Antioxidant and Antimicrobial Potencies of Chemically-Profiled Essential Oil from Asteriscus graveolens against Clinically-Important Pathogenic Microbial Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction of EOAG
2.3. Identification of Terpenic Compounds by GC/MS
2.3.1. Gas Chromatography-Flame Ionization Detector (CG-FID)
2.3.2. Gas Chromatography-Mass Spectrometry Analysis (GCMS)
2.4. Antioxidant Activity of EOAG
2.4.1. DPPH Test
2.4.2. FRAP Test
2.4.3. TAC Test
2.5. Evaluation of the Antimicrobial Activity of EOAG
2.5.1. Disc Diffusion Method
2.5.2. Determination of Minimum Inhibitory Concentration (MIC)
2.6. Statistical Analysis
3. Results and Discussion
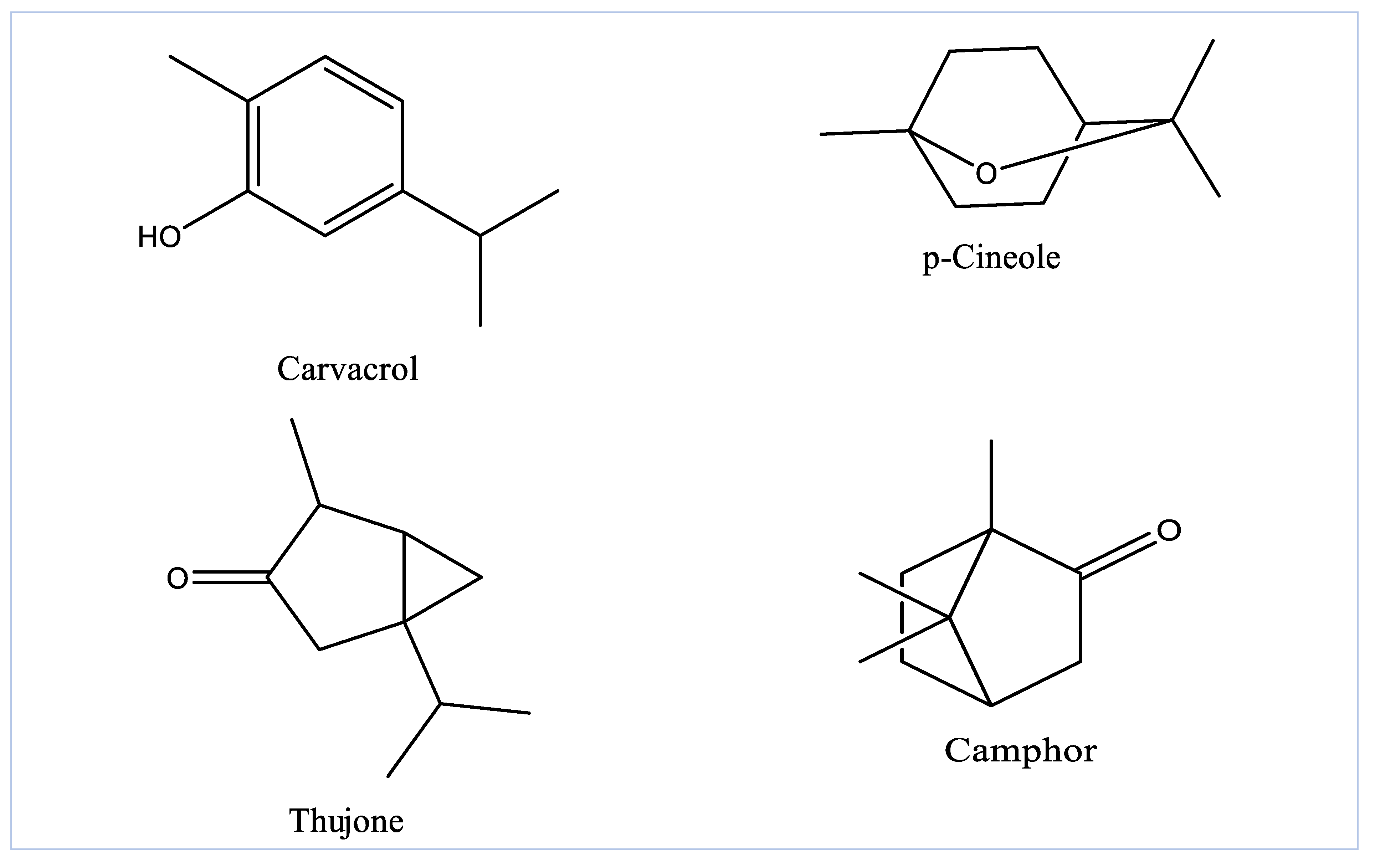
3.1. GC-MS Profiling of EOAG
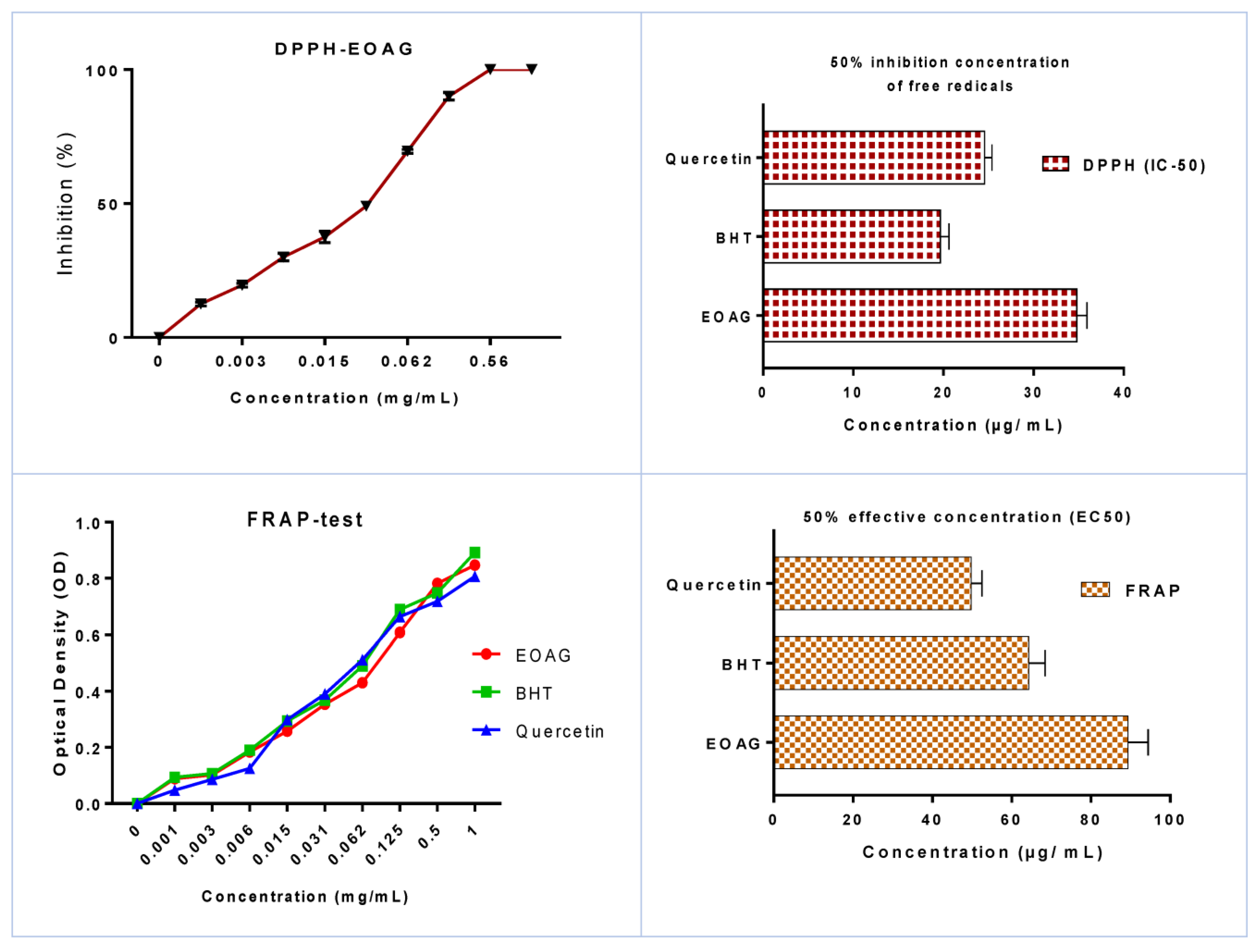
3.2. In Vitro Antioxidant Activity of EOAG
3.3. Evaluation of Antibacterial Activity of EOAG
3.4. Evaluation of Antifungal Activity of EOAG
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bellakhdar, J. The Traditional Moroccan Pharmacopoeia: Ancient Arabic Medicine and Popular Knowledge; Ibis Press: Paris, France, 1997. [Google Scholar]
- Bourhia, M.; Messaoudi, M.; Bakrim, H.; Mothana, R.A.; Sddiqui, N.A.; Almarfadi, O.M.; El Mzibri, M.; Gmouh, S.; Laglaoui, A.; Benbacer, L. Citrullus colocynthis (L.) Schrad: Chemical Characterization, Scavenging and Cytotoxic Activities. Open Chem. 2020, 18, 986–994. [Google Scholar] [CrossRef]
- Lardry, J.-M.; Haberkorn, V. L’aromathérapie et Les Huiles Essentielles. Kinésithérapie La Rev. 2007, 7, 14–17. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.M.; Scheffer, J.J.C.; Svendsen, A.B. Antimicrobial Activity of Essential Oils: A 1976-1986 Literature Review. Aspects Test Methods. Planta Med. 1987, 53, 395–398. [Google Scholar]
- Erdemoglu, N.; Turan, N.N.; Cakõcõ, I.; Sener, B.; Aydõn, A. Antioxidant Activities of Some Lamiaceae Plant Extracts. Phytotherapy Research: An International. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 9–13. [Google Scholar]
