Dually Responsive Poly(N-vinylcaprolactam)-b-poly(dimethylsiloxane)-b-poly(N-vinylcaprolactam) Polymersomes for Controlled Delivery
Abstract
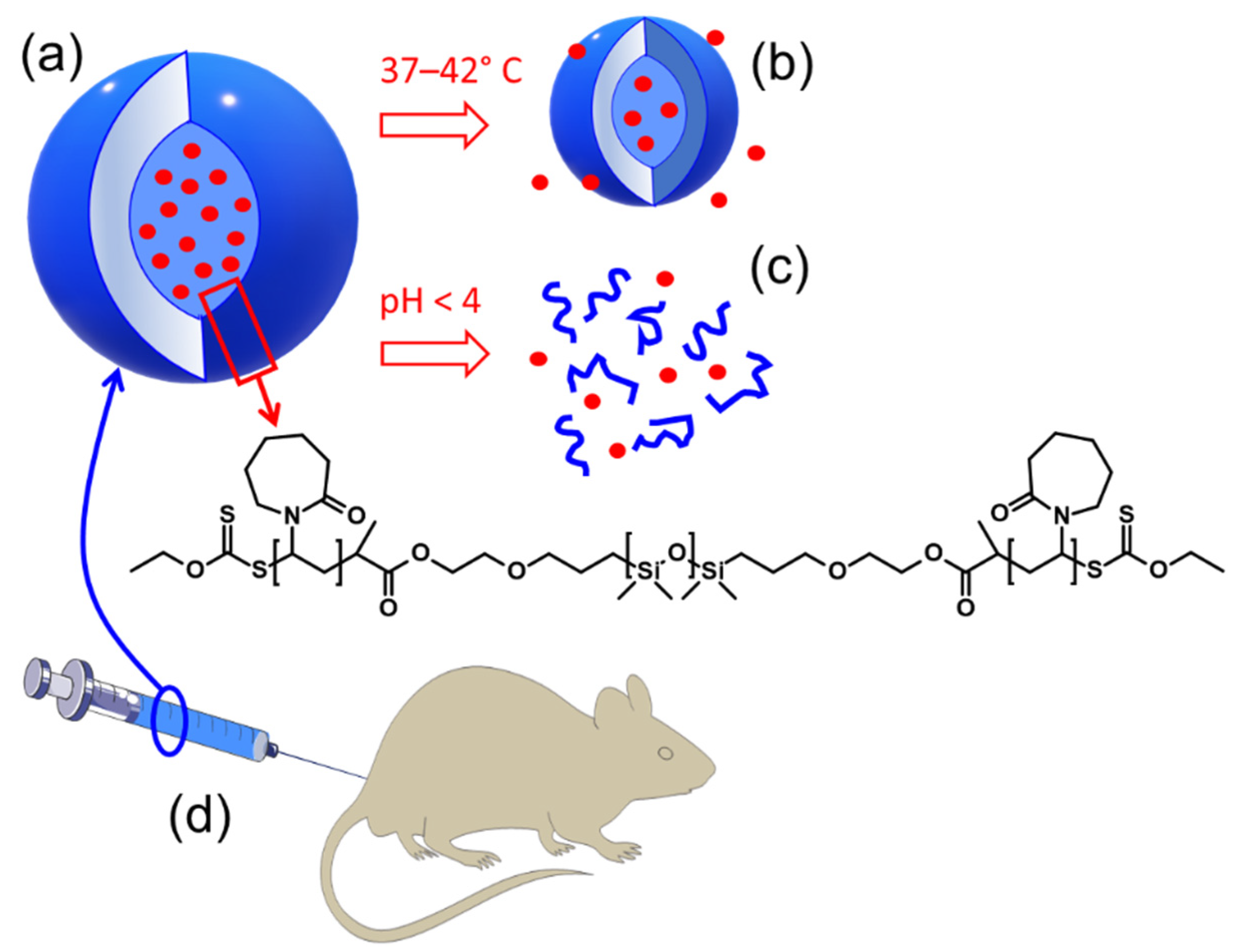
:1. Introduction
2. Results and Discussion
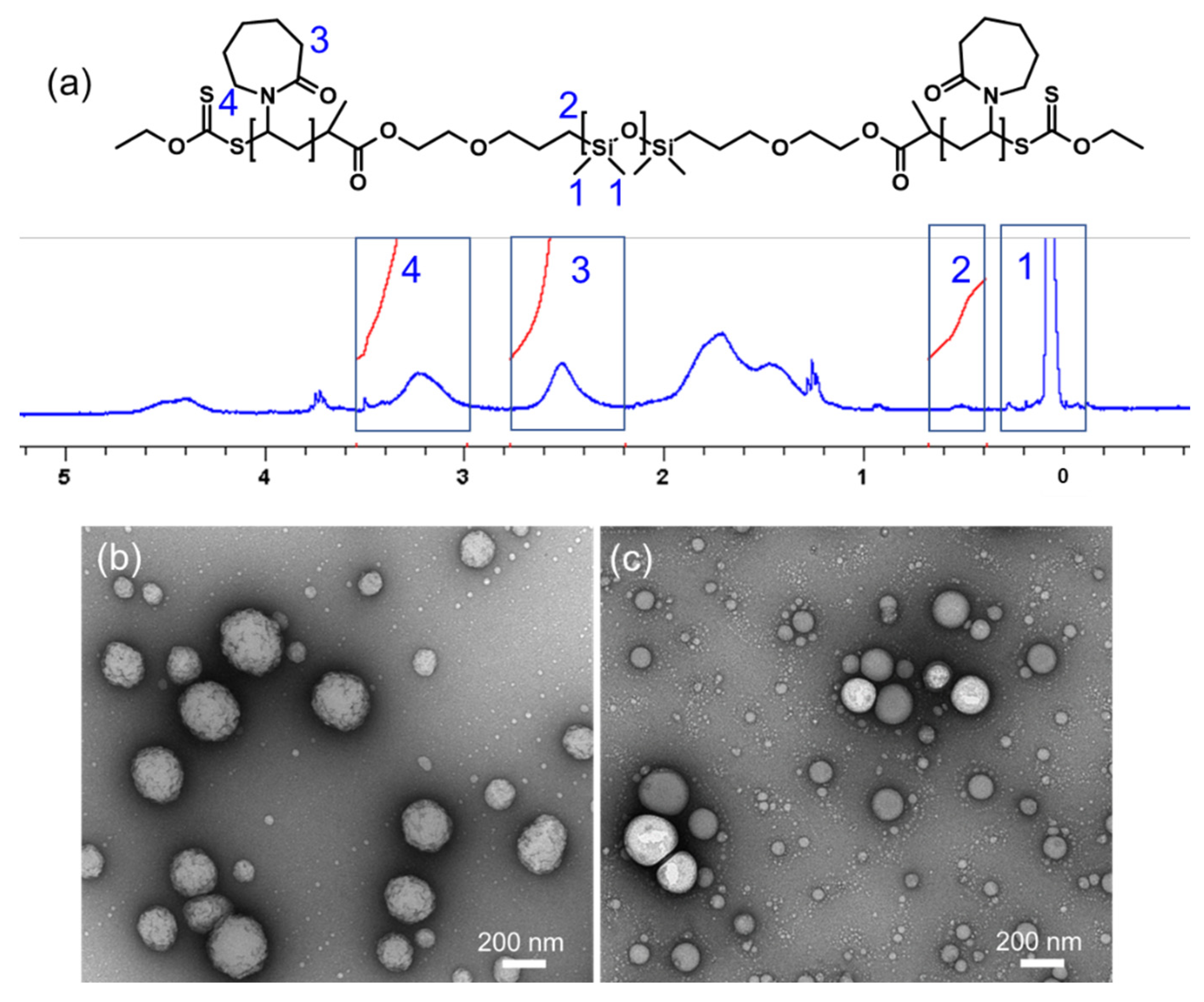
2.1. Synthesis of PVCL10-PDMS65-PVCL10 and PMOXA14-PDMS65-PMOXA14 Triblock Copolymers and Vesicle Assembly
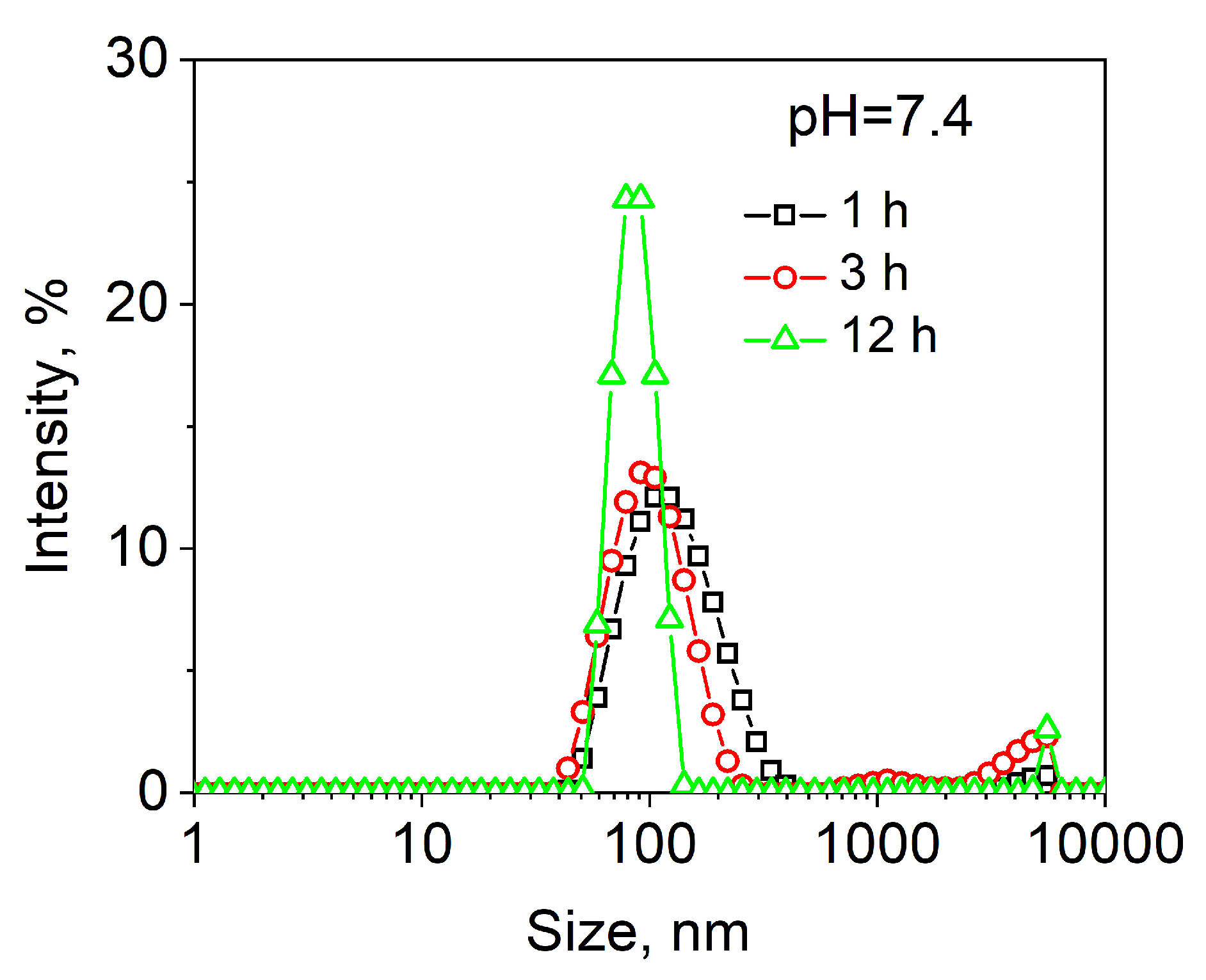
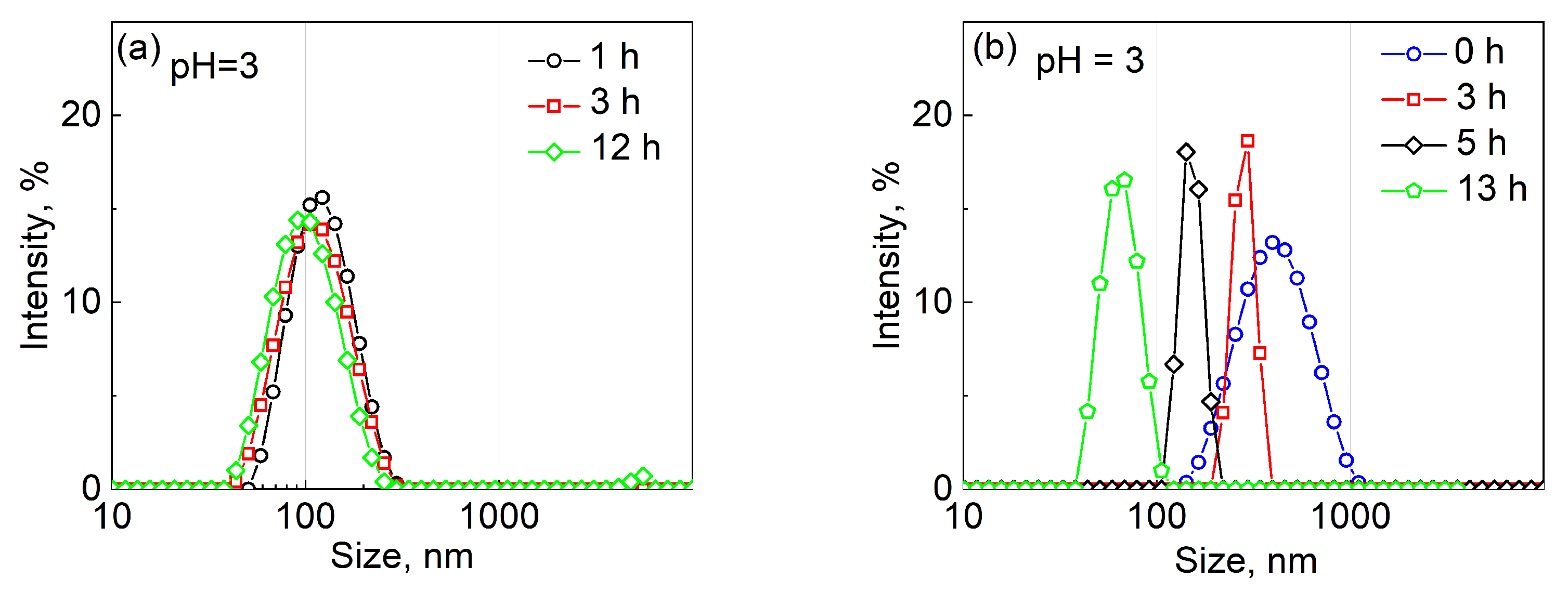
2.2. pH-Induced Degradation of PVCL10-PDMS65-PVCL10 Polymersomes
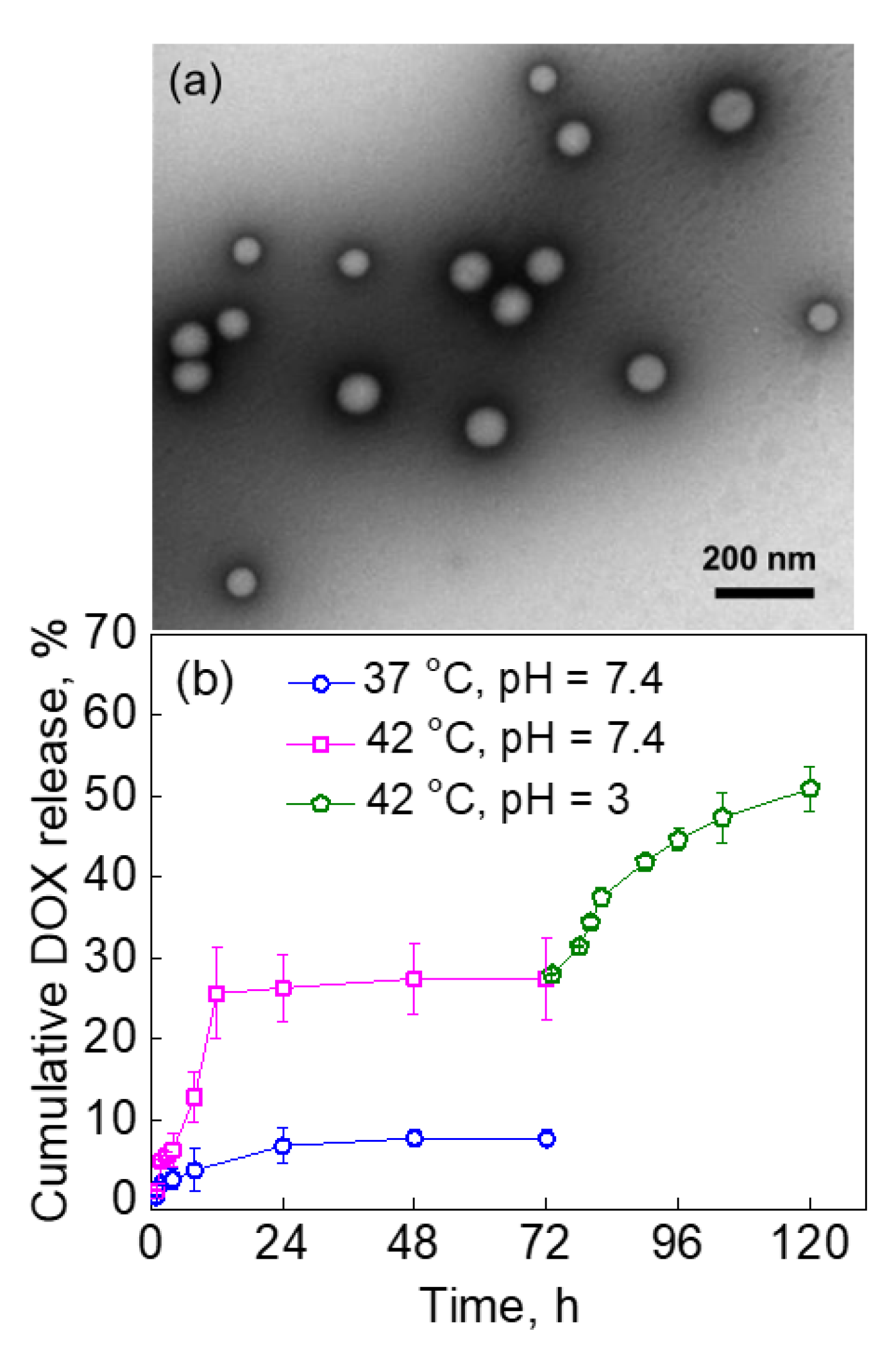
2.3. pH-Dependent Release of DOX from PVCL10-PDMS65-PVCL10 Vesicles
2.4. The Effect of PVCL-PDMS-PVCL Vesicle Shell Thickness on DOX Release via the Dual Response
2.5. In-Vivo Toxicity Study of PVCL10-PDMS65-PVCL10 Vesicle Treatment in Mice
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Poly(N-vinylcaprolactam)10-block-poly(dimethylsiloxane)65-block-poly(N-vinylcaprolactam)10 (PVCL10-PDMS65-PVCL10) and Poly(N-vinylcaprolactam)5-block-poly(dimethylsiloxane)30-block-poly(N-vinylcaprolactam)5 (PVCL5-PDMS30-PVCL5) Triblock Copolymers
3.3. Synthesis of Poly-(2-methyl-2-oxazoline)14-block-poly(dimethylsiloxane)65-block-poly-(2-methyl-2-oxazoline)14 (PMOXA14-PDMS65-PMOXA14) Triblock Copolymer
3.4. Assembly of PVCL-PDMS-PVCL and PMOXA14-PDMS65-PMOXA14 Polymersomes
3.5. Loading and Release of DOX into PVCL-PDMS-PVCL and PMOXA14-PDMS65-PMOXA14 Vesicles
3.6. Transmission Electron Microscopy (TEM)
3.7. Dynamic Light Scattering (DLS)
3.8. Animal Care, Compliance, and Treatment
3.9. Vesicle Toxicity Study
3.10. Gravimetric Necropsy Analysis
3.11. Histology Analysis
3.12. Hematology Analysis
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Kabb, C.P.; Sims, M.B.; Sumerlin, B.S. Architecture-transformable polymers: Reshaping the future of stimuli-responsive polymers. Prog. Polym. Sci. 2019, 89, 61–75. [Google Scholar] [CrossRef]
- Gundogdu, D.; Bütün, V.; Erel-Göktepe, I. Preparation of Layer-by-Layer Films with Remarkably Different pH-Stability and Release Properties Using Dual Responsive Block Copolymer Micelles. Macromol. Chem. Phys. 2018, 219, 1800128. [Google Scholar] [CrossRef]
