Pharmacological, Neurochemical, and Behavioral Mechanisms Underlying the Anxiolytic- and Antidepressant-like Effects of Flavonoid Chrysin
Abstract
1. Introduction
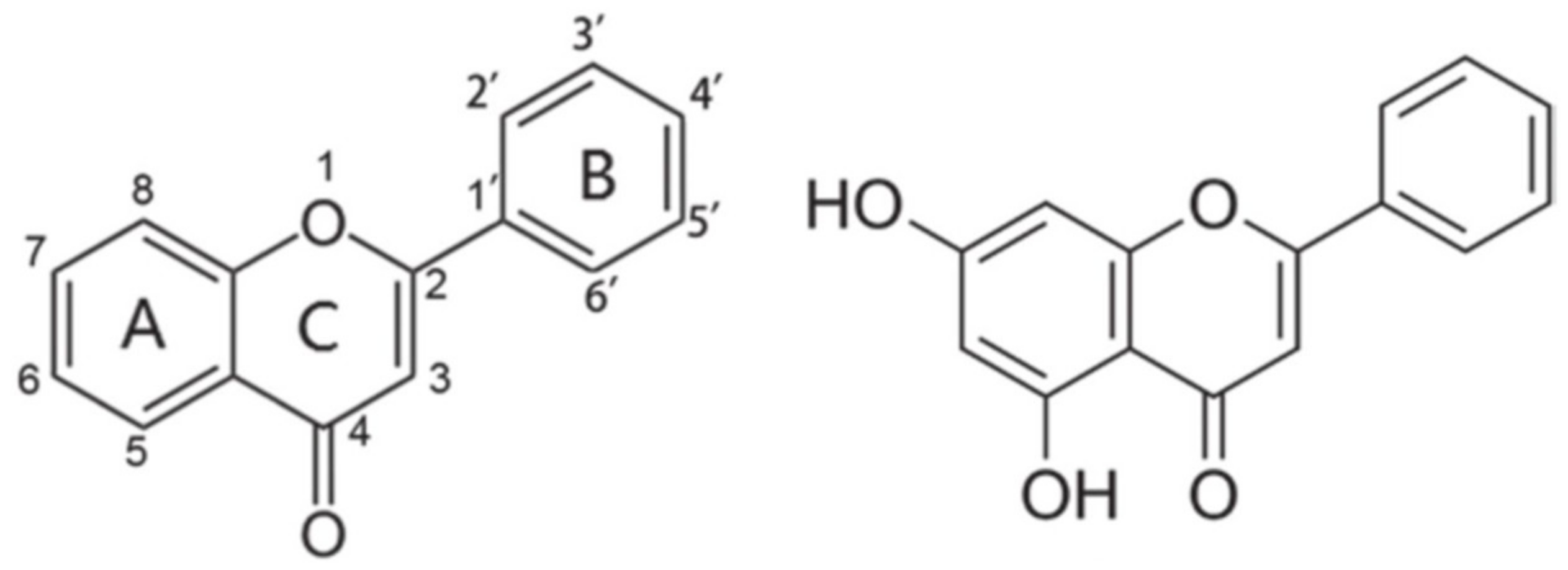
2. Generalities of the Flavonoid Chrysin
3. Biochemical and Pharmacological Activity of Flavonoid Chrysin
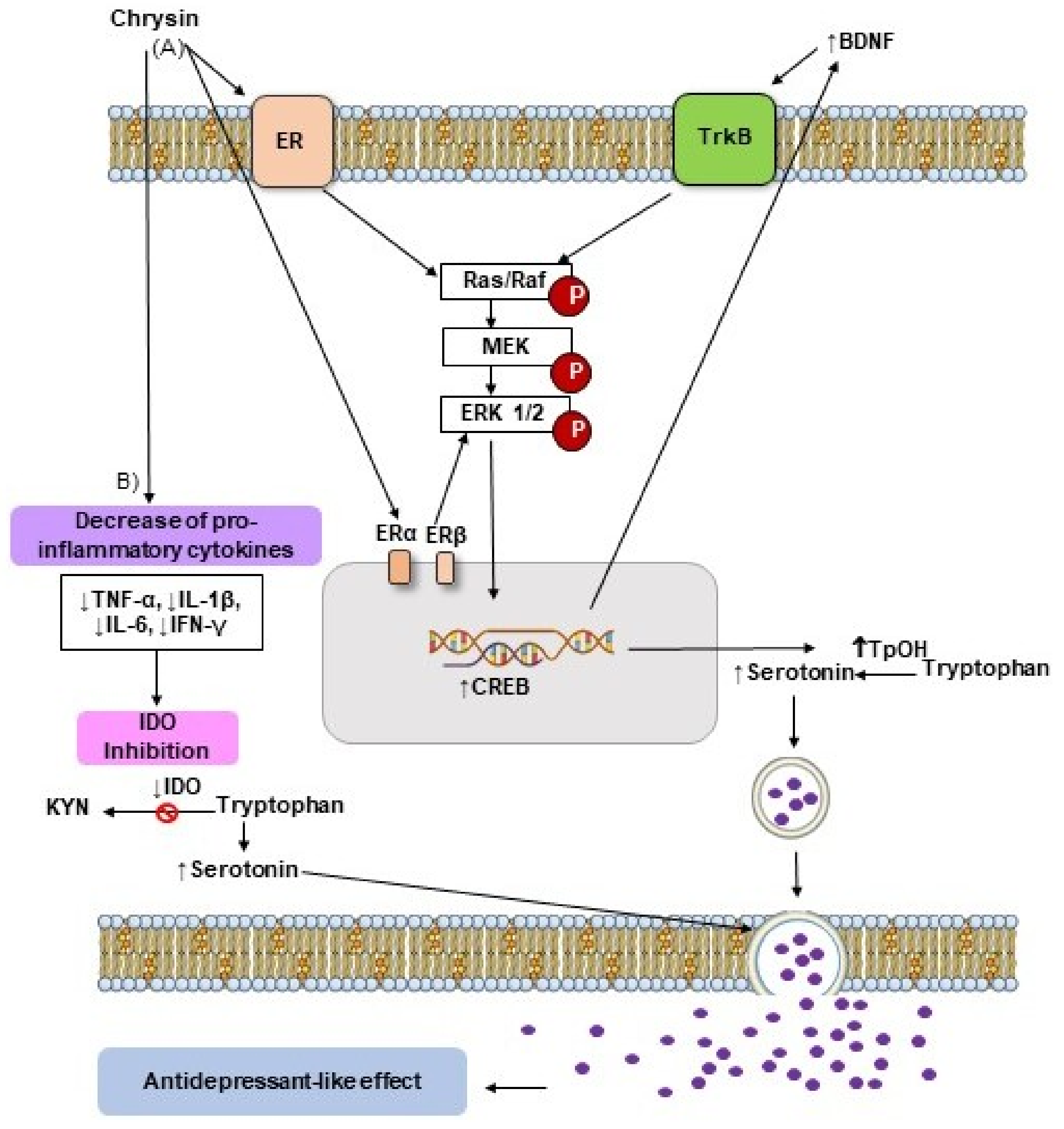
3.1. Action of Chrysin on Neurotransmission Systems
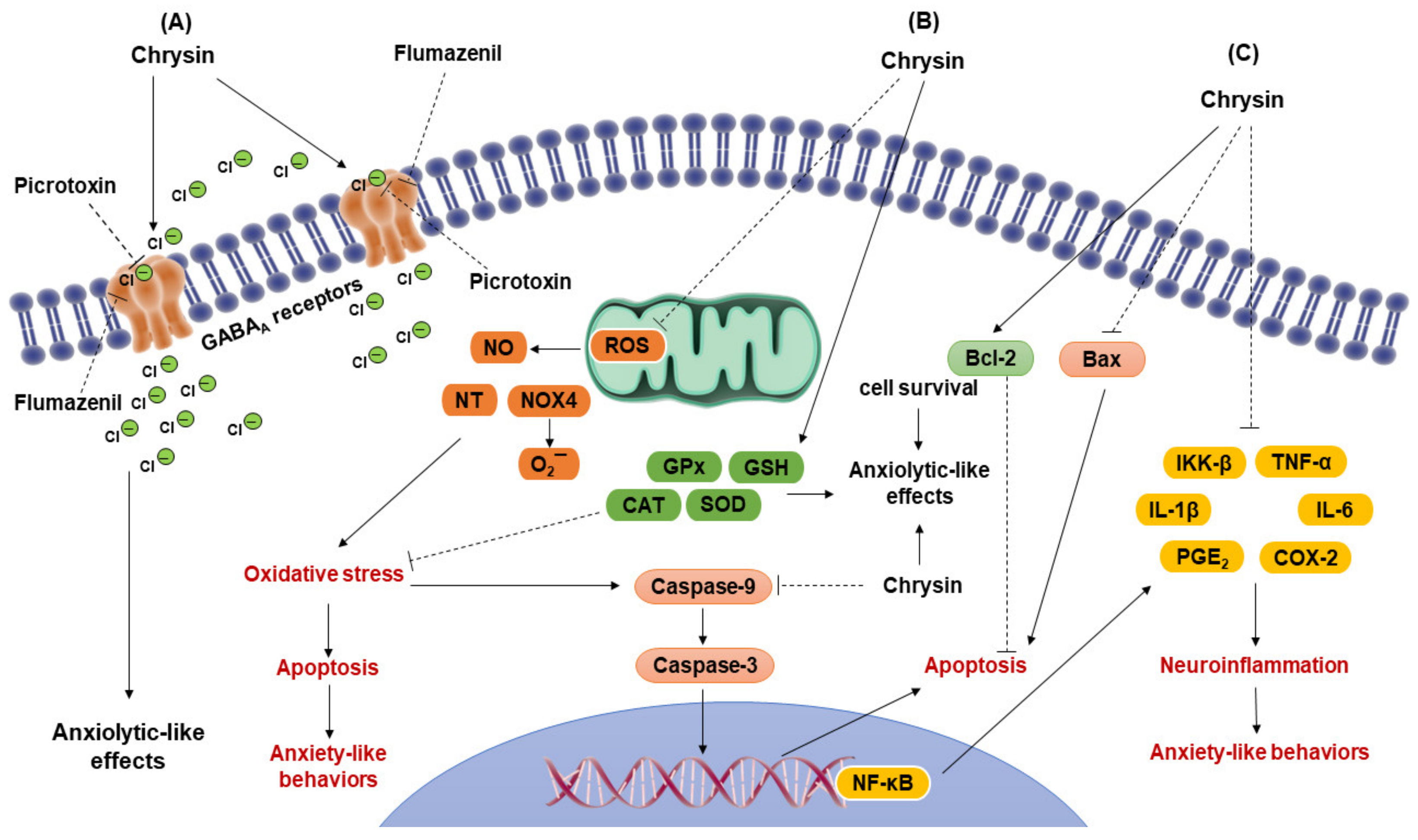
3.2. Antioxidant Activity of Chrysin
3.3. Anti-Inflammatory and Anti-Apoptotic Activity of Chrysin
3.4. Effects of Chrysin on Gut Microbiota
4. Anxiolytic-like Effects of Flavonoid Chrysin
5. Antidepressant-like Effects of Flavonoid Chrysin
6. Future Considerations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, M.J.U. Natural products in drug discovery and human health. Phytochem. Rev. 2021, 20, 1–4. [Google Scholar] [CrossRef]
- Tresina, P.S.; Selvam, M.S.; Rajesh, A.; Doss, A.; Mohan, V.R. Natural products in drug discovery: Approaches and development. J. Pharm. Res. Int. 2021, 33, 93–110. [Google Scholar] [CrossRef]
- Dzobo, K. The role of natural products as sources of therapeutic agents for innovative drug discovery. In Comprehensive Pharmacology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–15. [Google Scholar] [CrossRef]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From theory to practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef] [PubMed]
- German-Ponciano, L.J.; Rosas-Sánchez, G.U.; Rivadeneyra-Domínguez, E.; Rodríguez-Landa, J.F. Advances in the preclinical study of some flavonoids as potential antidepressant agents. Scientifica 2018, 2018, 2963565. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Demeneghi, R.; Rodríguez-Landa, J.F.; Guzmán-Gerónimo, R.I.; Acosta-Mesa, H.G.; Meza-Alvarado, E.; Vargas-Moreno, I.; Herrera-Meza, S. Effect of blackberry juice (Rubus fruticosus L.) on anxiety-like behaviour in Wistar rats. Int. J. Food Sci. Nutr. 2018, 70, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Jayaraman, A.; Chapkin, R.S.; Howard, M.; Mohankumar, K.; Shrestha, R. Flavonoids: Structure-function and mechanisms of action and opportunities for drug development. Toxicol. Res. 2021, 37, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Chaturvedi, S. A comprehensive review on chrysin: Emphasis on molecular targets, pharmacological actions and bio-pharmaceutical aspects. Curr. Drug Targets 2022, 23, 420–436. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Mishra, P.S.; Bandopadhyay, R.; Khurana, N.; Angelopoulou, E.; Paudel, Y.N.; Piperi, C. Neuroprotective potential of chrysin: Mechanistic insights and therapeutic potential for neurological disorders. Molecules 2021, 26, 6456. [Google Scholar] [CrossRef]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Kopustinskiene, D.M.; Simal-Gandara, J.; Bernatoniene, J.; Samarghandian, S. An updated review on the versatile role of chrysin in neurological diseases: Chemistry, pharmacology, and drug delivery approaches. Biomed. Pharm. 2021, 141, 111906. [Google Scholar] [CrossRef]
- Filho, C.B.; Jesse, C.R.; Donato, F.; Giacomeli, R.; Del Fabbro, L.; da Silva Antunes, M.; de Gomes, M.G.; Goes, A.T.R.; Boeira, S.P.; Prigol, M.; et al. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na+,K+-ATPase activity in the hippocampus and prefrontal cortex of mice: Antidepressant effect of chrysin. Neuroscience 2015, 289, 367–380. [Google Scholar] [CrossRef]
- Bortolotto, V.C.; Pinheiro, F.C.; Araujo, S.M.; Poetini, M.R.; Bertolazi, B.S.; de Paula, M.T.; Meichtry, L.B.; de Almeida, F.P.; de Freitas Couto, S.; Jesse, C.R.; et al. Chrysin reverses the depressive-like behavior induced by hypothyroidism in female mice by regulating hippocampal serotonin and dopamine. Eur. J. Pharmacol. 2018, 822, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Germán-Ponciano, L.J.; Rosas-Sánchez, G.U.; Ortiz-Guerra, S.I.; Soria-Fregozo, C.; Rodríguez-Landa, J.F. Effects of chrysin on mRNA expression of 5-HT1A and 5-HT2A receptors in the raphe nuclei and hippocampus. Rev. Bras. Farmacog. 2021, 31, 352–360. [Google Scholar] [CrossRef]
