Bicyclic Isoxazoline Derivatives: Synthesis and Evaluation of Biological Activity
Abstract
:1. Introduction
2. Results and Discussion
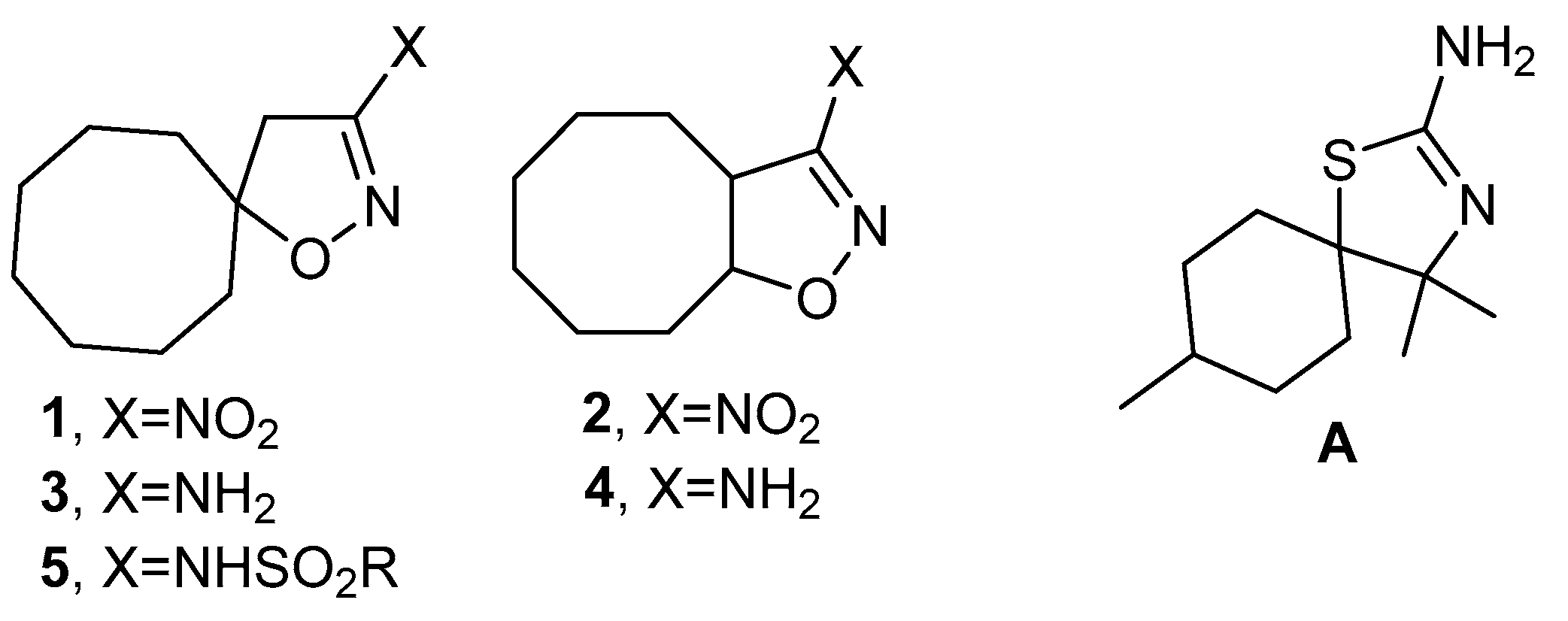
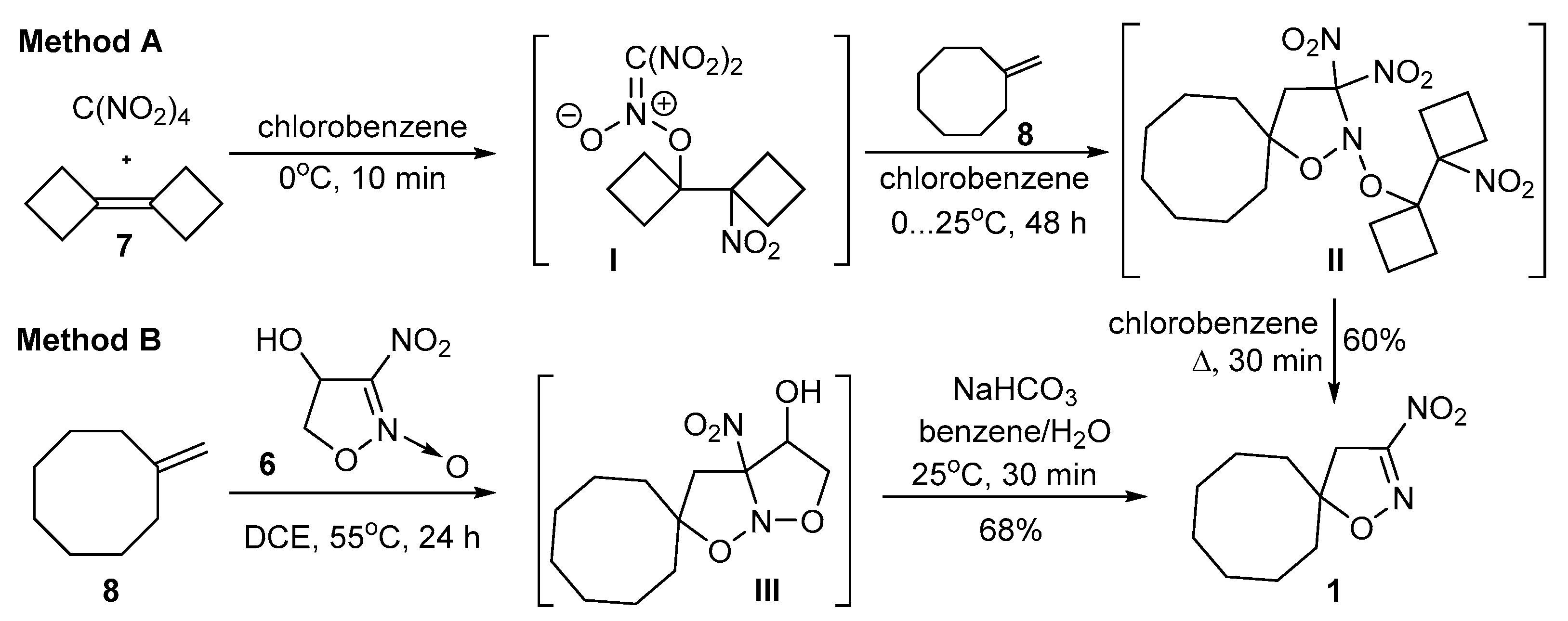
2.1. Synthesis of 3-Substituted Isoxazolines
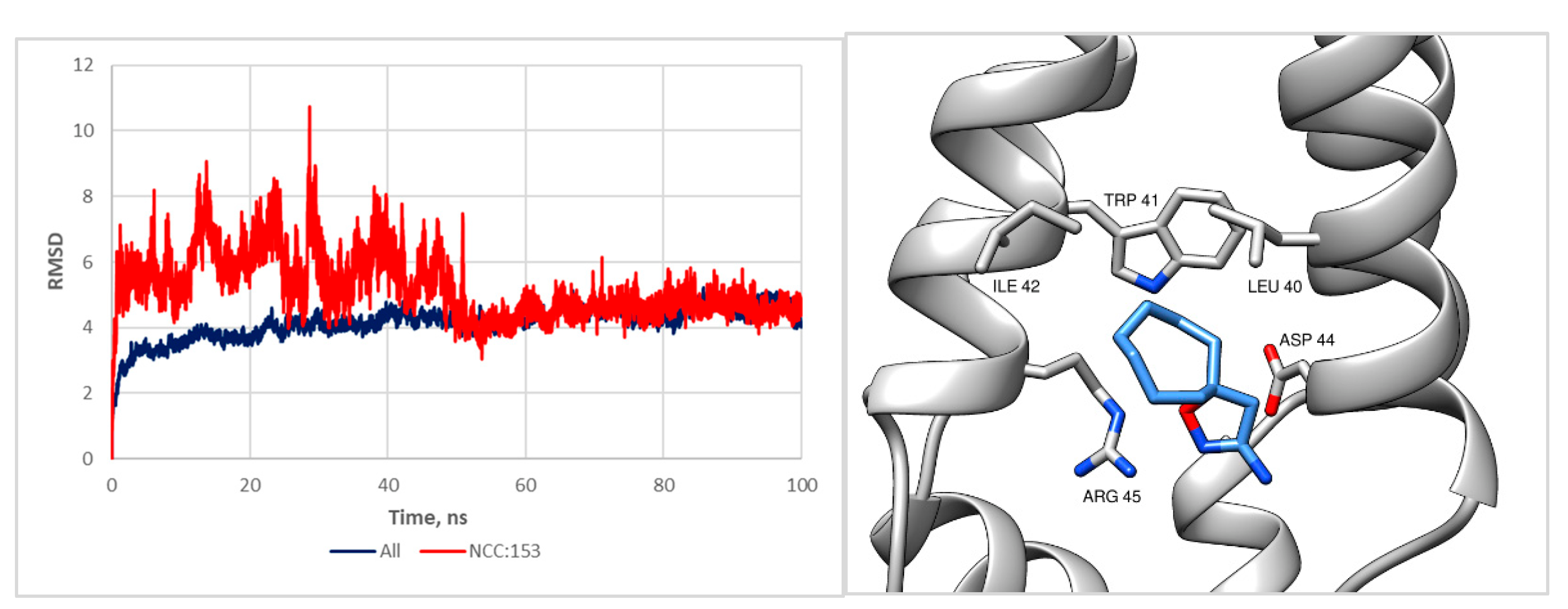
2.2. Bioscreening Results and Molecular Modeling
3. Materials and Methods
3.1. Chemistry
3.1.1. General Remarks
3.1.2. One-Pot Synthesis of 3-Nitroisoxazolines 1,2
General Method A
General Method B
3.1.3. Synthesis of 3-Aminoisoxazolines 7,8 (General Method)
3.1.4. Synthesis of Sulfonamides 5a-l (General Method)
3.2. Pharmacological Assays
3.2.1. Evaluation of Antiviral Activity against Influenza Virus
3.2.2. Screening of Antimicrobial Activity
3.2.3. MTT-Assay
3.3. Molecular Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Morley, A.D.; Pugliese, A.; Birchall, K.; Bower, J.; Brennan, P.; Brown, N.; Chapman, T.; Drysdale, M.; Gilbert, I.H.; Hoelder, S.; et al. Fragment-based hit identification: Thinking in 3D. Drug Discov. Today 2013, 18, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.J.; Dekker, T.; Klein, H.F.; Janssen, G.V.; Wijtmans, M.; O’Brien, P.; de Esch, I.J.P. Escape from planarity in fragment-based drug discovery: A physicochemical and 3D property analysis of synthetic 3D fragment libraries. Drug Discov. Today 2020, 38, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Talele, T.T. Opportunities for Tapping into Three-Dimensional Chemical Space through a Quaternary Carbon. J. Med. Chem. 2020, 63, 13291–13315. [Google Scholar] [CrossRef] [PubMed]
- Sveiczer, A.; North, A.J.P.; Mateu, N.; Kidd, S.L.; Sore, H.F.; Spring, D.R. Spirocycles as Rigidified sp3-Rich Scaffolds for a Fragment Collection. Org. Lett. 2019, 21, 4600–4604. [Google Scholar] [CrossRef]
