Alkaline Soil Degradation and Crop Safety of 5-Substituted Chlorsulfuron Derivatives
Abstract
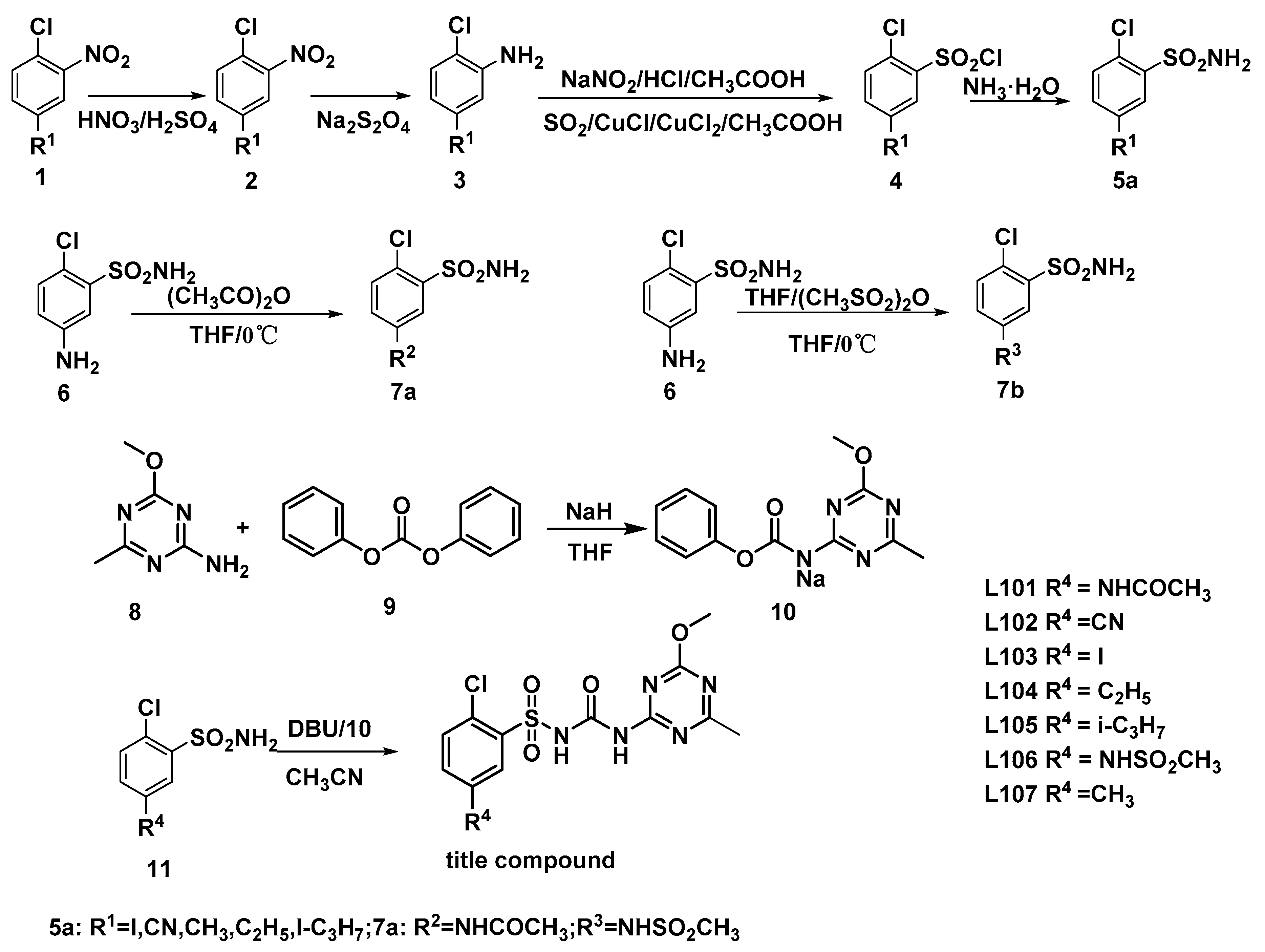
:1. Introduction
2. Materials and Methods
2.1. Instruments and Materials
2.2. Compounds L101–L107
2.3. Soil Degradation Assay
2.4. Crop Safety Assay
3. Results and Discussion
3.1. Soil Degradation
3.2. Crop Safety Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Levitt, G. Discovery of the Sulfonylurea Herbicides. In Synthesis and Chemistry of Agrochemicals II; ACS: Washington, DC, USA, 1991; Volume 443, pp. 16–31. [Google Scholar]
- Chipman, D.; Barak, Z.; Schloss, J.V. Biosynthesis of 2-aceto-2-hydroxy acids: Acetolactate synthases and acetohydroxyacid synthases. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1998, 1385, 401–419. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, W.; Liu, K.K. Action mechanisms of acetolactate synthase-inhibiting herbicides. Pestic. Biochem. Physiol. 2007, 89, 89–96. [Google Scholar] [CrossRef]
- Qu, R.-Y.; Yang, J.-F.; Devendar, P.; Kang, W.-M.; Liu, Y.-C.; Chen, Q.; Niu, C.W.; Xi, Z.; Yang, G.F. Discovery of New 2-[(4,6-Dimethoxy-1,3,5-triazin-2-yl)oxy]-6-(substituted phenoxy)benzoic Acids as Flexible Inhibitors of Arabidopsis thaliana Acetohydroxyacid Synthase and Its P197L Mutant. J. Agric. Food Chem. 2017, 65, 11170–11178. [Google Scholar] [CrossRef]
- Blair, A.M.; Martin, T.D. A review of the activity, fate and mode of action of sulfonylurea herbicides. Pestic. Sci. 1988, 22, 195–219. [Google Scholar] [CrossRef]
- Anderson, R.L.; Barrett, M.R.J. Residual Phytotoxicity of Chlorsulfuron in Two Soils. J. Environ. Qual. 1985, 14, 111–114. [Google Scholar] [CrossRef]
- Walker, A.; Cotterill, E.G.; Welch, S.J. Adsorption and Degradation of Chlorsulfuron and Metsulfuron-Methyl in Soils from Different Depths. Weed Res. 1989, 29, 281–287. [Google Scholar] [CrossRef]
- Rother, P.A.; Mckercher, R.B. Crop responses to applications of both chlorsulfuron and monoammonium phosphate. Plant Soil 1989, 116, 177–182. [Google Scholar] [CrossRef]
- Yang, Q.; Xue, S.; Zhu, R.; Han, S.; Yang, C. Conservation Tillage Techniques for Two Crops within One Year in North China. Trans. Chin. Soc. Agric. Eng. 2007, 23, 32–39. [Google Scholar]
- Ministry of Agriculture of the People’s Republic of China. Bulletin of the Ministry of Agriculture of the People’s Republic of China; Announcement No. 2032; Ministry of Agriculture: Beijing, China, 2014. [Google Scholar]
- Fredrickson, D.; Shea, P. Effect of Soil pH on Degradation, Movement, and Plant Uptake of Chlorsulfuron. Weed Sci. 1986, 34, 328–332. [Google Scholar] [CrossRef]