- Rodríguez-Landa, J.F.; Hernández-López, F.; Martínez-Mota, L.; Scuteri, D.; Bernal-Morales, B.; Rivadeneyra-Domínguez, E. GABAA/benzodiazepine receptor complex in the dorsal hippocampus mediates the effects of chrysin on anxiety-like behaviour in female rats. Front. Behav. Neurosci. 2022, 15, 789557. [Google Scholar] [CrossRef]
- Medina, J.H.; Paladini, A.C.; Wolfman, C.; Levi de Stein, M.; Calvo, D.; Diaz, L.E.; Peña, C. Chrysin (5,7-di-OH-flavone), a naturally-occurring ligand for benzodiazepine receptors, with anticonvulsant properties. Biochem. Pharmacol. 1990, 40, 2227–2231. [Google Scholar] [CrossRef]
- Salgueiro, J.B.; Ardenghi, P.; Dias, M.; Ferreira, M.B.; Izquierdo, I.; Medina, J.H. Anxiolytic natural and synthetic flavonoid ligands of the central benzodiazepine receptor have no effect on memory tasks in rats. Pharmacol. Biochem. Behav. 1997, 58, 887–891. [Google Scholar] [CrossRef]
- Zanoli, P.; Avallone, R.; Baraldi, M. Behavioral characterisation of the flavonoids apigenin and chrysin. Fitoterapia 2000, 71, S117–S123. [Google Scholar] [CrossRef]
- Filho, C.B.; Jesse, C.R.; Donato, F.; Del Fabbro, L.; de Gomes, M.G.; Goes, A.T.R.; Souza, L.C.; Giacomeli, R.; Antunes, M.; Luchese, C.; et al. Neurochemical factors associated with the antidepressant-like effect of flavonoid chrysin in chronically stressed mice. Eur. J. Pharmacol. 2016, 791, 284–296. [Google Scholar] [CrossRef]
- Goes, A.T.; Jesse, C.R.; Antunes, M.S.; Ladd, F.V.L.; Ladd, A.A.L.; Luchese, C.; Paroul, N.; Boeira, S.P. Protective role of chrysin on 6-hydroxydopamine-induced neurodegeneration a mouse model of Parkinson’s disease: Involvement of neuroinflammation and neurotrophins. Chem. Biol. Interact. 2018, 279, 111–120. [Google Scholar] [CrossRef]
- Souza, L.C.; Antunes, M.S.; Filho, C.B.; Del Fabbro, L.; de Gomes, M.G.; Goes, A.T.; Donato, F.; Prigol, M.; Boeira, S.P.; Jesse, C.R. Flavonoid chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol. Biochem. Behav. 2015, 134, 22–30. [Google Scholar] [CrossRef]
- Feiger, J.A.; Snyder, R.L.; Walsh, M.J.; Cissne, M.; Cwiek, A.; Al-Momani, S.I.; Chiou, K.S. The role of neuroinflammation in neuropsychiatric disorders following traumatic brain injury: A systematic review. J. Head Trauma Rehabil. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Sathiavelu, J.; Senapathy, G.J.; Devaraj, R.; Namasivayam, N. Hepatoprotective effect of chrysin on prooxidant-antioxidant status during ethanol-induced toxicity in female albino rats. J. Pharm. Pharmacol. 2009, 61, 809–817. [Google Scholar] [CrossRef] [PubMed]
- German-Ponciano, L.J.; Costa, B.P.D.; Feitosa, L.M.; dos Santos Campos, K.; da Silva Chaves, S.N.; Cueto-Escobedo, J.; Maximino, C. Chrysin, but not flavone backbone, decreases anxiety-like behavior in animal screens. Neurochem. Int. 2020, 140, 104850. [Google Scholar] [CrossRef]
- Wolfman, C.; Viola, H.; Paladini, A.; Dajas, F.; Medina, J.H. Possible anxiolytic effects of chrysin, a central benzodiazepine receptor ligand isolated from Passiflora Coerulea. Pharmacol. Biochem. Behav. 1994, 47, 1–4. [Google Scholar] [CrossRef]
- Filho, C.B.; Jesse, C.R.; Donato, F.; Del Fabbro, L.; Gomes de Gomes, M.; Rossito Goes, A.T.; Souza, L.C.; Boeira, S.P. Chrysin promotes attenuation of depressive-like behavior and hippocampal dysfunction resulting from olfactory bulbectomy in mice. Chem. Biol. Interact. 2016, 260, 154–162. [Google Scholar] [CrossRef]
- Rodríguez-Landa, J.F.; Hernández-López, F.; Cueto-Escobedo, J.; Herrera-Huerta, E.V.; Rivadeneyra-Domínguez, E.; Bernal-Morales, B.; Romero-Avendaño, E. Chrysin (5,7-dihydroxyflavone) exerts anxiolytic-like effects through GABAA receptors in a surgical menopause model in rats. Biomed. Pharmacother. 2019, 109, 2387–2395. [Google Scholar] [CrossRef]
