In Vitro Antithrombotic, Hematological Toxicity, and Inhibitor Studies of Protocatechuic, Isovanillic, and p-Hydroxybenzoic Acids from Maclura tricuspidata (Carr.) Bur
Abstract
:1. Introduction
2. Results
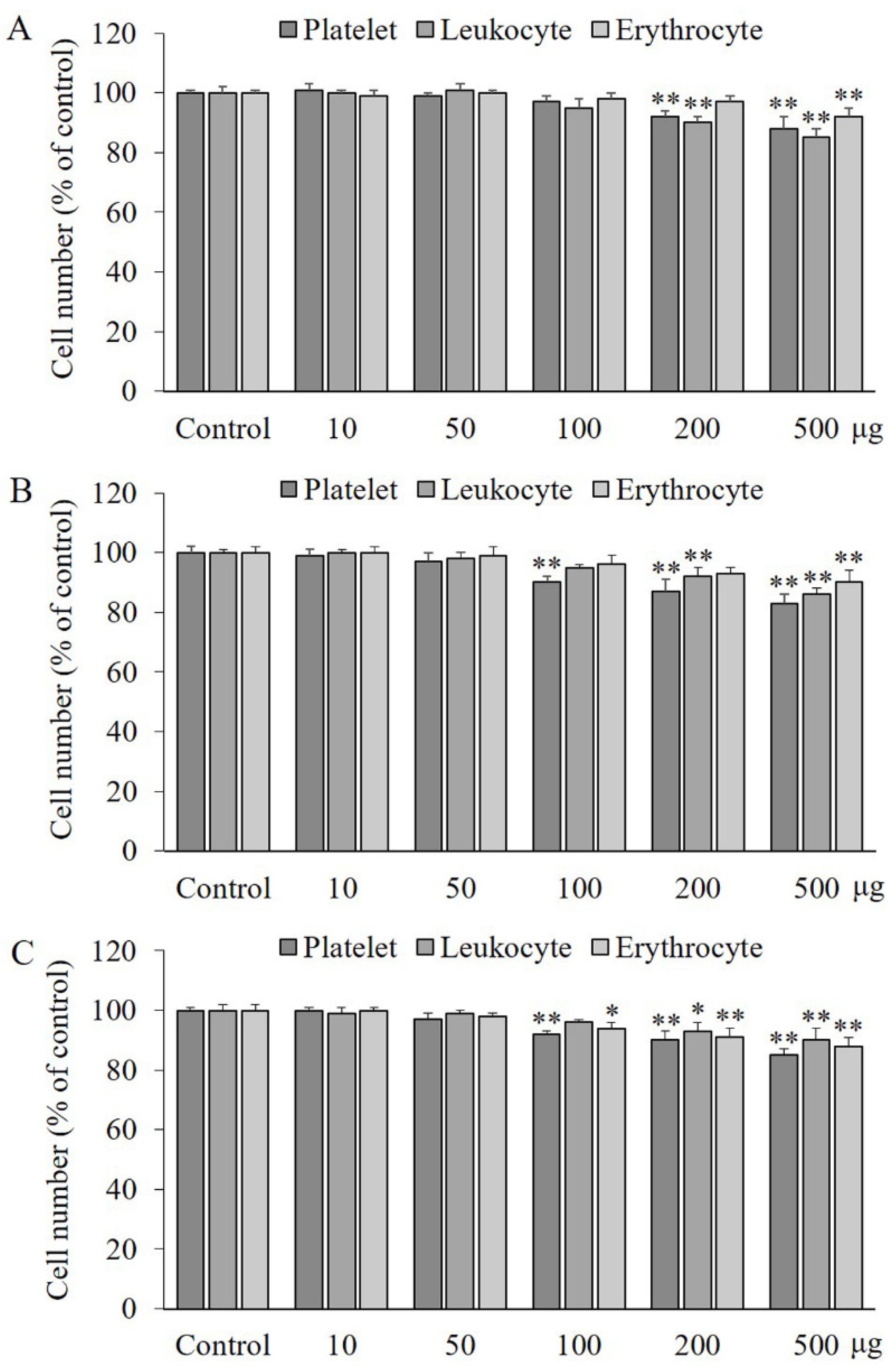
2.1. Effects of PA, IVA, and 4-HA on the Viability of Blood Cells
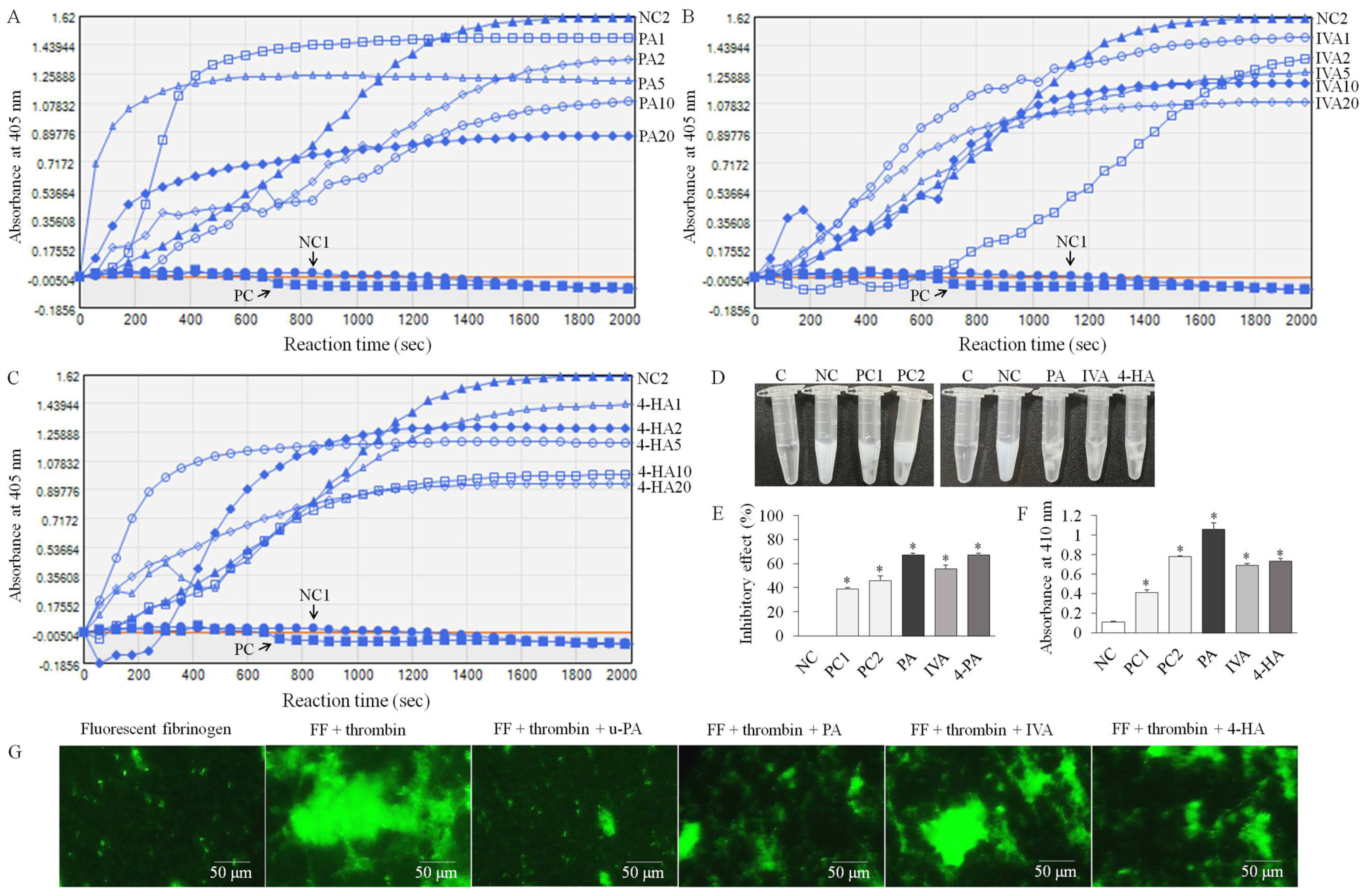
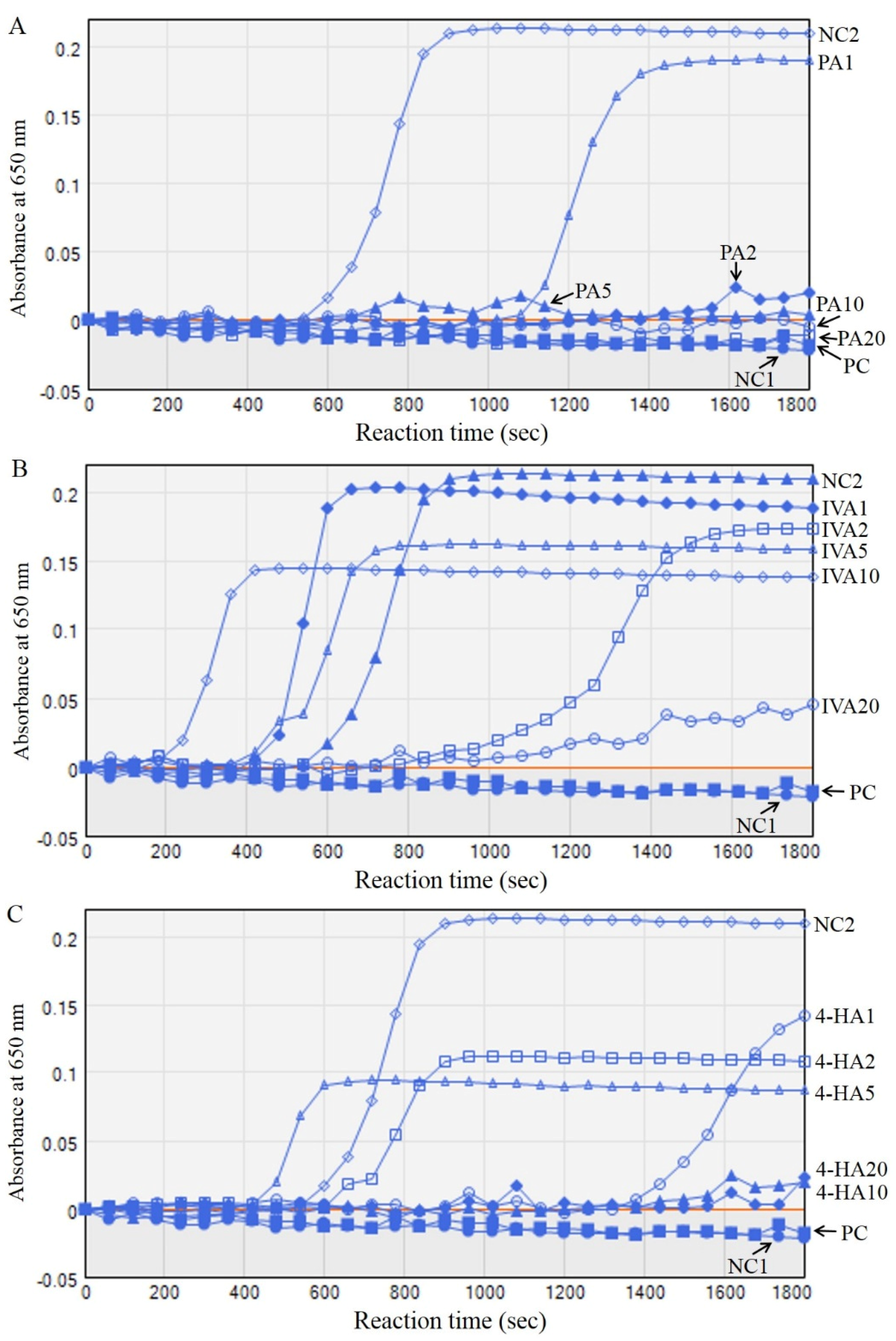
2.2. PA, IVA, and 4-HA Attenuate Fibrin/Blood Clot Formation
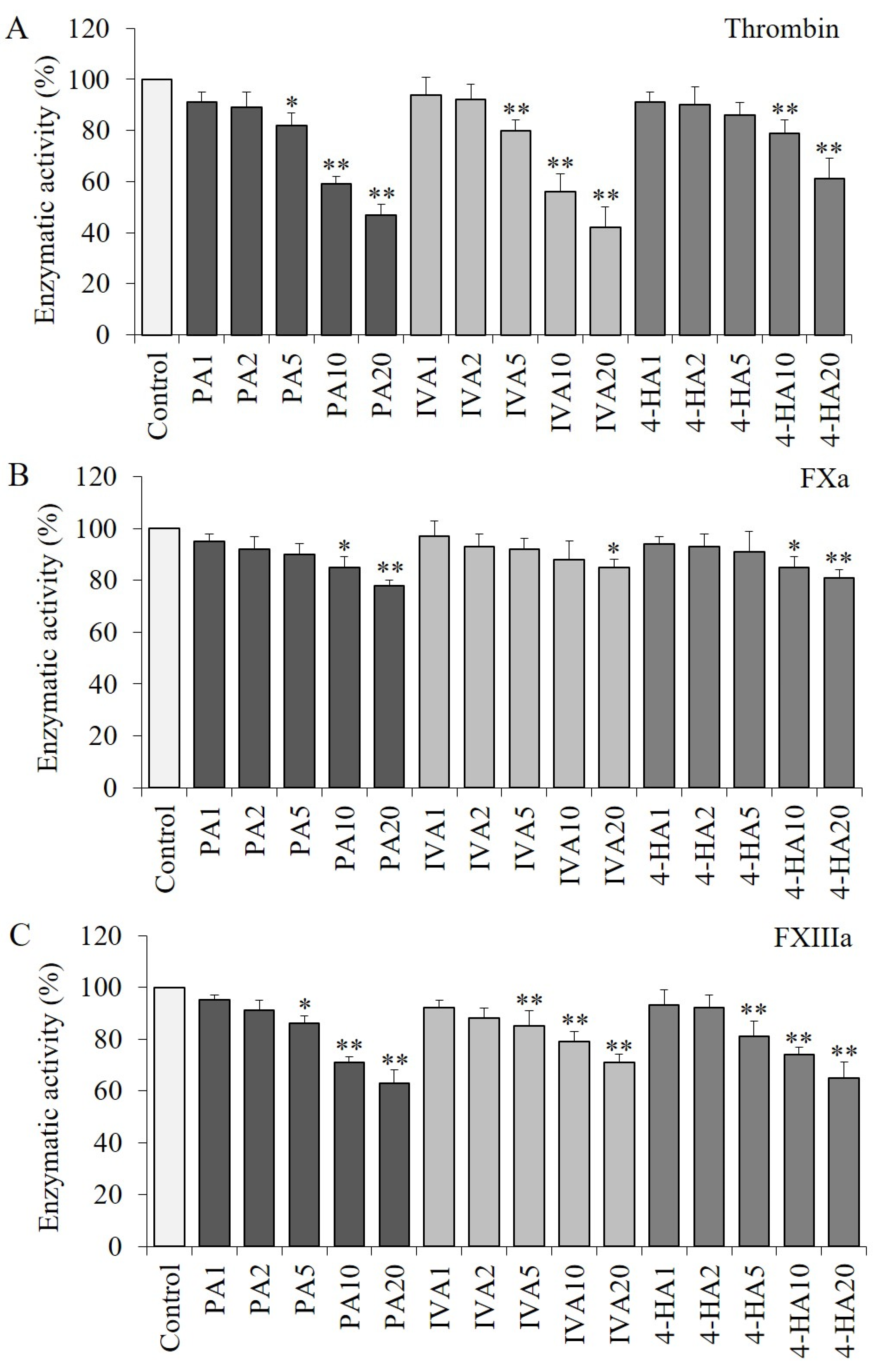
2.3. PA, IVA, and 4-HA Decrease the Enzymatic Activities of Procoagulant Proteinases
2.4. Effects of PA, IVA, and 4-HA on Plasma Recalcification
2.5. Effects of PA, IVA, and 4-HA on Coagulation Time
2.6. Effects of PA, IVA, and 4-HA on Collagen and Epinephrine-Activated Platelet Aggregation
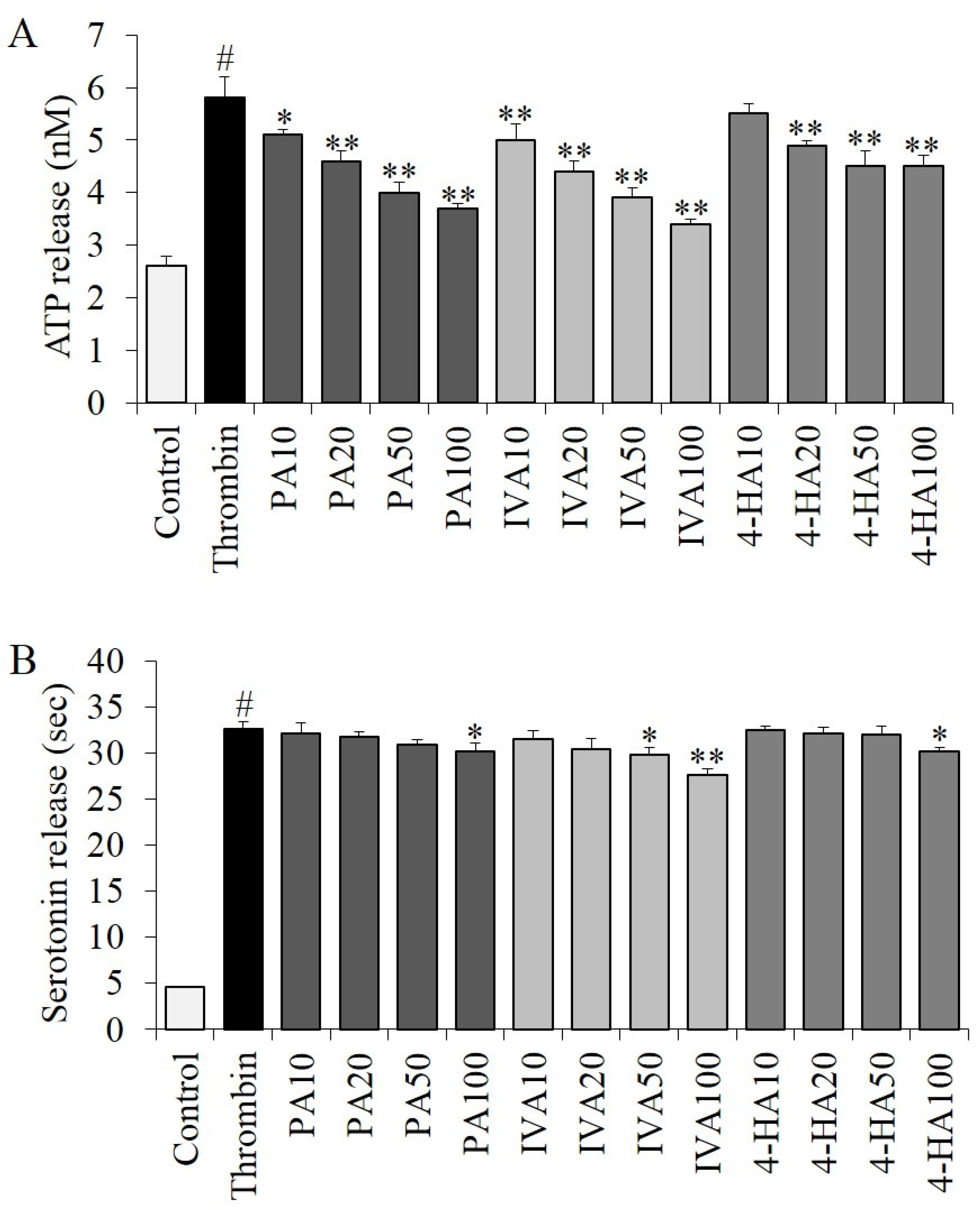
2.7. Effects of PA, IVA, and 4-HA on Granule Secretion
3. Materials and Methods
3.1. Materials
3.2. Turbidity Assay
3.3. Fibrin Clot Assay
3.4. In Vitro Antithrombotic Activity
3.5. Thrombin, FXa, and FXIIIa Assays
3.6. Procoagulant Proteases and Fibrinoligase Inhibitor Studies: Kinetic Assays
3.7. Recalcification Time Assay
3.8. Coagulation Assay
3.9. Platelet Function Assay
3.10. ATP Release Assay
3.11. Serotonin Release Assay
3.12. Hematological Study
3.13. Statistical Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Choi, J.H.; Kim, M.K.; Yeo, S.H.; Kim, S. Short-term Cudrania tricuspidata fruit vinegar administration attenuates obesity in high-fat diet-fed mice by improving fat accumulation and metabolic parameters. Sci. Rep. 2020, 10, 21102. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.K.; Jun, W.; Lee, J. Effect of Cudrania tricuspidata and kaempferol in endoplasmic reticulum stress-induced inflammation and hepatic insulin resistance in HepG2 cells. Nutrients 2016, 8, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Lee, S.; Chung, Y.W.; Kim, B.M.; Kim, H.; Kim, K.; Yang, K.M. Antiobesity and antidiabetes effects of a Cudrania tricuspidata hydrophilic extract presenting PTP1B inhibitory potential. Biomed. Res. Int. 2016, 2016, 8432759. [Google Scholar] [PubMed] [Green Version]
- Shin, J.H.; Rhee, M.H.; Kwon, H.W. Antiplatelet effect of cudraxanthone L isolated from Cudrania tricuspidata via inhibition of phosphoproteins. Nat. Prod. Sci. 2020, 26, 295–302. [Google Scholar]
- Voet, D.; Voet, J.G. Biochemistry, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1990; pp. 1087–1095. [Google Scholar]
- Choi, J.H.; Kim, S.J.; Kim, S. A novel anticoagulant protein with antithrombotic properties from the mosquito Culex pipiens pallens. Int. J. Biol. Macromol. 2016, 93, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Park, S.E.; Kim, S.J.; Kim, S. Kaempferol inhibits thrombosis and platelet activation. Biochimie 2015, 115, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W.; Stauffacher, C.V.; Bullitt, E.; Cohen, C. A model for fibrinogen: Domains and sequence. Science 1985, 230, 1388–1391. [Google Scholar] [CrossRef]
