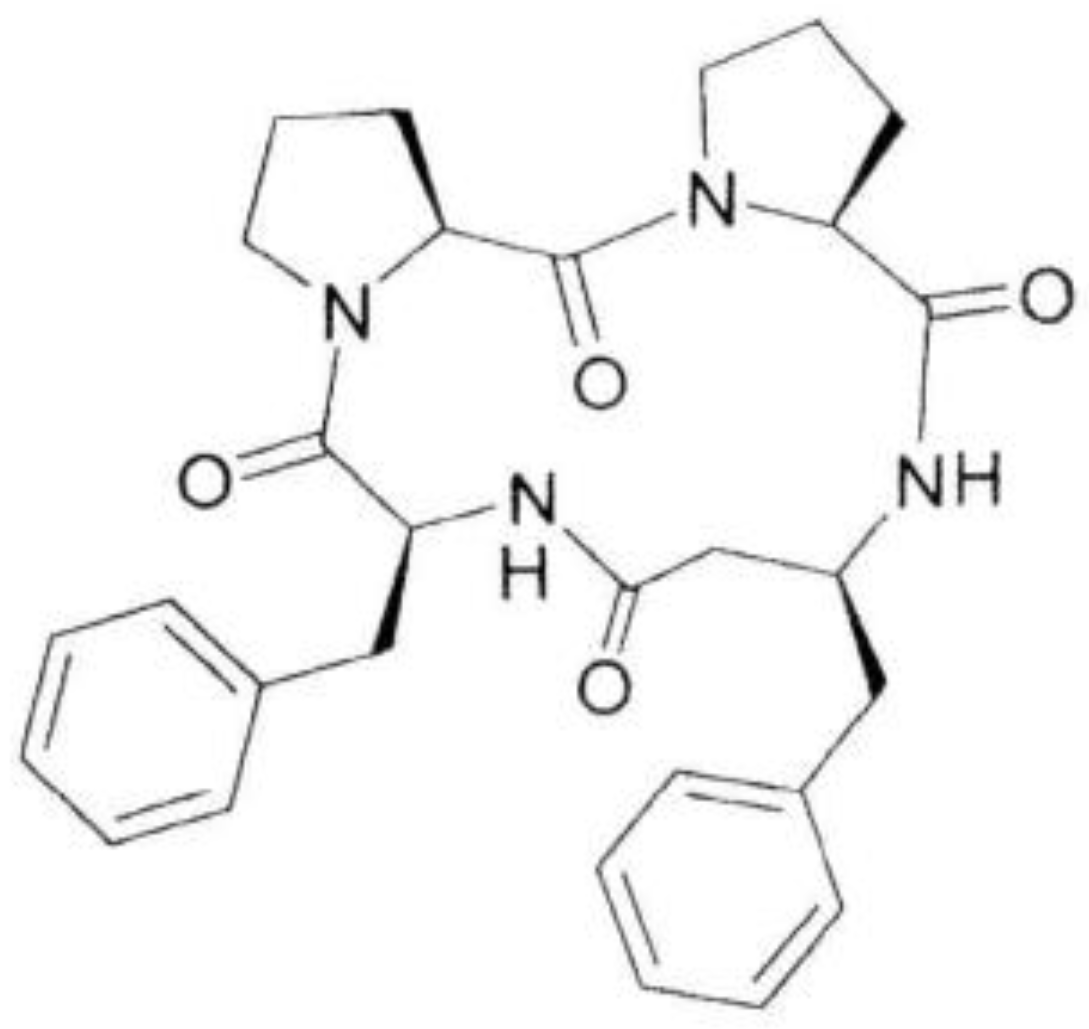
Antiviral Activity of a Cyclic Pro-Pro-β3-HoPhe-Phe Tetrapeptide against HSV-1 and HAdV-5
Abstract
1. Introduction
2. Results
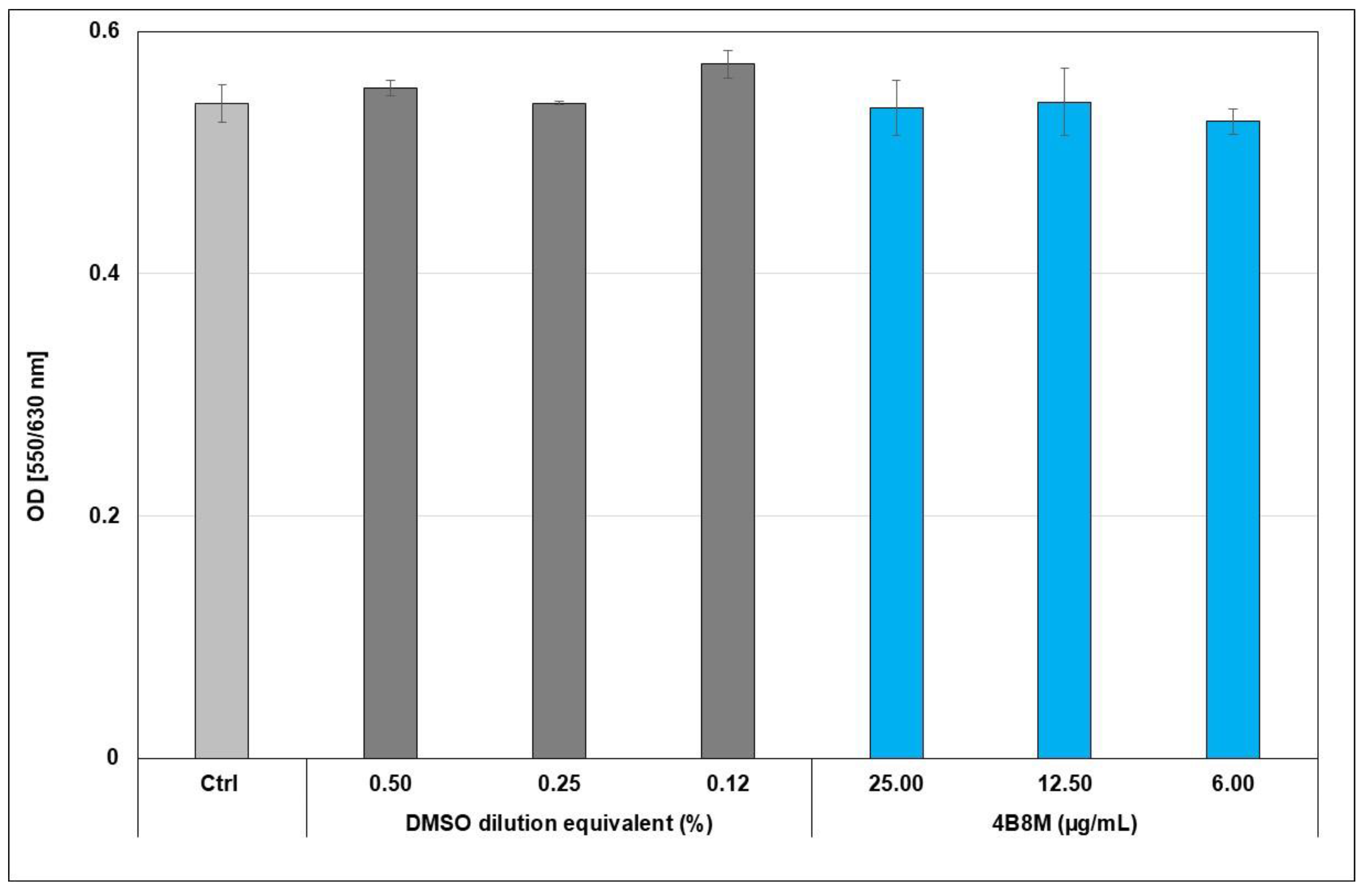
2.1. Toxicity of the 4B8M Peptide
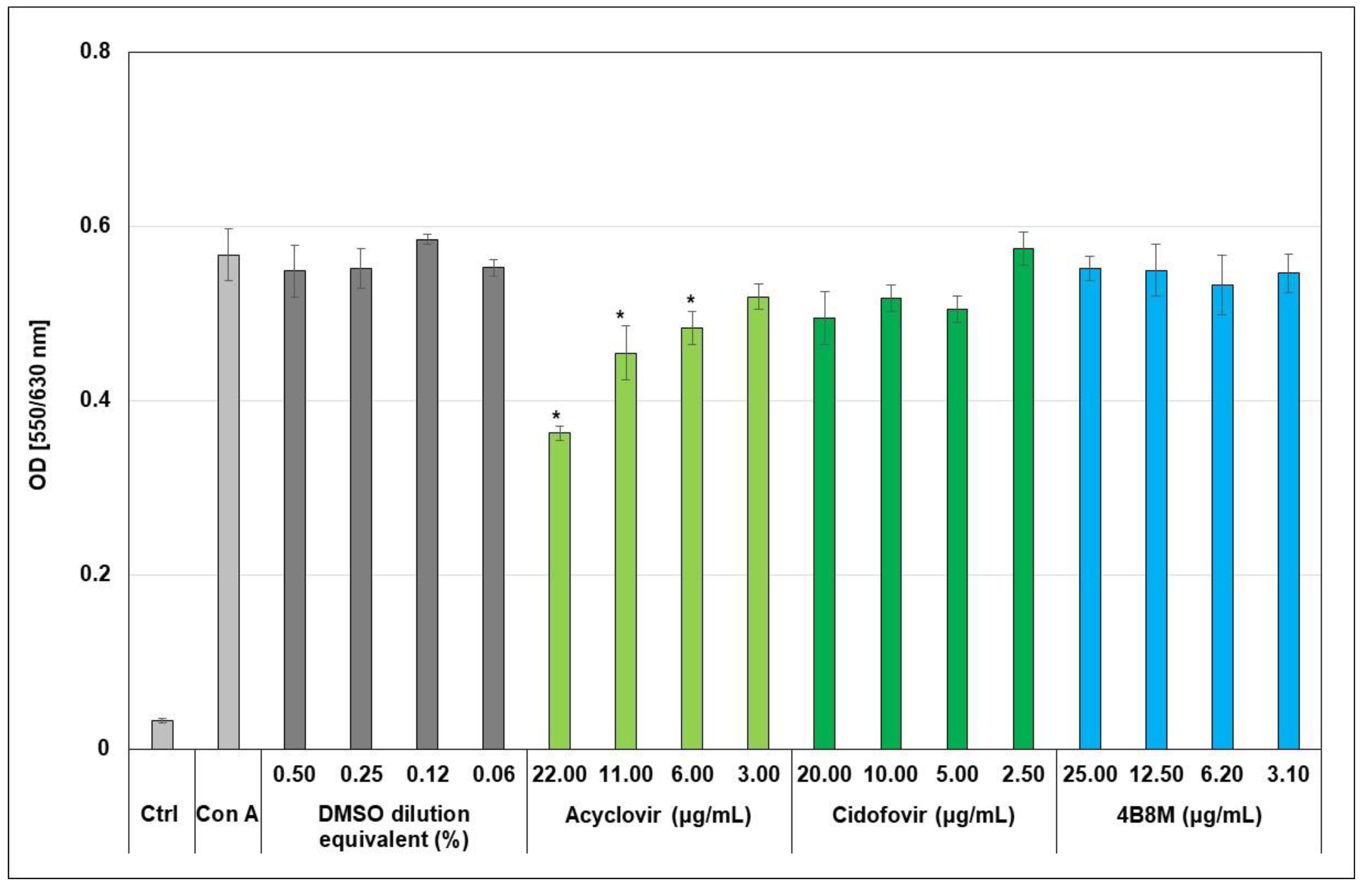
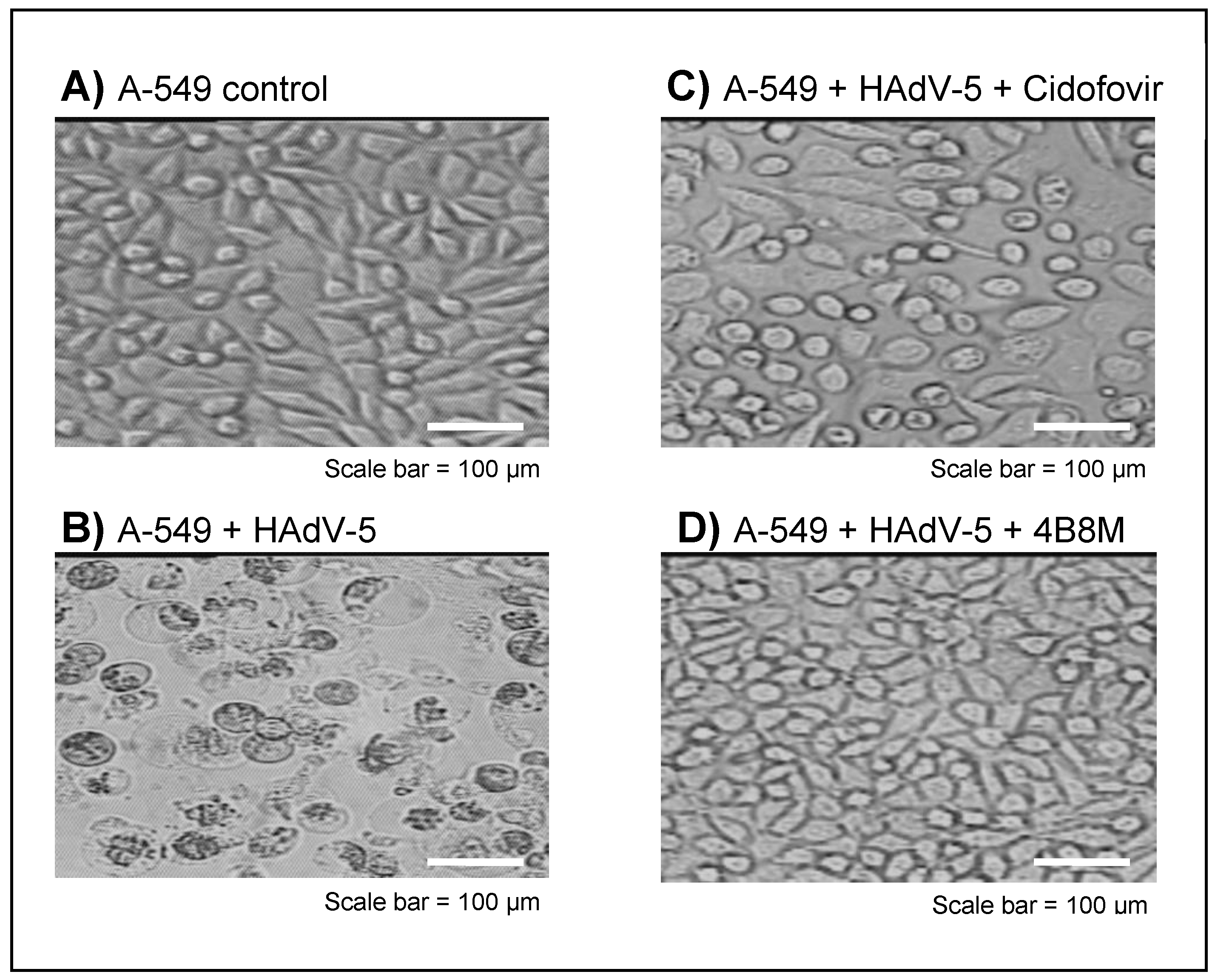
2.2. The Virus-Inhibiting Properties of the 4B8M Peptide against Human HAdV-5
2.3. The Virus-Inhibiting Properties of 4B8M Peptide against Human HSV-1
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. Reagents for 4B8M Peptide Synthesis, Cyclization and Purification
4.1.2. Synthesis of the 4B8M Peptide
4.1.3. Physicochemical Characteristics of 4B8M Peptide
4.2. Biology
4.2.1. Mice
4.2.2. Reagents for Biological Assays
4.2.3. Preparation of 4B8M Peptide and Reference Compounds for In Vitro Studies
4.2.4. Cell Line and Viruses
4.2.5. Evaluation of Toxicity of 4B8M Peptide against A-549 Cells
4.2.6. Evaluation of Toxicity of 4B8M Peptide against Mouse Splenocytes
4.2.7. Evaluation of the Activity of the 4B8M Peptide in the Proliferation Test of Mouse Splenocytes
4.2.8. Determination of 4B8M Peptide Antiviral Activity
4.2.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Abdalla, M.A. Medicinal significance of naturally occurring cyclotetrapeptides. J. Nat. Med. 2016, 70, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, M.; Xu, D.; Lai, D.; Zhou, L. Structural diversity and biological activities of fungal cyclic peptides, excluding cyclodipeptides. Molecules 2017, 22, 2069. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.A.; McGaw, L.J. Natural cyclic peptides as an attractive modality for therapeutics: A Mini Review. Molecules 2018, 23, 2080. [Google Scholar] [CrossRef]
