Alginate Lyases from Marine Bacteria: An Enzyme Ocean for Sustainable Future
Abstract
1. Introduction
2. Alginate and Alginate Lyases
3. Marine Sources of Alginate Lyase
4. Alginate Lyase-Producing Marine Bacteria
5. Enzymatic Properties of Alginate Lyases from Marine Bacteria
| Source | Localization | Substrate Specificity | Protein Name | Endo/Exolytic | PL | Main Products (DP) | Cleavage Site | References |
|---|---|---|---|---|---|---|---|---|
| Photobacterium sp. FC615 | Extracellular | polyG | AlyPB1 | endolytic | 6 | - | [83] | |
| Photobacterium sp. FC615 | Intracellular | polyM | AlyPB2 | exolytic | 15 | - | - | [83] |
| Vibrio sp. QY108 | - | polyM G | VsAly7D | exolytic | 7 | - | - | [106] |
| Streptomyces sp. ALG-5 | Extracellular | polyG | ALG-5 | 7 | - | - | [104] | |
| Cobetia sp. NAP1 | - | polyMG | AlgC-PL7 | 7 | - | - | [107] | |
| Sphingomonas sp. | - | polyMG | SALy | endolytic | 7 | 3 | G–G or G–M | [107] |
| Flavobacterium sp. | - | poly-(M) | FALy | endolytic | 7 | 5–6 | - | [111] |
| Microbulbifer sp. Q7. | Extracellular | polyG | AlyM | - | 7 | 2–5 | G–G or G–M | [112] |
| Pseudoalteromonas sp. SM0524 | - | polyGM | Aly-SJ02 | - | 18 | dimers and trimers from poly M, G3 and G4 from polyG | - | [93] |
| Pseudoalteromonas sp. SM0524 | - | polyM | AlyPM | endolytic | 7 | dimers and trimers | - | [113] |
| Microbulbifer sp. 6532A | - | polyG | AlgMsp | 7 | 2–5 | - | [46] | |
| BP-2 strain | - | polyM | Alg17B | endolytic and exolytic | 17 | 2–6 | - | [97] |
| Vibriofurnissii H1 | - | polyGM | AlyH1 | 7 | 2–4 | - | [114] | |
| Pseudoalteromonascarrageenovora ASY5 | extracellular | polyGM | Aly1281 | endolytic | 7 | 2 | - | [108] |
| Pseudoalteromonascarrageenovora ASY5 | extracellular | polyM | Alg823 | endolytic | 6 | 2 | - | [115] |
| Agarivorans sp. L11 | - | polyGM | AlyL1 | endolytic | 7 | 2–4 | - | [109] |
| Streptomycesluridiscabiei | - | polyGM | AlyDS44 | endolytic | 7 | 2–4 | - | [100] |
| Formosaalgae KMM 3553T | - | polyM | ALFA3 | endolytic | 7 | 1–20 | M–M, M–G, G–M | [110] |
| Formosaalgae KMM 3553T | - | polyGM | ALFA4 | endolytic | 6 | 1–20 | M–M | [110] |
| Vibrio. sp. QD-5 | - | polyG | Aly-IV | endolytic | 7 | 1–3 | - | [96] |
| Zobelliagalactanivorans | Intracellular | poly-MG | AlyA1 | endolytic | 7 | 4–20 | G–M, G–G | [90] |
| Zobelliagalactanivorans | Intracellular | polyG | AlyA5 | exolytic | 7 | - | M–M, M–G, G–G | [90] |
| Glaciecolachathamensis S18K6T | - | polyG | AlyGC | - | 6 | - | - | [116] |
| Vibrio sp. W2 | - | polyG | Alyw203 | endo-type | 7 | 1–2 | - | [105] |
6. Biochemical Properties of Marine Bacteria-Produced Alginate Lyases
7. Enzyme Kinetics of Alginate Lyases from Marine Bacteria
8. Application of Alginate Lyases from Marine Bacteria
8.1. Preparation of AOs
| Enzyme | Source | Application | References | Field of Application |
|---|---|---|---|---|
| ALFA3 | Formosa algae KMM 3553T | Preparation of alginate oligosaccharides | [110] | in agriculture, in feed production, to lower cholesterol levels in blood plasma |
| Aly1281 | Pseudoalteromonascarrageenovora ASY5 | Preparation of alginate oligosaccharides | [108] | in agriculture, feed production |
| AlgNJ-07 | Serratia marcescens NJ-07 | Preparation of alginate oligosaccharides | [81] | antimicrobials |
| AlgNJ-07 | Serratia marcescens NJ-07 | Preparation of alginate oligosaccharides | [81] | antimicrobials for the treatment of cystic fibrosis, in agriculture, in feed production, in medicine for the diagnosis of diseases, to lower cholesterol in blood plasma |
| FsAlgB | Flammeovirga sp. NJ–04 | Preparation of alginate oligosaccharides | [58] | in medicine for the diagnosis |
| Aly | Pseudomonas sp. HZJ 216 | Preparation of alginate oligosaccharides | [148] | antimicrobials, in medicine for the diagnosis of diseases |
| Alg2A | Flavobacterium sp. S20 | Preparation of alginate oligosaccharides | [146] | to lower plasma cholesterol levels |
| Aly5 | Flammeovirga sp. Strain MY04 | Preparation of alginate oligosaccharides | [149] | in medicine for the diagnosis |
| AlyPB1 and AlyPB2 | Photobacterium sp. FC615 | Preparation of unsaturated monosaccharide | [83] | antimicrobials for the treatment of cystic fibrosis |
| Alg7A | Vibrio sp. W13 | Preparation of alginate oligosaccharides | [144] | inhibition of lipid oxidation in food emulsions |
| Alginate lyase | Isoptericolahalotolerans CGMCC 5336 | Preparation of alginate oligosaccharides | [145] | in feed production |
| AlyP1400 | Pseudoalteromonas sp. 1400 | The degradation of biofilms | [150] | in biofuel production |
| AlyL1 | Agarivorans sp. L11 | Produce TPC for bioenergy production | [151] | inhibition of lipid oxidation in industrial emulsions |
| Alg7D | Saccharophagusdegradans 2–40T | Produce DEH for bioenergy production | [123] | inhibition of lipid oxidation in industrial emulsions |
| AlyPB2 | Photobacterium sp. FC615 | Alginate Sequencing | [83] | in the production of alginates |
| Aly SM0524 | Pseudoalteromonas sp. SM0524 | Preparation of bioethanol | [152] | antimicrobials for the treatment of cystic fibrosis, for lowering plasma cholesterol levels |
| Alg17C | Cobetia sp. NAP1 | Biofuels and chemicals production | [107] | in agriculture |
| Alginate lyase | Shewanella sp. Kz7 | Biofuel production | [153] | in agriculture |
| Alginate lyase | Gracilibacillus sp. A7 | Disposal of seaweed waste | [154] | in agriculture |
8.2. Anti-Biofilm Activity
8.3. Bioethanol Production
8.4. Disposal of Seaweed Waste
8.5. Elucidate the Structure of Alginate
9. Conclusions Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barzkar, N.; Jahromi, S.T.; Poorsaheli, H.B.; Vianello, F. Metabolites from marine microorganisms, micro, and macroalgae: Immense scope for pharmacology. Mar. Drugs 2019, 17, 464. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, S.T.; Barzkar, N. Marine bacterial chitinase as sources of energy, eco-friendly agent, and industrial biocatalyst. Int. J. Biol. Macromol. 2018, 120, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Barzkar, N.; Homaei, A.; Hemmati, R.; Patel, S. Thermostable marine microbial proteases for industrial applications: Scopes and risks. Extremophiles 2018, 22, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, S.T.; Barzkar, N. Future direction in marine bacterial agarases for industrial applications. Appl. Microbiol. Biotechnol. 2018, 102, 6847–6863. [Google Scholar] [CrossRef] [PubMed]
- Barzkar, N. Marine microbial alkaline protease: An efficient and essential tool for various industrial applications. Int. J. Biol. Macromol. 2020, 161, 1216–1229. [Google Scholar] [CrossRef]
