline separated. Tryptophanol’s low enantio-resolution was interpreted as a result of the stereogenic center’s increased distance from the aromatic moiety when compared with the structure of the rest of the amines [
64].
Gamithromycin is a second-generation macrolide used in veterinary medicine composed of a 15-ring structure with two sugar moieties. It has five hydroxyl groups, two tertiary amino groups, a methoxy group, and a propyl group associated with the N atom at the 7α position [
65].
Ren et al. tested gamithromycin, as a new CS in NACE for the enantio-separation of nine amines (1-aminoindan, amlodipine, ethylamine, α-methylbenzylamine, 1-(4-methoxyphenyl) ethylamine, mexiletine, octapamine, pimaquine, and 1,2,3,4-tetrahydro-1-naphthylamine). The influence of BGE composition, CS concentration, type, and concentration of non-aqueous solvents, and applied voltage on the enantio-resolution was investigated.
N-methylformamide was successfully used as a non-aqueous solvent, alone or in combination with methanol. The best results were obtained when using 100 mM Tris, 125 mM boric acid, and 80 mM CS in a mixture of methanol:
N-methylformamide (25:75). The separation mechanism was also discussed, and it was concluded that enantio-recognition is based on the formation of hydrogen bonds between the CS hydroxyl group and the primary amine of the enantiomers. It is noticeable that gamithromycin exhibited remarkable enantio-selectivity towards amlodipine (resolution over 15) [
65].
2.4. Lincosamides
Lincosamides are a small class of antibiotics that have amino acid and sugar moieties in their chemical composition. Lincomycin, the first native antibiotic of this class isolated from
Streptomyces lincolnensis, can be considered structurally as a 4-propyl-proline derivative; the carboxyl group from proline acylates the amine group of an aminose [
66].
Clindamycin, a semisynthetic derivative of lincomycin, a natural antibiotic produced by
Streptomyces lincolnensis, is the only member of this family that has been used as a CS in CE. Clindamycin chemical structure is presented in
Figure 9. In comparison to other macrocyclic antibiotics, clindamycin phosphate has a high solubility, as well as a low viscosity in water, and a low UV absorption due to the lack of aromatic rings in its structure [
66].
Chen et al. used clindamycin phosphate as CS for the CE separation of eight basic chiral model drugs (atenolol, chlorphenamine, citalopram, metoprolol, nefopam, propranolol, tryptophan, and tryptophan methyl ester). The chiral separation of some acidic drugs was also investigated, without positive results. The influence of CS concentration, BGE pH, organic modifier, capillary temperature, and applied voltage on both migration time and enantio-separation was studied. The best results were obtained using a 40 mM sodium tetraborate BGE at neutral or weak basic pH (7.0–7.6), and a CS concentration of 60 to 80 mM. The organic modifier concentration also influenced the resolution and migration times of analytes, as well as the solubility of the CS; methanol and ethanol, among several organic solvents utilized, allowing the higher resolution. Furthermore, the effects of the analyzed parameters were studied using Statistical Product and Service Solutions [