- Zimecki, M.; Kaczmarek, K. Effects of modifications on the immunosuppressive properties of cyclolinopeptide A and its linear analogs in animal experimental models. Molecules 2021, 26, 2538. [Google Scholar] [CrossRef] [PubMed]
- Zimecki, M.; Artym, J.; Kałas, W.; Strządała, L.; Kaleta-Kuratewicz, K.; Kuryszko, J.; Kaszuba, A.; Kaczmarek, K.; Zabrocki, J. Anti-inflammatory activity of a cyclic tetrapeptide in mouse and human experimental models. Pharmaceutics 2020, 12, 1030. [Google Scholar] [CrossRef]
- Wieczorek, Z.; Zimecki, M.; Pedyczak, A.; Lisowski, M.; Siemion, I.Z. Immunosuppressive activity of alanine analogs of cyclolinopeptide A. Arch. Immunol. Exp. 1993, 41, 291–296. [Google Scholar]
- Cebrat, M.; Lisowski, M.; Siemion, I.Z.; Wieczorek, Z. The cyclolinopeptide A analogues with D-Phe, D-Tyr, and L- and D-Trp residues. Pol. J. Chem. 1997, 71, 1401–1412. [Google Scholar]
- He, F.; Bao, J.; Zhang, X.-Y.; Tu, Z.-C.; Shi, Y.-M.; Qi, S.-H. Asperterrestide A, a cytotoxic cyclic tetrapeptide from the marine-derived fungus Aspergillus terreus SCSGAF0162. J. Nat. Prod. 2013, 76, 1182–1186. [Google Scholar] [CrossRef]
- Tan, Q.-W.; Gao, F.-L.; Wang, F.-R.; Chen, Q.-J. Anti-TMV activity of malformin A1, a cyclic penta-peptide produced by an endophytic fungus Aspergillus tubingensis FJBJ11. Int. J. Mol. Sci. 2015, 16, 5750–5761. [Google Scholar] [CrossRef]