- Mantawy, E.M.; El-Bakly, W.M.; Esmat, A.; Badr, A.M.; El-Demerdash, E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur. J. Pharmacol. 2014, 728, 107–118. [Google Scholar] [CrossRef]
- Rani, N.; Bharti, S.; Bhatia, J.; Tomar, A.; Nag, T.C.; Ray, R.; Arya, D.S. Inhibition of TGF-β by a novel PPAR-γ agonist, chrysin, salvages β-receptor stimulated myocardial injury in rats through MAPKs-dependent mechanism. Nutr. Metab. 2015, 12, 11. [Google Scholar] [CrossRef]
- Rani, N.; Bharti, S.; Bhatia, J.; Nag, T.C.; Ray, R.; Arya, D.S. Chrysin, a PPAR-γ agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem. Biol. Interact. 2016, 250, 59–67. [Google Scholar] [CrossRef]
- Campos, H.M.; da Costa, M.; da Silva Moreira, L.K.; da Silva Neri, H.F.; Branco da Silva, C.R.; Pruccoli, L.; Dos Santos, F.C.A.; Costa, E.A.; Tarozzi, A.; Ghedini, P.C. Protective effects of chrysin against the neurotoxicity induced by aluminium: In Vitro and In Vivo studies. Toxicology 2022, 465, 153033. [Google Scholar] [CrossRef]
- Harasstani, O.A.; Moin, S.; Tham, C.L.; Liew, C.Y.; Ismail, N.; Rajajendram, R.; Harith, H.H.; Zakaria, Z.A.; Mohamad, A.S.; Sulaiman, M.R.; et al. Flavonoid combinations cause synergistic inhibition of proinflammatory mediator secretion from lipopolysaccharide-induced RAW 264.7 cells. Inflamm. Res. 2010, 59, 711–721. [Google Scholar] [CrossRef]
- Feng, X.; Qin, H.; Shi, Q.; Zhang, Y.; Zhou, F.; Wu, H.; Ding, S.; Niu, Z.; Lu, Y.; Shen, P. Chrysin attenuates inflammation by regulating M1/M2 status via activating PPARγ. Biochem. Pharmacol. 2014, 89, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Harasstani, O.A.; Tham, C.L.; Israf, D.A. Kaempferol and chrysin synergies to improve septic mice survival. Molecules 2017, 22, 92. [Google Scholar] [CrossRef]
- Wang, H.; Hui, K.M.; Chen, Y.; Xu, S.; Wong, J.T.; Xue, H. Structure-activity relationships of flavonoids, isolated from Scutellaria baicalensis, binding to benzodiazepine site of GABAA receptor complex. Planta Med. 2002, 68, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Goutman, J.D.; Waxemberg, M.D.; Doñate-Oliver, F.; Pomata, P.E.; Calvo, D.J. Flavonoid modulation of ionic currents mediated by GABAA and GABAc receptors. Eur. J. Pharmacol. 2003, 461, 79–87. [Google Scholar] [CrossRef]
- Rodríguez-Landa, J.F.; Guillén-Ruiz, G.; Hernández-López, F.; Cueto-Escobedo, J.; Rivadeneyra-Domínguez, E.; Bernal-Morales, B.; Herrera-Huerta, E.V. Chrysin reduces anxiety-like behavior through actions on GABAA receptors during metestrus-diestrus in the rat. Behav. Brain Res. 2021, 397, 112952. [Google Scholar] [CrossRef]
- Cueto-Escobedo, J.; Andrade-Soto, J.; Lima-Maximino, M.; Maximino, C.; Hernández-López, F.; Rodríguez-Landa, J.F. Involvement of GABAergic system in the antidepressant-like effects of chrysin (5, 7-dihydroxyflavone) in ovariectomized rats in the forced swim test: Comparison with neurosteroids. Behav. Brain Res. 2020, 386, 112590. [Google Scholar] [CrossRef]
- Haider, M.; Salman, M.; Kaushik, P.; Bharadwaj, N.; Aggarwal, N.B.; Tabassum, H.; Parvez, S. Chrysin ameliorates 3 nitropropinoic acid induced neurotoxicity targeting behavioural, biochemical and histological alterations. Int. J. Neurosci. 2020, 1–9, Online ahead of print. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Cui, W.; Zhou, W.; Zhao, X. 5-HT1A receptor-mediated attenuation of heat hyperalgesia and mechanical allodynia by chrysin in mice with experimental mononeuropathy. Reg. Anesth. Pain Med. 2020, 45, 610–619. [Google Scholar] [CrossRef]
- Krishnamoorthy, A.; Sevanan, M.; Mani, S.; Balu, M.; Balaji, S.; Ramajayan, P. Chrysin restores MPTP induced neuroinflammation, oxidative stress and neurotrophic factors in an acute Parkinson’s disease mouse model. Neurosci. Lett. 2019, 709, 134382. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Samarghandian, S.; Azimin-Nezhad, M.; Samini, F. Effect of chrysin on nociception in formalin test and serum levels of noradrenalin and corticosterone in rats. Int. J. Clin. Exp. Med. 2015, 8, 2465. [Google Scholar]