- Pieroni, A.; Janiak, V.; Dürr, C.M.; Lüdeke, S.; Trachsel, E.; Heinrich, M. In Vitro Antioxidant Activity of Non-Cultivated Vegetables of Ethnic Albanians in Southern Italy. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2002, 16, 467–473. [Google Scholar]
- Ames, B.N. Dietary Carcinogens and Anticarcinogens: Oxygen Radicals and Degenerative Diseases. Science 1983, 221, 1256–1264. [Google Scholar] [CrossRef] [Green Version]
- Vickers, N.J. Animal Communication: When i’m Calling You, Will You Answer Too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential Oils as Antimicrobials in Food Systems–A Review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Sumathi, T.; Srilakshmi, A.; Kotakadi, V.S.; Saigopal, D.V.R. Role_of_Fungal_enzymes_in_Polymer_degradation n: A Mini review. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 1694–1711. [Google Scholar]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Collection: London, UK, 2016; Available online: https://wellcomecollection.org/works/thvwsuba (accessed on 20 April 2022).
- Maksimović, Z.; Malenčić, D.; Kovačević, N. Polyphenol Contents and Antioxidant Activity of Maydis Stigma Extracts. Bioresour. Technol. 2005, 96, 873–877. [Google Scholar] [CrossRef]
- Alvarenga, S.A.V.; Ferreira, M.J.P.; Emerenciano, V.; Cabrol-Bass, D. Chemosystematic Studies of Natural Compounds Isolated from Asteraceae: Characterization of Tribes by Principal Component Analysis. Chemom. Intell. Lab. Syst. 2001, 56, 27–37. [Google Scholar] [CrossRef]
- Ramdane, F.; Essid, R.; Mkadmini, K.; Hammami, M.; Fares, N.; Mahammed, M.H.; El Ouassis, D.; Tabbene, O.; Limam, F.; Hadj, M.D.O. Phytochemical Composition and Biological Activities of Asteriscus Graveolens (Forssk) Extracts. Process Biochem. 2017, 56, 186–192. [Google Scholar] [CrossRef]
- Znini, M.; Cristofari, G.; Majidi, L.; Mazouz, H.; Tomi, P.; Paolini, J.; Costa, J. Antifungal Activity of Essential Oil from Asteriscus Graveolens against Postharvest Phytopathogenic Fungi in Apples. Nat. Prod. Commun. 2011, 6, 1934578X1100601147. [Google Scholar] [CrossRef] [Green Version]