- Perrier, A.; Eluard, M.; Petitjean, M.; Vanet, A. In silico design of new inhibitors against hemagglutinin of influenza. J. Phys. Chem. B 2019, 123, 582–592. [Google Scholar] [CrossRef]
- Tamamura, H.; Otaka, A.; Fujii, N. Development of anti-HIV agents targeting dynamic supramolecular mechanism: Entry and fusion inhibitors based on CXCR4/CCR5 antagonists and gp41-C34-remodeling peptides. Curr. HIV Res. 2005, 3, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Grande, F.; Garofalo, A.; Neamati, N. Small molecules anti-HIV therapeutics targeting CXCR4. Curr. Pharm. Des. 2008, 14, 385–404. [Google Scholar] [CrossRef] [PubMed]
- Sadri, F.; Rezaei, Z.; Fereidouni, M. The significance of the SDF-1/CXCR4 signaling pathway in the normal development. Mol. Biol. Rep. 2022, 49, 3307–3320. [Google Scholar] [CrossRef] [PubMed]
- Mehrpouri, M. The contributory roles of the CXCL12/CXCR4/CXCR7 axis in normal and malignant hematopoiesis: A possible therapeutic target in hematologic malignancies. Eur. J. Pharmacol. 2022, 920, 174831. [Google Scholar] [CrossRef]
- Kinchington, P.R.; Romanowski, E.G.; Gordon, Y.J. Prospects for adenovirus antivirals. J. Antimicrob. Chemother. 2005, 55, 424–429. [Google Scholar] [CrossRef]
- Muruve, D.A. The innate immune response to adenovirus vectors. Hum. Gene Tam. 2004, 15, 1157–1166. [Google Scholar] [CrossRef]
- Schuhmacher, A.; Reichling, J.; Schnitzler, P. Virucidal effect of peppermint oil on the enveloped viruses herpes simplex virus type 1 and type 2 in vitro. Phytomedicine 2003, 10, 504–510. [Google Scholar] [CrossRef]