- Hiesinger, K.; Dar’in, D.; Proschak, E.; Krasavin, M. Spirocyclic Scaffolds in Medicinal Chemistry. J. Med. Chem. 2021, 64, 150–183. [Google Scholar] [CrossRef] [PubMed]
- Troelsen, N.S.; Shanina, E.; Gonzalez-Romero, D.; Danková, D.; Jensen, I.S.A.; Śniady, K.J.; Nami, F.; Zhang, H.; Rademacher, C.; Cuenda, A.; et al. The 3F Library: Fluorinated Fsp3-Rich Fragments for Expeditious 19F NMR Based Screening. Angew. Chem. Int. Ed. 2020, 59, 2204–2210. [Google Scholar] [CrossRef]
- Downes, T.D.; Jones, S.P.; Klein, H.F.; Wheldon, M.C.; Atobe, M.; Bond, P.S.; Firth, J.D.; Chan, N.S.; Waddelove, L.; Hubbard, R.E.; et al. Design and Synthesis of 56 Shape-Diverse 3D Fragments. Chem. Eur. J. 2020, 26, 8969–8975. [Google Scholar] [CrossRef]
- Atmika, P.; Hiroshi, H.; Suresh, P.; Keiichi, K.; Shigeki, M.; Motomu, K.; Yutaka, S.; Kazuhisa, S. A Novel Spiro-Heterocyclic Compound Identified by the Silkworm Infection Model Inhibits Transcription in Staphylococcus aureus. Front. Microbiol. 2017, 8, 712. [Google Scholar]
- Couturier, C.; Bauer, A.; Rey, A.; Schroif-Dufour, C.; Broenstrup, M. Armeniaspiroles, a new class of antibacterials: Antibacterial activities and total synthesis of 5-chloro-Armeniaspirole A. Bioorg. Med. Chem. Lett. 2012, 22, 6292–6296. [Google Scholar] [CrossRef]
- Arns, S.; Balgi, A.D.; Shimizu, Y.; Pfeifer, T.A.; Kumar, N.; Shidmoossavee, F.S.; Sun, S.; Tai, S.S.-H.; Agafitei, O.; Jaquith, J.B.; et al. Novel spirothiazamenthane inhibitors of the influenza A M2 proton channel. Eur. J. Med. Chem. 2016, 120, 64–73. [Google Scholar] [CrossRef]
