Gold Nanoparticles as Effective ion Traps in Poly(dimethylsiloxane) Cross-Linked by Metal-Ligand Coordination
Abstract
:1. Introduction
2. Materials
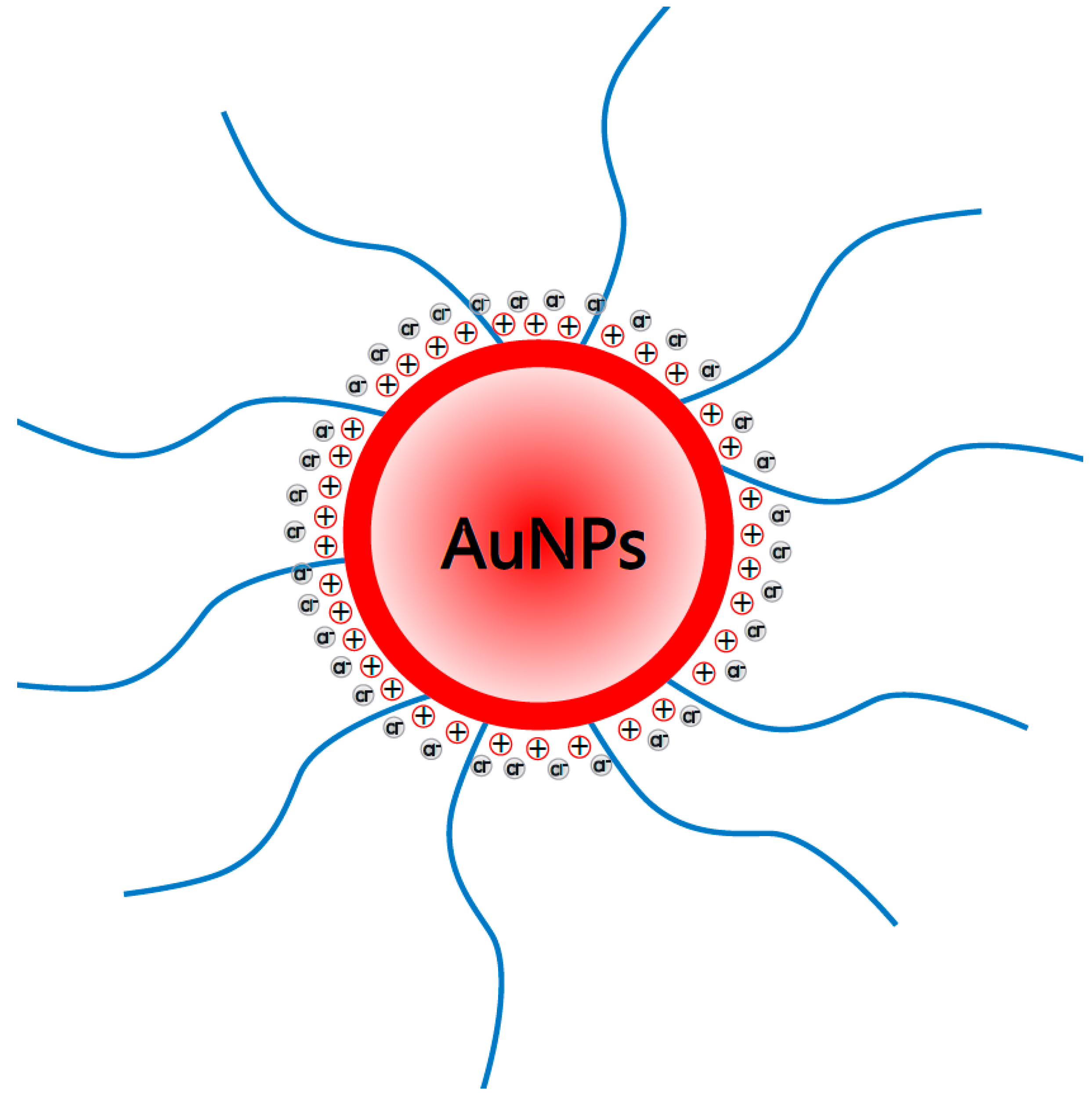
2.1. Synthesis, Functionalization, and Characterization of the Nanoparticles
2.2. Synthesis Poly(dimethylsiloxane) Cross-Linked by Metal-Ligand Coordination with the Addition of AuNPs
3. Techniques
3.1. Transmission Electron Microscopy (TEM)
3.2. Dynamic Light Scattering (DLS)
3.3. Broadband Dielectric Spectroscopy (BDS)
- -
- Relative permittivity (ε′) that is part of complex dielectric function ε* defined by:where ε ′(ω) is the real part and ε ″(ω) the imaginary part of the complex dielectric function, i = and ω denotes angular frequency.ε* (ω) = ε′ (ω) − iε″ (ω)
- -
- Tangent delta called loss tangent, which is described as tangents of the phase angle between ε′(ω) and ε″(ω)
- -
- Real part of conductivity (σ′)where is the dielectric permittivity of vacuum ( = 8.854 × 10−12 As·V−1·m−1).