- Barzkar, N.; Khan, Z.; Jahromi, S.T.; Pourmozaffar, S.; Gozari, M.; Nahavandi, R. A critical review on marine serine protease and its inhibitors: A new wave of drugs? Int. J. Biol. Macromol. 2021, 170, 674–687. [Google Scholar] [CrossRef]
- Barzkar, N.; Sohail, M. An overview on marine cellulolytic enzymes and their potential applications. Appl. Microbiol. Biotechnol. 2020, 104, 6873–6892. [Google Scholar] [CrossRef]
- Barzkar, N.; Sohail, M.; Jahromi, S.T.; Gozari, M.; Poormozaffar, S.; Nahavandi, R.; Hafezieh, M. Marine bacterial esterases: Emerging biocatalysts for industrial applications. Appl. Biochem. Biotechnol. 2021, 193, 1187–1214. [Google Scholar] [CrossRef]
- Barzkar, N.; Jahromi, S.T.; Vianello, F. Marine Microbial Fibrinolytic Enzymes: An Overview of Source, Production, Biochemical Properties and Thrombolytic Activity. Mar. Drugs 2022, 20, 46. [Google Scholar] [CrossRef]
- Barzkar, N.; Sohail, M.; Jahromi, S.T.; Nahavandi, R.; Khodadadi, M. Marine microbial l-glutaminase: From pharmaceutical to food industry. Appl. Microbiol. Biotechnol. 2021, 105, 4453–4466. [Google Scholar] [CrossRef]
- Barzkar, N.; Fariman, G.A.; Taheri, A. Proximate composition and mineral contents in the body wall of two species of sea cucumber from Oman Sea. Environ. Sci. Pollut. Res. 2017, 24, 18907–18911. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, S.T.; Pourmozaffar, S.; Jahanbakhshi, A.; Rameshi, H.; Gozari, M.; Khodadadi, M.; Sohrabipour, J.; Behzadi, S.; Barzkar, N.; Nahavandi, R. Corrigendum to “Effect of different levels of dietary Sargassum cristaefolium on growth performance, hematological parameters, histological structure of hepatopancreas and intestinal microbiota of Litopenaeus vannamei”. Aquaculture 2021, 535, 736376. [Google Scholar] [CrossRef]
- Choi, A.H.; Ben-Nissan, B. Marine-Derived Biomaterials for Tissue Engineering Applications; Springer: Berlin/Heidelberg, Germany, 2019; Volume 14. [Google Scholar]
- Ozturk, M.; Egamberdieva, D.; Pešić, M. Biodiversity and Biomedicine: Our Future; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Chakravarty, R.; Hong, H.; Cai, W. Positron Emission Tomography Image-Guided Drug Delivery: Current Status and Future Perspectives. Mol. Pharm. 2014, 11, 3777–3797. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.K.; Beardall, J.; Mehta, S.; Sahoo, D.; Stojkovic, S. Using marine macroalgae for carbon sequestration: A critical appraisal. J. Appl. Phycol. 2011, 23, 877–886. [Google Scholar] [CrossRef]
- Roesijadi, G.; Jones, S.B.; Snowden-Swan, L.J.; Zhu, Y. Macroalgae as a Biomass Feedstock: A Preliminary Analysis; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2010. [Google Scholar]
- Fleming, M.; Manners, D.; Masson, A. The enzymic degradation of laminarin. Biochem. J. 1967, 104, 32. [Google Scholar]
- Horn, S.; Aasen, I.; Østgaard, K. Production of ethanol from mannitol by Zymobacter palmae. J. Ind. Microbiol. Biotechnol. 2000, 24, 51–57. [Google Scholar] [CrossRef]
- Jensen, A.; Haug, A. Geographical and seasonal variation in the chemical composition of Laminaria hyperborea and Laminaria digitata from the Norwegian coast. Akademisk Trykningssentral 1956, 14, 20. [Google Scholar]
- Mabeau, S.; Kloareg, B. Isolation and analysis of the cell walls of brown algae: Fucus spiralis, F. ceranoides, F. vesiculosus, F. serratus, Bifurcaria bifurcata and Laminaria digitata. J. Exp. Bot. 1987, 38, 1573–1580. [Google Scholar] [CrossRef]
- Gacesa, P. Enzymic degradation of alginates. Int. J. Biochem. 1992, 24, 545–552. [Google Scholar] [CrossRef]
- Haug, A.; Larsen, B.; Smidsrod, O. Studies on the sequence of uronic acid residues in alginic acid. Acta Chem. Scand 1967, 21, 691–704. [Google Scholar] [CrossRef]
- Peteiro, C. Alginate production from marine macroalgae, with emphasis on kelp farming. In Alginates and Their Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2018; pp. 27–66. [Google Scholar]
- Wang, M.; Chen, L.; Zhang, Z.; Wang, X.; Qin, S.; Yan, P. Screening of alginate lyase-excreting microorganisms from the surface of brown algae. AMB Express 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gacesa, P. Alginates. Carbohydr. Polym. 1988, 8, 161–182. [Google Scholar] [CrossRef]
- Itoh, T.; Nakagawa, E.; Yoda, M.; Nakaichi, A.; Hibi, T.; Kimoto, H. Structural and biochemical characterisation of a novel alginate lyase from Paenibacillus sp. str. FPU-7. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Xu, F.; Wang, P.; Zhang, Y.-Z.; Chen, X.-L. Diversity of three-dimensional structures and catalytic mechanisms of alginate lyases. Appl. Environ. Microbiol. 2018, 84, e02040-17. [Google Scholar] [CrossRef] [PubMed]
- Osawa, T.; Matsubara, Y.; Muramatsu, T.; Kimura, M.; Kakuta, Y. Crystal structure of the alginate (poly α-l-guluronate) lyase from Corynebacterium sp. at 1.2 Å resolution. J. Mol. Biol. 2005, 345, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Mashino, C.; Uji, T.; Saga, N.; Mikami, K.; Ojima, T. Characterization of an eukaryotic PL-7 alginate lyase in the marine red alga Pyropia yezoensis. Curr. Biotechnol. 2015, 4, 240–258. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Ojima, T. Functional identification of alginate lyase from the brown alga Saccharina japonica. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Elyakova, L.A.; Favorov, V.V. Isolation and certain properties of alginate lyase VI from the mollusk Littorina sp. Biochim. Biophys. Acta (BBA)-Enzymol. 1974, 358, 341–354. [Google Scholar] [CrossRef]
- Hata, M.; Kumagai, Y.; Rahman, M.M.; Chiba, S.; Tanaka, H.; Inoue, A.; Ojima, T. Comparative study on general properties of alginate lyases from some marine gastropod mollusks. Fish. Sci. 2009, 75, 755–763. [Google Scholar] [CrossRef]
- Suda, K.; Tanji, Y.; Hori, K.; Unno, H. Evidence for a novel Chlorella virus-encoded alginate lyase. FEMS Microbiol. Lett. 1999, 180, 45–53. [Google Scholar] [CrossRef]
- Schaumann, K.; Weide, G. Enzymatic degradation of alginate by marine fungi. In Thirteenth International Seaweed Symposium; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Zhang, W.; Xia, X.; Zhang, Z. Alginate lyase of a novel algae fermentation strain. Chem. Biochem. Eng. Q. 2019, 33, 125–131. [Google Scholar] [CrossRef]
- Yamasaki, M.; Moriwaki, S.; Miyake, O.; Hashimoto, W.; Murata, K.; Mikami, B. Structure and function of a hypothetical Pseudomonas aeruginosa protein PA1167 classified into family PL-7, a novel alginate lyase with a β-sandwich fold. J. Biol. Chem. 2004, 279, 31863–31872. [Google Scholar] [CrossRef] [PubMed]