- Clarke, A.K.; Unsworth, W.P. A happy medium: The synthesis of medicinally important medium-sized rings via ring expansion. Chem. Sci. 2020, 11, 2876–2881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, N. The Nitro Group in Organic Synthesis; Wiley-VCH: New York, NY, USA, 2001; 372p. [Google Scholar]
- Vasilenko, D.A.; Sedenkova, K.N.; Kuznetsova, T.S.; Averina, E.B. Synthetic Approaches to Nitro-Substituted Isoxazoles. Synthesis 2019, 51, 1516–1528. [Google Scholar]
- Zhao, C.; Rakesh, K.P.; Ravidar, L.; Fang, W.-Y.; Qin, H.-L. Pharmaceutical and Medicinal Significance of Sulfur (SVI)-Containing Motifs for Drug Discovery: A Critical Review. Eur. J. Med. Chem. 2019, 162, 679–734. [Google Scholar] [CrossRef] [PubMed]
- Torssell, K.B.G. Nitrile Oxides, Nitrones and Nitronates in Organic Synthesis; VCH Publishers: New York, NY, USA, 1988; 753p. [Google Scholar]
- Ivanova, O.A.; Budynina, E.M.; Averina, E.B.; Kuznetsova, T.S.; Zefirov, N.S. Application of a Thermal β-Elimination Reaction to N-Alkoxy-3,3-dinitroisoxazolidines. Synthesis 2006, 2006, 706–710. [Google Scholar]
- Tartakovskii, V.A.; Onishchenko, A.A.; Chlenov, I.E.; Novikov, S.S. General Method of Synthesis of 3-Nitroisoxazolines. Dokl. Chem. 1966, 167, 406–409. [Google Scholar]
- Ivanova, O.A.; Averina, E.B.; Grishin, Y.K.; Kuznetsova, T.S.; Korlyukov, A.A.; Antipin, M.Y.; Zefirov, N.S. Study of 4-Hydroxy-3-nitroisoxazoline N-oxide in the Reactions of 1,3-Dipolar Cycloaddition to Small-Ring Olefins. Dokl. Chem. 2002, 382, 21–24. [Google Scholar] [CrossRef]
- Vasilenko, D.A.; Averina, E.B.; Zefirov, N.A.; Wobith, B.; Grishin, Y.K.; Rybakov, V.B.; Zefirova, O.N.; Kuznetsova, T.S.; Kuznetsov, S.A.; Zefirov, N.S. Synthesis and Antimitotic Activity of Novel 5-Aminoisoxazoles Bearing Alkoxyaryl Moieties. Mendeleev Commun. 2017, 27, 228–230. [Google Scholar] [CrossRef]