- -
- Imaginary part of modulus (M″)
- -
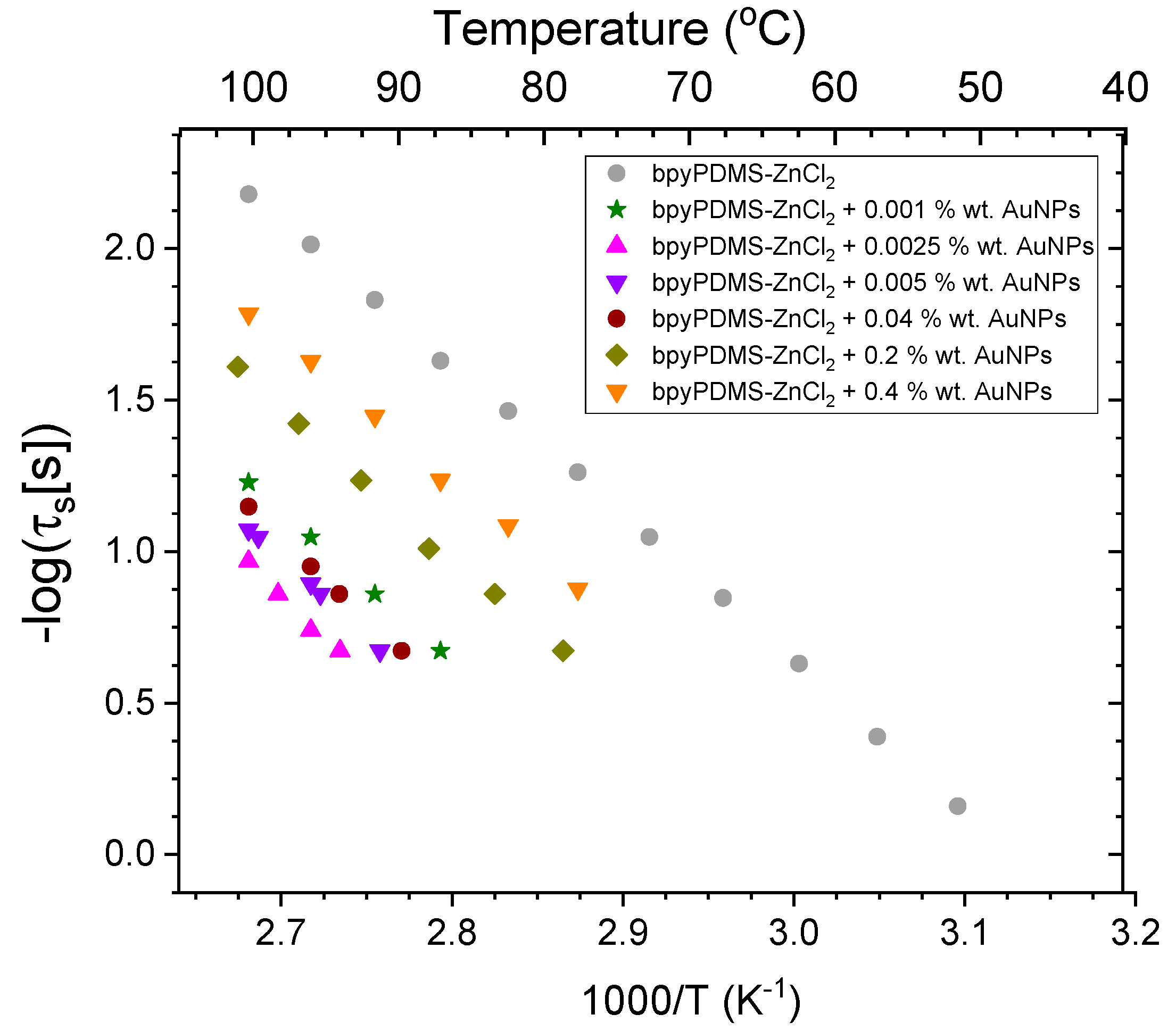
- Additionally, Arrhenius plots of conductivity relaxation times (τc) can be defined as follows:where τ0 denotes pro-exponential factor, EA denotes activation energy, kB denotes Boltzmann constant, and T denotes absolute temperature.