- Caswell, R.; Gacesa, P.; Lutrell, K.; Weightman, A. Molecular cloning and heterologous expression of a Klebsiella pneumoniae gene encoding alginate lyase. Gene 1989, 75, 127–134. [Google Scholar] [CrossRef]
- Haraguchi, K.; Kodama, T. Purification and propertes of poly (β-d-mannuronate) lyase from Azotobacter chroococcum. Appl. Microbiol. Biotechnol. 1996, 44, 576–581. [Google Scholar] [CrossRef]
- Yu, Z.; Zhu, B.; Wang, W.; Tan, H.; Yin, H. Characterization of a new oligoalginate lyase from marine bacterium Vibrio sp. Int. J. Biol. Macromol. 2018, 112, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.J.; Kim, H.S.; Kim, M.S.; Lee, E.Y.; Kim, H.S. Functional Characterization of a Novel Oligoalginate Lyase of Stenotrophomonas maltophilia KJ-2 Using Site-Specific Mutation Reveals Bifunctional Mode of Action, Possessing Both Endolytic and Exolytic Degradation Activity Toward Alginate in Seaweed Biomass. Front. Mar. Sci. 2020, 7, 420. [Google Scholar] [CrossRef]
- Chen, P.; Zhu, Y.; Men, Y.; Zeng, Y.; Sun, Y. Purification and characterization of a novel alginate lyase from the marine bacterium Bacillus sp. Alg07. Mar. Drugs 2018, 16, 86. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, L.; Chen, Y.; Ni, H.; Xiao, A.; Cai, H. Characterization of an extracellular biofunctional alginate lyase from marine Microbulbifer sp. ALW1 and antioxidant activity of enzymatic hydrolysates. Microbiol. Res. 2016, 182, 49–58. [Google Scholar] [CrossRef]
- Dou, W.; Wei, D.; Li, H.; Li, H.; Rahman, M.M.; Shi, J.; Xu, Z.; Ma, Y. Purification and characterisation of a bifunctional alginate lyase from novel Isoptericola halotolerans CGMCC 5336. Carbohydr. Polym. 2013, 98, 1476–1482. [Google Scholar] [CrossRef]
- Zhu, B.; Tan, H.; Qin, Y.; Xu, Q.; Du, Y.; Yin, H. Characterization of a new endo-type alginate lyase from Vibrio sp. W13. Int. J. Biol. Macromol. 2015, 75, 330–337. [Google Scholar] [CrossRef]
- Swift, S.M.; Hudgens, J.W.; Heselpoth, R.D.; Bales, P.M.; Nelson, D.C. Characterization of AlgMsp, an alginate lyase from Microbulbifer sp. 6532A. PLoS ONE 2014, 9, e112939. [Google Scholar] [CrossRef] [PubMed]
- Kitamikado, M.; Tseng, C.-H.; Yamaguchi, K.; Nakamura, T. Two types of bacterial alginate lyases. Appl. Environ. Microbiol. 1992, 58, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Ertesvåg, H. Alginate-modifying enzymes: Biological roles and biotechnological uses. Front. Microbiol. 2015, 6, 523. [Google Scholar] [PubMed]
- Jia, G.; Rao, Z.; Zhang, J.; Li, Z.; Chen, F. Tetraether biomarker records from a loess-paleosol sequence in the western Chinese Loess Plateau. Front. Microbiol. 2013, 4, 199. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Yin, H. Alginate lyase: Review of major sources and classification, properties, structure-function analysis and applications. Bioengineered 2015, 6, 125–131. [Google Scholar] [CrossRef]
- Rehm, B.H. Alginates: Biology and Applications; Springer: Berlin/Heidelberg, Germany, 2009; Volume 13. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in research on the bioactivity of alginate oligosaccharides. Mar. Drugs 2020, 18, 144. [Google Scholar] [CrossRef]
- Zong, W.-R.; Cheong, K.-L.; Wu, D.-T.; Li, J.; Zhao, J.; Li, S.-P. Preparation and purification of raffinose family oligosaccharides from Rehmannia glutinosa Libosch. by fast protein liquid chromatography coupled with refractive index detection. Sep. Purif. Technol. 2014, 138, 98–103. [Google Scholar] [CrossRef]
- Cheng, D.; Jiang, C.; Xu, J.; Liu, Z.; Mao, X. Characteristics and applications of alginate lyases: A review. Int. J. Biol. Macromol. 2020, 164, 1304–1320. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Zhang, X.; Li, Y.; Wang, L. Bacterial alginate metabolism: An important pathway for bioconversion of brown algae. Biotechnol. Biofuels 2021, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Ni, F.; Sun, Y.; Ning, L.; Yao, Z. Elucidation of degrading pattern and substrate recognition of a novel bifunctional alginate lyase from Flammeovirga sp. NJ-04 and its use for preparation alginate oligosaccharides. Biotechnol. Biofuels 2019, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, Q.; Lu, D.; Han, W.; Li, F. A novel bifunctional endolytic alginate lyase with variable alginate-degrading modes and versatile monosaccharide-producing properties. Front. Microbiol. 2018, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Preston, L.A.; Schiller, N.L. Alginate lyase: Review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Annu. Rev. Microbiol. 2000, 54, 289–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ji, S.-Q.; Lu, M.; Li, F.-L. Biochemical and structural characterization of alginate lyases: An update. Curr. Biotechnol. 2015, 4, 223–239. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, C.-G.; Lee, E.Y. Alginate lyase: Structure, property, and application. Biotechnol. Bioprocess Eng. 2011, 16, 843–851. [Google Scholar] [CrossRef]
- An, Q.D.; Zhang, G.L.; Wu, H.T.; Zhang, Z.C.; Zheng, G.S.; Luan, L.; Murata, Y.; Li, X. Alginate-deriving oligosaccharide production by alginase from newly isolated Flavobacterium sLXA and its potential application in protection against pathogens. J. Appl. Microbiol. 2009, 106, 161–170. [Google Scholar] [CrossRef]
- Courtois, J. Oligosaccharides from land plants and algae: Production and applications in therapeutics and biotechnology. Curr. Opin. Microbiol. 2009, 12, 261–273. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, M.; Yin, H.; Du, Y.; Ning, L. Enzymatic hydrolysis of alginate to produce oligosaccharides by a new purified endo-type alginate lyase. Mar. Drugs 2016, 14, 108. [Google Scholar] [CrossRef]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef]
- Martin, M.; Portetelle, D.; Michel, G.; Vandenbol, M. Microorganisms living on macroalgae: Diversity, interactions, and biotechnological applications. Appl. Microbiol. Biotechnol. 2014, 98, 2917–2935. [Google Scholar] [CrossRef] [PubMed]