- Jackson, S.P.; Nesbitt, W.S.; Kulkarni, S. Signaling events underlying thrombus formation. J. Thromb. Haemost. 2003, 1, 1602–1612. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, S. Investigation of the anticoagulant and antithrombotic effects of chlorogenic acid. J. Biochem. Mol. Toxicol. 2017, 31, e21865. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, H.J.; Kim, Y.S.; Yeo, S.H.; Kim, S. Effects of Maclura tricuspidata (Carr.) Bur fruits and its phytophenolics on obesity-related enzymes. J. Food Biochem. 2020, 44, e13110. [Google Scholar] [CrossRef]
- Yun, H.S.; Kang, S.S.; Kim, M.H.; Jung, K.H. Anti-thrombotic effects of analogs of protocatechuic acid and gallic acid. Yakhak Hoeji 1993, 37, 453–457. [Google Scholar]
- Kim, K.; Bae, O.N.; Lim, K.M.; Noh, J.Y.; Kang, S.; Chung, K.Y.; Chung, J.H. Novel antiplatelet activity of protocatechuic acid through the inhibition of high shear stress-induced platelet aggregation. J. Pharmacol. Exp. Ther. 2012, 343, 704–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.Y.; Huang, C.S.; Huang, C.Y.; Yin, M.C. Anticoagulatory, antiinflammatory, and antioxidative effects of protocatechuic acid in diabetic mice. J. Agric. Food Chem. 2009, 57, 6661–6667. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, B.; Choi, H.; Kim, W.; Kim, H.J.; Cheong, H. Antithrombosis activity of protocatechuic and shikimic acids from functional plant Pinus densiflora Sieb. et Zucc needles. J. Nat. Med. 2016, 70, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.K.; Kim, J.M.; Koo, J.Y.; Kang, S.S.; Bae, K.; Kim, Y.S.; Chung, J.H.; Yun-Choi, H.S. Platelet anti-aggregatory and blood anti-coagulant effects of compounds isolated from Paeonia lactiflora and Paeonia suffruticosa. Pharmazie 2010, 65, 624–628. [Google Scholar]
- Yun, H.S.; Kim, M.H.; Jung, K.H. Esters of substituted benzoic acids as anti-thrombotic agents. Arch. Pharm. Res. 1996, 19, 66–70. [Google Scholar]
- Yang, X.; Du, Z.; Pu, J.; Zhao, H.; Chen, H.; Liu, Y.; Li, Z.; Cheng, Z.; Zhong, H.; Liao, F. Classification of difference between inhibition constants of an inhibitor to facilitate identifying the inhibition type. J. Enzyme Inhib. Med. Chem. 2013, 28, 205–213. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, S. Mechanisms of attenuation of clot formation and acute thromboembolism by syringic acid in mice. J. Funct. Foods 2018, 43, 112–122. [Google Scholar] [CrossRef]
- Choi, J.H.; Park, J.K.; Kim, K.M.; Lee, H.J.; Kim, S. In vitro and in vivo antithrombotic and cytotoxicity effects of ferulic acid. J. Biochem. Mol. Toxicol. 2018, 32, e22004. [Google Scholar] [CrossRef]