- Fatahzadeh, M.; Schwartz, R.A. Human herpes simplex virus infections: Epidemiology, pathogenesis, symptomatology, diagnosis, and management. J. Am. Acad. Dermatol. 2007, 57, 737–763. [Google Scholar] [CrossRef]
- Hoffman, J.A.; Shah, A.J.; Ross, L.A.; Kapoor, N. Adenoviral infections and a prospective trial of cidofovir in pediatric hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2001, 7, 388–394. [Google Scholar] [CrossRef]
- Bacon, T.H.; Levin, M.J.; Leary, J.J.; Sarisky, R.T.; Sutton, D. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin. Microbiol. Rev. 2003, 16, 114–128. [Google Scholar] [CrossRef]
- Gallo, P.; Rossi, F.; Saviano, M.; Pedone, C.; Colonna, G.; Ragone, R. Specific interaction between bovine cyclophilin A and synthetic analogues of cyclolinopeptide A. J. Biochem. 1998, 124, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Fenizia, C.; Galbiati, S.; Vanetti, C.; Vago, R.; Clerici, M.; Tacchetti, C.; Tiziana, D.T. Cyclosporine A Inhibits Viral Infection and Release as Well as Cytokine Production in Lung Cells by Three SARS-CoV-2 Variants. Microbiol. Spectr. 2022, 10, e0150421. [Google Scholar] [CrossRef] [PubMed]
- Sander, W.J.; O’Neill, H.G.; Pohl, C.H. Prostaglandin E2 as a modulator of viral infections. Front. Physiol. 2017, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Hao, Q.; Liu, L.; Li, Y.; Wu, J.; Huo, X.; Zhu, Y. Epigenetic changes mediated by microRNA miR29 activate cyclooxygenase 2 and lambda-1 interferon production during viral infection. J. Virol. 