- Rashno, M.; Ghaderi, S.; Nesari, A.; Khorsandi, L.; Farbood, Y.; Sarkaki, A. Chrysin attenuates traumatic brain injury-induced recognition memory decline, and anxiety/depression-like behaviors in rats: Insights into underlying mechanisms. Psychopharmacology 2020, 237, 1607–1619. [Google Scholar] [CrossRef]
- Bortolotto, V.C.; Araujo, S.M.; Pinheiro, F.C.; Poetini, M.R.; Meichtry, L.B.; Fronza, M.G.; Boeira, S.P.; Savegnago, L.; Prigol, M. Chrysin restores memory deficit in hypothyroidism mice: Behavioral, neurochemical and computational approaches involving the neurotrophinergic system. J. Psychiatr. Res. 2021, 144, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Gargouri, B.; Bhatia, H.S.; Bouchard, M.; Fiebich, B.L.; Fetoui, H. Inflammatory and oxidative mechanisms potentiate bifenthrin-induced neurological alterations and anxiety-like behavior in adult rats. Toxicol. Lett. 2018, 294, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef]
- Smaga, I.; Niedzielska, E.; Gawlik, M.; Moniczewski, A.; Krzek, J.; Przegaliński, E.; Pera, J.; Filip, M. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol. Rep. 2015, 67, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Di Sarno, R.; Brigida, A.; Caprio, G.G.; Ciardiello, D.; Dallio, M.; Sangineto, M.; Fagoonee, S.; Abenavoli, L.; Luzza, F.; Gravina, A.G.; et al. Critical review on the use and abuse of alcohol. When the dose makes the difference. Minerva Med. 2020, 111, 344–353. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Hovatta, I.; Juhila, J.; Donner, J. Oxidative stress in anxiety and comorbid disorders. Neurosci. Res. 2010, 68, 261–275. [Google Scholar] [CrossRef]
- Sarwar, H.; Rafiqi, S.I.; Ahmad, S.; Jinna, S.; Khan, S.A.; Karim, T.; Qureshi, O.; Zahid, Z.A.; Elhai, J.D.; Levine, J.C.; et al. Hyperinsulinemia associated depression. Clin. Med. Insights Endocrinol. Diabetes 2022, 15, 11795514221090244. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Younos, C.; Soulimani, R. Positive correlation between peripheral blood granulocyte oxidative status and level of anxiety in mice. Eur. J. Pharmacol. 2007, 564, 146–149. [Google Scholar] [CrossRef]
- Salim, S.; Sarraj, N.; Taneja, M.; Saha, K.; Tejada-Simon, M.V.; Chugh, G. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav. Brain Res. 2010, 208, 545–552. [Google Scholar] [CrossRef]
- Kaufmann, F.N.; Gazal, M.; Mondin, T.C.; Cardoso, T.A.; Quevedo, L.Á.; Souza, L.D.; Ghisleni, G. Cognitive psychotherapy treatment decreases peripheral oxidative stress parameters associated with major depression disorder. Biol. Psychol. 2015, 110, 175–181. [Google Scholar] [CrossRef]
- Moccia, M.; Capacchione, A.; Lanzillo, R.; Carbone, F.; Micillo, T.; Perna, F.; Brescia Morra, V. Coenzyme Q10 supplementation reduces peripheral oxidative stress and inflammation in interferon-β1a-treated multiple sclerosis. Ther. Adv. Neurol. Disord. 2019, 12, 1756286418819074. [Google Scholar] [CrossRef]
- Nakagawa, Y.; To, M.; Saruta, J.; Yamamoto, Y.; Yamamoto, T.; Shimizu, T.; Kamata, Y.; Matsuo, M.; Tsukinoki, K. Effect of social isolation stress on saliva BDNF in rat. J. Oral Sci. 2019, 61, 516–520. [Google Scholar] [CrossRef]
- Khurana, K.; Bansal, N. Lacidipine attenuates caffeine-induced anxiety-like symptoms in mice: Role of calcium-induced oxido-nitrosative stress. Pharmacol. Rep. 2019, 71, 1264–1272. [Google Scholar] [CrossRef]
- Risbrough, V.B.; Vaughn, M.N.; Friend, S.F. Role of inflammation in traumatic brain injury-associated risk for neuropsychiatric disorders: State of the evidence and where do we go from here. Biol. Psychiatry 2022, 91, 438–448. [Google Scholar] [CrossRef]
- Zorzo, C.; Méndez-López, M.; Méndez, M.; Arias, J.L. Adult social isolation leads to anxiety and spatial memory impairment: Brain activity pattern of COx and c-Fos. Behav. Brain Res. 2019, 365, 170–177. [Google Scholar] [CrossRef]