- Achoub, H.; Mencherini, T.; Esposito, T.; Luca, R.; Aquino, R.; Gazzerro, P.; Zaiter, L.; Benayache, F.; Benayache, S. New sesquiterpenes from Asteriscus graveolens. Nat. Prod. Res. 2021, 35, 2190–2198. [Google Scholar] [CrossRef]
- Aouissi, H.; Gourine, N.; Wang, H.; Chen, X.; Bombarda, I.; Boudjeniba, M.; Yousfi, M. Chemical Composition, Antioxidative, Antimicrobial and Anti-Cancer Activities of Asteriscus graveolens (Forssk) Essential Oil. Orient. Pharm. Exp. Med. 2018, 18, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Chaib, F.; Allali, H.; Benali, O.; Flamini, G. Corrosion Inhibition Effects of the Essential Oils of Two Asteraceae Plants from South Algeria. Inter. J. Chem. Biochem. Sci. 2020, 18, 129–136. [Google Scholar]
- Cheriti, A.; Saad, A.; Belboukhari, N.; Ghezali, S. The Essential Oil Composition of Bubonium Graveolens (Forssk.) Maire from the Algerian Sahara. Flavour Fragr. J. 2007, 22, 286–288. [Google Scholar] [CrossRef]
- Fahmy, H.S. Chemical Composition and Antimicrobial Activity of the Volatile Oil of Nauplius Graveolens (Forssk.). Less. J. Environ. Sci. 2003, 26, 307–317. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatograpy/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 978-1-932()33-11-4. [Google Scholar]
- Moattar, F.S.; Sariri, R.; Yaghmaee, P.; Giahi, M. Enzymatic and Non-Enzymatic Antioxidants of Calamintha Officinalis Moench Extracts. J. Appl. Biotechnol. Rep. 2016, 3, 489–494. [Google Scholar]
- Mašković, P.Z.; Manojlović, N.T.; Mandić, A.I.; Mišan, A.Č.; Milovanović, I.L.; Radojković, M.M.; Cvijović, M.S.; Solujić, S.R. Phytochemical Screening and Biological Activity of Extracts of Plant Species Halacsya Sendtneri (Boiss.). Dörfl. Hem. Ind. 2012, 66, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Alilou, H.; Asdadi, A.; Hassani, L.; González-Mas, M.C.; Blázquez, M.A.; Akssira, M. Antifungal and Antioxidant Activity of Asteriscus graveolens subspodorus Essential Oil. J. Nat. Sci. Res. 2014, 10, 1–11. [Google Scholar]
