Asymmetrically Substituted Phospholes as Ligands for Coinage Metal Complexes
Abstract
1. Introduction
2. Results and Discussion
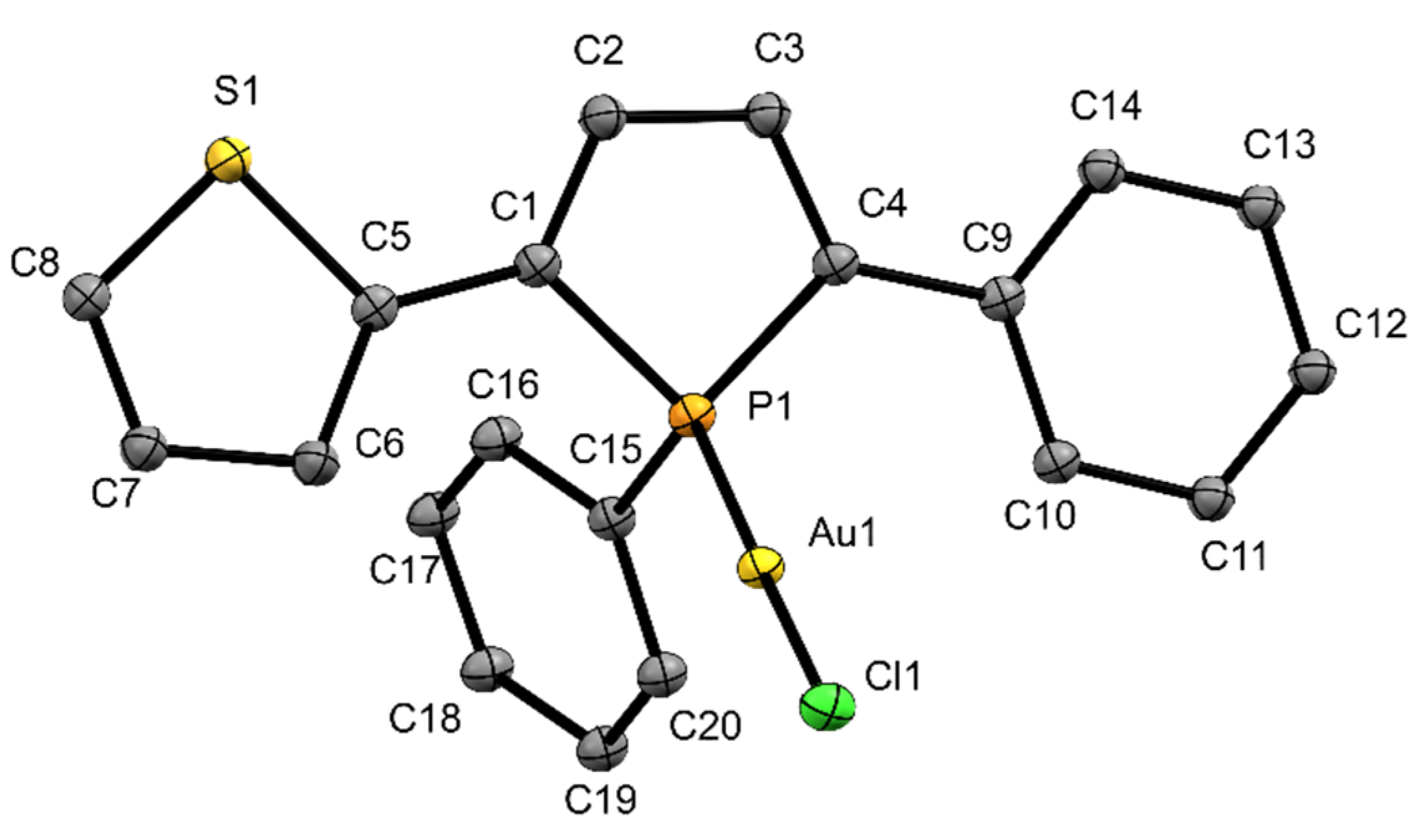
2.1. Characterization in the Solid State
2.2. Luminescence Properties
3. Materials and Methods
3.1. Synthesis of Phosphole 2
3.2. Synthesis of Copper Complex 3
3.3. Synthesis of Silver Complex 4
3.4. Synthesis of Gold Complex 5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mathey, F. The organic chemistry of phospholes. Chem. Rev. 1988, 88, 429–453. [Google Scholar] [CrossRef]
- Carmichael, D. Phospholes. In Phosphorus (III) Ligands in Homogeneous Catalysis: Design and Synthesis; Kamer, P.C.J., van Leeuwen, P.W.N.M., Eds.; Wiley-Blackwell: Oxford, UK, 2012; pp. 267–285. ISBN 9781118299715. [Google Scholar]
- Shameem, M.A.; Orthaber, A. Organophosphorus Compounds in Organic Electronics. Chem. Eur. J. 2016, 22, 10718–10735. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, T.; Réau, R. Organophosphorus pi-conjugated materials. Chem. Rev. 2006, 106, 4681–4727. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.P.; Delaunay, W.; Bouit, P.-A.; Hissler, M. π-Conjugated phospholes and their incorporation into devices: Components with a great deal of potential. Chem. Soc. Rev. 2016, 45, 5296–5310. [Google Scholar] [CrossRef]
- Fave, C.; Cho, T.-Y.; Hissler, M.; Chen, C.-W.; Luh, T.-Y.; Wu, C.-C.; Réau, R. First Examples of Organophosphorus-Containing Materials for Light-Emitting Diodes. J. Am. Chem. Soc. 2003, 125, 9254–9255. [Google Scholar] [CrossRef]
- Joly, D.; Bouit, P.-A.; Hissler, M. Organophosphorus derivatives for electronic devices. J. Mater. Chem. C 2016, 4, 3686–3698. [Google Scholar] [CrossRef]
- Matano, Y.; Imahori, H. Design and synthesis of phosphole-based pi systems for novel organic materials. Org. Biomol. Chem. 2009, 7, 1258–1271. [Google Scholar] [CrossRef]
- Regulska, E.; Romero-Nieto, C. Design of organophosphorus materials for organic electronics and bio-applications. Mater. Today Chem. 2021, 22, 100604. [Google Scholar] [CrossRef]
- Roesler, F.; Kaban, B.; Klintuch, D.; Ha, U.-M.; Bruhn, C.; Hillmer, H.; Pietschnig, R. Tailoring Phospholes for Imprint of Fluorescent 3D Structures. Eur. J. Inorg. Chem. 2019, 4820–4825. [Google Scholar] [CrossRef]
- Klintuch, D.; Höfler, M.V.; Wissel, T.; Bruhn, C.; Gutmann, T.; Pietschnig, R. Trifunctional Silyl Groups as Anchoring Units in the Preparation of Luminescent Phosphole–Silica Hybrids. Inorg. Chem. 2021, 60, 14263–14274. [Google Scholar] [CrossRef]
- Arkhypchuk, A.I.; Orthaber, A.; Ott, S. Tuning the Optical Properties of 1,1′-Biphospholes by Chemical Alterations of the P-P Bridge. Eur. J. Inorg. Chem. 2014, 2014, 1760–1766. [Google Scholar] [CrossRef]
