Characterization of Volatile Profiles and Correlated Contributing Compounds in Pan-Fried Steaks from Different Chinese Yellow Cattle Breeds through GC-Q-Orbitrap, E-Nose, and Sensory Evaluation
Abstract
:1. Introduction
2. Results and Discussion
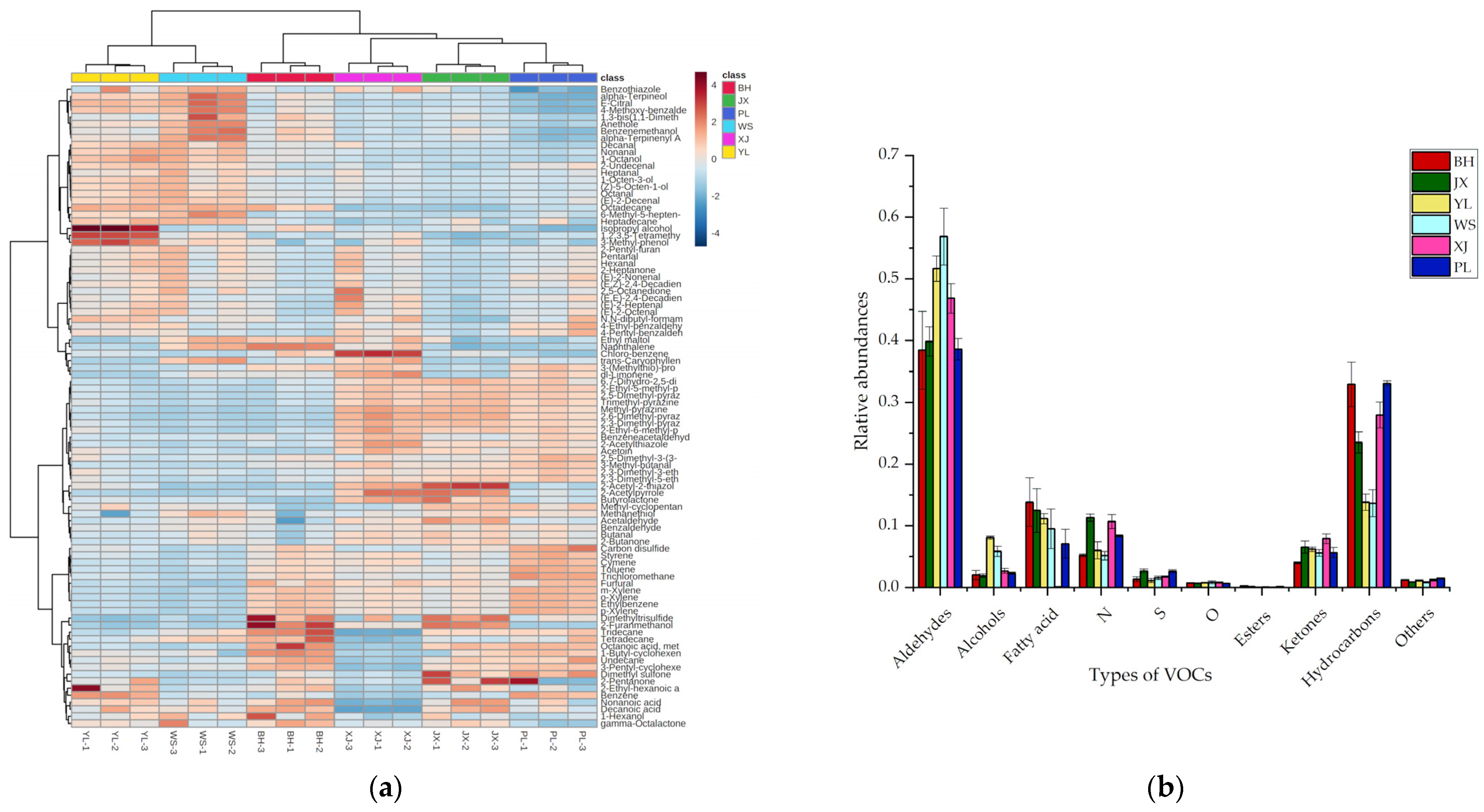