2012, 86, 1010–1020. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Z.; Chen, J.; Li, R.; Cao, Y.; Zhu, C.; Wu, K.; Wu, J.; Liu, F.; Zhu, Y. Influenza A virus induces interleukin-27 through cyclooxygenase-2 and protein kinase A signaling. J. Biol. Chem. 2012, 287, 11899–11910. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, H.; Shen, S.-M.; Wen, C.-X.; Xing, Z.; Shi, Y. Inhibition of autophagy and chemokine induction by sphingosine 1-phosphate receptor 1 through NF-κB signaling in human pulmonary endothelial cells infected with influenza A viruses. PLoS ONE 2018, 13, e0205344. [Google Scholar] [CrossRef]
- Wan, M.; Tang, X.; Rekha, R.S.; Muvva, J.R.; Brighenti, S.; Agerberth, B.; Haeggström, J.Z. Prostaglandin E2 suppresses hCAP18/LL-37 expression in human macrophages via EP2/EP4: Implications for treatment of Mycobacterium tuberculosis infection. FASEB J. 2018, 32, 2827–2840. [Google Scholar] [CrossRef]
- Yudhawati, R.; Amin, M.; Rantam, F.A.; Prasetya, R.R.; Dewantari, J.R.; Nastri, A.M.; Poetranto, E.D.; Wulandari, L.; Lusida, M.I.; Koesnowidagdo, S.; et al. Bone marrow-derived mesenchymal stem cells attenuate pulmonary inflammation and lung damage caused by highly pathogenic avian influenza A/H5N1 virus in BALB/c mice. BMC Infect. Dis. 2020, 20, 823. [Google Scholar] [CrossRef]
- Wu, Q.; Jorde, I.; Kershaw, O.; Jeron, A.; Bruder, D.; Schreiber, J.; Stegemann-Koniszewski, S. Resolved influenza a virus infection has extended effects on lung homeostasis and attenuates allergic airway inflammation in a mouse model. Microorganisms 2020, 8, 1878. [Google Scholar] [CrossRef]