- Alkawash, M.A.; Soothill, J.S.; Schiller, N.L. Alginate lyase enhances antibiotic killing of mucoid Pseudomonas aeruginosa in biofilms. Apmis 2006, 114, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Gacesa, P. Bacterial alginate biosynthesis–recent progress and future prospects. Microbiology 1998, 144, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, G.; Guan, H.; Zhao, X.; Du, Y.; Jiang, X. Preparation and structure elucidation of alginate oligosaccharides degraded by alginate lyase from Vibro sp. 510. Carbohydr. Res. 2004, 339, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Pilgaard, B.; Wilkens, C.; Herbst, F.-A.; Vuillemin, M.; Rhein-Knudsen, N.; Meyer, A.S.; Lange, L. Proteomic enzyme analysis of the marine fungus Paradendryphiella salina reveals alginate lyase as a minimal adaptation strategy for brown algae degradation. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ravanal, M.C.; Sharma, S.; Gimpel, J.; Reveco-Urzua, F.E.; Øverland, M.; Horn, S.J.; Lienqueo, M.E. The role of alginate lyases in the enzymatic saccharification of brown macroalgae, Macrocystis pyrifera and Saccharina latissima. Algal Res. 2017, 26, 287–293. [Google Scholar] [CrossRef]
- Madgwick, J. Alginate lyase in the brown alga Laminaria digitata (Huds.) Lamour. Acta Chem. Scand. 1973, 27, 711–712. [Google Scholar] [CrossRef]
- Madgwick, J.; Haug, A.; Larsen, B. Ionic requirements of alginate-modifying enzymes in the marine alga Pelvetia canaliculata (L.) Dcne. et Thur. Bot. Mar. 1978, 21, 1–4. [Google Scholar] [CrossRef]
- Watanabe, T.; Nisizawa, K. Enzymatic studies on alginate lyase from Undaria pinnatifida in relation to texture-softening prevention by ash-treatment (Haiboshi) [Algae]. Bull. Jpn. Soc. Sci. Fish. 1982, 2, 243–249. [Google Scholar] [CrossRef]
- Muramatsu, T.; Hirose, S.; Katayose, M. Isolation and properties of alginate lyase from the mid-gut gland of wreath shell Turbo cornutus. Agric. Biol. Chem. 1977, 41, 1939–1946. [Google Scholar]
- Nisizawa, K.; Fujibayashi, S.; Kashiwabara, Y. Alginate lyases in the hepatopancreas of a marine mollusc, Dolabella auricula Solander. J. Biochem. 1968, 64, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Seiderer, L.; LJ, S.; RC, N.; PA, C. Quantitative significance of style enzymes from two marine mussels (Choromytilus meridionalis Krauss and Perna perna Linnaeus) in relation to diet. Mar. Biol. Lett. 1982, 3, 257–272. [Google Scholar]
- Xue, X.; Zhou, Y.; Gao, X.; Yan, P. Advances in application of alginate lyase and its enzymatic hydrolysate. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Zhu, B.; Ni, F.; Sun, Y.; Yao, Z. Expression and characterization of a new heat-stable endo-type alginate lyase from deep-sea bacterium Flammeovirga sp. NJ-04. Extremophiles 2017, 21, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Hu, F.; Yuan, H.; Sun, Y.; Yao, Z. Biochemical characterization and degradation pattern of a unique pH-stable polyM-specific alginate lyase from newly isolated Serratia marcescens NJ-07. Mar. Drugs 2018, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Sasaki, S.F.; Kashiwabara, Y.; Suzuki, H.; Nisizawa, K. Multiple components of endo-polyguluronide lyase of Pseudomonas sp. J. Biochem. 1977, 81, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Zhang, Q.; Wang, S.; Guan, J.; Jiao, R.; Han, N.; Han, W.; Li, F. Biochemical characteristics and synergistic effect of two novel alginate lyases from Photobacterium sp. FC615. Biotechnol. Biofuels 2019, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, I.; Sawabe, T.; Ezura, Y. Cloning and sequence analysis of Vibrio halioticoli genes encoding three types of polyguluronate lyase. Mar. Biotechnol. 2000, 2, 65–73. [Google Scholar] [CrossRef]
- Ji, S.-Q.; Wang, B.; Lu, M.; Li, F.-L. Defluviitalea phaphyphila snov., a novel thermophilic bacterium that degrades brown algae. Appl. Environ. Microbiol. 2016, 82, 868–877. [Google Scholar] [CrossRef]
- Lange, B.; Wingender, J.; Winkler, U.K. Isolation and characterization of an alginate lyase from Klebsiella aerogenes. Arch. Microbiol. 1989, 152, 302–308. [Google Scholar] [CrossRef]
- Boyen, C.; Bertheau, Y.; Barbeyron, T.; Kloareg, B. Preparation of guluronate lyase from Pseudomonas alginovora for protoplast isolation in Laminaria. Enzym. Microb. Technol. 1990, 12, 885–890. [Google Scholar] [CrossRef]
- Zilda, D.S.; Yulianti, Y.; Sholihah, R.F.; Subaryono, S.; Fawzya, Y.N.; Irianto, H.E. A novel Bacillus sp. isolated from rotten seaweed: Identification and characterization alginate lyase its produced. Biodiversitas J. Biol. Divers. 2019, 20, 1166–1172. [Google Scholar] [CrossRef]
- Matsubara, Y.; Iwasaki, K.-I.; Muramatsu, T. Action of poly (α-l-guluronate) lyase from Corynebacterium sp. ALY-1 strain on saturated oligoguluronates. Biosci. Biotechnol. Biochem. 1998, 62, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Lundqvist, L.C.; Jam, M.; Jeudy, A.; Barbeyron, T.; Sandström, C.; Michel, G.; Czjzek, M. Comparative characterization of two marine alginate lyases from Zobellia galactanivorans reveals distinct modes of action and exquisite adaptation to their natural substrate. J. Biol. Chem. 2013, 288, 23021–23037. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Uchimura, K.; Miyazaki, M.; Nogi, Y.; Horikoshi, K. A new high-alkaline alginate lyase from a deep-sea bacterium Agarivorans sp. Extremophiles 2009, 13, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mo, K.; Zheng, Z.; Wang, Z.; Hu, Y.; Zou, X.; Gu, H.; Wang, H.; Bao, S.; Huang, H. Paenibacillus algicola sp. nov., a novel alginate lyase-producing marine bacterium. Int. J. Syst. Evol. Microbiol. 2020, 70, 5087–5092. [Google Scholar] [CrossRef]
- Li, J.-W.; Dong, S.; Song, J.; Li, C.-B.; Chen, X.-L.; Xie, B.-B.; Zhang, Y.-Z. Purification and characterization of a bifunctional alginate lyase from Pseudoalteromonas sp. SM0524. Mar. Drugs 2011, 9, 109. [Google Scholar] [CrossRef]
- Martin, M.; Barbeyron, T.; Martin, R.; Portetelle, D.; Michel, G.; Vandenbol, M. The cultivable surface microbiota of the brown alga Ascophyllum nodosum is enriched in macroalgal-polysaccharide-degrading bacteria. Front. Microbiol. 2015, 6, 1487. [Google Scholar] [CrossRef]
- Dong, S.; Yang, J.; Zhang, X.-Y.; Shi, M.; Song, X.-Y.; Chen, X.-L.; Zhang, Y.-Z. Cultivable alginate lyase-excreting bacteria associated with the arctic brown alga Laminaria. Mar. Drugs 2012, 10, 2481. [Google Scholar] [CrossRef]
- Chao, Y.; Wang, S.; Wu, S.; Wei, J.; Chen, H. Cloning and characterization of an alginate lyase from marine Vibrio. sp. QD-5. 2017; preprint. [Google Scholar]
- Huang, G.; Wen, S.; Liao, S.; Wang, Q.; Pan, S.; Zhang, R.; Lei, F.; Liao, W.; Feng, J.; Huang, S. Characterization of a bifunctional alginate lyase as a new member of the polysaccharide lyase family 17 from a marine strain BP-2. Biotechnol. Lett. 2019, 41, 1187–1200. [Google Scholar] [CrossRef]