- Chebbac, K.; Ghneim, H.K.; El Moussaoui, A.; Bourhia, M.; El Barnossi, A.; Benziane Ouaritini, Z.; Salamatullah, A.M.; Alzahrani, A.; Aboul-Soud, M.A.; Giesy, J.P.; et al. Antioxidant and Antimicrobial Activities of Chemically-Characterized Essential Oil from Artemisia aragonensis Lam. against Drug-Resistant Microbes. Molecules 2022, 27, 1136. [Google Scholar] [CrossRef]
- Chebbac, K.; Moussaoui, A.E.L.; Bourhia, M.; Salamatullah, A.M.; Alzahrani, A.; Guemmouh, R. Chemical Analysis and Antioxidant and Antimicrobial Activity of Essential Oils from Artemisia Negrei, L. Against Drug-Resistant Microbes. Evid.-Based Complementary Altern. Med. 2021, 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chaib, F.; Allali, H.; Bennaceur, M.; Flamini, G. Chemical Composition and Antimicrobial Activity of Essential Oils from the Aerial Parts of Asteriscus Graveolens (Forssk.) Less. and Pulicaria Incisa (Lam.) DC.: Two Asteraceae Herbs Growing Wild in the Hoggar. Chem. Biodivers. 2017, 14, e1700092. [Google Scholar] [CrossRef] [PubMed]
- Cristofari, G.; Znini, M.; Majidi, L.; Mazouz, H.; Tomi, P.; Costa, J.; Paolini, J. Chemical Diversity of Essential Oils from Asteriscus Graveolens (Forssk.) Less.: Identification of Cis-8-Acetoxychrysanthenyl Acetate as a New Natural Component. Chem. Biodivers. 2012, 9, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Zhigzhitzhapova, S.V.; Radnaeva, L.D.; Chen, S.L.; Fu, P.C.; Zhang, F.Q. Chemical Composition of the Essential Oil of Artemisia Hedinii Ostenf. et Pauls. from the Qinghai-Tibetan Plateau. Ind. Crops Prod. 2014, 62, 293–298. [Google Scholar] [CrossRef]
- Moussaoui, A.E.; Bourhia, M.; Jawhari, F.Z.; Khalis, H.; Chedadi, M.; Agour, A.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Alotaibi, A.; et al. Responses of Withania Frutescens (L.) Pauquy (Solanaceae) Growing in the Mediterranean Area to Changes in the Environmental Conditions: An Approach of Adaptation. Front. Ecol. Evol. 2021, 9, 666005. [Google Scholar] [CrossRef]
- Aeschbach, R.; Löliger, J.; Scott, B.C.; Murcia, A.; Butler, J.; Halliwell, B.; Aruoma, O.I. Antioxidant Actions of Thymol, Carvacrol, 6-Gingerol, Zingerone and Hydroxytyrosol. Food Chem. Toxicol. 1994, 32, 31–36. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical Scavenging Ability of Polyphenolic Compounds towards DPPH Free Radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