- Park, S.S.; Park, H.S.; Kim, C.J.; Baek, S.S.; Kim, T.W. Exercise attenuates maternal separation-induced mood disorder-like behaviors by enhancing mitochondrial functions and neuroplasticity in the dorsal raphe. Behav. Brain Res. 2019, 372, 112049. [Google Scholar] [CrossRef]
- Zhang, L.X.; Levine, S.; Dent, G.; Zhan, Y.; Xing, G.; Okimoto, D.; Kathleen Gordon, M.; Post, R.M.; Smith, M.A. Maternal deprivation increases cell death in the infant rat brain. Brain Res. Dev. Brain Res. 2002, 133, 1–11. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, X.; Wang, Y.; Liu, M.; Sun, A.; Li, N.; Lin, Y.; Geng, Z.; Jin, Y.; Li, X. Adolescent escitalopram prevents the effects of maternal separation on depression- and anxiety-like behaviours and regulates the levels of inflammatory cytokines in adult male mice. Int. J. Dev. Neurosci. 2017, 62, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rivera, N.M.; Fernández-Guasti, A.; Ramírez-Rodríguez, G.; Estrada-Camarena, E. Acute stress further decreases the effect of ovariectomy on immobility behavior and hippocampal cell survival in rats. Psychoneuroendocrinology 2013, 38, 1407–1417. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, J.; Wang, X.; Xie, K.; Luan, Q.; Wan, N.; Zhang, Q.; Jiang, H.; Liu, D. Antidepressant-like activity of resveratrol treatment in the forced swim test and tail suspension test in mice: The HPA axis, BDNF expression and phosphorylation of ERK. Pharmacol. Biochem. Behav. 2013, 112, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Abd Al Haleem, E.N.; Ahmed, H.I.; El-Naga, R.N. Lycopene and chrysin through mitigation of neuroinflammation and oxidative stress exerted antidepressant effects in clonidine-induced depression-like behavior in rats. J. Diet Suppl. 2021, 1–20, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bear, T.L.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The role of the gut microbiota in dietary interventions for depression and anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef] [PubMed]
- Sonali, S.; Ray, B.; Ahmed Tousif, H.; Rathipriya, A.G.; Sunanda, T.; Mahalakshmi, A.M.; Rungratanawanich, W.; Essa, M.M.; Qoronfleh, M.W.; Chidambaram, S.B.; et al. Mechanistic insights into the link between gut dysbiosis and major depression: An extensive review. Cells 2022, 11, 1362. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Dinan, T.G. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Qiao, Y.; Xie, R.; Lin, L.; Jiang, J.; Wang, C.; Li, G. Fecal microbiota transplantation ameliorates stress-induced depression-like behaviors associated with the inhibition of glial and NLRP3 inflammasome in rat brain. J. Psychiatr. Res. 2021, 137, 147–157. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; Goñi, I. Dietary polyphenols and human gut microbiota: A review. Food Rev. Int. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Hong, M.; Zhang, R.; Liu, Y.; Wu, Z.; Weng, P. The interaction effect between tea polyphenols and intestinal microbiota: Role in ameliorating neurological diseases. J. Food Biochem. 2022, 46, e13870. [Google Scholar] [CrossRef]
- Zhou, N.; Gu, X.; Zhuang, T.; Xu, Y.; Yang, L.; Zhou, M. Gut microbiota: A pivotal hub for polyphenols as antidepressants. J. Agric. Food Chem. 2020, 68, 6007–6020. [Google Scholar] [CrossRef]
- Cheng, N.; Chen, S.; Liu, X.; Zhao, H.; Cao, W. Impact of schisandra chinensis bee pollen on nonalcoholic fatty liver disease and gut microbiota in high fat diet induced obese mice. Nutrients 2019, 11, 346. [Google Scholar] [CrossRef]
- Wu, W.T.; Wang, Z.H. Effects of chrysin on intestinal inflammation and gut microbiota in lipopolysaccharides-induced mice. FASEB J. 2019, 33, 764–768. [Google Scholar] [CrossRef]
- Wen, X.; Walle, T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab. Dispos. 2006, 34, 1786–1792. [Google Scholar] [CrossRef]
- Paladini, A.C.; Marder, M.; Viola, H.; Wolfman, C.; Wasowski, C.; Medina, J.H. Flavonoids and the central nervous system: From forgotten factors to potent anxiolytic compounds. J. Pharm. Pharmacol. 1999, 51, 519–526. [Google Scholar] [CrossRef]