- Lu, W.; Le, X.; Zhang, J.; Huang, Y.; Chen, T. Supramolecular shape memory hydrogels: A new bridge between stimuli-responsive polymers and supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C 2016, 60, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Marguet, M.; Sandre, O.; Lecommandoux, S. Polymersomes in “Gelly” Polymersomes: Toward Structural Cell Mimicry. Langmuir 2012, 28, 2035–2043. [Google Scholar] [CrossRef]
- Wang, Y.; Sukhishvili, S.A. All-Aqueous Nanoprecipitation: Spontaneous Formation of Hydrogen-Bonded Nanoparticles and Nanocapsules Mediated by Phase Separation of Poly(N-Isopropylacrylamide). Macromol. Rapid Commun. 2017, 38, 1700242. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-Assembly of Block Copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef]
- Liao, J.F.; Wang, C.; Wang, Y.J.; Luo, F.; Qian, Z.Y. Recent Advances in Formation, Properties, and Applications of Polymersomes. Curr. Pharm. Des. 2012, 18, 3432–3441. [Google Scholar] [CrossRef]
- Bej, R.; Achazi, K.; Haag, R.; Ghosh, S. Polymersome Formation by Amphiphilic Polyglycerol-b-polydisulfide-b-polyglycerol and Glutathione-Triggered Intracellular Drug Delivery. Biomacromolecules 2020, 21, 3353–3363. [Google Scholar] [CrossRef]
- Wang, Y.; Sukhishvili, S.A. Hydrogen-Bonded Polymer Complexes and Nanocages of Weak Polyacids Templated by a Pluronic® Block Copolymer. Soft Matter 2016, 12, 8744–8754. [Google Scholar] [CrossRef] [PubMed]
- Ogunsola, O.A.; Kraeling, M.E.; Zhong, S.; Pochan, D.J.; Bronaugh, R.L.; Raghavan, S.R. Structural Analysis of “Flexible” Liposome Formulations: New Insights into the Skin-Penetrating Ability of Soft Nanostructures. Soft Matter 2012, 8, 10226–10232. [Google Scholar] [CrossRef]
- Aibani, N.; Nesbitt, H.; Marino, N.; Jurek, J.; O’Neill, C.; Martin, C.; Di Bari, I.; Sheng, Y.; Logan, K.; Hawthorne, S.; et al. Electroneutral polymersomes for combined cancer chemotherapy. Acta Biomater. 2018, 80, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Tanner, P.; Baumann, P.; Enea, R.; Onaca, O.; Palivan, C.; Meier, W. Polymeric Vesicles: From Drug Carriers to Nanoreactors and Artificial Organelles. Acc. Chem. Res. 2011, 44, 1039–1049. [Google Scholar] [CrossRef]