- Ruggeri, Z.M.; Mendolicchio, G.L. Adhesion mechanisms in platelet function. Circ. Res. 2007, 100, 1673–1685. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Shi, X.; Zhu, M.; Wang, J.; Huang, S.; Huang, X.; Wang, H.; Li, L.; Deng, H.; et al. Platelet integrin αIIbβ3: Signal transduction, regulation, and its therapeutic targeting. J. Hematol. Oncol. 2019, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shattil, S.J.; Kim, C.; Ginsberg, M.H. The final steps of integrin activation: The end game. Nat. Rev. Mol. Cell Biol. 2010, 11, 288–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolberg, A.S. Thrombin generation and fibrin clot structure. Blood Rev. 2007, 21, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Graefe-Mody, E.U.; Schühly, U.; Rathgen, K.; Stähle, H.; Leitner, J.M.; Jilma, B. Pharmacokinetics and pharmacodynamics of BIBT 986, a novel small molecule dual inhibitor of thrombin and factor Xa. J. Thromb. Haemost. 2006, 4, 1502–1509. [Google Scholar] [CrossRef]
- Walsmann, P.; Markwardt, F. On the isolation of the thrombin inhibitor hirudin. Thromb. Res. 1985, 40, 563–569. [Google Scholar] [CrossRef]
- Salzet, M.; Chopin, V.; Baert, J.; Matias, I.; Malecha, J. Theromin, a novel leech thrombin inhibitor. J. Biol. Chem. 2000, 275, 30774–30780. [Google Scholar] [CrossRef] [Green Version]
- Francischetti, I.M.; Monteiro, R.Q.; Guimarães, J.A. Identification of glycyrrhizin as a thrombin inhibitor. Biochem. Biophys. Res. Commun. 1997, 235, 259–263. [Google Scholar]
- Koh, C.Y.; Kazimirova, M.; Trimnell, A.; Takac, P.; Labuda, M.; Nuttall, P.A.; Kini, R.M. Variegin, a novel fast and tight binding thrombin inhibitor from the tropical bont tick. J. Biol. Chem. 2007, 282, 29101–29113. [Google Scholar] [CrossRef] [Green Version]
- Zingali, R.B.; Jandrot-Perrus, M.; Guillin, M.C.; Bon, C. Bothrojaracin, a new thrombin inhibitor isolated from Bothrops jararaca venom: Characterization and mechanism of thrombin inhibition. Biochemistry 1993, 32, 10794–10802. [Google Scholar] [CrossRef]
- Xiao, Z.; Théroux, P. Platelet activation with unfractionated heparin at therapeutic concentrations and comparisons with a low-molecular-weight heparin and with a direct thrombin inhibitor. Circulation 1998, 97, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Elg, M.; Gustafsson, D.; Carlsson, S. Antithrombotic effects and bleeding time of thrombin inhibitors and warfarin in the rat. Thromb. Res. 1999, 94, 187–197. [Google Scholar] [CrossRef]
- Gladwell, T.D. Bivalirudin: A direct thrombin inhibitor. Clin. Ther. 2002, 24, 38–58. [Google Scholar] [CrossRef]
- Walenga, J.M. An overview of the direct thrombin inhibitor argatroban. Pathophysiol. Haemost. Thromb. 2002, 32, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, D.; Antonsson, T.; Bylund, R.; Eriksson, U.; Gyzander, E.; Nilsson, I.; Elg, M.; Mattsson, C.; Deinum, J.; Pehrsson, S.; et al. Effects of melagatran, a new low-molecular-weight thrombin inhibitor, on thrombin and fibrinolytic enzymes. Thromb. Haemost. 1998, 79, 108–110. [Google Scholar]