- Brown, E.; Hurd, N.S.; McCall, S.; Ceremuga, T.E. Evaluation of the anxiolytic effects of chrysin, a Passiflora incarnata extract, in the laboratory rat. AANA J. 2007, 75, 333–337. [Google Scholar]
- Germán-Ponciano, L.J.; Puga-Olguín, A.; Rovirosa-Hernández, M.J.; Caba, M.; Meza, E.; Rodríguez-Landa, J.F. Differential effects of acute and chronic treatment with the flavonoid chrysin on anxiety-like behavior and Fos immunoreactivity in the lateral septal nucleus in rats. Acta Pharm. 2020, 70, 387–397. [Google Scholar] [CrossRef]
- Ognibene, E.; Bovicelli, P.; Adriani, W.; Saso, L.; Laviola, G. Behavioral effects of 6-bromoflavanone and 5-methoxy-6,8-dibromoflavanone as anxiolytic compounds. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 128–134. [Google Scholar] [CrossRef]
- Albert, K.; Pruessner, J.; Newhouse, P. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology 2015, 59, 14–24. [Google Scholar] [CrossRef]
- Rocca, W.A.; Grossardt, B.R.; Geda, Y.E.; Gostout, B.S.; Bower, J.H.; Maraganore, D.M.; de Andrade, M.; Melton, L.J. 3rd Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause 2018, 25, 1275–1285. [Google Scholar] [CrossRef]
- Giannini, A.; Caretto, M.; Genazzani, A.R.; Simoncini, T. Neuroendocrine changes during menopausal transition. Endocrines 2021, 2, 405–416. [Google Scholar] [CrossRef]
- Pinna, G. Allopregnanolone, the neuromodulator turned therapeutic agent: Thank you, next? Front. Endocrinol. 2020, 11, 236. [Google Scholar] [CrossRef]
- Puga-Olguín, A.; Rodríguez-Landa, J.F.; Rovirosa-Hernández, M.J.; Germán-Ponciano, L.J.; Caba, M.; Meza, E.; Guillén-Ruiz, G.; Olmos-Vázquez, O.J. Long-term ovariectomy increases anxiety- and despair-like behaviors associated with lower Fos immunoreactivity in the lateral septal nucleus in rats. Behav. Brain Res. 2019, 360, 185–195. [Google Scholar] [CrossRef]
- Lovick, T.A. GABA in the female brain—Oestrous cycle-related changes in GABAergic function in the periaqueductal grey matter. Pharmacol. Biochem. Behav. 2008, 90, 43–50. [Google Scholar] [CrossRef]
- Rodríguez-Landa, J.F. Considerations of timing post-ovariectomy in mice and rats in studying anxiety- and depression-like behaviors associated with surgical menopause in women. Front. Behav. Neurosci. 2022, 16, 829274. [Google Scholar] [CrossRef]
- Paul, S.M.; Pinna, G.; Guidotti, A. Allopregnanolone: From molecular pathophysiology to therapeutics. A historical perspective. Neurobiol. Stress 2020, 12, 100215. [Google Scholar] [CrossRef]
- Georgieva, I.; Lepping, P.; Bozev, V.; Lickiewicz, J.; Pekara, J.; Wikman, S.; Lantta, T. Prevalence, new incidence, course, and risk factors of PTSD, depression, anxiety, and panic disorder during the COVID-19 pandemic in 11 countries. Healthcare 2021, 9, 664. [Google Scholar] [CrossRef]
- Zhukov, D.A.; Vinogradova, E.P. Neurosteroids and depression. Neurochem. J. 2021, 15, 240–246. [Google Scholar] [CrossRef]
- Perez-Caballero, L.; Torres-Sanchez, S.; Romero-López-Alberca, C.; González-Saiz, F.; Mico, J.A.; Berrocoso, E. Monoaminergic system and depression. Cell Tissue Res. 2019, 377, 107–113. [Google Scholar] [CrossRef]
- Rana, T.; Behl, T.; Sehgal, A.; Srivastava, P.; Bungau, S. Unfolding the role of BDNF as a biomarker for treatment of depression. J. Mol. Neurosci. 2021, 71, 2008–2021. [Google Scholar] [CrossRef]
- Zhou, C.; Zhong, J.; Zou, B.; Fang, L.; Chen, J.; Deng, X.; Zhang, L.; Zhao, X.; Qu, Z.; Lei, Y.; et al. Meta-analyses of comparative efficacy of antidepressant medications on peripheral BDNF concentration in patients with depression. PLoS ONE 2017, 12, e0172270. [Google Scholar] [CrossRef] [PubMed]
- Sagud, M.; Nikolac Perkovic, M.; Vuksan-Cusa, B.; Maravic, A.; Svob Strac, D.; Mihaljevic Peles, A.; Zivkovic, M.; Kusevic, Z.; Pivac, N. A prospective, longitudinal study of platelet serotonin and plasma brain-derived neurotrophic factor concentrations in major depression: Effects of vortioxetine treatment. Psychopharmacol 2016, 233, 3259–3267. [Google Scholar] [CrossRef] [PubMed]