- Ulusan, S.; Bütün, V.; Banerjee, S.; Erel-Goktepe, I. Biologically Functional Ultrathin Films Made of Zwitterionic Block Copolymer Micelles. Langmuir 2019, 35, 1156–1171. [Google Scholar] [CrossRef]
- Kostyurina, E.; De Mel, J.U.; Vasilyeva, A.; Kruteva, M.; Frielinghaus, H.; Dulle, M.; Barnsley, L.; Förster, S.; Schneider, G.J.; Biehl, R.; et al. Controlled LCST Behavior and Structure Formation of Alternating Amphiphilic Copolymers in Water. Macromolecules 2022, 55, 1552–1565. [Google Scholar] [CrossRef]
- Rijcken, C.; Soga, O.; Hennink, W.; Van Nostrum, C. Triggered destabilisation of polymeric micelles and vesicles by changing polymers polarity: An attractive tool for drug delivery. J. Control Release 2007, 120, 131–148. [Google Scholar] [CrossRef]
- Agut, W.; Brûlet, A.; Schatz, C.; Taton, D.; Lecommandoux, S. pH and temperature responsive polymeric micelles and polymersomes by self-assembly of poly[2-(dimethylamino) ethyl methacrylate]-b-poly(glutamic acid) double hydrophilic block copolymers. Langmuir 2010, 26, 10546–10554. [Google Scholar] [CrossRef]
- Li, Y.; Lokitz, B.S.; McCormick, C.L. Thermally Responsive Vesicles and Their Structural “Locking” through Polyelectrolyte Complex Formation. Angew. Chem. 2006, 118, 5924–5927. [Google Scholar] [CrossRef]
- Liang, X.; Liu, F.; Kozlovskaya, V.; Palchak, Z.; Kharlampieva, E. Thermoresponsive Micelles from Double LCST-Poly(3-methyl-N-vinylcaprolactam) Block Copolymers for Cancer Therapy. ACS Macro Lett. 2015, 4, 308–311. [Google Scholar] [CrossRef]
- Nehate, C.; Nayal, A.; Koul, V. Redox responsive polymersomes for enhanced doxorubicin delivery. ACS Biomater. Sci. Eng. 2018, 5, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug. Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Jiang, Y.; Cao, J.; Yang, W.; Zhang, J.; Meng, F.; Zhong, Z. Boosting RNAi therapy for orthotopic glioblastoma with nontoxic brain-targeting chimaeric polymersomes. J. Control. Release 2018, 292, 163–171. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Xie, Z.; Jing, X.; Bellotti, A.; Gu, Z. Stimuli-responsive polymersomes for biomedical applications. Biomacromolecules 2017, 18, 649–673. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Tang, Y.; Lewis, A.L.; Armes, S.P. pH-sensitive vesicles based on a biocompatible zwitterionic diblock copolymer. J. Am. Chem. Soc. 2005, 127, 17982–17983. [Google Scholar] [CrossRef] [PubMed]