67].
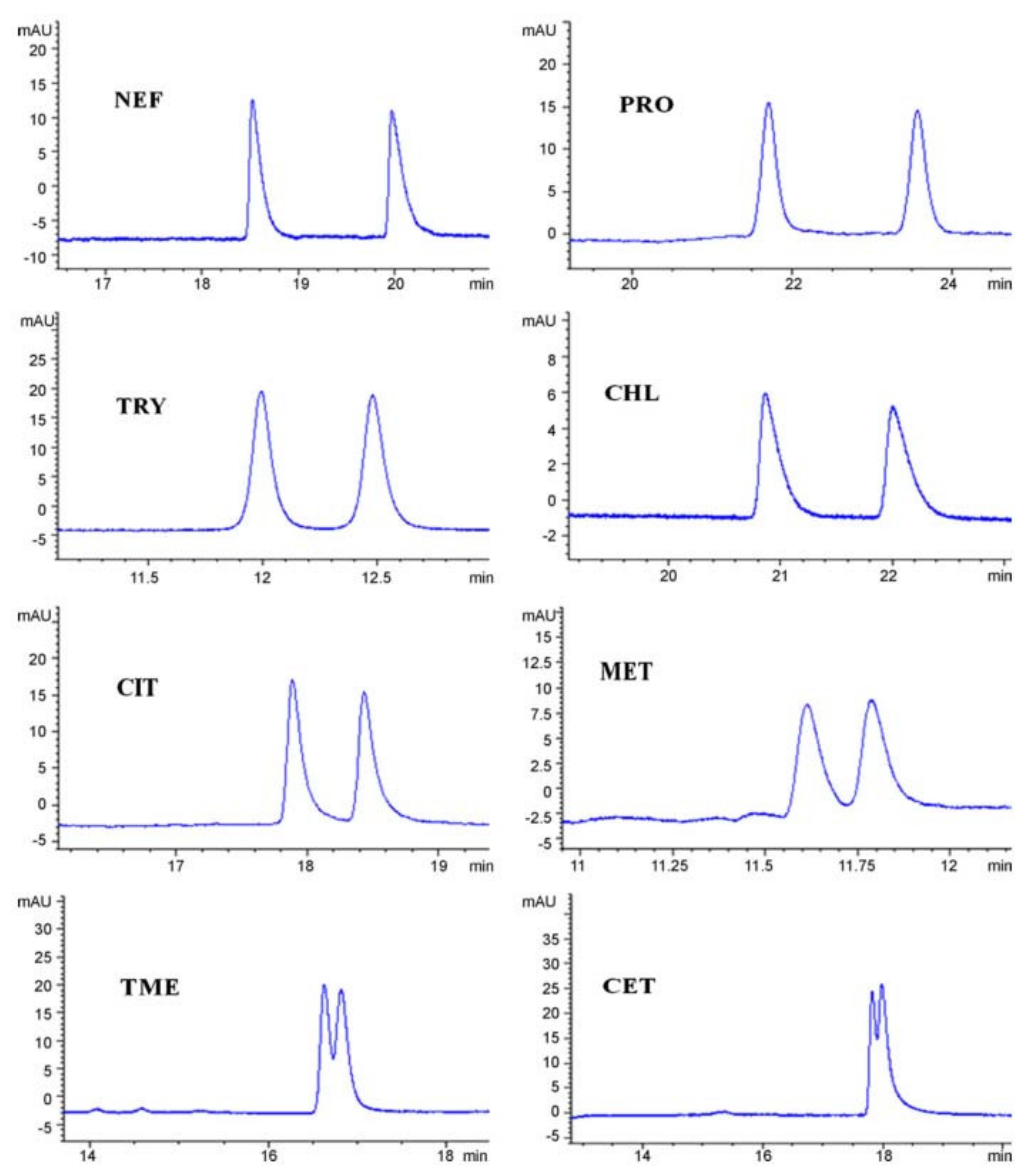
The same research group used clindamycin phosphate as CS in micellar electrokinetic chromatography (MEKC) for the enantio-separation of model basic drugs (cetirizine, chlorphenamine, citalopram, metoprolol, nefopam, tryptophan, tryptophan methyl ester). In MEKC, an anionic surfactant is added to the BGE, generating a “pseudostationary” phase; differential partitioning of analytes between the “pseudostationary” phase and the bulk aqueous phase generating separation. Different types of anionic surfactants, organic additives, and BGE were tested, the best results were achieved using sodium dodecyl sulfate (SDS) as the surfactant, 2-propanol as an organic additive, and phosphate BGE. The influence of BGE pH and concentration, CS concentration, SDS concentration, 2-propanol concentration, and applied voltage on both migration times and enantio-resolution was examined. Satisfactory enantio-separations of the studied analytes were achieved, when using a 40 mM phosphate BGE at a pH range of 7.2–8 containing 40 mM SDS, 25% 2-propanol, and 60 mM or 80 mM clindamycin phosphate as CS (
Figure 10) [
68].
Wu et al. synthetized and used clindamycin succinate as a new CS in CE. The CS was tested on six basic model drugs (chlorphenamine, metoprolol, ofloxacin, propranolol, sotalol, tryptophan). Clindamycin succinate has better solubility in water or organic solvent than clindamycin phosphate, which has been used before for chiral separation of several basic drugs [
67,
68]. The effects of BGE pH, CS concentration, organic modifier, capillary temperature, and applied voltage on the enantio-separation were investigated; it was observed that enantio-resolution was strongly influenced by BGE pH and CS concentration. Good results were obtained when using a 50 mM Tris BGE at pH 4.0 containing 10% methanol and 50 mM clindamycin succinate as CS [
69].
Wu et al. used a copper (II)-clindamycin CS for the enantio-separation of eight model basic drugs (atenolol, bisoprolol, epinephrine, esmolol, metoprolol, propranolol, sotalol, and tropicamide). The effects of the type of metal ion, the ratio of clindamycin and Cu(II), BGE pH, CS concentration, capillary temperature, and applied voltage on the enantio-separation were studied. The best results were obtained when using a BGE composed of 20 mM clindamycin/10 mM Cu
2+ at pH 9.06. The high adsorption of the complex to the capillary inner surface is the fundamental drawback of this chiral separation method [
70].