2.1. Volatile Profiles of Pan-Fried Steaks Obtained by GC-Q-Orbitrap
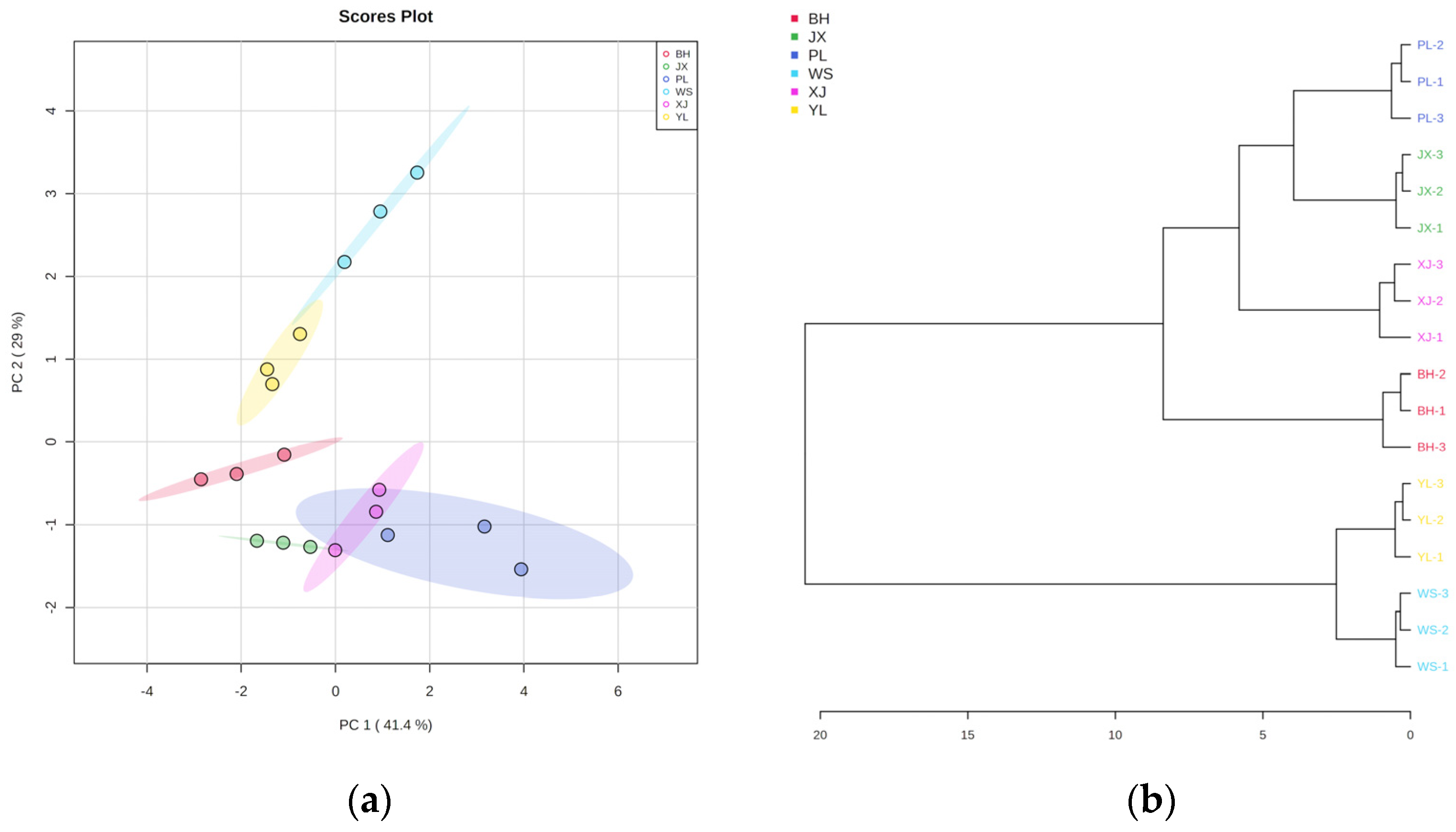
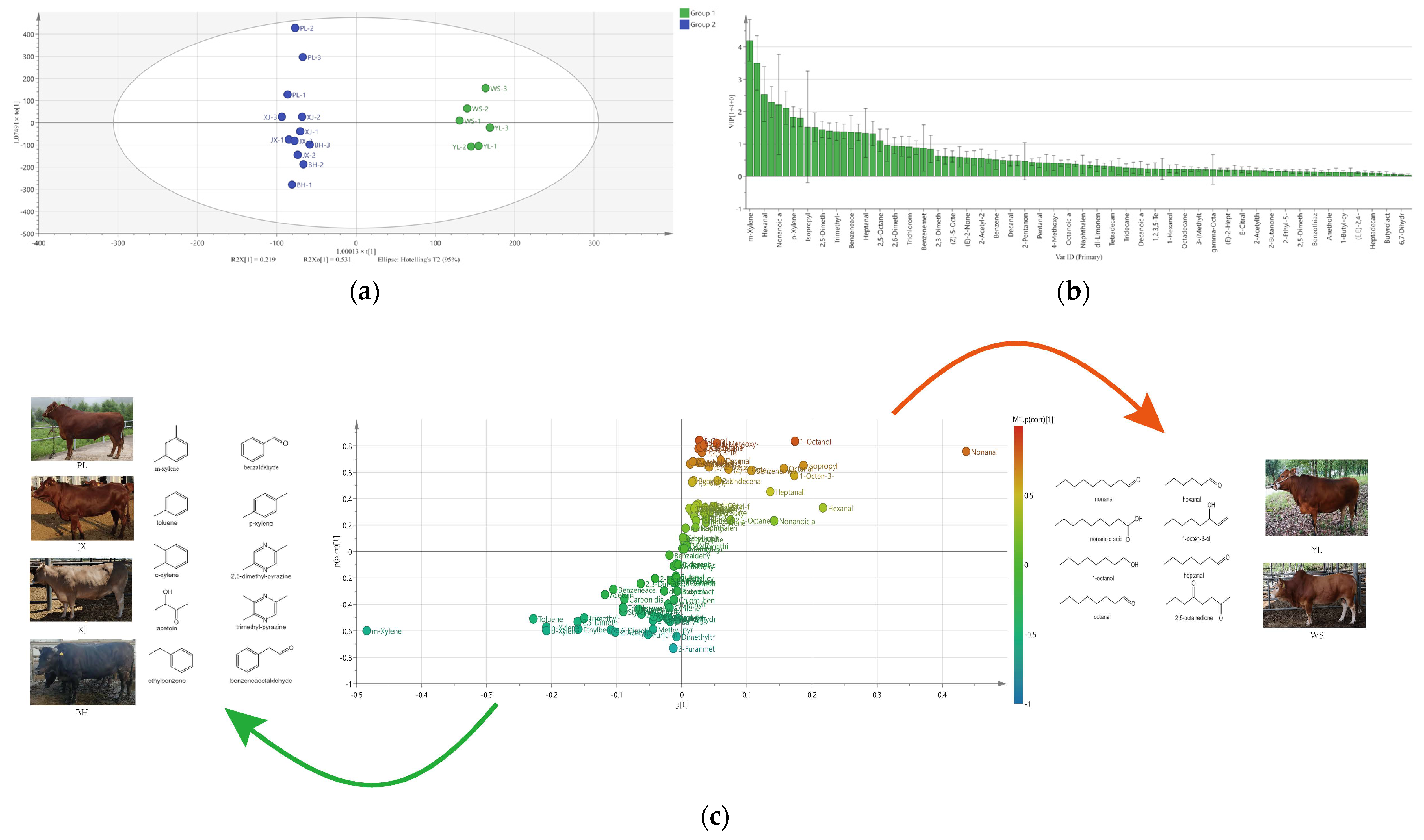
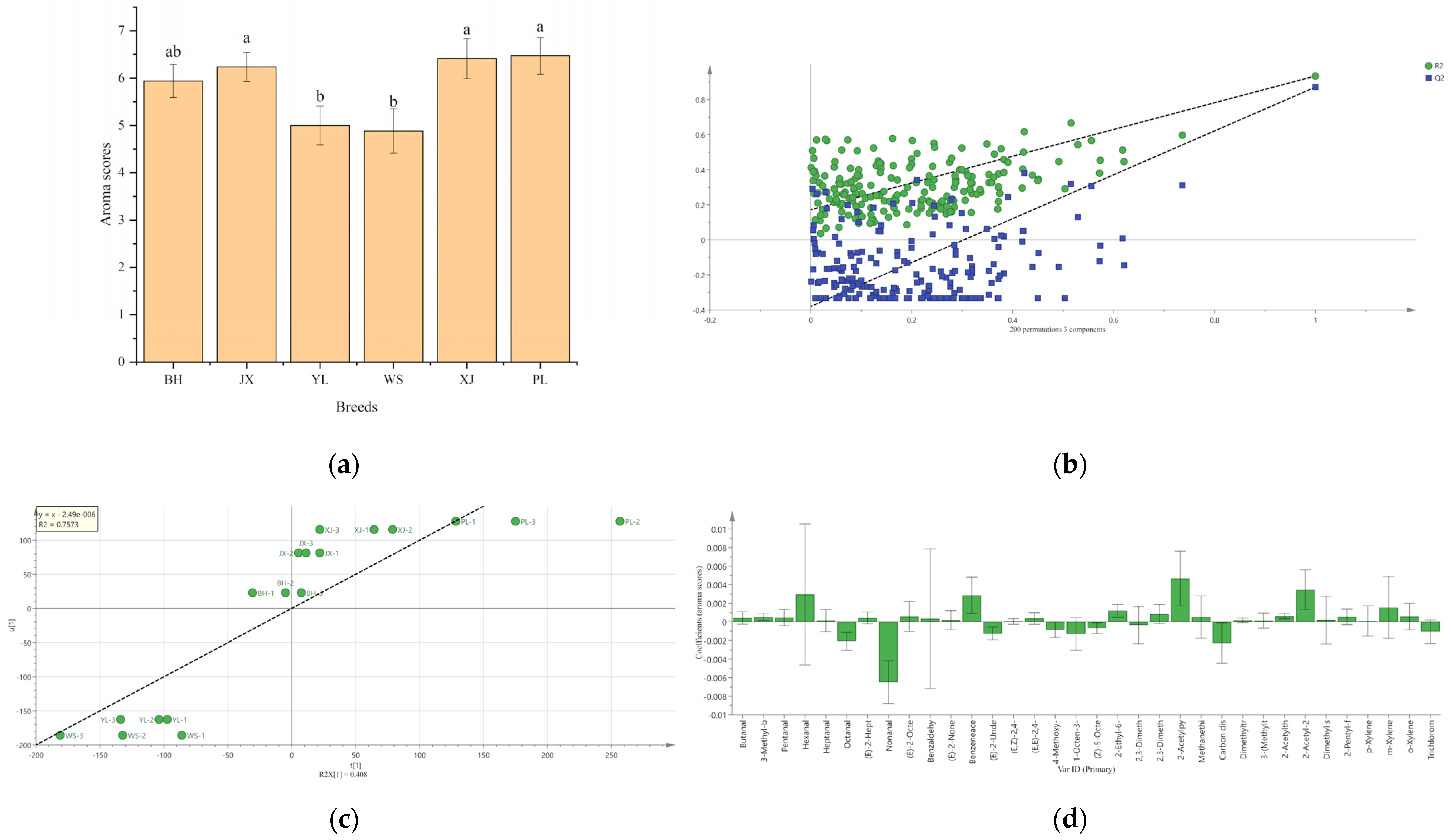
2.2. Discrimination of Pan-Fried Steaks by PCA, HCA, and OPLS-DA Analysis