- Bärnthaler, T.; Maric, J.; Platzer, W.; Konya, V.; Theiler, A.; Hasenöhrl, C.; Gottschalk, B.; Trautmann, S.; Schreiber, Y.; Graier, W.F.; et al. The role of PGE 2 in alveolar epithelial and lung microvascular endothelial crosstalk. Sci. Rep. 2017, 7, 7923. [Google Scholar] [CrossRef]
- Surh, I.; Rundhaug, J.E.; Pavone, A.; Mikulec, C.; Abel, E.; Simper, M.; Fischer, S.M. The EP1 receptor for prostaglandin E2 promotes the development and progression of malignant murine skin tumors. Mol. Carcinog. 2012, 51, 553–564. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bojarska, J.; Breza, M.; Remko, M.; Czyz, M.; Gajos-Michniewicz, A.; Zimecki, M.; Kaczmarek, K.; Madura, I.; Wojciechowski, J.; Wolf, W. Structural and Biofunctional Insights into the Cyclo(Pro-Pro-Phe-Phe-) Scaffold from Experimental and In Silico Studies: Melanoma and Beyond. Int. J. Mol. Sci. 2022; submitted. [Google Scholar]
- Bojarska, J.; Mieczkowski, A.; Ziora, Z.M.; Skwarczynski, M.; Toth, I.; Shalash, A.O.; Parang, K.; El-Mowafi, S.A.; Mohammed, E.H.M.; Elnagdy, S.; et al. Cyclic dipeptides: The biological and structural landscape with special focus on the anti-cancer proline-based scaffold. Biomolecules 2021, 11, 1515. [Google Scholar] [CrossRef] [PubMed]