- Vorozhtsov, N.O.; Yarovaya, O.I.; Roznyatovskii, V.A.; Tarasevich, B.N.; Kozlovskaya, Y.A.; Petkova, A.I.; Slita, A.V.; Sinegubova, E.O.; Zarubaev, V.V.; Salakhutdinov, N.F.; et al. Synthesis and antiviral activity of novel 3-substituted pyrazolinium salts. Chem. Heterocycl. Compd. 2021, 57, 432–441. [Google Scholar] [CrossRef]
- Klimochkin, Y.N.; Shiryaev, V.A.; Petrov, P.V.; Radchenko, E.V.; Palyulin, V.A.; Zefirov, N.S. Design of Broad-Spectrum Inhibitors of Influenza A Virus M2 Proton Channels: A Molecular Modeling Approach. Curr. Comput. Aided Drug Des. 2016, 12, 154–164. [Google Scholar] [CrossRef]
- Rosenberg, M.R.; Casarotto, M.G. Coexistence of two adamantane binding sites in the influenza A M2 ion channel. Proc. Natl. Acad. Sci. USA 2010, 107, 13866–13871. [Google Scholar] [CrossRef] [Green Version]
- Shiner, V.J.; Tai, J.J. Deuterium Isotope Effects for Migrating and Nonmigrating Groups in the Solvolysis of Neopentyl-Type Esters. J. Am. Chem. Soc. 1981, 103, 436–442. [Google Scholar] [CrossRef]
- Liang, P. Tetranitromethane. Org. Synth. 1941, 21, 105. [Google Scholar]
- Niks, M.; Otto, M. Towards an optimized MTT assay. J. Immunol. Methods 1990, 130, 149–151. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A Web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Wu, E.L.; Cheng, X.; Jo, S.; Rui, H.; Song, K.C.; Davila-Contreras, E.M.; Qi, Y.; Lee, J.; Monje-Galvan, V.; Venable, R.M.; et al. CHARMM-GUI Membrane Builder Toward realistic biological membrane simulations. J. Comput. Chem. 2014, 35, 1997–2004. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; MacKerell, A.D. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef] [Green Version]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [Green Version]