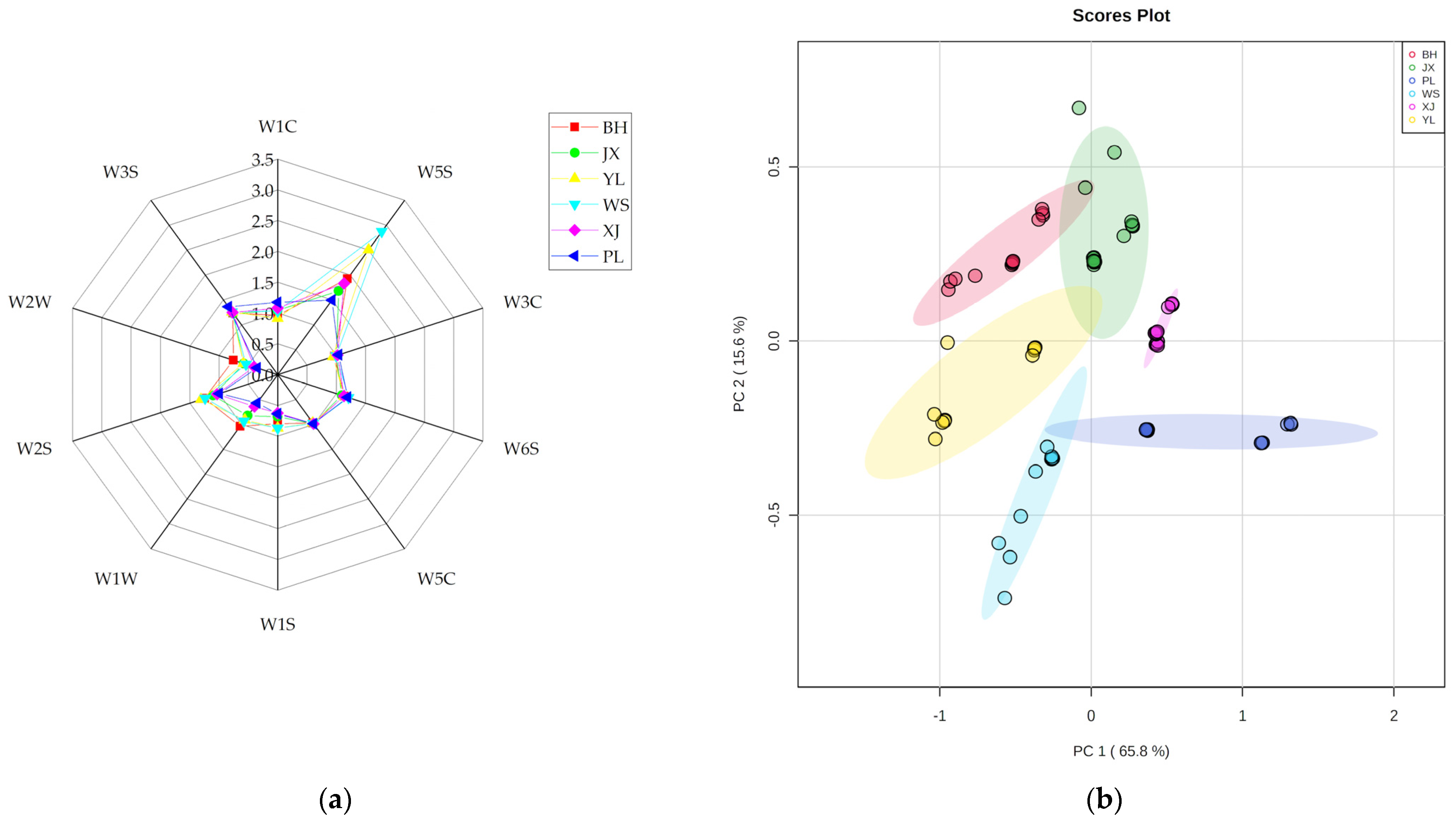
2.3. Volatile Profiling of Pan-Fried Steaks Obtained by E-Nose
2.4. PLSR Analysis between Beef Aroma Intensities and Odor-Active Volatiles
2.5. The Difference and Preference Testing for Recombination Samples
3. Materials and Methods
3.1. Chemicals
3.2. Samples Preparation
3.3. GC-Q-Orbitrap Measurement
3.4. E-Nose Measurement
3.5. Sensory Evaluation
3.5.1. Evaluation of Aroma Intensities
3.5.2. The Difference and Preference Testing for Recombination
3.6. Statistical Analysis
4. Conclusions, Limitations, and Future Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Notice on the Publication of the National list of Breeds of Livestock and Poultry Genetic Resources (2021 Edition). Available online: http://www.moa.gov.cn/govpublic/nybzzj1/202101/t20210114_6359937.htm (accessed on 12 August 2021).