4. Results and Discussion
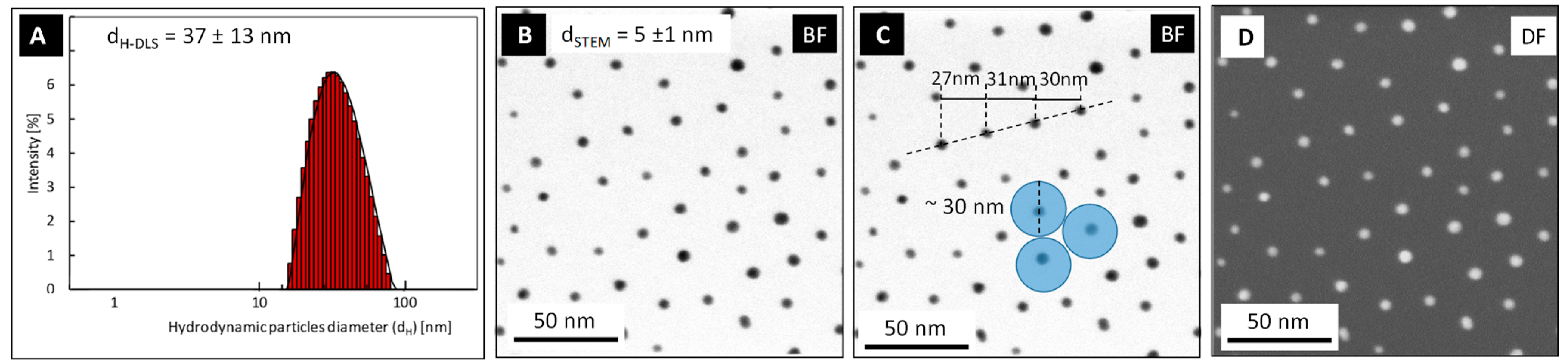
4.1. AuNPs Characterization by DLS and HR-STEM
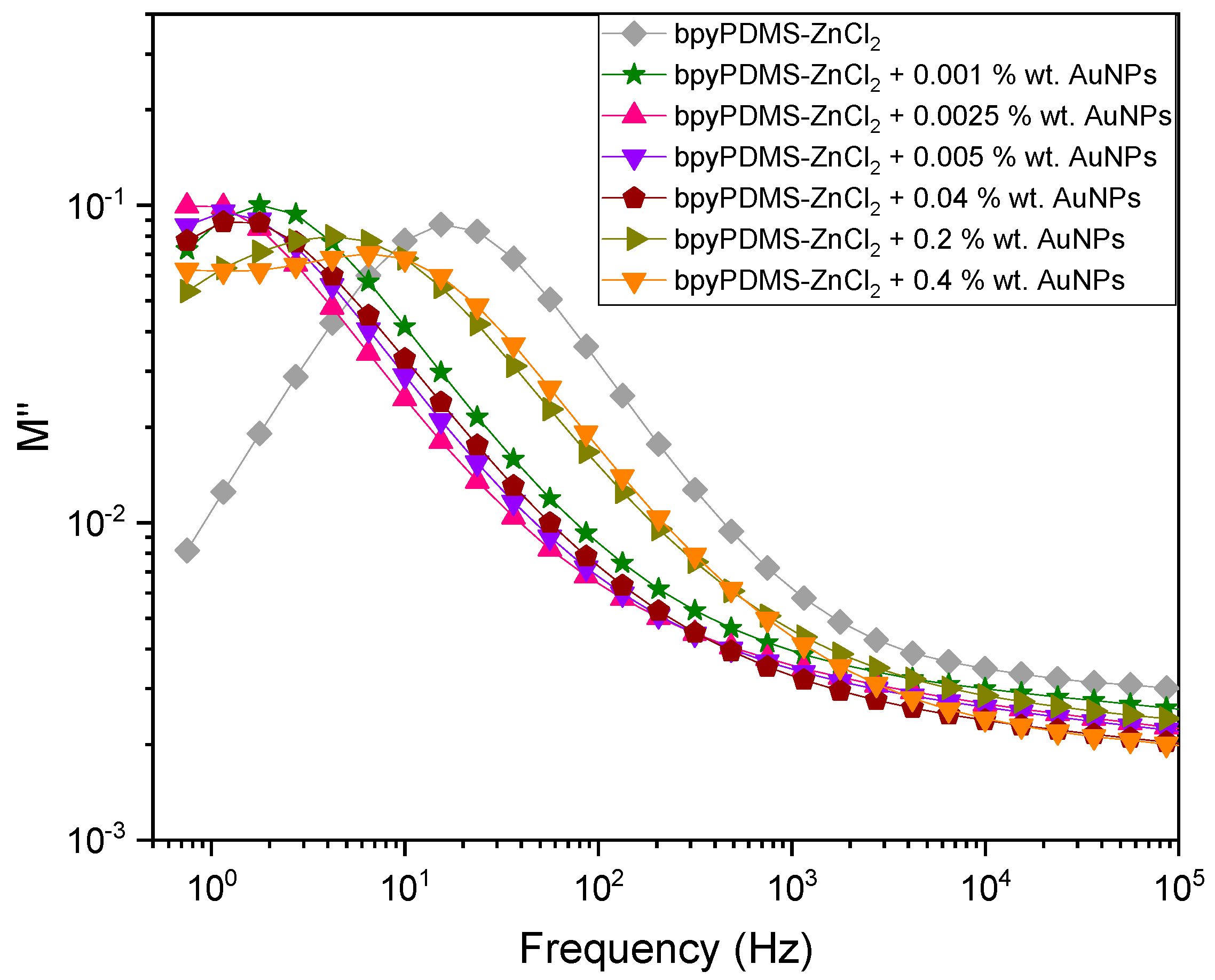
4.2. Broadband Dielectric Spectroscopy
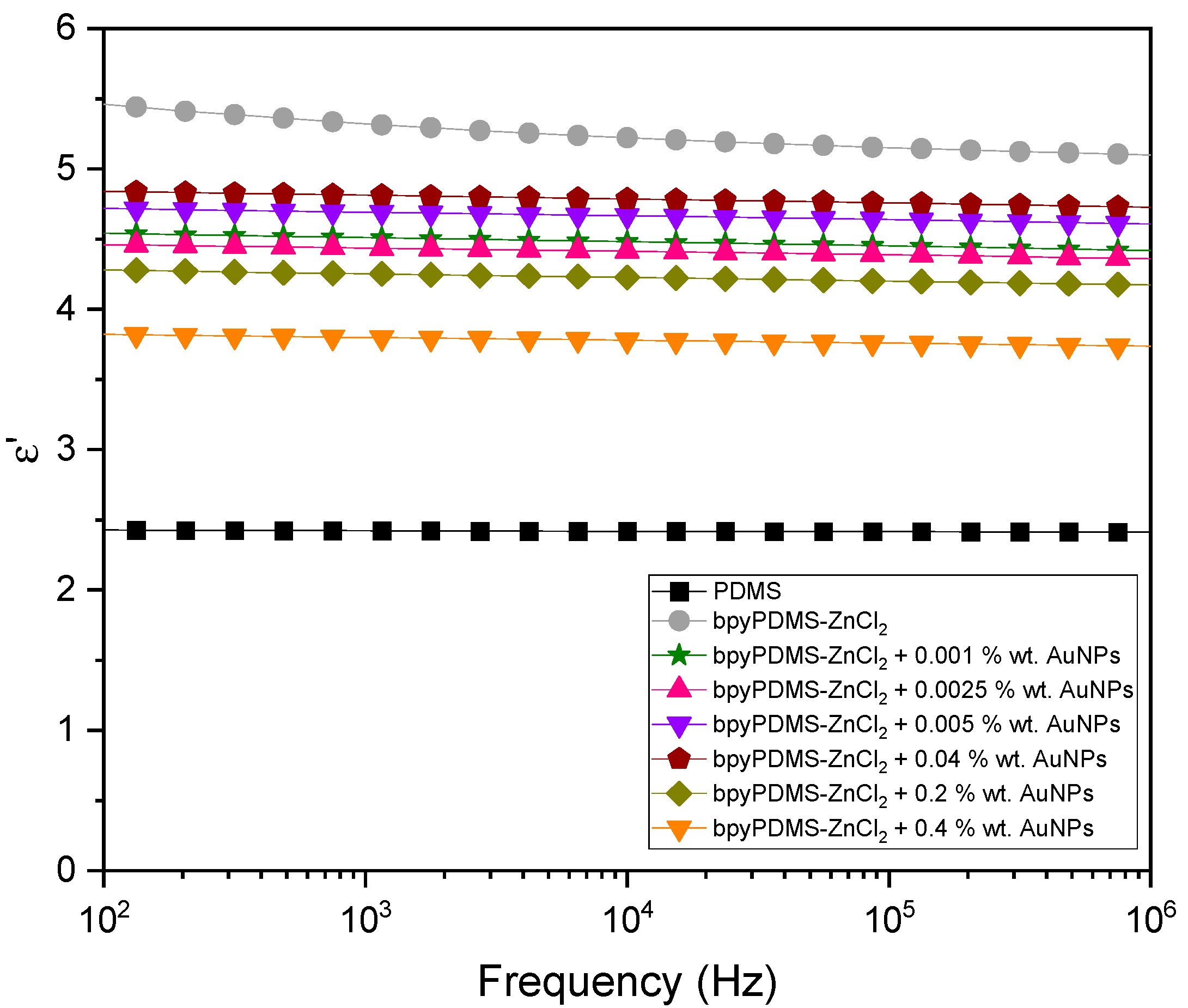
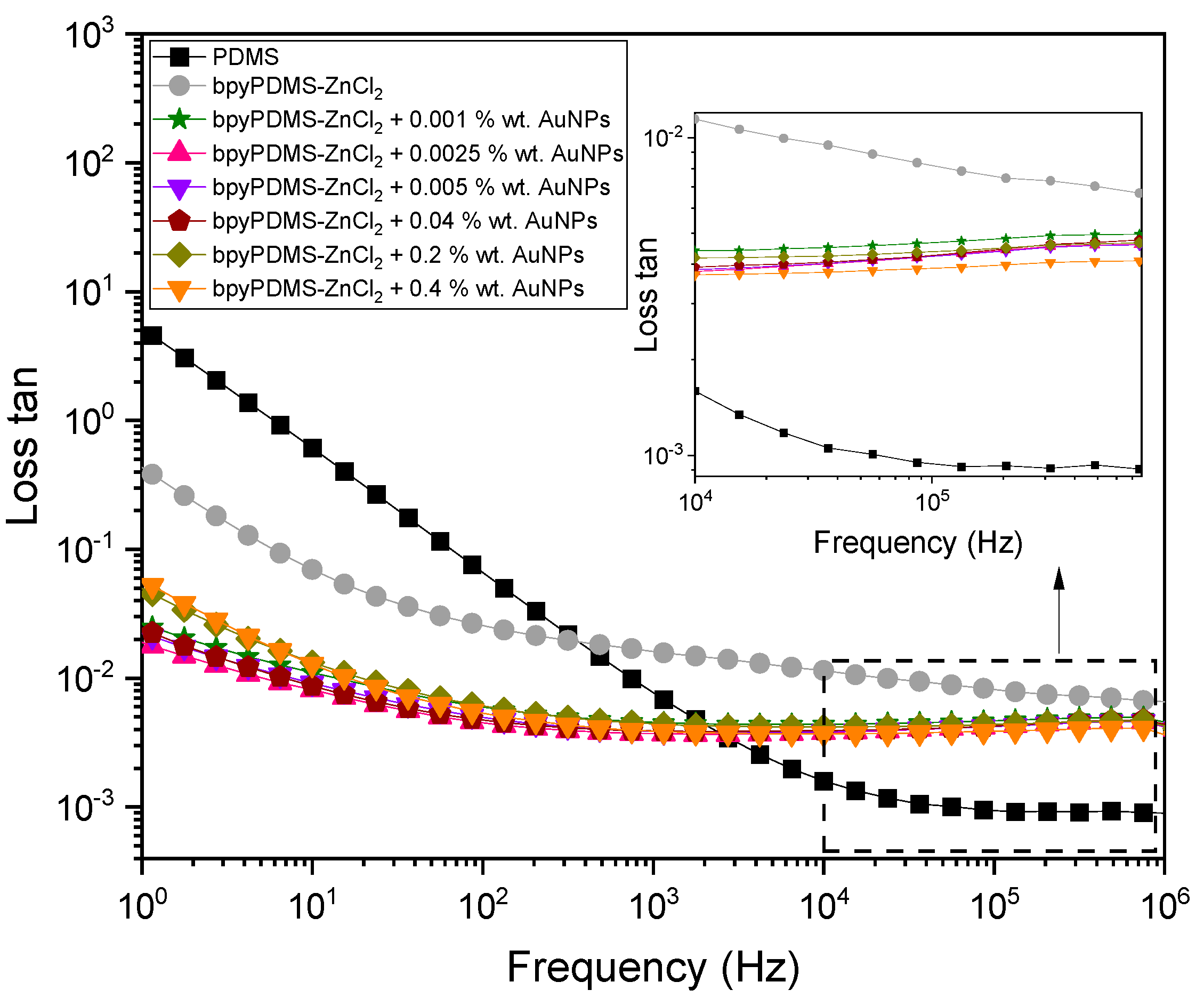
4.2.1. Isothermal Representation of Dielectric Properties of the Systems Investigated
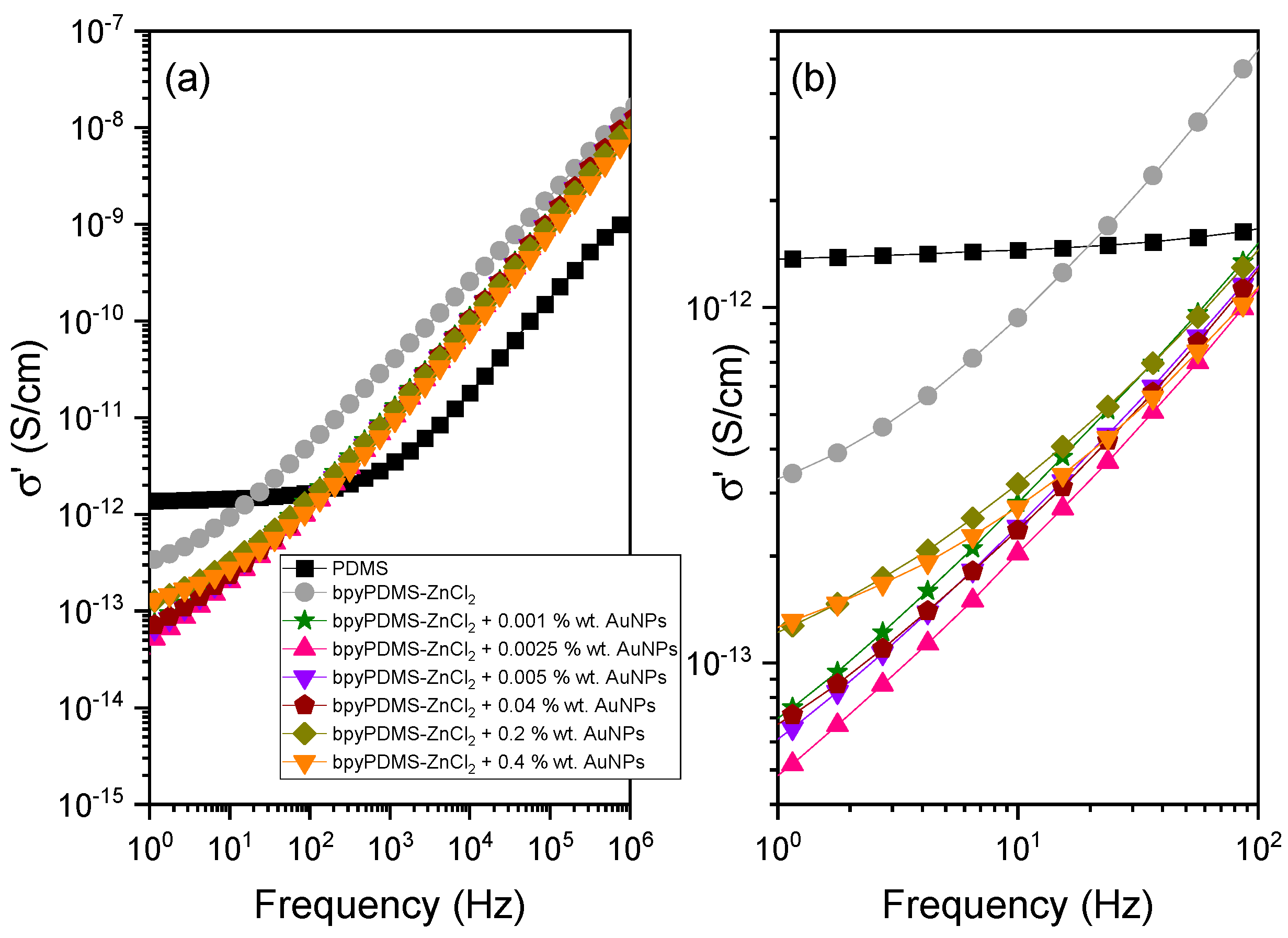
4.2.2. Electrical Conductivity of Neat PDMS, Its Metalloorganic Complex bpyPDMS-ZnCl2, and the Materials Containing Gold Nanoparticles
4.2.3. DC and AC Electrical Conductivity Contribution to System Complex Conductivity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, B.; Huang, W.; Chi, L.; Al-Hashimi, M.; Marks, T.J.; Facchetti, A. High-k Gate Dielectrics for Emerging Flexible and Stretchable Electronics. Chem. Rev. 2018, 118, 5690–5754. [Google Scholar] [CrossRef]
- Yan, J.; Pietrasik, J.; Wypych-Puszkarz, A.; Ciekanska, M.; Matyjaszewski, K. Synthesis of High k Nanoparticles by Controlled Radical Polymerization. In Solution-Processable Components for Organic Electronic Devices; Luszczynska, B., Matyjaszewski, K., Ulanski, J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; pp. 181–226. ISBN 978-3-527-34442-0. [Google Scholar]
- Ha, Y.G.; Everaerts, K.; Hersam, M.C.; Marks, T.J. Hybrid gate dielectric materials for unconventional electronic circuitry. Acc. Chem. Res. 2014, 47, 1019–1028. [Google Scholar] [CrossRef]
- Guo, S.; Li, S.; Shen, T.; Tang, W.; Guo, X.; Xin, J.; Jin, J.; Wei, H.; Wang, X.; Li, J. Fröhlich polaron effect in flexible low-voltage organic thin-film transistors gated with high-k polymer dielectrics. J. Phys. D Appl. Phys. 2021, 54, 444001. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, C.; Jiao, H.; Zhang, M. Polyvinyl Alcohol/SiO2 Hybrid Dielectric for Transparent Flexible/Stretchable All-Carbon-Nanotube Thin-Film-Transistor Integration. Adv. Electron. Mater. 2020, 6, 1901133. [Google Scholar] [CrossRef]