- Schulman, S.; Wåhlander, K.; Lundström, T.; Clason, S.B.; Eriksson, H.; THRIVE III Investigators. Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. N. Engl. J. Med. 2003, 349, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Blech, S.; Ebner, T.; Ludwig-Schwellinger, E.; Stangier, J.; Roth, W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab. Dispos. 2008, 36, 386–399. [Google Scholar] [CrossRef] [Green Version]
- Tulinsky, A. Molecular Interactions of Thrombin. Semin. Thromb. Hemost. 1996, 22, 117–124. [Google Scholar] [CrossRef]
- Graff, J.; Harder, S. Anticoagulant therapy with the oral direct factor Xa inhibitors rivaroxaban, apixaban and edoxaban and the thrombin inhibitor dabigatran etexilate in patients with hepatic impairment. Clin. Pharmacokinet. 2013, 52, 243–254. [Google Scholar] [CrossRef]
- Steinberg, B.A.; Becker, R.C. Structure-function relationships of factor Xa inhibitors: Implications for the practicing clinician. J. Thromb. Thromb. 2014, 37, 234–241. [Google Scholar] [CrossRef]
- Chen, A.; Stecker, E.; Warden, B.A. Direct oral anticoagulant use: A practical guide to common clinical challenges. J. Am. Heart Assoc. 2020, 9, e017559. [Google Scholar] [CrossRef]
- Santana-Romo, F.; Lagos, C.F.; Duarte, Y.; Castillo, F.; Moglie, Y.; Maestro, M.A.; Charbe, N.; Zacconi, F.C. Innovative three-step microwave-promoted synthesis of N-propargyltetrahydroquinoline and 1,2,3-triazole derivatives as a potential factor Xa (FXa) inhibitors: Drug design, synthesis, and biological evaluation. Molecules 2020, 25, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.F.; Chen, N.H.; Wang, W. Advance in the mechanism of anti-platelet aggregation drugs. Chin. J. Rehabil. Theory Pract. 2010, 16, 954–957. [Google Scholar]
- Nithyanandam, S.; Joseph, M.; Mathew, T. Clinical profile of cerebral venous thrombosis and the role of imaging in its diagnosis in patients with presumed idiopathic intracranial hypertension. Indian J. Ophthalmol. 2011, 59, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collen, D.; Lijnen, H.R. Basic and clinical aspects of fibrinolysis and thrombolysis. Blood 1991, 78, 3114–3124. [Google Scholar] [CrossRef] [PubMed]
- Raccah, B.H.; Kalish, Y.; Jabara, R.; Herzog, E.; Jelinek, B.R. Pharmacologic treatment of pulmonary embolism. In Pulmonary Embolism; Herzog, E., Ed.; Springer: Cham, Switzerland, 2022; pp. 143–170. [Google Scholar]
- Potla, N.; Ganti, L. Tenecteplase vs. alteplase for acute ischemic stroke: A systematic review. Int. J. Emerg. Med. 2022, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Wang, Y.; Hu, F.; Lv, L.; Chen, K.; Xing, G. Thrombolytic agents: Nanocarriers in targeted release. Molecules 2021, 26, 6776. [Google Scholar] [CrossRef]