- Roe, D.R.; Cheatham, T.E.J. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Salomon-Ferrer, R.; Case, D.A.; Walker, R.C. An overview of the Amber biomolecular simulation package. WIREs Comput. Mol. Sci. 2013, 3, 198–210. [Google Scholar] [CrossRef]


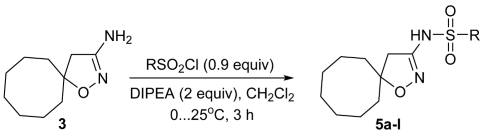 | |||||
|---|---|---|---|---|---|
| Compound | R | Yield, % * | Compound | R | Yield, % * |
| 5a | Me | 56 | 5g | 4-(MeC(O)NH)C6H4 | 31 |
| 5b | Bn | 33 | 5h | 2,4-F2C6H3 | 77 |
| 5c | Ph | 88 | 5i | 2-(NO2)C6H4 | 70 |
| 5d | 4-MeC6H4 | 50 | 5j | 3-naphthyl | 81 |
| 5e | 2,4,6-Me3C6H2 | 47 | 5k | 2-thienyl | 53 |
| 5f |  | 37 | 5l | 3-pyridyl | 66 |
| Compound | IC50, μM * | CC50, μM ** | SI *** |
|---|---|---|---|
| 3 | 6.6 | >550 | 83 |
| 4 | 22.0 | >1790 | 81 |
| Rimantadine | 67 ± 8 | 406 ± 26 | 6 |
| Compound | MIK, μg/mL (Zone of Inhibition Ø, mm) | ||||
|---|---|---|---|---|---|
| A. niger | C. albicans | B. subtilis | St. aureus | E. coli | |
| 1 | 8 (15) | 8 (24) | 32 (12) | 32 (15) | ˃256 (—) |
| 2 | 16 (15) | 32 (19) | 32 (16) | 8–16 (25) | ˃256 (—) |
| Amphotericin B | 0.04–1.5 | 2 | — | — | — |
| Clotrimazole | — | 4 | — | — | — |
| Vancomycin | — | — | 0.25–1 | 0.5–2 | — |
| Ampicillin | — | — | — | — | 2–8 |
| Compound | IC50, µM * | ||||
|---|---|---|---|---|---|
| MCF-7 | HCT-116 | A-549 | WI38 | Hek293t | |
| 1 | 58.8 ± 5 | 20.8 ± 5 | 57.1 ± 7 | 42.2 ± 13 | n/a |
| 2 | 35.4 ± 4.5 | 17.7 ± 5 | 40.4 ± 2.5 | 27.4 ± 4 | n/a |
| 3 | >150 | >500 | >150 | >150 | n/a |
| 4 | >150 | >500 | >150 | >150 | n/a |
| 5b | 138.3 ± 25 | >200 | n/a | >500 | n/a |
| 5c | 115.6 ± 30 | 63 ± 18 | 163 | 113.1 | n/a |
| 5d | 94 ± 30 | n/a | 143 ± 50 | 54.6 ± 7 | n/a |
| 5e | 79 ± 36 | 70.1 ± 11.2 | 61 ± 21 | 95.5 ± 14.3 | n/a |