- Kim, H.T.; Ko, H.-J.; Kim, N.; Kim, D.; Lee, D.; Choi, I.-G.; Woo, H.C.; Kim, M.D.; Kim, K.H. Characterization of a recombinant endo-type alginate lyase (Alg7D) from Saccharophagus degradans. Biotechnol. Lett. 2012, 34, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Kurachi, M.; Nakashima, T.; Kim, D.; Yamaguchi, K.; Oda, T.; Iwamoto, Y.; Muramatsu, T. Structure–activity relationship of alginate oligosaccharides in the induction of cytokine production from RAW264. 7 cells. FEBS Lett. 2005, 579, 4423–4429. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.T.; Chataway, T.; Araujo, R.; Puri, M.; Franco, C.M.M. Purification and Characterization of a Novel Alginate Lyase from a Marine Streptomyces Species Isolated from Seaweed. Mar. Drugs 2021, 19, 590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhou, J.; Jia, Z.; Zhang, Y.; Gu, G. Hypoglycemic effect of Rehmannia glutinosa oligosaccharide in hyperglycemic and alloxan-induced diabetic rats and its mechanism. J. Ethnopharmacol. 2004, 90, 39–43. [Google Scholar] [CrossRef]
- Hirayama, M.; Hashimoto, W.; Murata, K.; Kawai, S. Comparative characterization of three bacterial exo-type alginate lyases. Int. J. Biol. Macromol. 2016, 86, 519–524. [Google Scholar] [CrossRef][Green Version]
- Yang, J.; Cui, D.; Ma, S.; Chen, W.; Chen, D.; Shen, H. Characterization of a novel PL 17 family alginate lyase with exolytic and endolytic cleavage activity from marine bacterium Microbulbifer sp. SH-1. Int. J. Biol. Macromol. 2021, 169, 551–563. [Google Scholar] [CrossRef]
- Kim, D.E.; Lee, E.Y.; Kim, H.S. Cloning and characterization of alginate lyase from a marine bacterium Streptomyces sp. ALG-5. Mar. Biotechnol. 2009, 11, 10–16. [Google Scholar] [CrossRef]
- LLiu, L.; Wang, Z.; Zheng, Z.; Li, Z.; Ji, X.; Cong, H.; Wang, H. Secretory Expression of an Alkaline Alginate Lyase with Heat Recovery Property in Yarrowia lipolytica. Front. Microbiol. 2021, 12, 710533. [Google Scholar] [CrossRef]
- Zhang, F.; Fu, Z.; Tang, L.; Zhang, Z.; Han, F.; Yu, W. Biochemical Characterization of a Novel Exo-Type PL7 Alginate Lyase VsAly7D from Marine Vibrio sp. QY108. Int. J. Mol. Sci. 2021, 22, 8402. [Google Scholar] [CrossRef]
- Yagi, H.; Fujise, A.; Itabashi, N.; Ohshiro, T. Purification and characterization of a novel alginate lyase from the marine bacterium Cobetia sp. NAP1 isolated from brown algae. Biosci. Biotechnol. Biochem. 2016, 80, 2338–2346. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Shao, Y.; Jiao, C.; Yang, Q.-M.; Weng, H.-F.; Xiao, A.-F. Characterization and application of an alginate lyase, Aly1281 from marine bacterium Pseudoalteromonas carrageenovora ASY5. Mar. Drugs 2020, 18, 95. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, X.; Zhang, L.; Yu, W.; Han, F. Cloning, expression, and characterization of a cold-adapted and surfactant-stable alginate lyase from marine bacterium Agarivorans sp. L11. J. Microbiol. Biotechnol. 2015, 25, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Belik, A.; Silchenko, A.; Malyarenko, O.; Rasin, A.; Kiseleva, M.; Kusaykin, M.; Ermakova, S. Two new alginate lyases of PL7 and PL6 families from polysaccharide-degrading bacterium Formosa algae KMM 3553T: Structure, properties, and products analysis. Mar. Drugs 2020, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Manns, D.; Nyffenegger, C.; Saake, B.; Meyer, A.S. Impact of different alginate lyases on combined cellulase–lyase saccharification of brown seaweed. RSC Adv. 2016, 6, 45392–45401. [Google Scholar] [CrossRef]
- Yang, M.; Yu, Y.; Yang, S.; Shi, X.; Mou, H.; Li, L. Expression and characterization of a new polyG-specific alginate lyase from marine bacterium Microbulbifer sp. Q7. Front. Microbiol. 2018, 9, 2894. [Google Scholar] [CrossRef]
- Chen, X.-L.; Dong, S.; Xu, F.; Dong, F.; Li, P.-Y.; Zhang, X.-Y.; Zhou, B.-C.; Zhang, Y.-Z.; Xie, B.-B. Characterization of a new cold-adapted and salt-activated polysaccharide lyase family 7 alginate lyase from Pseudoalteromonas sp. SM0524. Front. Microbiol. 2016, 7, 1120. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Shi, H.; Zhou, J.; Tan, Z.; Yuan, M.; Yao, P.; Liu, X. Characterization of a novel alginate lyase from marine bacterium Vibrio furnissii H1. Mar. Drugs 2018, 16, 30. [Google Scholar] [CrossRef]
- Zeng, J.; An, D.; Jiao, C.; Xiao, Q.; Weng, H.; Yang, Q.; Xiao, A. Cloning, expression, and characterization of a new pH- and heat-stable alginate lyase from Pseudoalteromonas carrageenovora ASY5. J. Food Biochem. 2019, 43, e12886. [Google Scholar] [CrossRef]
- Xu, F.; Dong, F.; Wang, P.; Cao, H.-Y.; Li, C.-Y.; Li, P.-Y.; Pang, X.-H.; Zhang, Y.-Z.; Chen, X.-L. Novel molecular insights into the catalytic mechanism of marine bacterial alginate lyase AlyGC from polysaccharide lyase family 6. J. Biol. Chem. 2017, 292, 4457–4468. [Google Scholar] [CrossRef]
- Dong, S.; Wei, T.-D.; Chen, X.-L.; Li, C.-Y.; Wang, P.; Xie, B.-B.; Qin, Q.-L.; Zhang, X.-Y.; Pang, X.-H.; Zhou, B.-C. Molecular insight into the role of the N-terminal extension in the maturation, substrate recognition, and catalysis of a bacterial alginate lyase from polysaccharide lyase family 18. J. Biol. Chem. 2014, 289, 29558–29569. [Google Scholar] [CrossRef]
- Tomoo, S.; Yoshio, E.; Takahisa, K. Purification and Characterization of an Alginate Lyase from Marine Alteromonass. Nippon. Suisan Gakkaishi 1992, 58, 521–527. [Google Scholar]
- Ma, Y.; Li, J.; Zhang, X.-Y.; Ni, H.-D.; Wang, F.-B.; Wang, H.-Y.; Wang, Z.-P. Characterization of a New Intracellular Alginate Lyase with Metal Ions-Tolerant and pH-Stable Properties. Mar. Drugs 2020, 18, 416. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Fathoni, A.; Jeong, G.-T.; Do Jeong, H.; Nam, T.-J.; Kong, I.-S.; Kim, J.K. Microbacterium oxydans, a novel alginate-and laminarin-degrading bacterium for the reutilization of brown-seaweed waste. J. Environ. Manag. 2013, 130, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, P.; Zeng, Y.; Men, Y.; Mu, S.; Zhu, Y.; Chen, Y.; Sun, Y. The characterization and modification of a novel bifunctional and robust alginate lyase derived from Marinimicrobium sp. H1. Mar. Drugs 2019, 17, 545. [Google Scholar] [CrossRef]
- McHugh, D.J. Production, properties and uses of alginates. Production and Utilization of Products from Commercial Seaweeds. FAO. Fish. Tech. Pap 1987, 288, 58–115. [Google Scholar]
- Wang, D.M.; Kim, H.T.; Yun, E.J.; Kim, D.H.; Park, Y.-C.; Woo, H.C.; Kim, K.H. Optimal production of 4-deoxy-l-erythro-5-hexoseulose uronic acid from alginate for brown macro algae saccharification by combining endo-and exo-type alginate lyases. Bioprocess Biosyst. Eng. 2014, 37, 2105–2111. [Google Scholar] [CrossRef]