- Green, D.; Ts’ao, C.; Reynolds, N.; Kahn, D.; Kohl, H.; Cohen, I. In vitro studies of a new synthetic thrombin inhibitor. Thromb. Res. 1985, 37, 145–153. [Google Scholar] [CrossRef]
- Lee, C.J.; Ansell, J.E. Direct thrombin inhibitors. Br. J. Clin. Pharmacol. 2011, 72, 581–592. [Google Scholar] [CrossRef]
- Campbell, C.L.; Smyth, S.; Montalescot, G.; Steinhubl, S.R. Aspirin dose for the prevention of cardiovascular disease: A systematic review. JAMA 2007, 297, 2018–2024. [Google Scholar] [CrossRef] [Green Version]
- Guerra, D.R.; Tcheng, J.E. Prasugrel: Clinical development and therapeutic application. Adv. Ther. 2009, 26, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Li, S.; Zhi, Z.J.; Yan, L.F.; Ye, X.Q.; Ding, T.; Yan, L.; Linhardt, R.J.; Chen, S.G. Depolymerization of fucosylated chondroitin sulfate with a modified fenton-system and anticoagulant activity of the resulting fragments. Mar. Drugs 2016, 14, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, D.Y.; Mellick, G.D.; Masci, P.P.; Whitaker, A.N.; Whitehouse, M.W.; Roberts, M.S. Focused antithrombotic therapy: Novel anti-platelet salicylates with reduced ulcerogenic potential and higher first-pass detoxification than aspirin in rats. J. Lab. Clin. Med. 1998, 132, 469–477. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Rai, S.K.; Thakur, R.; Chattopadhyay, P.; Kar, S.K. Bafibrinase: A non-toxic, non-hemorrhagic, direct-acting fibrinolytic serine protease from Bacillus sp. strain AS-S20-I exhibits in vivo anticoagulant activity and thrombolytic potency. Biochimie 2012, 94, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, J.; Chidambaram, R.; Sukumaran, S. Sulfated polysaccharides and its commercial applications in food industries-A review. J. Food Sci. Technol. 2021, 58, 2453–2466. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.C.; Yin, M.C.; Chao, W.J. Effect of diallyl trisulfide-rich garlic oil on blood coagulation and plasma activity of anticoagulation factors in rats. Food Chem Toxicol. 2007, 45, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef] [Green Version]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: Review on the basis of in vitro and in vivo studies in rodents and humans. Nutr. Neurosci. 2019, 22, 72–82. [Google Scholar] [CrossRef]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological properties of protocatechuic acid and its potential roles as complementary medicine. Evid. Based Complement Alternat. Med. 2015, 2015, 593902. [Google Scholar] [CrossRef] [Green Version]
- Masella, R.; Santangelo, C.; D’Archivio, M.; LiVolti, G.; Giovannini, C.; Galvano, F. Protocatechuic acid and human disease prevention: Biological activities and molecular mechanisms. Curr. Med. Chem. 2012, 19, 2901–2917. [Google Scholar] [CrossRef]