- Najafi-Ashtiani, H.; Rahdar, A. Flexibility investigation of free-silicon organic–inorganic (ZrTiHfO2-PVP) hybrid films as a gate dielectric. Appl. Phys. A Mater. Sci. Process. 2021, 127, 217. [Google Scholar] [CrossRef]
- Jang, M.; Park, S.Y.; Kim, S.K.; Jung, D.; Song, W.; Myung, S.; Lee, S.S.; Yoon, D.H.; An, K.S. Strategic Customization of Polymeric Nanocomposites Modified by 2D Titanium Oxide Nanosheet for High-k and Flexible Gate Dielectrics. Small 2021, 17, 2007213. [Google Scholar] [CrossRef]
- You, I.; Kong, M.; Jeong, U. Block Copolymer Elastomers for Stretchable Electronics. Acc. Chem. Res. 2019, 52, 63–72. [Google Scholar] [CrossRef]
- Jiao, H.; Zhang, M.; Du, C.; Zhang, Z.; Huang, W.; Huang, Q. Intrinsically stretchable all-carbon-nanotube transistors with styrene-ethylene-butylene-styrene as gate dielectrics integrated by photolithography-based process. RSC Adv. 2020, 10, 8080–8086. [Google Scholar] [CrossRef] [Green Version]
- Qi, D.; Zhang, K.; Tian, G.; Jiang, B.; Huang, Y. Stretchable Electronics Based on PDMS Substrates. Adv. Mater. 2021, 33, 2003155. [Google Scholar] [CrossRef]
- Raveendran, R.; Nagaraj, M.; Namboothiry, M.A.G. High-performance, transparent solution-processed organic field-effect transistor with low-k elastomeric gate dielectric and liquid crystalline semiconductor: Promises and challenges. ACS Appl. Electron. Mater. 2020, 2, 3336–3345. [Google Scholar] [CrossRef]
- Tantraviwat, D.; Ngamyingyoud, M.; Sripumkhai, W.; Pattamang, P.; Rujijanagul, G.; Inceesungvorn, B. Tuning the Dielectric Constant and Surface Engineering of a BaTiO3/Porous PDMS Composite Film for Enhanced Triboelectric Nanogenerator Output Performance. ACS Omega 2021, 6, 29765–29773. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.L.; Chortos, A.; Pfattner, R.; Lissel, F.; Chiu, Y.C.; Feig, V.; Xu, J.; Kurosawa, T.; Gu, X.; Wang, C.; et al. Stretchable self-healing polymeric dielectrics cross-linked through metal-ligand coordination. J. Am. Chem. Soc. 2016, 138, 6020–6027. [Google Scholar] [CrossRef] [PubMed]