- O’Quinn, T.G.; Legako, J.F.; Brooks, J.C.; Miller, M.F. Evaluation of the contribution of tenderness, juiciness, and flavor to the overall consumer beef eating experience. Transl. Anim. Sci. 2018, 2, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Ueda, S.; Yamanoue, M.; Sirai, Y.; Iwamoto, E. Exploring the Characteristic Aroma of Beef from Japanese Black Cattle (Japanese Wagyu) via Sensory Evaluation and Gas Chromatography-Olfactometry. Metabolites 2021, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Ball, A.; Hughes, J.; Krishnamurthy, R.; Piyasiri, U.; Stark, J.; Watkins, P.; Warner, R. Sensory and Flavor Chemistry Characteristics of Australian Beef: Influence of Intramuscular Fat, Feed, and Breed. J. Agric. Food Chem. 2016, 64, 4299–4311. [Google Scholar] [CrossRef] [PubMed]
- Gorraiz, C.; Beriain, M.J.; Chasco, J.; Insausti, K. Effect of Aging Time on Volatile Compounds, Odor, and Flavor of Cooked Beef from Pirenaica and Friesian Bulls and Heifers. J. Food Sci. 2002, 67, 916–922. [Google Scholar] [CrossRef]
- Piao, M.Y.; Lee, H.J.; Yong, H.I.; Beak, S.; Kim, H.J.; Jo, C.; Wiryawan, K.G.; Baik, M. Comparison of reducing sugar content, sensory traits, and fatty acids and volatile compound profiles of the longissimus thoracis among Korean cattle, Holsteins, and Angus steers. Asian Austral. J. Anim. 2019, 32, 126–136. [Google Scholar] [CrossRef]
- Specht, K.; Baltes, W. Identification of Volatile Flavor Compounds with High Aroma Values from Shallow-Fried Beef. J. Agric. Food Chem. 1994, 42, 2246–2253. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit Rev. Food Sci. Nutr. 2005, 45, 49–59. [Google Scholar] [CrossRef]
- Sohail, A.; Al-Dalali, S.; Wang, J.; Xie, J.; Shakoor, A.; Asimi, S.; Shah, H.; Patil, P. Aroma compounds identified in cooked meat: A review. Food Res. Int. 2022, 111385. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Hayat, K.; Duhoranimana, E.; Zhang, X.; Xia, S.; Yu, J.; Xing, F. Characterization of odor-active compounds of chicken broth and improved flavor by thermal modulation in electrical stewpots. Food Res. Int. 2018, 109, 72–81. [Google Scholar] [CrossRef]
- Dubrow, G.A.; Forero, D.P.; Peterson, D.G. Identification of volatile compounds correlated with consumer acceptability of strawberry preserves: Untargeted GC–MS analysis. Food Chem. 2022, 378, 132043. [Google Scholar] [CrossRef]
- Guo, C.; Gong, L.; Wang, W.; Leng, J.; Zhou, L.; Xing, S.; Zhao, Y.; Xian, R.; Zhang, X.; Shi, F. Rapid screening and identification of targeted or non-targeted antitussive adulterants in herbal medicines by Q-Orbitrap HRMS and screening database. Int. J. Mass Spectrom. 2020, 447, 116250. [Google Scholar] [CrossRef]
- Weidt, S.; Haggarty, J.; Kean, R.; Cojocariu, C.I.; Silcock, P.J.; Rajendran, R.; Ramage, G.; Burgess, K.E. A novel targeted/untargeted GC-Orbitrap metabolomics methodology applied to Candida albicans and Staphylococcus aureus biofilms. Metabolomics 2016, 12, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belarbi, S.; Vivier, M.; Zaghouani, W.; Sloovere, A.D.; Agasse-Peulon, V.; Cardinael, P. Comparison of new approach of GC-HRMS (Q-Orbitrap) to GC–MS/MS (triple-quadrupole) in analyzing the pesticide residues and contaminants in complex food matrices. Food Chem. 2021, 359, 129932. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hui, T.; Zheng, X.; Li, S.; Wei, X.; Li, P.; Zhang, D.; Wang, Z. Characterization of key lipids for binding and generating aroma compounds in roasted mutton by UPLC-ESI-MS/MS and Orbitrap Exploris GC. Food Chem. 2022, 374, 131723. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, M.; Adhikari, B. Advances of electronic nose and its application in fresh foods: A review. Crit Rev. Food Sci. Nutr. 2018, 58, 2700–2710. [Google Scholar] [CrossRef]
- Jin, Y.; Cui, H.; Yuan, X.; Liu, L.; Liu, X.; Wang, Y.; Ding, J.; Xiang, H.; Zhang, X.; Liu, J.; et al. Identification of the main aroma compounds in Chinese local chicken high-quality meat. Food Chem. 2021, 359, 129930. [Google Scholar] [CrossRef]
- Kerth, C. Determination of volatile aroma compounds in beef using differences in steak thickness and cook surface temperature. Meat Sci. 2016, 117, 27–35. [Google Scholar] [CrossRef]