- Krga, I.; Vidovic, N.; Milenkovic, D.; Konic-Ristic, A.; Stojanovic, F.; Morand, C.; Glibetic, M. Effects of anthocyanins and their gut metabolites on adenosine diphosphate-induced platelet activation and their aggregation with monocytes and neutrophils. Arch. Biochem. Biophys. 2018, 645, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.L.; Liu, J.X.; Dong, W.; Li, P.; Li, L.; Lin, C.R.; Zheng, Y.Q.; Cong, W.H.; Hou, J.C. Cardioprotective effect of protocatechuic acid on myocardial ischemia/reperfusion injury. J. Pharmacol. Sci. 2014, 125, 176–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serreli, G.; Deiana, M. In vivo formed metabolites of polyphenols and their biological efficacy. Food Funct. 2019, 10, 6999–7021. [Google Scholar] [CrossRef] [PubMed]
- Krga, I.; Milenkovic, D. Anthocyanins: From sources and bioavailability to cardiovascular-health benefits and molecular mechanisms of action. J. Agric. Food Chem. 2019, 67, 1771–1783. [Google Scholar] [CrossRef] [PubMed]
- Peungvicha, P.; Thirawarapan, S.S.; Watanabe, H. Possible mechanism of hypoglycemic effect of 4-hydroxybenzoic acid, a constituent of Pandanus odorus root. Jpn. J. Pharmacol. 1998, 78, 395–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Ma, H.; Yang, N.; Tang, Y.; Guo, J.; Tao, W.; Duan, J. A series of natural flavonoids as thrombin inhibitors: Structure-activity relationships. Thromb. Res. 2010, 126, e365–e378. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Wu, Z.Y.; Xia, F.B.; Zhang, H.; Wang, X.; Gao, J.L.; Yang, F.Q.; Wan, J.B. Characterization of thrombin/factor Xa inhibitors in Rhizoma Chuanxiong through UPLC-MS-based multivariate statistical analysis. Chin. Med. 2020, 15, 93. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
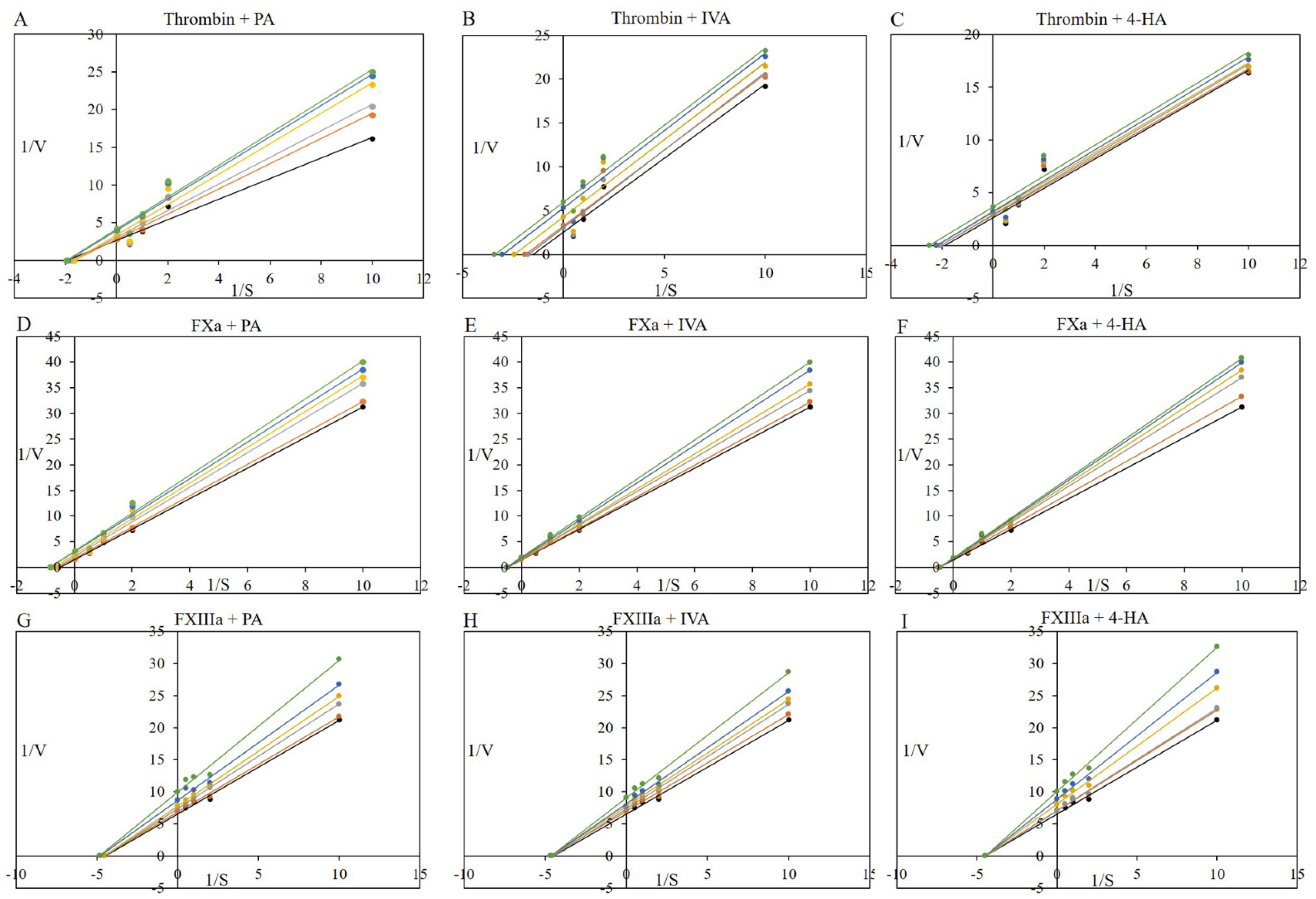

| Compound (mM) | Km (mM) | Vmax (mU/min) | Kcat (s−1) | Kcat/Km (mM−1s−1) | Kik | Kiv | Kik/Kiv | |
|---|---|---|---|---|---|---|---|---|
| Non-treated | 0.566 ± 0.025 | 0.381 ± 0.011 | 109.87 ± 3.16 | 196.33 ± 12.61 | - | - | - | |
| PA | 0.065 | 0.588 ± 0.017 | 0.352 ± 0.019 | 101.67 ± 1.44 | 172.79 ± 4.83 | 0.7831 | 0.0062 | 125.4 |
| 0.13 | 0.569 ± 0.011 | 0.323 ± 0.021 | 93.29 ± 1.60 | 163.85 ± 3.19 | ||||
| 0.325 | 0.595 ± 0.023 | 0.295 ± 0.015 | 85.00 ± 2.13 | 142.88 ± 3.05 | ||||
| 0.65 | 0.516 ± 0.015 | 0.249 ± 0.009 | 71.98 ± 1.95 | 139.46 ± 2.33 | ||||
| 1.3 | 0.506 ± 0.021 | 0.239 ± 0.008 | 69.08 ± 1.15 | 136.46 ± 3.35 | ||||
| IVA | 0.065 | 0.525 ± 0.013 | 0.304 ± 0.013 | 87.72 ± 1.32 | 166.94 ± 2.96 | 0.0028 | 0.0026 | 1.1 |
| 0.13 | 0.577 ± 0.015 | 0.326 ± 0.018 | 94.13 ± 2.08 | 163.13 ± 3.88 | ||||
| 0.325 | 0.410 ± 0.028 | 0.233 ± 0.010 | 67.14 ± 0.93 | 163.92 ± 2.54 | ||||