- Rajitha, G.; Dash, R.K. Optically transparent and high dielectric constant reduced graphene oxide (RGO)-PDMS based flexible composite for wearable and flexible sensors. Sens. Actuators A Phys. 2018, 277, 26–34. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, S.; Miao, J.; Yu, Z.; Wang, C. Fully Printed Stretchable Thin-Film Transistors and Integrated Logic Circuits. ACS Nano 2016, 10, 11459–11468. [Google Scholar] [CrossRef] [PubMed]
- Bertasius, P.; Schaefer, S.; Macutkevic, J.; Banys, J.; Selskis, A.; Fierro, V.; Celzard, A. Dielectric properties of polydimethylsiloxane composites filled with SrTiO3 nanoparticles. Polym. Compos. 2021, 42, 2982–2988. [Google Scholar] [CrossRef]
- Feng, P.; Zhong, M.; Zhao, W. Stretchable multifunctional dielectric nanocomposites based on polydimethylsiloxane mixed with metal nanoparticles. Mater. Res. Express 2019, 7, 015007. [Google Scholar] [CrossRef] [Green Version]
- Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications Toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Kempa, T.; Carnahan, D.; Olek, M.; Correa, M.; Giersig, M.; Cross, M.; Benham, G.; Sennett, M.; Ren, Z.; Kempa, K. Dielectric media based on isolated metallic nanostructures. J. Appl. Phys. 2005, 98, 034310. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, J.; Liu, Y.; Wang, Z.; Yan, C.; Song, C.; Gao, C.; Wu, Y. A NIR laser induced self-healing PDMS/Gold nanoparticles conductive elastomer for wearable sensor. J. Colloid Interface Sci. 2021, 599, 360–369. [Google Scholar] [CrossRef]
- Chaudhuri, B.; Uddin, M.J.; Chaudhuri, B.; Pramanik, K.; Middya, T.R. Black tea leaf extract derived Ag nanoparticle-PVA composite film: Structural and dielectric properties. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2012, 177, 1741–1747. [Google Scholar] [CrossRef]
- Wrzesińska, A.; Bobowska, I.; Maczugowska, P.; Małolepsza, J.; Błazewska, K.M.; Wypych-Puszkarz, A. Effect of metal-ligand coordination complexes on molecular dynamics and structure of cross-linked poly(dimethylosiloxane). Polymers 2020, 12, 1680. [Google Scholar] [CrossRef] [PubMed]