Ma et al. synthesized and used an ionic liquid (IL) CS based on tetramethylammonium clindamycin phosphate for CE enantio-separation of eight racemic analytes (chloroquine, chlorpheniramine, citalopram, hydroxychloroquine, nefopam, propranolol, tryptophan, tryptophanol). The influence of IL concentration, BGE pH, organic modifier proportion, and applied voltage on the enantio-separation was studied. The results were compared with the ones obtained with another IL CS, based on clindamycin phosphate, to investigate the influence of cation structure on the enantio-separation. Compared to native clindamycin phosphate, the results demonstrated that the tetramethylammonium clindamycin phosphate IL was superior as CS. In addition, molecular modeling was used to investigate the chiral recognition process of the IL CS, which indicated that the associated form of the IL has an important role in the enantio-separation. Moreover, the enantiomeric migration order could be predicted based on the calculated binding free energies of the enantiomers. This was the first time that an antibiotic-based IL CS has been used as the single CS in CE [
71].
Xu et al. synthetized an IL CS containing cholinium-clindamycin phosphate and employed it as a sole CS in CE. The CS was tested on five model drugs (chlorpheniramine, citalopram, nefopam, propranolol, tryptophan). The influence of BGE pH, CS concentration, organic modifier, and applied voltage on the enantio-separation was verified. The IL selector demonstrated better enantio-separation capabilities and enhanced peak shapes under optimized conditions compared to clindamycin phosphate. The best results were obtained when using a 40 mM Tris BGE at pH 8.0 containing 20–40% methanol and 60 mM cholinium-clindamycin phosphate as CS. Molecular docking was employed in elucidating the chiral recognition process. Both the calculated free binding energies and the identified enantiomer-CS interactions indicated greater enantiomer affinities and enantioselectivity in the case of the IL CS in comparison to clindamycin. The computing results were in accordance with the experimental results [
72].
2.6. Other Antibiotics
Benzylpenicillin (Penicillin G) is a natural β-lactam antibiotic. It has three chiral centers, a carboxylic group, and two amide groups. Low stability in acidic and basic environments represents one of the drawbacks of benzylpenicillin [
75].
Benzylpenicillin potassium salt was tested as ion-pair CS in CE by Dixit and Park for the chiral separation of five basic drugs (citalopram, darifenacin, metoprolol, propranolol, and sertraline). The effects of BGE composition, capillary temperature, and applied voltage on the enantio-separation were evaluated. The addition of methanol in the BGE was essential to obtain enantio-resolution. The best results were obtained when using a BGE composed of water:methanol (90:10,
v/v) and 10.7 or 16.1 mM benzylpenicillin as CS. The analytes were measured using indirect UV spectrophotometric detection, due to the strong UV absorption of benzylpenicillin. The stereoselective formation of diastereomeric ion-pairs between the negatively charged carboxylate group of the CS and the protonated amino group of the basic drugs was credited for benzylpenicillin enantio-separation ability [
75].
Doxycycline is a synthetic oxytetracycline derivative. It has six chiral centers, five hydroxyl groups, two carbonyl groups, and an amide group in its structure [
76].
Jang et al. evaluated doxycycline as CS in NACE for the enantio-separation of 10 acidic model substances (carprofen, flurbiprofen, ibuprofen, ketoprofen, mandelic acid, suprofen, tropic acid, warfarin, and two amino acids:
N-(3,5-dinitrobenzoyl)-leucine,
N-(3,5-dinitrobenzoyl)-phenylglycine). The effects of CS concentration, BGE composition (containing acetonitrile/acetic acid/methanol/triethylamine), organic solvents (acetonitrile/methanol ratio, acetic acid, and triethylamine concentration), and applied voltage on the enantio-separation were studied. The best results were obtained when using a BGE composed of acetonitrile:methanol (50:50), 214 mM acetic acid, 63 mM triethylamine, and 38 mM doxycycline as CS. Because of doxycycline UV light absorption, indirect detection was used; this mode of detection monitors a small difference between two relatively big absorbance signals, resulting in high baseline noises, which is problematic in quantitative studies [
76].
Fusidic acid is a steroid type of topic antibiotic isolated from the fungus
Fusidium coccineum. It has a steroid-like nucleus, 10 stereogenic centers, 2 hydroxyl groups, an acetyloxy group, and a carboxyl group. It has a pKa of 5.35, a neutral or basic BGE would be preferable for its CE use, as the ionization of the carboxyl group might enable complexation between fusidic acid and enantiomers through electrostatic interactions. It is insoluble in water (its sodium salt is soluble in water) but soluble in organic solvents [
77].