- Lomas, H.; Canton, I.; MacNeil, S.; Du, J.; Armes, S.P.; Ryan, A.J.; Lewis, A.L.; Battaglia, G. Biomimetic pH sensitive polymersomes for efficient DNA encapsulation and delivery. Adv. Mater. 2007, 19, 4238–4243. [Google Scholar] [CrossRef]
- Borchert, U.; Lipprandt, U.; Bilang, M.; Kimpfler, A.; Rank, A.; Peschka-Süss, R.; Schubert, R.; Lindner, P.; Förster, S. pH-induced release from P2VP-PEO block copolymer vesicles. Langmuir 2006, 22, 5843–5847. [Google Scholar] [CrossRef]
- Meng, F.; Hiemstra, C.; Engbers, G.H.; Feijen, J. Biodegradable polymersomes. Macromolecules 2003, 36, 3004–3006. [Google Scholar] [CrossRef]
- Ahmed, F.; Discher, D.E. Self-porating polymersomes of PEG-PLA and PEG-PCL: Hydrolysis-triggered controlled release vesicles. J. Control Release 2004, 96, 37–53. [Google Scholar] [CrossRef]
- Rijpkema, S.J.; Langens, S.G.; van der Kolk, M.R.; Gavriel, K.; Toebes, B.J.; Wilson, D.A. Modular approach to the functionalization of polymersomes. Biomacromolecules 2020, 21, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Yang, S.R.; An, E.J.; Kim, J.-D. Biodegradable polymersomes from poly(2-hydroxyethyl aspartamide) grafted with lactic acid oligomers in aqueous solution. Macromolecules 2006, 39, 4938–4940. [Google Scholar] [CrossRef]
- Brinkhuis, R.P.; Visser, T.R.; Rutjes, F.P.; van Hest, J.C. Shedding the hydrophilic mantle of polymersomes. Polym. Chem. 2011, 2, 550–552. [Google Scholar] [CrossRef] [Green Version]
- Brinkhuis, R.P.; De Graaf, F.; Hansen, M.B.; Visser, T.R.; Rutjes, F.P.; van Hest, J.C. Dynamically functionalized polymersomes via hydrazone exchange. Polym. Chem. 2013, 4, 1345–1350. [Google Scholar] [CrossRef] [Green Version]
- Bourgat, Y.; Tiersch, B.; Koetz, J.; Menzel, H. Enzyme Degradable Polymersomes from Chitosan-g-[poly-l-lysine-block-ε-caprolactone] Copolymer. Macromol. Biosci. 2021, 21, 2000259. [Google Scholar] [CrossRef]
- Liu, F.; Kozlovskaya, V.; Medipelli, S.; Xue, B.; Ahmad, F.; Saeed, M.; Cropek, D.; Kharlampieva, E. Temperature-sensitive polymersomes for controlled delivery of anticancer drugs. Chem. Mater. 2015, 27, 7945–7956. [Google Scholar] [CrossRef]