- Wrzesińska, A.; Wypych-Puszkarz, A.; Bobowska, I.; Ulański, J. Effects of counter anions on AC and DC electrical conductivity in poly(dimethylsiloxane) crosslinked by metal-ligand coordination. Polymers 2021, 13, 956. [Google Scholar] [CrossRef] [PubMed]
- Tkacz-Szczesna, B.; Soliwoda, K.; Rosowski, M.; Tomaszewska, E.; Celichowski, G.; Grobelny, J. Modification of gold and silver nanoparticles with n-dialkyldithiophosphates. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 468, 219–225. [Google Scholar] [CrossRef]
- Soliwoda, K.; Tomaszewska, E.; Tkacz-Szczesna, B.; Mackiewicz, E.; Rosowski, M.; Bald, A.; Blanck, C.; Schmutz, M.; Novák, J.; Schreiber, F.; et al. Effect of the alkyl chain length of secondary amines on the phase transfer of gold nanoparticles from water to toluene. Langmuir 2014, 30, 6684–6693. [Google Scholar] [CrossRef] [PubMed]
- Soliwoda, K.; Tomaszewska, E.; Tkacz-Szczesna, B.; Rosowski, M.; Celichowski, G.; Grobelny, J. The influence of the chain length and the functional group steric accessibility of thiols on the phase transfer efficiency of gold nanoparticles from water to toluene. Polish J. Chem. Technol. 2014, 16, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Ranoszek-Soliwoda, K.; Girleanu, M.; Tkacz-Szczȩsna, B.; Rosowski, M.; Celichowski, G.; Brinkmann, M.; Ersen, O.; Grobelny, J. Versatile Phase Transfer Method for the Efficient Surface Functionalization of Gold Nanoparticles: Towards Controlled Nanoparticle Dispersion in a Polymer Matrix. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]
- Williams, Z.H.; Burwell, E.D.; Chiomento, A.E.; Demsko, K.J.; Pawlik, J.T.; Harris, S.O.; Yarolimek, M.R.; Whitney, M.B.; Hambourger, M.; Schwab, A.D. Rubber-elasticity and electrochemical activity of iron(II) tris(bipyridine) crosslinked poly(dimethylsiloxane) networks. Soft Matter 2017, 13, 6542–6554. [Google Scholar] [CrossRef]
- Kremer, F.; Schonhals, A. Broadband Dielectric Spectroscopy; Springer: Cham, Switzerland, 2003. [Google Scholar]
- Shivashankar, H.; Kevin, A.M.; Manohar, S.B.S.; Kulkarni, S.M. Investigation on dielectric properties of PDMS based nanocomposites. Phys. B Condens. Matter 2021, 602, 412357. [Google Scholar] [CrossRef]