- Wall, K.R.; Kerth, C.R.; Miller, R.K.; Alvarado, C. Grilling temperature effects on tenderness, juiciness, flavor and volatile aroma compounds of aged ribeye, strip loin, and top sirloin steaks. Meat Sci. 2019, 150, 141–148. [Google Scholar] [CrossRef]
- Ba, H.V.; Park, K.; Dashmaa, D.; Hwang, I. Effect of muscle type and vacuum chiller ageing period on the chemical compositions, meat quality, sensory attributes and volatile compounds of Korean native cattle beef. Anim. Sci. J. 2014, 85, 164–173. [Google Scholar] [CrossRef]
- Calkins, C.R.; Hodgen, J.M. A fresh look at meat flavor. Meat Sci. 2007, 77, 63–80. [Google Scholar] [CrossRef]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Influence of thermal treatment on formation of volatile compounds, cooking loss and lipid oxidation in foal meat. LWT Food Sci. Technol. 2014, 58, 439–445. [Google Scholar] [CrossRef]
- Ma, Q.L.; Hamid, N.; Bekhit, A.E.D.; Robertson, J.; Law, T.F. Evaluation of pre-rigor injection of beef with proteases on cooked meat volatile profile after 1day and 21days post-mortem storage. Meat Sci. 2012, 92, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Bassam, S.M.; Noleto-Dias, C.; Farag, M.A. Dissecting grilled red and white meat flavor: Its characteristics, production mechanisms, influencing factors and chemical hazards. Food Chem. 2022, 371, 131139. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.B.; Kemp, R.; Samuelsson, L.M. Effects of dry-aging on meat quality attributes and metabolite profiles of beef loins. Meat Sci. 2016, 111, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Kamada, G.; Imanari, M.; Shiba, N.; Yonai, M.; Muramoto, T. Effect of aging on volatile compounds in cooked beef. Meat Sci. 2015, 107, 12–19. [Google Scholar] [CrossRef]
- Setyabrata, D.; Cooper, B.R.; Sobreira, T.J.P.; Legako, J.F.; Martini, S.; Kim, Y.H.B. Elucidating mechanisms involved in flavor generation of dry-aged beef loins using metabolomics approach. Food Res. Int. 2021, 139, 109969. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Dashdorj, D.; Amna, T.; Hwang, I. Influence of specific taste-active components on meat flavor as affected by intrinsic and extrinsic factors: An overview. Eur. Food Res. Technol. 2015, 241, 157–171. [Google Scholar] [CrossRef]
- Flores, M. Understanding the implications of current health trends on the aroma of wet and dry cured meat products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef]
- Rochat, S.; Laumer, J.D.S.; Chaintreau, A. Analysis of sulfur compounds from the in-oven roast beef aroma by comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2007, 1147, 85–94. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Guo, Y.; Luo, X.; Du, M.; Dong, L.; Yu, P.; Xu, X. Reinvestigation of 2-acetylthiazole formation pathways in the Maillard reaction. Food Chem. 2021, 345, 128761. [Google Scholar] [CrossRef]
- Purriños, L.; Franco, D.; Carballo, J.; Lorenzo, J.M. Influence of the salting time on volatile compounds during the manufacture of dry-cured pork shoulder “lacón”. Meat Sci. 2012, 92, 627–634. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, G.; Coppock, B.M. A comparison of the chemical composition of boiled and roasted aromas of heated beef. J. Agr. Food Chem. 1977, 25, 113–117. [Google Scholar] [CrossRef]
- Muriel, E.; Antequera, T.; Petron, M.J.; Andres, A.I.; Ruiz, J. Volatile compounds in Iberian dry-cured loin. Meat Sci. 2004, 68, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Plagemann, I.; Zelena, K.; Krings, U.; Berger, R.G. Volatile flavours in raw egg yolk of hens fed on different diets. J. Sci. Food Agr. 2011, 91, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.; Song, H. Variation of aroma components during frozen storage of cooked beef balls by SPME and SAFE coupled with GC-O-MS. J. Food Process. Preserv. 2021, 45, e15036. [Google Scholar] [CrossRef]
- Li, Z.; Ha, M.; Frank, D.; Mcgilchrist, P.; Warner, R.D. Volatile Profile of Dry and Wet Aged Beef Loin and Its Relationship with Consumer Flavour Liking. Foods 2021, 10, 3113. [Google Scholar] [CrossRef]