- Bourhia, M.; Bouothmany, K.; Bakrim, H.; Hadrach, S.; Salamatullah, A.M.; Alzahrani, A.; Khalil Alyahya, H.; Albadr, N.A.; Gmouh, S.; Laglaoui, A. Chemical Profiling, Antioxidant, Antiproliferative, and Antibacterial Potentials of Chemically Characterized Extract of Citrullus colocynthis L. Seeds. Separations 2021, 8, 114. [Google Scholar] [CrossRef]
- Moussaoui, A.E.; Bourhia, M.; Jawhari, F.Z.; Salamatullah, A.M.; Ullah, R.; Bari, A.; Mahmood, H.M.; Sohaib, M.; Serhii, B.; Rozhenko, A.; et al. Chemical Profiling, Antioxidant, and Antimicrobial Activity against Drug-Resistant Microbes of Essential Oil from Withania frutescens L. Appl. Sci. 2021, 11, 5168. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. 2001, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; Von Wright, A. Characterization of the Action of Selected Essential Oil Components on Gram-Negative Bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Jawhari, F.Z.; Moussaoui, A.E.; Bourhia, M.; Imtara, H.; Saghrouchni, H.; Ammor, K.; Ouassou, H.; Elamine, Y.; Ullah, R.; Ezzeldin, E. Anacyclus Pyrethrum Var. Pyrethrum (L.) and Anacyclus Pyrethrum Var. Depressus (Ball) Maire: Correlation between Total Phenolic and Flavonoid Contents with Antioxidant and Antimicrobial Activities of Chemically Characterized Extracts. Plants 2021, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Aimad, A.; Sanae, R.; Anas, F.; Abdelfattah, E.M.; Bourhia, M.; Mohammad Salamatullah, A.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Abdelkrim, A. Chemical Characterization and Antioxidant, Antimicrobial, and Insecticidal Properties of Essential Oil from Mentha pulegium L. Evid.-Based Complement. Altern. Med. 2021, 2021, 1108133. [Google Scholar] [CrossRef] [PubMed]
- El Abdali, Y.; Agour, A.; Allali, A.; Bourhia, M.; El Moussaoui, A.; Eloutassi, N.; Salamatullah, A.M.; Alzahrani, A.; Ouahmane, L.; Aboul-Soud, M.A. Lavandula dentata L.: Phytochemical Analysis, Antioxidant, Antifungal and Insecticidal Activities of Its Essential Oil. Plants 2022, 11, 311. [Google Scholar] [CrossRef]