- Li, C.H.; Wang, C.; Keplinger, C.; Zuo, J.L.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C.; et al. A highly stretchable autonomous self-healing elastomer. Nat. Chem. 2016, 8, 618–624. [Google Scholar] [CrossRef]
- Oh, I.; Jeon, S.I.; Chung, I.J.; Ahn, C.H. Self-Healable Dielectric Polydimethylsiloxane Composite Based on Zinc-Imidazole Coordination Bond. Macromol. Res. 2019, 27, 435–443. [Google Scholar] [CrossRef]
- Wang, R.; Xie, C.; Luo, S.; Gou, B.; Xu, H.; Zeng, L. The influence mechanism of nanoparticles on the dielectric properties of epoxy resin. RSC Adv. 2019, 9, 19648–19656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrøder, T.B.; Dyre, J.C. Scaling and universality of ac conduction in disordered solids. Phys. Rev. Lett. 2000, 84, 310–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyre, J.C.; Schrøder, T.B. Hopping models and ac universality. Phys. Status Solidi Basic Res. 2002, 230, 5–13. [Google Scholar] [CrossRef]
- Jonscher, A.K. Universal Relaxation Law; Chelsea Dielectrics Press: London, UK, 1992; Chapter 5. [Google Scholar]
- Dyre, J.C. The random free-energy barrier model for ac conduction in disordered solids. J. Appl. Phys. 1988, 64, 2456–2468. [Google Scholar] [CrossRef] [Green Version]
- Mauri, M.; Peterson, A.; Senol, A.; Elamin, K.; Gitsas, A.; Hjertberg, T.; Matic, A.; Gkourmpis, T.; Prieto, O.; Müller, C. Byproduct-free curing of a highly insulating polyethylene copolymer blend: An alternative to peroxide crosslinking. J. Mater. Chem. C 2018, 6, 11292–11302. [Google Scholar] [CrossRef] [Green Version]
- Roling, B. Conductivity spectra of disordered ionic conductors: Calculating the time- dependent mean square displacement of the mobile ions introduction. Dielectr. Newsl. 2002, 1, 1–8. [Google Scholar]