- Ajitha, A.A.; SivaKumar, S.; Viswanathan, G.; Baby, S.; Biju, P.G. Therapeutic Properties of PDMS Nanoparticles: A Promising New Drug Delivery Vehicle Against Inflammatory Conditions. Comb. Chem. High Throughput Screen. 2021. [Google Scholar] [CrossRef]
- Thompson, A.J.; Ma, L.J.; Major, T.; Jeakle, M.; Lautner-Csorba, O.; Goudie, M.J.; Handa, H.; Rojas-Peña, A.; Potkay, J.A. Assessing and improving the biocompatibility of microfluidic artificial lungs. Acta Biomater. 2020, 112, 190–201. [Google Scholar] [CrossRef]
- Vihola, H.; Laukkanen, A.; Valtola, L.; Tenhu, H.; Hirvonen, J. Cytotoxicity of Thermosensitive Polymers Poly(N-Isopropylacrylamide), Poly(N-Vinylcaprolactam) and Amphiphilically Modified Poly(N-Vinylcaprolactam). Biomaterials 2005, 26, 3055–3064. [Google Scholar] [CrossRef]
- Winninger, J.; Iurea, D.M.; Atanase, L.I.; Salhi, S.; Delaite, C.; Riess, G. Micellization of novel biocompatible thermo-sensitive graft copolymers based on poly(ε-caprolactone), poly(N-vinylcaprolactam) and poly(N-vinylpyrrolidone). Eur. Polym. J. 2019, 119, 74–82. [Google Scholar] [CrossRef]
- Yu, Y.C.; Kang, H.U.; Youk, J.H. Synthesis and micellar characterization of thermosensitive amphiphilic poly(ε-caprolactone)-b-poly(N-vinylcaprolactam) block copolymers. Colloid Polym. Sci. 2012, 290, 1107–1113. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Liu, F.; Xue, B.; Ahmad, F.; Alford, A.; Saeed, M.; Kharlampieva, E. Polyphenolic Polymersomes of Temperature-Sensitive Poly(N-vinylcaprolactam)-block-Poly(N-vinylpyrrolidone) for Anticancer Therapy. Biomacromolecules 2017, 18, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Alford, A.; Kozlovskaya, V.; Zhao, S.; Joshi, H.; Kim, E.; Qian, S.; Urban, V.; Cropek, D.; Aksimentiev, A. Effect of Temperature and Hydrophilic Ratio on the Structure of Poly(N-vinylcaprolactam)-block-poly(dimethylsiloxane)-block-poly(N-vinylcaprolactam) Polymersomes. ACS Appl. Polym. Mater. 2019, 1, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, V.; Liu, F.; Yang, Y.; Ingle, K.; Qian, S.; Halade, G.V.; Urban, V.S.; Kharlampieva, E. Temperature-Responsive Polymersomes of Poly(3-methyl-N-vinylcaprolactam)-block-poly(N-vinylpyrrolidone) To Decrease Doxorubicin-Induced Cardiotoxicity. Biomacromolecules 2019, 20, 3989–4000. [Google Scholar] [CrossRef] [PubMed]