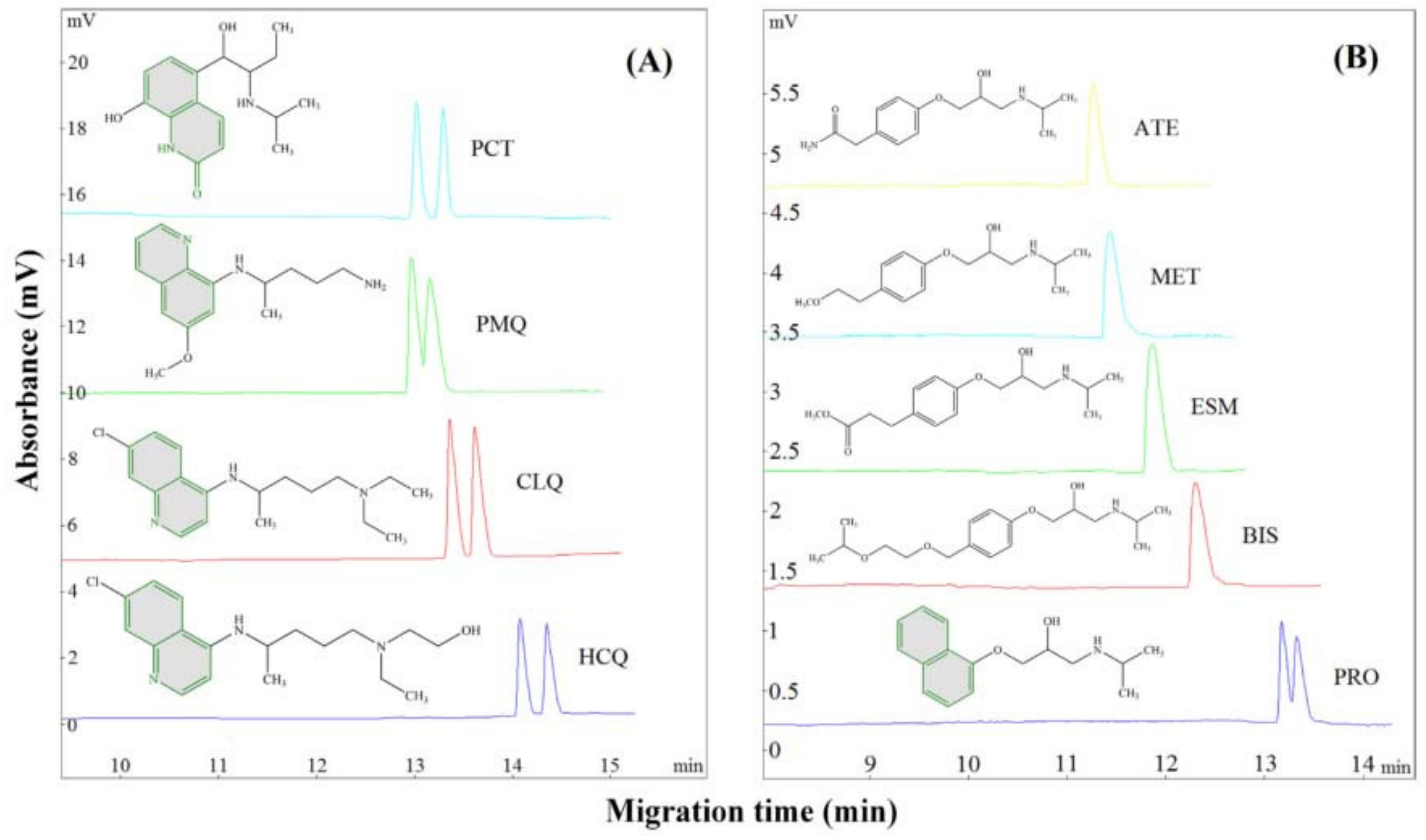
Zhang et al. tested fusidic acid as a CS in CE, using as model molecules quinolone derivatives (chloroquine, hydroxychloroquine, and primaquine) and β-blockers (atenolol, bisoprolol, esmolol, metoprolol, and propranolol). The effects of BGE pH and concentration, CS concentration, organic modifier type and concentration on the enantio-resolution were studied. The best results were obtained with 50 mM phosphate buffer at pH 8.0 containing 60–70 mM CS and 50% methanol. Fusidic acid is particularly well suited to chiral analytes with a stiff planar structure (e.g., quinolone ring), according to a preliminary investigation of the separation process. One possible explanation is that the enantiomers with a rigid planar structure can induce a strong steric hindrance effect when interacting with the unique chair-boat-chair conformation fusidic acid. Among the five β-blockers, only propranolol contains a rigid planar structure (because of its naphthyloxy group); as expected, split peaks were obtained only in the case of propranolol using fusidic acid as CS (
Figure 11) [
77].