| 5f | 64.1 ± 8 | 23.6 ± 4.5 | 119 | 42 ± 8.6 | n/a |
| 5g | 54.5 ± 15 | 41.3 ± 17 | 54 ± 14 | n/a | 30.36 ± 14.5 |
| 5h | 94.7 ± 15.5 | 97.1 ± 34 | 77.1 ± 10.2 | n/a | 60 ± 17 |
| 5j | 26.8 ± 3.5 | 19 ± 3 | 27.5 ± 4.2 | n/a | 23.1 ± 6.5 |
| 5k | >150 | 131 ± 80 | 111 ± 20 | n/a | 65.5 ± 13.5 |
| 5l | >150 | 133 ± 68 | >150 | n/a | >500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedenkova, K.N.; Andriasov, K.S.; Eremenko, M.G.; Grishin, Y.K.; Alferova, V.A.; Baranova, A.A.; Zefirov, N.A.; Zefirova, O.N.; Zarubaev, V.V.; Gracheva, Y.A.; et al. Bicyclic Isoxazoline Derivatives: Synthesis and Evaluation of Biological Activity. Molecules 2022, 27, 3546. https://doi.org/10.3390/molecules27113546
Sedenkova KN, Andriasov KS, Eremenko MG, Grishin YK, Alferova VA, Baranova AA, Zefirov NA, Zefirova ON, Zarubaev VV, Gracheva YA, et al. Bicyclic Isoxazoline Derivatives: Synthesis and Evaluation of Biological Activity. Molecules. 2022; 27(11):3546. https://doi.org/10.3390/molecules27113546
Chicago/Turabian StyleSedenkova, Kseniya N., Kristian S. Andriasov, Marina G. Eremenko, Yuri K. Grishin, Vera A. Alferova, Anna A. Baranova, Nikolay A. Zefirov, Olga N. Zefirova, Vladimir V. Zarubaev, Yulia A. Gracheva, and et al. 2022. "Bicyclic Isoxazoline Derivatives: Synthesis and Evaluation of Biological Activity" Molecules 27, no. 11: 3546. https://doi.org/10.3390/molecules27113546
APA StyleSedenkova, K. N., Andriasov, K. S., Eremenko, M. G., Grishin, Y. K., Alferova, V. A., Baranova, A. A., Zefirov, N. A., Zefirova, O. N., Zarubaev, V. V., Gracheva, Y. A., Milaeva, E. R., & Averina, E. B. (2022). Bicyclic Isoxazoline Derivatives: Synthesis and Evaluation of Biological Activity. Molecules, 27(11), 3546. https://doi.org/10.3390/molecules27113546