- Hannestad, J.; Dellagioia, N.; Bloch, M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: A meta-analysis. Neuropsychopharmacol 2011, 36, 2452. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.A.; Freitas, T.H.; Stubbs, B.; Maes, M.; Solmi, M.; Veronese, N.; de Andrade, N.Q.; Morris, G.; Fernandes, B.S.; Brunoni, A.R.; et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: Systematic review and meta-analysis. Mol. Neurobiol. 2018, 55, 4195–4206. [Google Scholar] [CrossRef] [PubMed]
- Więdłocha, M.; Marcinowicz, P.; Krupa, R.; Janoska-Jaździk, M.; Janus, M.; Dębowska, W.; Mosiołek, A.; Waszkiewicz, N.; Szulc, A. Effect of antidepressant treatment on peripheral inflammation markers-A meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Patatanian, E.; Nguyen, D.R. Brexanolone: A novel drug for the treatment of postpartum depression. J. Pharm. Pract. 2020, 0897190020979627, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.; Krohn, H.; Richardson, E.; Kimmel, M.; Meltzer-Brody, S.A. Brexanolone treatment program at an academic medical center: Patient selection, 90-day posttreatment outcomes, and lessons learned. J. Acad. Consult. Liaison Psychiatry 2022, 63, 14–22. [Google Scholar] [CrossRef]
| Activity | Chrysin Treatment | Effects | Reference |
|---|---|---|---|
| Antioxidant | 20 mg/kg/30 days, p.o. | ↓ TBARS, lipid hydroperoxides, conjugated dienes tissue, circulatory levels ↑ SOD, CAT, GPx, Gsr activity, GSH, GSTs, vitamin C and vitamin E levels in ethanol-induced toxicity in rats | [22] |
| 50 mg/kg/12 days, i.p. | ↑ GHS levels and CAT and SOD activity in heart homogenate in male rats | [27] | |
| 30 and 60 mg/kg/28 days, p.o. | ↑ NO and GHS levels, GSHPx, CAT, and SOD activity in rat heart homogenate | [28] | |
| 60 mg/kg/28 days, p.o | ↑ 8-OHdG, TBARS levels ↓ GSH, CAT, NO levels | [29] | |
| 1 and 10 mg/kg/60 days, p.o. | ↑ SOD, CAT and GPx activity in PFC and HP of aged mice | [20] | |
| 1.25, 2.5, and 5 µM/30 min exposure | ↓ ROS formation in neuronal SH-SY5Y and microglial THP-1 cells in vitro | [30] | |
| 10, 30, and 100 mg/kg/44 days, p.o. | Protects against aluminum-induced oxidative stress by restored LPO levels and SOD and CAT activity in cortex and HP of male Swiss mice | [30] | |
| Anti-inflammatory | 7.50, 4.75, and 120.90 µM, 18 h exposure | ↓ NO, PGE2 and TNF-α biosynthesis in CLP-induced RAW 264.7 cells | [31] |
| 30 mg/kg/2 weeks, i.p. | ↓ ALT and AST activity ↓ TNF-α and IL-1β levels ↑ IL-10 and adiponectin in high-fat feeding mice | [32] | |
| 25 and 50 mg/kg/12 days, i.p. | ↓ NF-κB, iNOS, COX-2, and TNF-α expression in heart homogenate of DOX-induced cardiotoxicity mice | [27] | |
| 30 and 60 mg/kg/28 days, p.o. | ↑ PPAR-γ and TGF-β expression ↓ NF-κBp65 and IKK-β expression and TNF-α level in heart homogenate of isoproterenol-induced myocardial injury rats | [28,29] | |
| 5 and 20 mg/kg/28 days, p.o. | ↓ TNF-α, IL-1β and IL-6 levels in PFC and HP of chronically stressed mice | [18] | |
| 5 mg/kg/1 h before LP, i.p. | ↓ AST and TNF-α serum levels in septic mice survival | [33] | |
| 5 µM/24 h exposure | ↓ iNOS, IL-1β, and TNF-α expression in microglial THP-1 cells exposed to LPS | [30] | |
| GABAergic/BZD | 3 µM, 60 min exposure | Acts as competitive ligand for central BZD site in bovine cerebral cortical membranes in vitro | [15] |
| 13 µM, 60 min exposure | Acts as competitive ligand for peripheral BZD binding site in rat kidneys membranes in vitro | [15] | |
| 1 mg/kg, i.p. | Activates the GABAA/BZD receptor complex in male CF1 mice | [24] | |
| 1 mg/kg, i.p. | Activates the GABAA/BZD receptor complex in male Sprague Dawley rats | [17] | |
| 0.62 µM, 2 h exposure | Acts as competitive ligand for central BZD site in synaptosomal fractions of rat brain in vitro | [34] | |
| 10 and 30 µM, 30 s exposure | Modulates the activity of Cl− ion channel in the GABAA receptor expressed in Xenopus oocytes in vitro | [35] | |