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef] [PubMed]
- Nardin, C.; Thoeni, S.; Widmer, J.; Winterhalter, M.; Meier, W. Nanoreactors based on (polymerized) ABA-triblock copolymer vesicles. Chem. Commun. 2000, 1433–1434. [Google Scholar] [CrossRef]
- Brož, P.; Benito, S.M.; Saw, C.; Burger, P.; Heider, H.; Pfisterer, M.; Marsch, S.; Meier, W.; Hunziker, P. Cell targeting by a generic receptor-targeted polymer nanocontainer platform. J. Control Release 2005, 102, 475–488. [Google Scholar] [CrossRef]
- Moquin, A.; Ji, J.; Neibert, K.; Winnik, F.M.; Maysinger, D. Encapsulation and delivery of neutrophic proteins and hydrophobic agents using PMOXA–PDMS–PMOXA triblock polymersomes. ACS Omega 2018, 3, 13882–13893. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Kozlovskaya, V.; Zhang, Z.; Xing, C.; Zaharias, S.; Dolmat, M.; Qian, S.; Zhang, J.; Warram, J.M.; Yang, E.S.; et al. Poly(N-vinylpyrrolidone)-block-poly(dimethylsiloxane)-block-poly(N-vinylpyrrolidone) Triblock Copolymer Polymersomes for Delivery of PARP1 siRNA to Breast Cancers. ACS Appl. Bio Mater. 2022, 5, 1670–1682. [Google Scholar] [CrossRef]
- Agrawal, K. Doxorubicin. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2007; pp. 1–5. [Google Scholar] [CrossRef]
- Kyropoulou, M.; Avsar, S.Y.; Schoenenberger, C.-A.; Palivan, C.G.; Meier, W.P. From spherical compartments to polymer films: Exploiting vesicle fusion to generate solid supported thin polymer membranes. Nanoscale 2021, 13, 6944–6952. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Kharlampieva, E. Self-Assemblies of Thermoresponsive Poly(N-vinylcaprolactam) Polymers for Applications in Biomedical Field. ACS Appl. Polym. Mater. 2020, 2, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Morarasu, S.; Morarasu, B.C.; Ghiarasim, R.; Coroaba, A.; Tirón, C.; Iliescu, R.; Dimofte, G.-M. Targeted Cancer Therapy via pH-Functionalized Nanoparticles: A Scoping Review of Methods and Outcomes. Gels 2022, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, V.; Chen, J.; Tedjo, C.; Liang, X.; Campos-Gomez, J.; Oh, J.; Saeed, M.; Lungu, C.T.; Kharlampieva, E. pH-Responsive Hydrogel Cubes for Release of Doxorubicin in Cancer Cells. J. Mater. Chem. B 2014, 2, 2494–2507. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Attia, A.B.E.; Tan, J.P.K.; Ke, X.Y.; Gao, S.J. The Role of Non-Covalent Interactions in Anticancer Drug Loading and Kinetic Stability of Polymeric Micelles. Biomaterials 2012, 33, 2971–2979. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Kozlovskaya, V.; Cox, C.P.; Wang, Y.; Saeed, M.; Kharlampieva, E. Synthesis and Self-Assembly of Thermosensitive Double-hydrophilic Poly(N-vinylcaprolactam)-b-poly(N-vinyl-2-pyrrolidone) Diblock Copolymers. J. Polym. Sci. A Polym. Chem. 2014, 52, 2725–2737. [Google Scholar] [CrossRef]
- Munday, R.; Smith, B.L.; Munday, C.M. Structure-activity relationships in the haemolytic activity and nephrotoxicity of derivatives of 1,2- and 1,4-naphthoquinone. J. Appl. Toxicol. 2007, 27, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kozlovskaya, V.; Dolmat, M.; Song, Y.; Qian, S.; Urban, V.; Cropek, D.; Kharlampieva, E. Temperature Controlled Transformations of Giant Unilamellar Vesicles of Amphiphilic Triblock Copolymers Synthesized via Microfluididc Mixing. Appl. Surf. Sci. Adv. 2021, 5, 100101. [Google Scholar] [CrossRef]

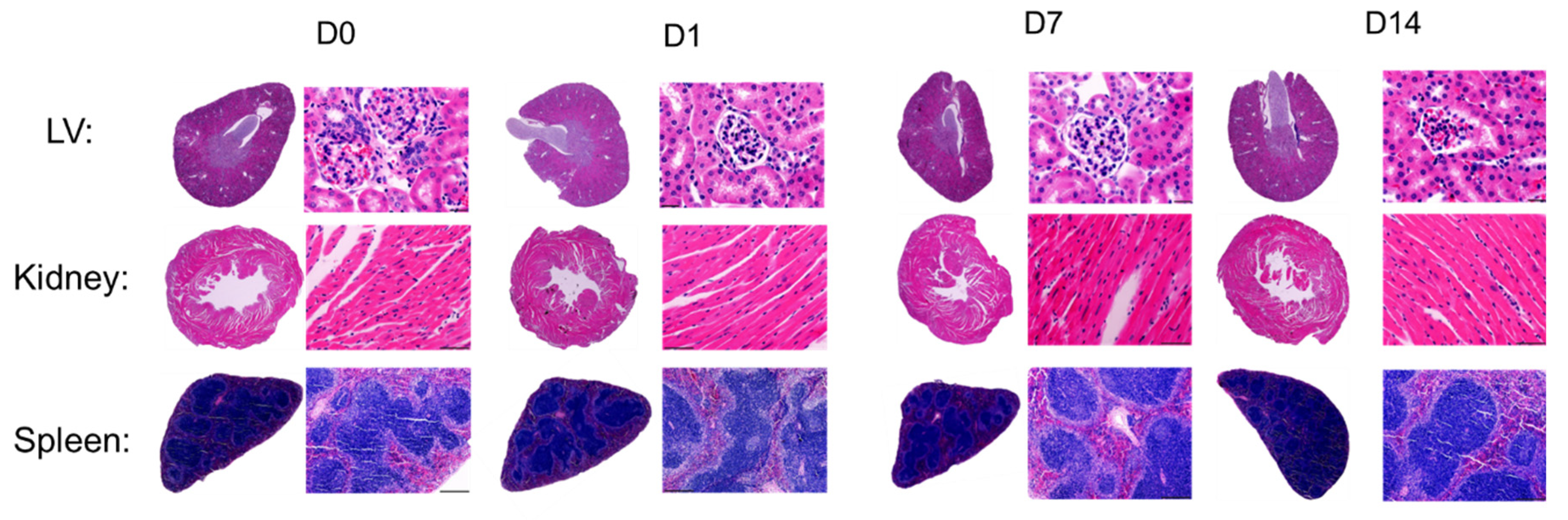
| Parameters | D0 (n = 5) | D1 (n = 5) | D7 (n = 5) | D14 (n = 5) |
|---|---|---|---|---|
| Mortality | 0 | 0 | 0 | 0 |
| Body weight (g) | 1 23.7 ± 0.4 | 30.3 ± 0.7 * | 27.4 ± 0.5 * | 32.1 ± 1.16 * |
| 2 LV/3 BW (mg/g) | 3 ± 0.14 | 2.9 ± 0.06 | 3 ± 0.04 | 3.1± 0.05 |
| 4 RV/BW (mg/g) | 0.80 ± 0.04 | 0.7 ± 0.03 * | 0.80 ± 0.02 | 0.8 ± 0.01 |
| Lung weight/BW (mg/g) | 5.4 ± 0.23 | 4.6 ± 0.13 * | 4.6 ± 0.17 * | 4.2 ± 0.26 * |
| Dry lung/BW (mg/g) | 1.2 ± 0.05 | 1.0 ± 0.05 | 1.1 ± 0.05 | 0.9 ± 0.06 |
| Tibia (mm) | 16.2 ± 0.2 | 17.4 ± 0.24 * | 17.1 ± 0.1 * | 17.4 ± 0.02 * |
| LV/BW:LV/Tibia | 0.2 ± 0.007 | 0.2 ± 0.005 | 0.2 ± 0.002 | 0.2 ± 0.002 |
| Spleen Weight/BW (mg/g) | 3.0 ± 0.30 | 2.6 ± 0.16 | 2.7 ± 0.10 | 2.6 ± 0.07 |
| Right Kidney/BW (mg/g) | 5.6 ± 0.11 | 5.2 ± 0.46 | 5.2 ± 0.12 | 5.6 ± 0.14 |
| Left Kidney/BW (mg/g) | 5.5 ± 0.24 | 5.3 ± 0.43 | 5.3 ± 0.08 | 5.3 ± 0.21 |