- Wiens, J.R.; Vasil, A.I.; Schurr, M.J.; Vasil, M.L. Iron-regulated expression of alginate production, mucoid phenotype, and biofilm formation by Pseudomonas aeruginosa. MBio 2014, 5, e01010-13. [Google Scholar] [CrossRef]
- Daboor, S.M.; Rohde, J.R.; Cheng, Z. Disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate lyase enhances pathogen eradication by antibiotics. J. Cyst. Fibros. 2021, 20, 264–270. [Google Scholar] [CrossRef]
- Yin, R.; Yi, Y.-J.; Chen, Z.; Wang, B.-X.; Li, X.-H.; Zhou, Y.-X. Characterization of a New Biofunctional, Exolytic Alginate Lyase from Tamlana sp. s12 with High Catalytic Activity and Cold-Adapted Features. Mar. Drugs 2021, 19, 191. [Google Scholar] [CrossRef]
- Xu, F.; Chen, X.-L.; Sun, X.-H.; Dong, F.; Li, C.-Y.; Li, P.-Y.; Ding, H.; Chen, Y.; Zhang, Y.-Z.; Wang, P. Structural and molecular basis for the substrate positioning mechanism of a new PL7 subfamily alginate lyase from the arctic. J. Biol. Chem. 2020, 295, 16380–16392. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Zhang, L.; Wang, Y.; Wang, S.; Zhang, Y.; Guo, H.; Ji, D.; Wang, Y. Alginate oligosaccharide DP5 exhibits antitumor effects in osteosarcoma patients following surgery. Front. Pharmacol. 2017, 8, 623. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Hao, C.; Zhang, L.; Liu, X.; Zhou, X.; Dun, Y.; Li, H.; Li, G.; Zhao, X.; An, Y. OM2, a novel oligomannuronate-chromium (III) complex, promotes mitochondrial biogenesis and lipid metabolism in 3T3-L1 adipocytes via the AMPK-PGC1α pathway. PLoS ONE 2015, 10, e0131930. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Tamura, Y.; Toda, N.; Yoshinaga, M.; Terakado, S.; Otsuka, K.; Numabe, A.; Kawabata, Y.; Murota, I.; Sato, N. Sodium alginate oligosaccharides attenuate hypertension in spontaneously hypertensive rats fed a low-salt diet. Clin. Exp. Hypertens. 2012, 34, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Tøndervik, A.; Sletta, H.; Klinkenberg, G.; Emanuel, C.; Onsøyen, E.; Myrvold, R.; Howe, R.A.; Walsh, T.R.; Hill, K.E. Overcoming drug resistance with alginate oligosaccharides able to potentiate the action of selected antibiotics. Antimicrob. Agents Chemother. 2012, 56, 5134–5141. [Google Scholar] [CrossRef]
- Saigusa, M.; Nishizawa, M.; Shimizu, Y.; Saeki, H. In vitro and in vivo anti-inflammatory activity of digested peptides derived from salmon myofibrillar protein conjugated with a small quantity of alginate oligosaccharide. Biosci. Biotechnol. Biochem. 2015, 79, 1518–1527. [Google Scholar] [CrossRef]
- Powell, L.C.; Pritchard, M.F.; Emanuel, C.; Onsøyen, E.; Rye, P.D.; Wright, C.J.; Hill, K.E.; Thomas, D.W. A nanoscale characterization of the interaction of a novel alginate oligomer with the cell surface and motility of Pseudomonas aeruginosa. Am. J. Respir. Cell Mol. Biol. 2014, 50, 483–492. [Google Scholar] [CrossRef]
- Tusi, S.K.; Khalaj, L.; Ashabi, G.; Kiaei, M.; Khodagholi, F. Alginate oligosaccharide protects against endoplasmic reticulum-and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials 2011, 32, 5438–5458. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Zhang, Z. Potential applications of alginate oligosaccharides for biomedicine—A mini review. Carbohydr. Polym. 2021, 271, 118408. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.; Jiang, Z. Alginate oligosaccharides: Production, biological activities, and potential applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1859–1881. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, X.; Wu, L.; Li, H.; Chen, Y.; Li, L.; Ni, H.; Li, Q.; Zhu, Y. Exolytic products of alginate by the immobilized alginate lyase confer antioxidant and antiapoptotic bioactivities in human umbilical vein endothelial cells. Carbohydr. Polym. 2021, 251, 116976. [Google Scholar] [CrossRef]
- Gao, J.; Lin, L.; Sun, B.; Zhao, M. A comparison study on polysaccharides extracted from Laminaria japonica using different methods: Structural characterization and bile acid-binding capacity. Food Funct. 2017, 8, 3043–3052. [Google Scholar] [CrossRef] [PubMed]
- Kawada, A.; Hiura, N.; Shiraiwa, M.; Tajima, S.; Hiruma, M.; Hara, K.; Ishibashi, A.; Takahara, H. Stimulation of human keratinocyte growth by alginate oligosaccharides, a possible co-factor for epidermal growth factor in cell culture. FEBS Lett. 1997, 408, 43–46. [Google Scholar] [CrossRef]
- Wilcox, M.D.; Brownlee, I.A.; Richardson, J.C.; Dettmar, P.W.; Pearson, J.P. The modulation of pancreatic lipase activity by alginates. Food Chem. 2014, 146, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Anraku, M.; Nakagawa, S.; Ojima, T. Discovery of a novel alginate lyase from Nitratiruptor sp. SB155-2 thriving at deep-sea hydrothermal vents and identification of the residues responsible for its heat stability. J. Biol. Chem. 2016, 291, 15551–15563. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Bi, X.; Ren, Y.; Han, Q.; Zhou, Y.; Han, Y.; Yao, R.; Li, S. Characterization of an alkaline alginate lyase with pH-stable and thermo-tolerance property. Mar. Drugs 2019, 17, 308. [Google Scholar] [CrossRef]
- Vasudevan, U.M.; Lee, O.K.; Lee, E.Y. Alginate derived functional oligosaccharides: Recent developments, barriers, and future outlooks. Carbohydr. Polym. 2021, 267, 118158. [Google Scholar] [CrossRef]
- Zhu, B.; Li, K.; Wang, W.; Ning, L.; Tan, H.; Zhao, X.; Yin, H. Preparation of trisaccharides from alginate by a novel alginate lyase Alg7A from marine bacterium Vibrio sp. W13. Int. J. Biol. Macromol. 2019, 139, 879–885. [Google Scholar] [CrossRef]
- Chen, Y.; Dou, W.; Li, H.; Shi, J.; Xu, Z. The alginate lyase from Isoptericola halotolerans CGMCC 5336 as a new tool for the production of alginate oligosaccharides with guluronic acid as reducing end. Carbohydr. Res. 2018, 470, 36–41. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, J.; Li, X.; Peng, Q.; Lu, H.; Du, Y. Characterization of a new alginate lyase from newly isolated Flavobacterium sp. S20. J. Ind. Microbiol. Biotechnol. 2013, 40, 113–122. [Google Scholar] [CrossRef]
- Tøndervik, A.; Klinkenberg, G.; Aarstad, O.A.; Drabløs, F.; Ertesvåg, H.; Ellingsen, T.E.; Skjåk-Bræk, G.; Valla, S.; Sletta, H. Isolation of mutant alginate lyases with cleavage specificity for di-guluronic acid linkages. J. Biol. Chem. 2010, 285, 35284–35292. [Google Scholar] [CrossRef]