- Klintuch, D.; Kirchmeier, A.; Bruhn, C.; Pietschnig, R. Synthetic access and luminescence tuning in a series of β-H and β-silyl substituted phospholes. Dyes Pigm. 2020, 180, 108443. [Google Scholar] [CrossRef]
- Hay, C.; Hissler, M.; Fischmeister, C.; Rault-Berthelot, J.; Toupet, L.; Nyulászi, L.; Réau, R. Phosphole-Containing π-Conjugated Systems: From Model Molecules to Polymer Films on Electrodes. Chem. Eur. J. 2001, 7, 4222–4236. [Google Scholar] [CrossRef]
- Klintuch, D.; Krekić, K.; Bruhn, C.; Benkő, Z.; Pietschnig, R. A Rational Synthetic Approach to 2,5-Diphenyl-β-silyl Phospholes. Eur. J. Inorg. Chem. 2016, 718–725. [Google Scholar] [CrossRef]
- Ren, Y.; Orthaber, A.; Pietschnig, R.; Baumgartner, T. Perfluorophenylene-bridged bisphospholes: Synthesis and unexpected photophysical properties. Dalton Trans. 2013, 42, 5314–5321. [Google Scholar] [CrossRef] [PubMed]
- Roesler, F.; Kovács, M.; Bruhn, C.; Kelemen, Z.; Pietschnig, R. Phosphetes via transition metal free ring closure - taking the proper turn at a thermodynamic crossing. Chem. Eur. J. 2021, 9782–9790. [Google Scholar] [CrossRef] [PubMed]
- Egan, W.; Tang, R.; Zon, G.; Mislow, K. Barriers to pyramidal inversion at phosphorus in phospholes, phosphindoles, and dibenzophospholes: Erstes asymmetrisches Phosphol. J. Am. Chem. Soc. 1971, 93, 6205–6216. [Google Scholar] [CrossRef]
- Dhbaibi, K.; Favereau, L.; Crassous, J. Enantioenriched Helicenes and Helicenoids Containing Main-Group Elements (B, Si, N, P). Chem. Rev. 2019, 119, 8846–8953. [Google Scholar] [CrossRef]
- Boursalian, G.B.; Nijboer, E.R.; Dorel, R.; Pfeifer, L.; Markovitch, O.; Blokhuis, A.; Feringa, B.L. All-Photochemical Rotation of Molecular Motors with a Phosphorus Stereoelement. J. Am. Chem. Soc. 2020, 142, 16868–16876. [Google Scholar] [CrossRef]
- Märkl, G.; Potthast, R. A Simple Synthesis of Phospholes. Angew. Chem. Int. Ed. Engl. 1967, 6, 86. [Google Scholar] [CrossRef]
- Bai, L.; Zheng, Z.; Wang, Z.; He, F.; Xue, Y.; Wang, N. Acetylenic bond-driven efficient hydrogen production of a graphdiyne based catalyst. Mater. Chem. Front. 2021, 5, 2247–2254. [Google Scholar] [CrossRef]
- Anzo, T.; Suzuki, A.; Sawamura, K.; Motozaki, T.; Hatta, M.; Takao, K.; Tadano, K. Formal synthesis of tubelactomicins via a transannular Diels–Alder approach. Tetrahedron Lett. 2007, 48, 8442–8448. [Google Scholar] [CrossRef]
- Attar, S.; Bowmaker, G.A.; Alcock, N.W.; Frye, J.S.; Bearden, W.H.; Nelson, J.H. Phosphole complexes of copper(I) halides. Investigations of structure and bonding by x-ray crystallography, infrared spectroscopy, and CP/MAS phosphorus-31 NMR spectroscopy. Inorg. Chem. 1991, 30, 4743–4753. [Google Scholar] [CrossRef]
- Attar, S.; Bearden, W.H.; Alcock, N.W.; Alyea, E.C.; Nelson, J.H. Phosphole complexes of gold(I) halides: Comparison of solution and solid-state structures by a combination of solution and CP/MAS phosphorus-31 NMR spectroscopy and x-ray crystallography. Inorg. Chem. 1990, 29, 425–433. [Google Scholar] [CrossRef]