- Gao, X.; Huang, Y.; He, X.; Fan, X.; Liu, Y.; Xu, H.; Wu, D.; Wan, C. Mechanically enhanced electrical conductivity of polydimethylsiloxane-based composites by a hot embossing process. Polymers 2019, 11, 56. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S. Recent progress in gold nanoparticle-based non-volatile memory devices. Gold Bull. 2010, 43, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Broadbent, S.R.; Hammersley, J.M. Percolation processes: I. Crystals and mazes. Math. Proc. Camb. Philos. Soc. 1957, 53, 629–641. [Google Scholar] [CrossRef]
- Isichenko, M.B. Percolation, statistical topography, and transport in random media. Rev. Mod. Phys. 1992, 64, 961–1043. [Google Scholar] [CrossRef] [Green Version]
- Bergman, D.J.; Stroud, D. Physical Properties of Macroscopically Inhomogeneous Media. Solid State Phys. Adv. Res. Appl. 1992, 46, 147–269. [Google Scholar] [CrossRef]
- Dyre, J.C. Universal low-temperature ac conductivity of macroscopically disordered nonmetals. Phys. Rev. B 1993, 48, 12511–12526. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrzesińska, A.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Bobowska, I.; Grobelny, J.; Ulański, J.; Wypych-Puszkarz, A. Gold Nanoparticles as Effective ion Traps in Poly(dimethylsiloxane) Cross-Linked by Metal-Ligand Coordination. Molecules 2022, 27, 3579. https://doi.org/10.3390/molecules27113579
Wrzesińska A, Tomaszewska E, Ranoszek-Soliwoda K, Bobowska I, Grobelny J, Ulański J, Wypych-Puszkarz A. Gold Nanoparticles as Effective ion Traps in Poly(dimethylsiloxane) Cross-Linked by Metal-Ligand Coordination. Molecules. 2022; 27(11):3579. https://doi.org/10.3390/molecules27113579
Chicago/Turabian StyleWrzesińska, Angelika, Emilia Tomaszewska, Katarzyna Ranoszek-Soliwoda, Izabela Bobowska, Jarosław Grobelny, Jacek Ulański, and Aleksandra Wypych-Puszkarz. 2022. "Gold Nanoparticles as Effective ion Traps in Poly(dimethylsiloxane) Cross-Linked by Metal-Ligand Coordination" Molecules 27, no. 11: 3579. https://doi.org/10.3390/molecules27113579
APA StyleWrzesińska, A., Tomaszewska, E., Ranoszek-Soliwoda, K., Bobowska, I., Grobelny, J., Ulański, J., & Wypych-Puszkarz, A. (2022). Gold Nanoparticles as Effective ion Traps in Poly(dimethylsiloxane) Cross-Linked by Metal-Ligand Coordination. Molecules, 27(11), 3579. https://doi.org/10.3390/molecules27113579