- Li, L.; Jiang, X.; Guan, H.; Wang, P. Preparation, purification and characterization of alginate oligosaccharides degraded by alginate lyase from Pseudomonas sp. HZJ 216. Carbohydr. Res. 2011, 346, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Gu, J.; Cheng, Y.; Liu, H.; Li, Y.; Li, F. Novel alginate lyase (Aly5) from a polysaccharide-degrading marine bacterium, Flammeovirga sp. strain MY04, effects of module truncation on biochemical characteristics, alginate degradation patterns, and oligosaccharide-yielding properties. Appl. Environ. Microbiol. 2016, 82, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Daboor, S.M.; Raudonis, R.; Cohen, A.; Rohde, J.R.; Cheng, Z. Marine bacteria, a source for alginolytic enzyme to disrupt Pseudomonas aeruginosa biofilms. Mar. Drugs 2019, 17, 307. [Google Scholar] [CrossRef] [PubMed]
- Dharani, S.R.; Srinivasan, R.; Sarath, R.; Ramya, M. Recent progress on engineering microbial alginate lyases towards their versatile role in biotechnological applications. Folia Microbiol. 2020, 65, 937–954. [Google Scholar] [CrossRef]
- Wargacki, A.J.; Leonard, E.; Win, M.N.; Regitsky, D.D.; Santos, C.N.S.; Kim, P.B.; Cooper, S.R.; Raisner, R.M.; Herman, A.; Sivitz, A.B. An engineered microbial platform for direct biofuel production from brown macroalgae. Science 2012, 335, 308–313. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Yu, W.; Gong, Q. Cloning, overexpression and characterization of a new oligoalginate lyase from a marine bacterium, Shewanella sp. Biotechnol. Lett. 2015, 37, 665–671. [Google Scholar] [CrossRef]
- Tang, J.C.; Taniguchi, H.; Chu, H.; Zhou, Q.; Nagata, S. Isolation and characterization of alginate-degrading bacteria for disposal of seaweed wastes. Lett. Appl. Microbiol. 2009, 48, 38–43. [Google Scholar] [CrossRef]
- Maurice, N.M.; Bedi, B.; Sadikot, R.T. Pseudomonas aeruginosa biofilms: Host response and clinical implications in lung infections. Am. J. Respir. Cell Mol. Biol. 2018, 58, 428–439. [Google Scholar] [CrossRef]
- Tavafi, H.; Ali, A.A.; Ghadam, P.; Gharavi, S. Screening, cloning and expression of a novel alginate lyase gene from P. aeruginosa TAG 48 and its antibiofilm effects on P. aeruginosa biofilm. Microb. Pathog. 2018, 124, 356–364. [Google Scholar] [CrossRef]
- Preiss, J.; Ashwell, G. Alginic acid metabolism in bacteria: I. Enzymatic formation of unsaturated oligosaccharides and 4-deoxy-l-erythro-5-hexoseulose uronic acid. J. Biol. Chem. 1962, 237, 309–316. [Google Scholar] [CrossRef]
- Skriptsova, A.; Khomenko, V.; Isakov, V. Seasonal changes in growth rate, morphology and alginate content in Undaria pinnatifida at the northern limit in the Sea of Japan (Russia). J. Appl. Phycol. 2004, 16, 17–21. [Google Scholar] [CrossRef]
- Kokoulin, M.S.; Tomshich, S.V.; Kalinovsky, A.I.; Komandrova, N.A. O-antigens of marine gram-negative bacteria. Bull. Far East. Branch Russ. Acad. Sci. 2015, 6, 132–139. (In Russian) [Google Scholar]
- Park, D.; Jagtap, S.; Nair, S.K. Structure of a PL17 family alginate lyase demonstrates functional similarities among exotype depolymerases. J. Biol. Chem. 2014, 289, 8645–8655. [Google Scholar] [CrossRef] [PubMed]
- Gacesa, P. Alginate-modifying enzymes. A proposed unified mechanism of action for the lyases and epimerases. Fed. Eur. Biochem. Soc. Lett. 1987, 212, 199–202. [Google Scholar] [CrossRef]
- Garron, M.L.; Cygler, M. Uronic polysaccharide degrading enzymes. Curr. Opin. Struct. Biol. 2014, 28, 87–95. [Google Scholar] [CrossRef]
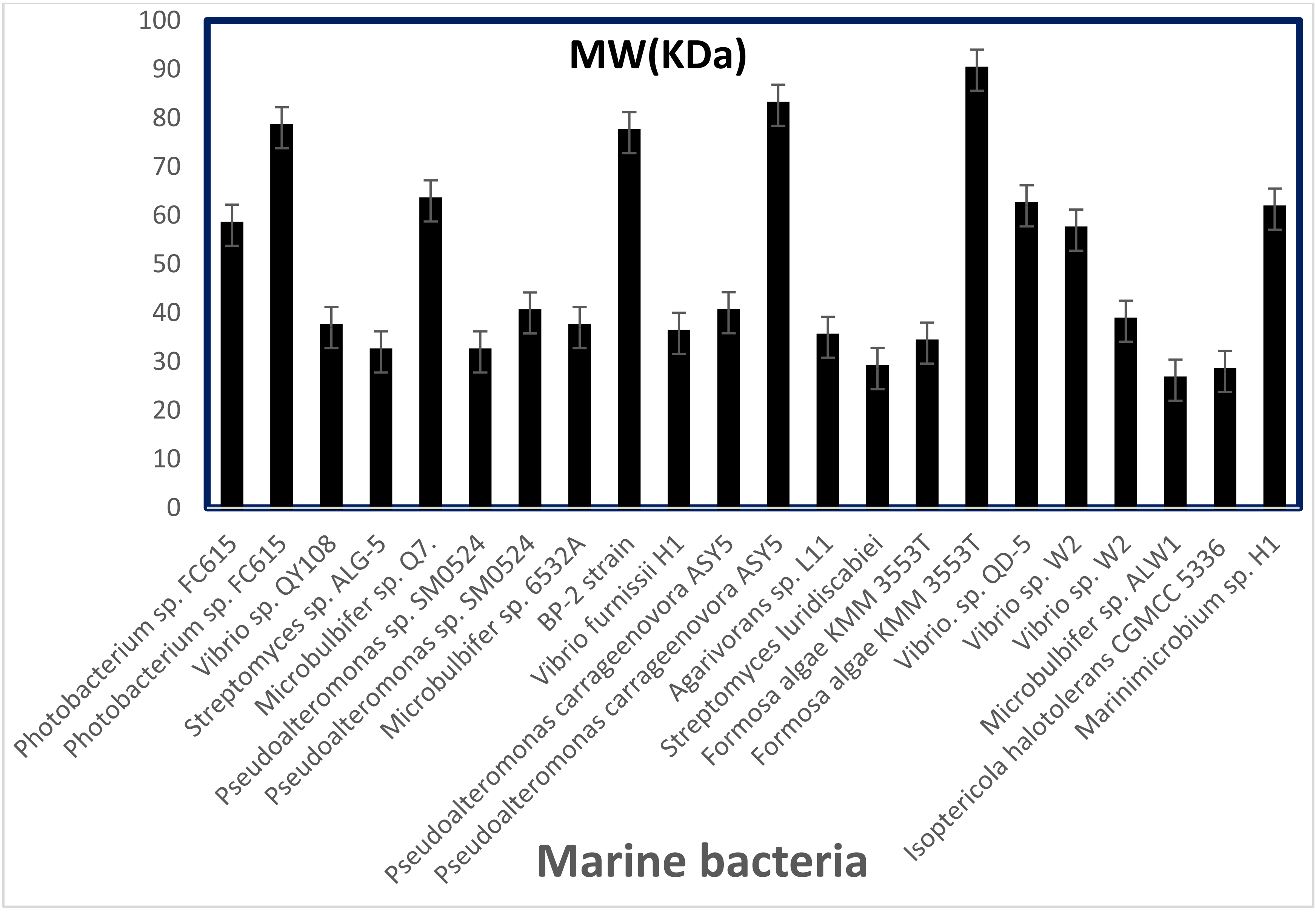

| Source | Enzyme | Opt. pH | pH Stability | Opt. Temp (°C) | Thermal Stability | PI | Activators | Inhibitors | Gen Bank Accession No. | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Photobacterium sp. FC615 | AlyPB1 | 8.0 | - | 30 | - | 4.88 | - | Hg2+, Ni2+, Mn2+, Zn2+, Cu2+, SDS, Co2+ | MN116685 | [83] |
| Photobacterium sp. FC615 | AlyPB2 | 8.0 | - | 20 | - | 5.01 | Co2+, DTT, β-mercaptoethanol | Hg2+, Ni2+, Mn2+, Zn2+, Cu2+, SDS, Ag+, Mg2+ | MN116686 | [83] |
| Vibrio sp. QY108 | VsAly7D | 7.6 | stable at pH 7.6~10.6, stable at pH 9.0~10.0 (12 h, with 80% activity) | 35 | 46.5% (20 °C) and 83.1% (30 °C) of the initial enzyme activities | 5.65 | - | Zn2+, Fe3+, Cu2+, SDS and EDTA | QPB15428 | [106] |
| Streptomyces sp. ALG-5 | ALG-5 | 8.0 | - | 30 | - | - | - | - | EU137870 | [104] |
| Cobetia sp. NAP1 | AlgC-PL7 | 8.0 | ~50% lyase activity at pH 6~9. | 45 | >90% of the initial enzyme activity (heating at 70~80 °C for 15 min), 80% (heating at 90 °C for 15 min). | - | - | - | - | [107] |
| Sphingomonas sp. | SALy | 6.5 | - | - | 70% of the initial enzyme activity at 55 °C | - | - | - | 2CWS | [107] |
| Flavobacterium sp. | FALy | 7.5 | - | - | 30–40% of the initial enzyme activity at 55 °C for 4 h; lost its activity at 60 °C | - | - | - | JF412659 | [111] |
| Microbulbifer sp. Q7. | AlyM | 7 | - | 55 | 32% of initial enzyme activity at 45 °C for 2 h; 14.7% at 55 °C for 1 h | 4.4 | K+, Ca2+, Mg2+, glycine | Zn2+, Cu2+, Li+, Fe3+, Fe2+, Mn2+, EDTA, SDS | WP066959628.1 | [112] |
| Pseudoalteromonas sp. SM0524 | Aly-SJ02 | 8.5 | stable at pH 8.0 ~50% activity at pH 7.0–10 for 20 min | 50 | Remain stable for 41 min at 40 °C and 20 min at 50 °C | Na+, K+, Mg2+, Ca2+, Co2+, Ba2+, Ni2+, Sr2+ | Cu2+, Sn2+, EDTA | EU548075 | [93] | |
| Pseudoalteromonas sp. SM0524 | AlyPM | 8.5 | >70% of its highest activity at pH 7.0~9.5 | 30 | 19% of the highest activity at 5 °C. unstable at >30 °C low Tm at 37 °C. | Cu2+,Co2+ | Ni2+ | EU548076 | [113] | |
| Microbulbifer sp. 6532A | AlgMsp | 8.0 | - | 50 | activity down by 86 at 60 °C, no activity at 70 °C | - | - | Ni2+, Ca2+ | AB603802 | [46] |
| BP-2 strain | Alg17B | 7.5–8.0 | stable at pH 7.0–8.0, enzyme activity was reduced to 33% at pH 8.5 | 40–45 | stable at 25–35 °C. 90% of the enzyme activity at 40 °C | - | Na+ | Ca2+, Zn2+ | MH820150.1 | [97] |
| Bacillus sp. | - | 8.0 | stable at pH 4.0–9.0 | 50 | stable at 45 °C. 50% at 50 °C for 105 min and maintain 100% activity at 45 °C after 180 min | - | Mg2+, Ca2+, K+ | Zn2+, Co2+, Li+, EDTA, PMSF | LC457966 | [88] |
| Vibrio furnissii H1 | AlyH1 | 7.5 | stable at pH 7.0–8.0 for 12 h, >60% activity at pH 6.5–8.5, 80% activity at pH 7.0–8.0 | 40 | stable at <30 °C. >60% of activity at 40 °C for 30 min | - | Na+, Mg2+, K+ | Zn2+, Fe2+, Cu2+, Mn2+, Ag+ | MG214325 | [114] |
| Pseudoalteromonascarrageenovora ASY5 | Aly1281 | 8.0 | >65% enzyme activity at pH 6.0–9.5. >70% of the enzyme activities at pH 7.0–9.0 | 50 | >50% of the activity at 45–55 °C | 9.06 | - | - | - | [108] |