- Zalegh, I.; Akssira, M.; Bourhia, M.; Mellouki, F.; Rhallabi, N.; Salamatullah, A.M.; Alkaltham, M.S.; Khalil Alyahya, H.; Mhand, R.A. A Review on Cistus Sp.: Phytochemical and Antimicrobial Activities. Plants 2021, 10, 1214. [Google Scholar] [CrossRef]
- Bourhia, M.; Aimad, A.; Youness, E.A.; Sanae, R.; El Moussaoui, A.; Salamatullah, A.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Ouahmane, L. Chemistry, Insecticidal and Repellent Activities of Essential Oils From Origanum Compactum Benth Used in the Mediterranean Diet. Front. Plant Sci. 2022, 13, 798259. [Google Scholar] [CrossRef]
- Lagrouh, F.; Dakka, N.; Bakri, Y. The Antifungal Activity of Moroccan Plants and the Mechanism of Action of Secondary Metabolites from Plants. J. De Mycol. Med. 2017, 27, 303–311. [Google Scholar] [CrossRef]
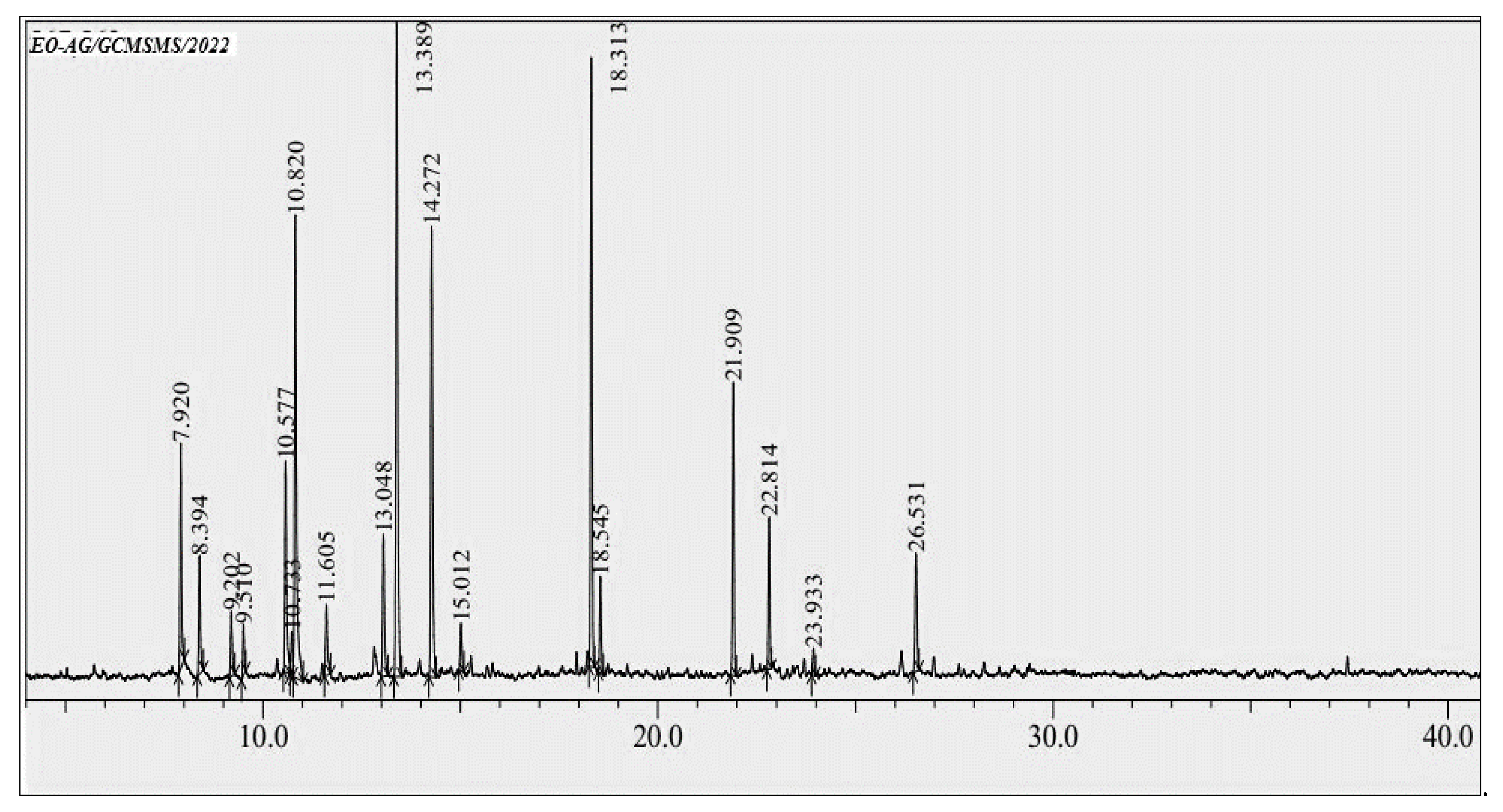



| RT | Compound | Retention Index | Chemical Class | Area (%) | |
|---|---|---|---|---|---|
| 1 | 7.92 | α-Pinene | 938 | MO.H | 5.10 |
| 2 | 8.39 | Camphene | 949 | MO.H | 2.91 |
| 3 | 9.20 | β-Pinene | 974 | MO.H | 1.58 |
| 4 | 9.51 | Myrcene | 988 | MO.H | 1.16 |
| 5 | 10.57 | o-Cymene | 1022 | MO.H | 5.70 |
| 6 | 10.73 | Limonene | 1028 | MO.H | 1.16 |
| 7 | 10.82 | p-Cineole | 1039 | MO.O | 13.83 |
| 8 | 11.60 | γ-Terpinene | 1058 | MO.H | 1.98 |
| 9 | 13.04 | Isothujone | 1002 | MO.O | 3.98 |
| 10 | 13.38 | α-Thujone | 1102 | MO.O | 17.92 |
| 11 | 14.27 | Camphor | 1141 | MO.O | 12.71 |
| 12 | 15.01 | Borneol | 1168 | MO.O | 1.23 |
| 13 | 18.31 | Carvacrol | 1297 | MO.O | 14.14 |
| 14 | 18.54 | Thymol acetate | 1357 | MO.O | 2.26 |
| 15 | 21.90 | Caryophyllene | 1404 | ST.H | 6.71 |
| 16 | 22.81 | α-Humulene | 1459 | ST.H | 3.51 |
| 17 | 23.93 | Eugenol acetate | 1525 | O | 0.61 |
| 18 | 26.53 | Pogostol | 1651 | ST.O | 3.43 |
| Chemical Class | |||||
| Monterpene oxygenated (MO.O) | 66.07 | ||||
| Monoterpene hydrocarbons (MO.H) | 19.59 | ||||
| Other (O) | 0.61 | ||||
| Sesquiterpene oxygenated (ST.O) | 3.43 | ||||