| Liver/BW (mg/g) | 41.5 ± 2.36 | 40.0 ± 1.85 | 44.5 ± 1.69 | 42.12 ± 0.77 |
| Hematological Profile | D0 (n = 5) 1 | D7 (n = 5) 1 | D14 (n = 14) 1 | Normal |
|---|---|---|---|---|
| Total leucocytes (K/µL) | 4.70 ± 0.46 | 5.4 ± 0.87 | 3.74 ± 0.44 * | 1.8–10.7 |
| Neutrophils (%) | 9.55 ± 1.02 | 21.55 ± 2.45 * | 20.58 ± 2.08 * | 6.6–38.9 |
| Lymphocytes (%) | 86.38 ± 1.18 | 71.81 ± 2.26 * | 72.43 ± 1.94 * | 55.8–91.6 |
| Monocytes (%) | 3.77 ± 0.785 | 5.87 ± 0.15 * | 5.56 ± 0.82 * | 0.0–7.5 |
| Eosinophils (%) | 0.27 ± 0.13 | 0.66 ± 0.23 * | 1.12 ± 0.59 * | 0.0–3.9 |
| Platelets (K/µL) | 741.25 ± 31.66 | 665.8 ± 149.68 | 905.8 ± 23.31 * | 592–2972 |
| Red Blood Cells (M/µL) | 9.042 ± 0.24 | 8.91 ± 0.09 | 8.58 ± 0.09 * | 6.36–9.42 |
| Hemoglobin (g/dL) | 10.88 ± 0.40 | 11.84 ± 0.12 * | 10.28 ± 0.13 * | 11.0–15.1 |
| Hematocrit (%) | 40.18 ± 1.09 | 39.58 ± 0.37 | 37.02 ± 0.55 * | 35.1–45.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlovskaya, V.; Yang, Y.; Liu, F.; Ingle, K.; Ahmad, A.; Halade, G.V.; Kharlampieva, E. Dually Responsive Poly(N-vinylcaprolactam)-b-poly(dimethylsiloxane)-b-poly(N-vinylcaprolactam) Polymersomes for Controlled Delivery. Molecules 2022, 27, 3485. https://doi.org/10.3390/molecules27113485
Kozlovskaya V, Yang Y, Liu F, Ingle K, Ahmad A, Halade GV, Kharlampieva E. Dually Responsive Poly(N-vinylcaprolactam)-b-poly(dimethylsiloxane)-b-poly(N-vinylcaprolactam) Polymersomes for Controlled Delivery. Molecules. 2022; 27(11):3485. https://doi.org/10.3390/molecules27113485
Chicago/Turabian StyleKozlovskaya, Veronika, Yiming Yang, Fei Liu, Kevin Ingle, Aftab Ahmad, Ganesh V. Halade, and Eugenia Kharlampieva. 2022. "Dually Responsive Poly(N-vinylcaprolactam)-b-poly(dimethylsiloxane)-b-poly(N-vinylcaprolactam) Polymersomes for Controlled Delivery" Molecules 27, no. 11: 3485. https://doi.org/10.3390/molecules27113485
APA StyleKozlovskaya, V., Yang, Y., Liu, F., Ingle, K., Ahmad, A., Halade, G. V., & Kharlampieva, E. (2022). Dually Responsive Poly(N-vinylcaprolactam)-b-poly(dimethylsiloxane)-b-poly(N-vinylcaprolactam) Polymersomes for Controlled Delivery. Molecules, 27(11), 3485. https://doi.org/10.3390/molecules27113485