| 0.65 | 0.333 ± 0.026 | 0.189 ± 0.007 | 54.42 ± 0.81 | 163.53 ± 2.07 | ||||
| 1.3 | 0.293 ± 0.011 | 0.167 ± 0.008 | 48.31 ± 1.05 | 164.76 ± 3.13 | ||||
| 4-HA | 0.065 | 0.484 ± 0.015 | 0.349 ± 0.013 | 100.59 ± 1.41 | 207.85 ± 4.06 | 0.0111 | 0.0092 | 1.2 |
| 0.13 | 0.485 ± 0.022 | 0.341 ± 0.015 | 98.38 ± 1.24 | 202.80 ± 3.55 | ||||
| 0.325 | 0.449 ± 0.019 | 0.317 ± 0.016 | 91.57 ± 0.98 | 203.81 ± 3.86 | ||||
| 0.65 | 0.453 ± 0.020 | 0.309 ± 0.020 | 89.25 ± 0.77 | 197.20 ± 3.15 | ||||
| 1.3 | 0.400 ± 0.014 | 0.273 ± 0.013 | 78.83 ± 1.01 | 196.93 ± 2.81 | ||||
| Compound (mM) | Km (mM) | Vmax (mU/min) | Kcat (s−1) | Kcat/Km (mM−1s−1) | Kik | Kiv | Kik/Kiv | |
|---|---|---|---|---|---|---|---|---|
| Non-treated | 2.04 ± 0.09 | 0.69 ± 0.05 | 25.27 ± 0.71 | 12.38 ± 0.45 | - | - | - | |
| PA | 0.065 | 1.77 ± 0.05 | 0.58 ± 0.03 | 21.36 ± 0.54 | 12.08 ± 0.48 | 0.036 | 0.023 | 1.6 |
| 0.13 | 1.59 ± 0.08 | 0.47 ± 0.02 | 17.35 ± 0.48 | 10.93 ± 0.33 | ||||
| 0.325 | 1.38 ± 0.04 | 0.40 ± 0.02 | 14.67 ± 0.25 | 10.61 ± 0.29 | ||||
| 0.65 | 1.17 ± 0.03 | 0.33 ± 0.01 | 12.09 ± 0.29 | 10.34 ± 0.35 | ||||
| 1.3 | 1.17 ± 0.04 | 0.32 ± 0.01 | 11.62 ± 0.31 | 9.94 ± 0.30 | ||||
| IVA | 0.065 | 2.02 ± 0.07 | 0.66 ± 0.04 | 24.20 ± 0.27 | 12.00 ± 0.41 | 0.418 | 0.075 | 5.6 |
| 0.13 | 1.99 ± 0.05 | 0.61 ± 0.02 | 22.34 ± 0.41 | 11.23 ± 0.40 | ||||
| 0.325 | 2.05 ± 0.05 | 0.60 ± 0.03 | 22.23 ± 0.35 | 10.83 ± 0.31 | ||||
| 0.65 | 2.06 ± 0.04 | 0.56 ± 0.03 | 20.67 ± 0.32 | 10.05 ± 0.27 | ||||
| 1.3 | 1.92 ± 0.06 | 0.50 ± 0.04 | 18.56 ± 0.30 | 9.69 ± 0.25 | ||||
| 4-HA | 0.065 | 1.90 ± 0.09 | 0.60 ± 0.03 | 22.14 ± 0.22 | 11.65 ± 0.36 | 0.575 | 0.109 | 5.3 |
| 0.13 | 2.09 ± 0.08 | 0.59 ± 0.02 | 21.77 ± 0.26 | 10.44 ± 0.22 | ||||
| 0.325 | 2.17 ± 0.10 | 0.59 ± 0.04 | 21.79 ± 0.44 | 10.03 ± 0.28 | ||||
| 0.65 | 2.20 ± 0.09 | 0.57 ± 0.02 | 21.18 ± 0.42 | 9.64 ± 0.21 | ||||
| 1.3 | 2.14 ± 0.06 | 0.55 ± 0.02 | 20.22 ± 0.37 | 9.46 ± 0.23 | ||||
| Compound (mM) | Km (mM) | Vmax (mU/min) | Kcat (s−1) | Kcat/Km (mM−1s−1) | Kik | Kiv | Kik/Kiv | |
|---|---|---|---|---|---|---|---|---|
| Non-treated | 0.223 ± 0.010 | 0.153 ± 0.03 | 95.3 ± 0.05 | 427.3 ± 11.7 | - | - | - | |
| PA | 0.065 | 0.217 ± 0.011 | 0.146 ± 0.03 | 91.1 ± 0.03 | 420.1 ± 10.2 | 0.019 | 0.003 | 6.1 |
| 0.13 | 0.220 ± 0.009 | 0.135 ± 0.02 | 84.4 ± 0.06 | 382.9 ± 10.5 | ||||
| 0.325 | 0.220 ± 0.007 | 0.128 ± 0.03 | 80.1 ± 0.05 | 364.3 ± 7.1 | ||||
| 0.65 | 0.207 ± 0.010 | 0.115 ± 0.04 | 71.8 ± 0.07 | 347.4 ± 6.3 | ||||
| 1.3 | 0.205 ± 0.009 | 0.100 ± 0.02 | 62.5 ± 0.04 | 304.6 ± 6.9 | ||||
| IVA | 0.065 | 0.215 ± 0.012 | 0.143 ± 0.02 | 89.3 ± 0.09 | 415.2 ± 7.5 | 0.043 | 0.0041 | 10.4 |
| 0.13 | 0.221 ± 0.008 | 0.135 ± 0.03 | 84.5 ± 0.07 | 383.2 ± 6.5 | ||||
| 0.325 | 0.214 ± 0.009 | 0.129 ± 0.04 | 80.5 ± 0.05 | 375.3 ± 5.3 | ||||
| 0.65 | 0.214 ± 0.006 | 0.129 ± 0.03 | 80.5 ± 0.09 | 375.3 ± 4.9 | ||||