| Sesquiterpene hydrocarbons (ST.H) | 10.22 | ||||
| Total | 99.92% | ||||
| S. aureus | E. coli | B. subtilis | P. aeruginosa | ||
|---|---|---|---|---|---|
| EOAG | Inhibition diameter (mm) | 27.41 ± 1.54 a | 19.68 ± 1.25 b | 17.48 ± 1.75 b | 28.47 ± 1.44 a |
| MIC (µg/mL) | 12.18 ± 0.98 b | 14.57 ± 0.87 b | 22.48 ± 0.69 a | 14.65 ± 1.28 a | |
| Stp | Inhibition diameter (mm) | 10.73± 0.45 a | 0 b | 0 b | 0 b |
| MIC (µg/mL) | 15.83 ± 0.20 a | 0 b | 0 b | 0 | |
| Kan | Inhibition diameter (mm) | 0 b | 17.24 ± 1.34 a | 0 b | 0 b |
| MIC (µg/mL) | 0 b | 13.47 ± 0.92 a | 0 b | 0 b | |
| C. albicans | A. niger | A. flavus | F. oxysporum | ||
|---|---|---|---|---|---|
| EOAG | Inhibition diameter (mm) | 26.41 ± 1.90 a | 17.01 ± 1.08 b | 16.76 ± 1.02 b | 33.62 ± 2.14 c |
| MIC (µg/mL) | 19.39 ± 1.0 a | 24.50 ± 1.30 b | 23.74 ± 1.81 b | 18.29 ± 1.21 a | |
| Flu | Inhibition diameter | 0 a | 11.41 ± 1.31 b | 0 a | 16.18 ± 2.43 c |
| MIC (µg/mL) | 0 a | 10.27 ± 0.84 b | 0 a | 33.12 ± 1.38 c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljeldah, M.M. Antioxidant and Antimicrobial Potencies of Chemically-Profiled Essential Oil from Asteriscus graveolens against Clinically-Important Pathogenic Microbial Strains. Molecules 2022, 27, 3539. https://doi.org/10.3390/molecules27113539
Aljeldah MM. Antioxidant and Antimicrobial Potencies of Chemically-Profiled Essential Oil from Asteriscus graveolens against Clinically-Important Pathogenic Microbial Strains. Molecules. 2022; 27(11):3539. https://doi.org/10.3390/molecules27113539
Chicago/Turabian StyleAljeldah, Mohammed M. 2022. "Antioxidant and Antimicrobial Potencies of Chemically-Profiled Essential Oil from Asteriscus graveolens against Clinically-Important Pathogenic Microbial Strains" Molecules 27, no. 11: 3539. https://doi.org/10.3390/molecules27113539
APA StyleAljeldah, M. M. (2022). Antioxidant and Antimicrobial Potencies of Chemically-Profiled Essential Oil from Asteriscus graveolens against Clinically-Important Pathogenic Microbial Strains. Molecules, 27(11), 3539. https://doi.org/10.3390/molecules27113539