| Pseudoalteromonascarrageenovora ASY5 | Alg823 | 8.0 | >80% activity at pH 6.0–10.0 (4 °C for 24 h) | 55 | ~75% of the optimal activity at 50 °C for 30 min | - | Mg2+, Ca2+, Na+, and K+ | CTAB | - | [115] |
| Agarivorans sp. L11 | AlyL1 | 8.6 | stable at pH 6.0–9.6 | 40 | 54.5% and 72.1% of optimal activity at 15 °C and 20 °C, respectively | - | - | - | KM018274 | [109] |
| Streptomycesluridiscabiei | AlyDS44 | 8.5 | >70% of the maximum activity at pH 6.5–9.5. | 45 | >80% enzyme activity at 35 °C to 55 °C. | - | Mn2+, Co2+, Fe2+ | Zn2+, Cu2+ | OK169607 | [100] |
| Alteromonas sp. H-4 | - | 7.5 | stable at pH 6.6–9.0, <20% activity at pH < 5.0 | 30 | 20% and 40% decrease in the enzyme activity at 30 and 40° C for 5 min, respectively. | - | MnCl2 or BaCl2, | EDTA. Na+, ZnSO4, or CdCl2 | - | [118] |
| Formosaalgae KMM 3553T | ALFA3 | 6.0 | - | 35 | 50% activity at 42 °C for 30 min | - | - | - | PRJNA299442 | [110] |
| Formosaalgae KMM 3553T | ALFA4 | 8.0 | - | 30 | stable up to 30 °C; 50% activity at 37 °C for 1 h 40 min. | - | - | PRJNA299442 | [110] | |
| Vibrio. sp. QD-5 | Aly-IV | 8.9 | >80% activity at pH 7.0–10.0. | 35 | stable at <30 °C for 30 min | 5.12 | K+, Mg2+ | Ba2+, Al3+, Ni2+, Zn2+, Pb2+, EDTA | PRJNA382465 | [96] |
| Zobelliagalactanivorans | AlyA1 | 7.0 | - | 30 | - | - | - | - | - | [90] |
| Zobelliagalactanivorans | AlyA5 | 7.0 | - | - | - | - | - | - | - | [90] |
| Vibrio sp. W2 | Alyw203 | 10 | >80% of the highest activity at pH 4.0–10.0. | 45 | >90% of its initial activity at 10 °C for 20 min >80% activity at 40–55 °C | 6.09 | Fe3+, Cu2+, Zn2+, Al3+ | SDS, EDTA | [105] | |
| Vibrio sp. W2 | Alyw202 | 9 | >80% activity at pH 5.0–9.0 (4 °C) for 12 h, >60% activity at pH 3.0–10.0 (4 °C) for 12 h | 45 | - | 5.10 | Mn2+ and Co2+ | Na+, Mg2+ and Ba2+, EDTA and SDS | - | [119] |
| Enzyme | Source | Substrate Preference | Km | Vmax | kcat | References |
|---|---|---|---|---|---|---|
| AlyPM | Pseudoalteromonas sp. SM0524 | polyM | 3.15 mg/mL (0.5 M NaCl) and 74.39 mg/mL (0 M NaCl) for sodium alginate | - | - | [113] |
| ALFA3 | Formosa algae KMM 3553T | polyGM | 0.12 ± 0.01 mg/mL | 0.128 × 10−3 M/min for G, 0.150 × 10−3 M/min for MG, 0.211 × 10−3 M/min for M | 3.52 s−1 for G, 4.13 s−1 for MG and 5.80 s−1 for M | [111] |
| ALFA4 | Formosa algae KMM 3553T | polyM | 3.01 ± 0.05 mg/mL for polyM | 0.314 × 10−3 M/min for MG | 2.88 s−1 for MG | [111] |
| ALW1 | Microbulbifer sp. ALW1 | - | 1.03 mg/mL for sodium alginate | 4.63 U/mg for sodium alginate | 69.38 s−1 for sodium alginate | [42] |
| Aly1281 | Pseudoalteromonascarrageenovora ASY5 | - | 0.3180 (0.3 M NaCl) and 0.1810 mg/mL (1.0 M NaCl), respectively, 0.2805 (0.3 M KCl) and 0.1631 (1.0 M KCl) for sodium alginate | - | 2.185 s−1 (in 0.3 NaCl), 2.095 s−1 (in 1.0 M NaCl), 1.875 s−1 (in 0.3 KCl), 1.502 s−1 (in 1.0 M KCl) for sodium alginate | [123] |
| AlgNJ–07 | Serratia marcescens NJ-07 | - | 0.53 mM for sodium alginate, 0.27 mM for polyM | 74, 67 nmol/s for sodium alginate and polyM | 34 for sodium alginate, and 31 s−1 for polyM | [81] |
| Aly-IV | Vibrio. sp. QD-5 | - | 0.2223 g/mL for sodium alginate, 0.3274 g/mL for polyG | 3.6 OD235/h for sodium alginate, 2.8321 OD235/h for polyG | - | [97] |
| Aly-SJ02 | Pseudoalteromonas sp. SM0524 | bifunctional | 1.086 for sodium alginate, 0.465 for polyG, 2.751 mg/mL for polyM, | 8.074 OD235/h for sodium alginate, 5.318 OD235/h for polyG, 7.131 for polyM | - | [93] |
| Alg823 | Pseudoalteromonascarrageenovora ASY5 | - | 0.15 mg/mL for sodium alginate | 1.84 U/g for sodium alginate | 1.19 × 106 s−1 for sodium alginate | [93] |
| VsAly7D | Vibrio sp. QY108 | - | 0.217 mM for alginate | - | 42.26 s−1 for sodium alginate | [107] |
| AlgM4 | Vibrio weizhoudaoensis M0101 | bifunctional | 2.72 mg/mL, for sodium alginate | 2.75 nmol/s for sodium alginate | 30.25 s−1 for sodium alginate | [124] |
| AlgH | Marinimicrobium sp. H1 | - | 6.6 ± 2.2 mg·mL−1 for sodium alginate, 7.6 ± 1.6 mg·mL−1 for polyG, 9.1 ± 2.4 mg·mL−1 for polyM | 224.6 ± 33.6, 146.6 ± 15.6, 62.6 ± 8.8 U/mg of protein, respectively, for sodium alginate, polyG and polyM | 260.6 ± 36.2 s−1 for sodium alginate, 155.7 ± 17.1 s−1 for polyG, 66.8 ± 6.7 s−1 for polyM | [121] |
| AlyH1 | Vibrio furnissii H1 | 2.28 mg/mL for sodium alginate | 2.81 U/mg for sodium alginate | - | [115] | |
| AlgNJU-03 | Vibrio sp. NJU-03 | bifunctional | 8.50 mM for sodium alginate,, 10.94 mM for polyM, 4.00 mM for polyG | 1.67 nmol/s for sodium alginate, 0.30 nmol/s for polyM, 2.50 nmol/s for polyG | 30.64, 5.50, 45.87 s−1, respectively for sodium alginate, polyM and polyG | [125] |
| AlgNJ–04 | Vibrio sp. NJ04 | - | 0.49 mM for alginate, 0.86 mM for polyM, 0.24 mM for polyG | 72 pmol/s for alginate, 95 for polyM, 35 pmol/s for polyG | 59 s−1 for alginate, 77 s−1 for polyM, 29 s−1 for polyG | [124] |
| Alys1 | Tamlana sp. S12 | polyM | 0.20 ± 0.01 mM for sodium alginate | - | 4.43 ± 0.027 s−1 for sodium alginate | [126] |
| AlyC3 | Psychromonas sp. C-3 | polyM | 0.24 ± 0.05 mg/mL for polyM | 19,704.73 ± 1865.49 U/mg of protein for polyM | - | [127] |
| AlgMsp | Microbulbifer sp. 6532A | polyG | 3.46 ± 0.9 mM for alginate, 1.8 ± 0.4 mM for polyG, 6.8 ± 2.1 mM for polyM | 5765, 3562, 6368 U/mg of protein for alginate, polyG and polyM, respectively | 42 s−1 for alginate,26 s−1 for polyG, 46 s−1 for polyM | [46] |
| A1m | Agarivorans sp. JAM-A1m | - | - | 38.4, 285.7, 416.7, and 526.3 U/mg of protein (0, 0.1, 0.2, and 0.5 M NaCl, respectively) for sodium alginate | - | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barzkar, N.; Sheng, R.; Sohail, M.; Jahromi, S.T.; Babich, O.; Sukhikh, S.; Nahavandi, R. Alginate Lyases from Marine Bacteria: An Enzyme Ocean for Sustainable Future. Molecules 2022, 27, 3375. https://doi.org/10.3390/molecules27113375
Barzkar N, Sheng R, Sohail M, Jahromi ST, Babich O, Sukhikh S, Nahavandi R. Alginate Lyases from Marine Bacteria: An Enzyme Ocean for Sustainable Future. Molecules. 2022; 27(11):3375. https://doi.org/10.3390/molecules27113375
Chicago/Turabian StyleBarzkar, Noora, Ruilong Sheng, Muhammad Sohail, Saeid Tamadoni Jahromi, Olga Babich, Stanislav Sukhikh, and Reza Nahavandi. 2022. "Alginate Lyases from Marine Bacteria: An Enzyme Ocean for Sustainable Future" Molecules 27, no. 11: 3375. https://doi.org/10.3390/molecules27113375
APA StyleBarzkar, N., Sheng, R., Sohail, M., Jahromi, S. T., Babich, O., Sukhikh, S., & Nahavandi, R. (2022). Alginate Lyases from Marine Bacteria: An Enzyme Ocean for Sustainable Future. Molecules, 27(11), 3375. https://doi.org/10.3390/molecules27113375