- Orthaber, A.; Belaj, F.; Pietschnig, R. Synthesis, structure and π-delocalization of a phosphaalkenyl based neutral PNP-pincer. Inorg. Chim. Acta 2011, 374, 211–215. [Google Scholar] [CrossRef][Green Version]
- Attar, S.; Alcock, N.W.; Bowmaker, G.A.; Frye, J.S.; Bearden, W.H.; Nelson, J.H. Phosphole complexes of silver(I). Investigations of structure and bonding by x-ray crystallography, infrared spectroscopy, and CP/MAS and solution phosphorus-31 NMR spectroscopy. Inorg. Chem. 1991, 30, 4166–4176. [Google Scholar] [CrossRef]
- Orthaber, A.; Borucki, S.; Shen, W.; Réau, R.; Lescop, C.; Pietschnig, R. Coordination Behaviour of a Hexadentate 1,1′-Ferrocenylene-Bridged Bisphosphole towards Coinage Metal Centres. Eur. J. Inorg. Chem. 2014, 2014, 1751–1759. [Google Scholar] [CrossRef]
- Alyea, E.C.; Malito, J.; Nelson, J.H. Identification by stopped-exchange solution phosphorus-31 NMR spectroscopy of the stepwise formation of [AgLn]PF6 (n = 1-4). Comparison of metal-phosphorus coupling constants for triphenylphosphine and 5-phenyldibenzophosphole. Inorg. Chem. 1987, 26, 4294–4296. [Google Scholar] [CrossRef]
- Orthaber, A.; Fuchs, M.; Belaj, F.; Rechberger, G.N.; Kappe, C.O.; Pietschnig, R. Bis(diethylamino)(pentafluorophenyl)phosphane—a Push–Pull Phosphane Available for Coordination. Eur. J. Inorg. Chem. 2011, 2588–2596. [Google Scholar] [CrossRef][Green Version]
- Twigg, M.V.; Spencer, M.S. Deactivation of supported copper metal catalysts for hydrogenation reactions. Appl. Catal. A 2001, 212, 161–174. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Fourmy, K.; Gouygou, M.; Dechy-Cabaret, O.; Benoit-Vical, F. Gold(I) complexes bearing phosphole ligands: Synthesis and antimalarial activity. C. R. Chim. 2017, 20, 333–338. [Google Scholar] [CrossRef]
- Shamsieva, A.V.; Musina, E.I.; Gerasimova, T.P.; Fayzullin, R.R.; Kolesnikov, I.E.; Samigullina, A.I.; Katsyuba, S.A.; Karasik, A.A.; Sinyashin, O.G. Intriguing Near-Infrared Solid-State Luminescence of Binuclear Silver(I) Complexes Based on Pyridylphospholane Scaffolds. Inorg. Chem. 2019, 58, 7698–7704. [Google Scholar] [CrossRef] [PubMed]
- Musina, E.I.; Shamsieva, A.V.; Strelnik, I.D.; Gerasimova, T.P.; Krivolapov, D.B.; Kolesnikov, I.E.; Grachova, E.V.; Tunik, S.P.; Bannwarth, C.; Grimme, S.; et al. Synthesis of novel pyridyl containing phospholanes and their polynuclear luminescent copper(i) complexes. Dalton. Trans. 2016, 45, 2250–2260. [Google Scholar] [CrossRef]
- Ishidoshiro, M.; Matsumura, Y.; Imoto, H.; Irie, Y.; Kato, T.; Watase, S.; Matsukawa, K.; Inagi, S.; Tomita, I.; Naka, K. Practical Synthesis and Properties of 2,5-Diarylarsoles. Org. Lett. 2015, 17, 4854–4857. [Google Scholar] [CrossRef]