| 1.3 | 0.215 ± 0.009 | 0.110 ± 0.04 | 68.7 ± 0.08 | 319.4 ± 4.5 | ||||
| 4-HA | 0.065 | 0.223 ± 0.010 | 0.142 ± 0.04 | 88.6 ± 0.10 | 427.3 ± 6.1 | 17.875 | 0.003 | 6005.6 |
| 0.13 | 0.223 ± 0.007 | 0.141 ± 0.04 | 87.7 ± 0.09 | 397.3 ± 5.2 | ||||
| 0.325 | 0.223 ± 0.011 | 0.124 ± 0.03 | 77.2 ± 0.05 | 346.1 ± 4.6 | ||||
| 0.65 | 0.223 ± 0.009 | 0.113 ± 0.03 | 70.5 ± 0.07 | 316.2 ± 7.7 | ||||
| 1.3 | 0.223 ± 0.010 | 0.099 ± 0.04 | 62.0 ± 0.06 | 277.7 ± 6.2 | ||||
| Half Time at 1/2 Maximum (s) | Fold of Half Time | Absorbance after Reaction | ||
|---|---|---|---|---|
| NC1 | - | - | 0.0224 ± 0.0018 | |
| NC2 | 740 ± 20 | 1.00 | 0.2094 ± 0.0305 # | |
| PC | - | - | 0.0184 ± 0.0016 ** | |
| PA | 1 | 1220 ± 90 * | 1.65 ± 0.12 * | 0.1898 ± 0.0227 |
| 2–20 | - | - | 0.0201 ± 0.0012 **(2), 0.0044 ± 0.0009 **(5), −0.0047 ± 0.0004 **(10), −0.0103 ± 0.0011 **(20) | |
| IVA | 1 | 510 ± 25 * | 0.69 ± 0.03 * | 0.1882 ± 0.0097 |
| 2 | - | - | 0.1732 ± 0.0106 * | |
| 5 | 615 ± 20 | 0.83 ± 0.03 | 0.1575 ± 0.0052 ** | |
| 10 | 355 ± 30 * | 0.48 ± 0.04 * | 0.1377 ± 0.0071 ** | |
| 20 | - | - | 0.0462 ± 0.0035 ** | |
| 4-HA | 1 | - | - | 0.1424 ± 0.0122 ** |
| 2 | 950 ± 70 * | 1.28 ± 0.09 * | 0.1086 ± 0.0083 ** | |
| 5–20 | - | - | 0.0866 ± 0.0051 **(5), 0.0233 ± 0.0014 **(10), 0.0205 ± 0.0029 **(20) | |
| Control | PA (μg) | IVA (μg) | 4-HA (μg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 50 | 100 | 10 | 20 | 50 | 100 | 10 | 20 | 50 | 100 | ||
| Closure time (s) | 118.6 ± 2.1 | 119.9 ± 2.4 | 122.1 ± 1.4 | 126.3 ± 2.9 | 133.7 ± 5.2 ** | 118.5 ± 1.8 | 119.7 ± 2.8 | 123.5 ± 2.2 | 129.4 ± 3.4 * | 121.2 ± 1.9 | 127.7 ± 3.1 | 134.6 ± 4.5 ** | 147.3 ± 5.8 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-H.; Kim, S. In Vitro Antithrombotic, Hematological Toxicity, and Inhibitor Studies of Protocatechuic, Isovanillic, and p-Hydroxybenzoic Acids from Maclura tricuspidata (Carr.) Bur. Molecules 2022, 27, 3496. https://doi.org/10.3390/molecules27113496
Choi J-H, Kim S. In Vitro Antithrombotic, Hematological Toxicity, and Inhibitor Studies of Protocatechuic, Isovanillic, and p-Hydroxybenzoic Acids from Maclura tricuspidata (Carr.) Bur. Molecules. 2022; 27(11):3496. https://doi.org/10.3390/molecules27113496
Chicago/Turabian StyleChoi, Jun-Hui, and Seung Kim. 2022. "In Vitro Antithrombotic, Hematological Toxicity, and Inhibitor Studies of Protocatechuic, Isovanillic, and p-Hydroxybenzoic Acids from Maclura tricuspidata (Carr.) Bur" Molecules 27, no. 11: 3496. https://doi.org/10.3390/molecules27113496
APA StyleChoi, J.-H., & Kim, S. (2022). In Vitro Antithrombotic, Hematological Toxicity, and Inhibitor Studies of Protocatechuic, Isovanillic, and p-Hydroxybenzoic Acids from Maclura tricuspidata (Carr.) Bur. Molecules, 27(11), 3496. https://doi.org/10.3390/molecules27113496