- Li, J.; Tang, C.; Zhao, Q.; Yang, Y.; Li, F.; Qin, Y.; Liu, X.; Yue, X.; Zhang, J. Integrated lipidomics and targeted metabolomics analyses reveal changes in flavor precursors in psoas major muscle of castrated lambs. Food Chem. 2020, 333, 127451. [Google Scholar] [CrossRef]
- Legako, J.F.; Dinh, T.T.N.; Miller, M.F.; Brooks, J.C. Effects of USDA beef quality grade and cooking on fatty acid composition of neutral and polar lipid fractions. Meat Sci. 2015, 100, 246–255. [Google Scholar] [CrossRef]
- Zhang, G.X.; Wang, Z.G.; Chen, W.S.; Wu, C.X.; Han, X.; Chang, H.; Zan, L.S.; Li, R.L.; Wang, J.H.; Song, W.T.; et al. Genetic diversity and population structure of indigenous yellow cattle breeds of China using 30 microsatellite markers. Anim. Genet. 2007, 38, 550–559. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Li, C.; Ye, H.; Wang, Z.; Wu, X.; Xu, B. Evolution of Volatile Compounds and Spoilage Bacteria in Smoked Bacon during Refrigeration Using an E-Nose and GC-MS Combined with Partial Least Squares Regression. Molecules 2018, 23, 3286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, S.; Wang, Y.; Luo, R.; Ding, D.; Bai, H.; Shen, F. Characterization of flavor volatile compounds in industrial stir-frying mutton sao zi by GC-MS, E-nose, and physicochemical analysis. Food Sci. Nutr. 2021, 9, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Zhang, C.; Fauconnier, M.; Mi, S. Characterization and differentiation of boiled pork from Tibetan, Sanmenxia and Duroc × (Landrac × Yorkshire) pigs by volatiles profiling and chemometrics analysis. Food Res. Int. 2020, 130, 108910. [Google Scholar] [CrossRef] [PubMed]
- Cerny, C.; Grosch, W. Evaluation of potent odorants in roasted beef by aroma extract dilution analysis. Zeitschrift für Lebensmittel-Untersuchung und Forschung 1992, 194, 322–325. [Google Scholar] [CrossRef]
- Gao, L.; Liu, T.; An, X.; Zhang, J.; Ma, X.; Cui, J. Analysis of volatile flavor compounds influencing Chinese-type soy sauces using GC–MS combined with HS-SPME and discrimination with electronic nose. J. Food Sci. Technol. 2017, 54, 130–143. [Google Scholar] [CrossRef]
- Dikeman, M.E.; Obuz, E.; Gök, V.; Akkaya, L.; Stroda, S. Effects of dry, vacuum, and special bag aging; USDA quality grade; and end-point temperature on yields and eating quality of beef Longissimus lumborum steaks. Meat Sci. 2013, 94, 228–233. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Y.P.; Deng, S.; Li, C.; Xu, X.; Zhou, G.; Liu, Y. Application of sensory evaluation, GC-ToF-MS, and E-nose to discriminate the flavor differences among five distinct parts of the Chinese blanched chicken. Food Res. Int. 2020, 137, 109669. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, M.; Liu, X.; Xie, P.; Lei, Y.; Yu, H.; Han, A.; Xie, L.; Jia, H.; Lin, S.; Bai, Y.; et al. Characterization of Volatile Profiles and Correlated Contributing Compounds in Pan-Fried Steaks from Different Chinese Yellow Cattle Breeds through GC-Q-Orbitrap, E-Nose, and Sensory Evaluation. Molecules 2022, 27, 3593. https://doi.org/10.3390/molecules27113593
Wei M, Liu X, Xie P, Lei Y, Yu H, Han A, Xie L, Jia H, Lin S, Bai Y, et al. Characterization of Volatile Profiles and Correlated Contributing Compounds in Pan-Fried Steaks from Different Chinese Yellow Cattle Breeds through GC-Q-Orbitrap, E-Nose, and Sensory Evaluation. Molecules. 2022; 27(11):3593. https://doi.org/10.3390/molecules27113593
Chicago/Turabian StyleWei, Meng, Xiaochang Liu, Peng Xie, Yuanhua Lei, Haojie Yu, Aiyun Han, Libin Xie, Hongliang Jia, Shaohua Lin, Yueyu Bai, and et al. 2022. "Characterization of Volatile Profiles and Correlated Contributing Compounds in Pan-Fried Steaks from Different Chinese Yellow Cattle Breeds through GC-Q-Orbitrap, E-Nose, and Sensory Evaluation" Molecules 27, no. 11: 3593. https://doi.org/10.3390/molecules27113593
APA StyleWei, M., Liu, X., Xie, P., Lei, Y., Yu, H., Han, A., Xie, L., Jia, H., Lin, S., Bai, Y., Sun, B., & Zhang, S. (2022). Characterization of Volatile Profiles and Correlated Contributing Compounds in Pan-Fried Steaks from Different Chinese Yellow Cattle Breeds through GC-Q-Orbitrap, E-Nose, and Sensory Evaluation. Molecules, 27(11), 3593. https://doi.org/10.3390/molecules27113593