- Ziegler, C.B.; Harris, S.M.; Baldwin, J.E. 1,4-Dehydrobromination of 1-bromo-1,2,3-butatrienes: An efficient synthesis of 1,4-disubstituted 1,3-diynes: Thienyl-Phenyl-Diin. J. Org. Chem. 1987, 52, 443–446. [Google Scholar] [CrossRef]
- Neufeld, R.; Stalke, D. Accurate molecular weight determination of small molecules via DOSY-NMR by using external calibration curves with normalized diffusion coefficients. Chem. Sci. 2015, 6, 3354–3364. [Google Scholar] [CrossRef]
- Bachmann, S.; Neufeld, R.; Dzemski, M.; Stalke, D. New External Calibration Curves (ECCs) for the Estimation of Molecular Weights in Various Common NMR Solvents. Chem. Eur. J. 2016, 22, 8462–8465. [Google Scholar] [CrossRef]
- Bachmann, S.; Gernert, B.; Stalke, D. Solution structures of alkali metal cyclopentadienides in THF estimated by ECC-DOSY NMR-spectroscopy (incl. software). Chem. Commun. 2016, 52, 12861–12864. [Google Scholar] [CrossRef]
- Kreyenschmidt, A.-K.; Bachmann, S.; Niklas, T.; Stalke, D. Molecular Weight Estimation of Molecules Incorporating Heavier Elements from van-der-Waals Corrected ECC-DOSY. ChemistrySelect 2017, 2, 6957–6960. [Google Scholar] [CrossRef]
- Neufeld, R.; John, M.; Stalke, D. The Donor-Base-Free Aggregation of Lithium Diisopropyl Amide in Hydrocarbons Revealed by a DOSY Method. Angew. Chem. Int. Ed. Engl. 2015, 54, 6994–6998. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.H.; Chen, A.D.; Johnson, C.S. An improved diffusion-ordered spectroscopy experiment incorporating bipolar-gradient pulses. J. Magn. Reson. A 1995, 115, 260–264. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]





| DCM | Solid | ||||||
|---|---|---|---|---|---|---|---|
| Compound | λabs [nm] | λEm [nm] | Ф [%] | εmax·104 [l·cm−1·mol−1] | λabs [nm] | λEm [nm] | Ф [%] |
| 2 | 395 [10] | 487 [10] | 33.8 [10] | - | 446 | 523 | 44.5 |
| 3 | 411 | 497 | 6.2 | 2.84 | 451 | 536 | 4.7 |
| 4 | 402 | 496 | 21.5 | 3.69 | 428 | 496 | 0.7 |
| 5 | 418 | 519 | 7.7 | 2.62 | 450 | 552 | 22.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roesler, F.; Bruhn, C.; Pietschnig, R. Asymmetrically Substituted Phospholes as Ligands for Coinage Metal Complexes. Molecules 2022, 27, 3368. https://doi.org/10.3390/molecules27113368
Roesler F, Bruhn C, Pietschnig R. Asymmetrically Substituted Phospholes as Ligands for Coinage Metal Complexes. Molecules. 2022; 27(11):3368. https://doi.org/10.3390/molecules27113368
Chicago/Turabian StyleRoesler, Fabian, Clemens Bruhn, and Rudolf Pietschnig. 2022. "Asymmetrically Substituted Phospholes as Ligands for Coinage Metal Complexes" Molecules 27, no. 11: 3368. https://doi.org/10.3390/molecules27113368
APA StyleRoesler, F., Bruhn, C., & Pietschnig, R. (2022). Asymmetrically Substituted Phospholes as Ligands for Coinage Metal Complexes. Molecules, 27(11), 3368. https://doi.org/10.3390/molecules27113368