- Dard, C.; Leforestier, B.; Hilário, F.F.; Traoré, M.D.M.; Lespinasse, M.-A.; Pérès, B.; Molina, M.-C.; Pereira de Freitas, R.; Milet, A.; Maubon, D.; et al. Crossing of the cystic barriers of Toxoplasma gondii by the fluorescent coumarin tetra-cyclopeptide. Molecules 2021, 26, 7506. [Google Scholar] [CrossRef] [PubMed]
- Zabrocki, J.; Zimecki, M.; Kaszuba, A.; Kaczmarek, K. Cyclic Tetrapeptides and Therapeutic Applications Thereof. U.S. Patent 09382292, 5 July 2016. [Google Scholar]
- Smith, C.D.; Craft, D.W.; Shiromoto, R.S.; Yan, P.O. Alternative cell line for virus isolation. J. Clin. Microbiol. 1986, 24, 265–268. [Google Scholar] [CrossRef]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaczyńska, E.; Kaczmarek, K.; Zabrocki, J.; Artym, J.; Zimecki, M. Antiviral Activity of a Cyclic Pro-Pro-β3-HoPhe-Phe Tetrapeptide against HSV-1 and HAdV-5. Molecules 2022, 27, 3552. https://doi.org/10.3390/molecules27113552
Zaczyńska E, Kaczmarek K, Zabrocki J, Artym J, Zimecki M. Antiviral Activity of a Cyclic Pro-Pro-β3-HoPhe-Phe Tetrapeptide against HSV-1 and HAdV-5. Molecules. 2022; 27(11):3552. https://doi.org/10.3390/molecules27113552
Chicago/Turabian StyleZaczyńska, Ewa, Krzysztof Kaczmarek, Janusz Zabrocki, Jolanta Artym, and Michał Zimecki. 2022. "Antiviral Activity of a Cyclic Pro-Pro-β3-HoPhe-Phe Tetrapeptide against HSV-1 and HAdV-5" Molecules 27, no. 11: 3552. https://doi.org/10.3390/molecules27113552
APA StyleZaczyńska, E., Kaczmarek, K., Zabrocki, J., Artym, J., & Zimecki, M. (2022). Antiviral Activity of a Cyclic Pro-Pro-β3-HoPhe-Phe Tetrapeptide against HSV-1 and HAdV-5. Molecules, 27(11), 3552. https://doi.org/10.3390/molecules27113552