| 2 mg/kg, i.p. | ↓ Anxiety-like behavior by modulating Cl− ion channel in the GABAA receptor of cycling female rats | [36] | |
| 2 mg/kg, i.p. | ↓ Depression-like behavior by modulating GABA-binding site in the GABAA receptor of ovariectomized female rats | [37] | |
| 0.5 µg/rat, i.h. | ↓ Anxiety-like behavior by modulating GABAA/BZD receptor complex in the dorsal hippocampus of cycling female rats | [14] | |
| Serotonergic | 5 and 20 mg/kg, p.o. | ↑ 5-HT levels and 5-HIAA/5-HT ratio in HP of chronic stressed mice | [18] |
| 20 mg/kg/28 days, p.o. | ↑ 5-HT levels in PFC and HP in female mice with hypothyroidism | [12] | |
| 50 mg/kg twice a day per 4 days, p.o. | ↑ 5-HT levels in the striatum of the rat brain | [38] | |
| 10 and 30 mg/kg/2 weeks, p.o. | ↑ 5-HT spinal levels ↓ 5-HIAA/5-HT ratio in male mice with experimental neuropathy | [39] | |
| 5 mg/kg/28 days, i.p. | ↓ 5-HT1A receptor expression in the dorsal raphe ↑ 5-HT1A and 5-HT2A in the hippocampus of male rats | [13] | |
| Dopaminergic | 10 mg/kg/28 days, p.o | ↑ DA striatal levels in mice | [19] |
| 50, 100 and 200 mg/kg/5 days, p.o. | ↑ DA levels in striatum of mice treated with 1-methyl-1,2,3,6-tetrahidropidine | [40] | |
| 20 mg/kg/28 days, p.o. | ↑ DA levels in PFC and HP in a hypothyroidism model in female mice | [12] | |
| Noradrenergic | 50, 100 and 150 mg/kg, i.p. | ↓ NE serum levels in rats with pain induced by formalin | [41] |
| 20 mg/kg/28 days, p.o. | No effects | [12] | |
| Anti-apoptotic | 25 and 50 mg/kg/12 days, i.p. | ↓ Bax, caspase-3, and cytochrome c activity ↑ Bcl-2 expression in rat heart tissue extract | [27] |
| 30 and 60 mg/kg/28 days, p.o. | ↑ Bcl-2 expression ↓ Bax and caspase-3 activity | [29] | |
| 5 and 20 mg/kg/28 days, p.o. | ↓ Caspase-3 and caspase-9 activity in HP and PFC of chronically stressed mice | [18] | |
| 25, 50 and 100 mg/kg/3 days, p.o. | ↓ Apoptotic index in cerebral cortex and HP of rats with traumatic brain injury | [42] | |
| Neuroendocrine | 5 and 20 mg/kg/28 days, p.o. | ↓ Corticosterone plasma levels in chronically stressed mice | [11] |
| 5 and 20 mg/kg/28 days, p.o. | ↓ CRH and ACTH in chronically stressed mice | [18] | |
| 50, 100 and 150 mg/kg, i.p | ↓ Corticosterone serum levels in rats with pain induced by formalin | [41] | |
| Neurotrophic | 5 and 20 mg/kg/28 days, p.o. | ↑ BDNF and NGF levels in PFC and HP in chronically stressed mice | [11] |
| 1 and 10 mg/kg/60 days, p.o. | ↑ BDNF levels in HP and PFC in aged mice | [20] | |
| 10 mg/kg/28 days, p.o. | ↑ BDNF and NGF levels in striatum in a Parkinson’s disease model in mice | [19] | |
| 20 mg/kg/28 days, p.o. | ↑ BDNF and NGF in HP and PFC in mice subjected to a hypothyroidism model | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Landa, J.F.; German-Ponciano, L.J.; Puga-Olguín, A.; Olmos-Vázquez, O.J. Pharmacological, Neurochemical, and Behavioral Mechanisms Underlying the Anxiolytic- and Antidepressant-like Effects of Flavonoid Chrysin. Molecules 2022, 27, 3551. https://doi.org/10.3390/molecules27113551
Rodríguez-Landa JF, German-Ponciano LJ, Puga-Olguín A, Olmos-Vázquez OJ. Pharmacological, Neurochemical, and Behavioral Mechanisms Underlying the Anxiolytic- and Antidepressant-like Effects of Flavonoid Chrysin. Molecules. 2022; 27(11):3551. https://doi.org/10.3390/molecules27113551
Chicago/Turabian StyleRodríguez-Landa, Juan Francisco, León Jesús German-Ponciano, Abraham Puga-Olguín, and Oscar Jerónimo Olmos-Vázquez. 2022. "Pharmacological, Neurochemical, and Behavioral Mechanisms Underlying the Anxiolytic- and Antidepressant-like Effects of Flavonoid Chrysin" Molecules 27, no. 11: 3551. https://doi.org/10.3390/molecules27113551
APA StyleRodríguez-Landa, J. F., German-Ponciano, L. J., Puga-Olguín, A., & Olmos-Vázquez, O. J. (2022). Pharmacological, Neurochemical, and Behavioral Mechanisms Underlying the Anxiolytic- and Antidepressant-like Effects of Flavonoid Chrysin. Molecules, 27(11), 3551. https://doi.org/10.3390/molecules27113551