- Hua, X.W.; Zhou, S.; Chen, M.G.; Wei, W.; Liu, M.; Lei, K.; Zhou, S.; Li, Y.H.; Wang, B.L.; Li, Z.M. Controllable Effect of Structural Modification of Sulfonylurea Herbicides on Soil Degradation. Chin. J. Chem. 2016, 34, 1135–1142. [Google Scholar] [CrossRef]
- Hua, X.W.; Chen, M.G.; Zhou, S.; Zhang, D.K.; Liu, M.; Zhou, S.; Liu, J.B.; Lei, K.; Song, H.B.; Li, Y.H.; et al. Research on Controllable Degradation of Sulfonylurea Herbicides. RSC Adv. 2016, 6, 23038–23047. [Google Scholar] [CrossRef]
- Meng, F.F.; Wu, L.; Gu, Y.C.; Zhou, S.; Li, Y.H.; Chen, M.G.; Zhou, S.; Zhao, Y.Y.; Ma, Y.; Li, Z.M. Research on the Controllable Degradation of N-methylamido and Dialkylamino Substituted at the 5th Position of the Benzene Ring in Chlorsulfuron in Acidic Soil. RSC Adv. 2020, 10, 17870–17880. [Google Scholar] [CrossRef] [PubMed]
- Thirunarayanan, K.; Zimdahl, R.; Smika, D. Chlorsulfuron Adsorption and Degradation in Soil. Weed Sci. 1985, 33, 558–563. [Google Scholar] [CrossRef]
- Zhou, S. The Research on Novel RyR Insecticides and Sulfonylurea Herbicides with Controllable Degradation. Ph.D. Thesis, Nankai University, Tianjin, China, 2018. (In Chinese). [Google Scholar]
- Zhou, S.; Hua, X.W.; Wei, W.; Chen, M.G.; Gu, Y.C.; Zhou, S.; Song, H.B.; Li, Z.M. Research on Controllable Alkaline Soil Degradation of 5-Substituted Chlorsulfuron. Chin. Chem. Lett. 2018, 29, 945–948. [Google Scholar] [CrossRef]
- Wu, L.; Gu, Y.-C.; Li, Y.-H.; Meng, F.-F.; Zhou, S.; Li, Z.-M. Degradation of 5-Dialkylamino Substituted Chlorsulfuron Derivatives in Alkaline Soil. Molecules 2022, 27, 1486. [Google Scholar] [CrossRef]
- GB/T 16631-2008; General Rules for High Performance Liquid Chromatography. Standardization Administration: Beijing, China, 2008.
- GB/T 31270.1-2014; Test Guidelines on Environmental Safety Assessment for Chemical Pesticides—Part 1: Transformation in Soils. Ministry of Agriculture: Beijing, China, 2014.
- Singles, S.K.; Dean, G.M.; Kirkpatrick, D.M.; Mayo, B.C.; Langford-Pollard, A.D.; Barefoot, A.C.; Bramble, F.Q. Fate and Behaviour of Flupyrsulfuron-Methyl in Soil and Aquatic Systems. Pest Manag. Sci. 1999, 55, 288–300. [Google Scholar] [CrossRef]
- Villaverde, J.; Kah, M.; Brown, C.-D. Adsorption and degradation of four acidic herbicides in soils from southern Spain. Pest Manag. Sci. 2008, 64, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Rouchaud, J.; Moulard, C.; Eelen, H.; Bulcke, R. Persistence of the sulfonylurea herbicide iodosulfuron-methyl in the soil of winter wheat crops. Toxicol. Environ. Chem. 2003, 85, 103–120. [Google Scholar] [CrossRef]
- Tang, M.Z.; Guo, Z.Y.; Yuan, M.; Yang, R.B. Degradation Dynamics of Iodosulfuron-Methyl-Sodium in Soils. J. Agro-Environ. Sci. 2005, 24, 724–727. [Google Scholar]
- Wu, W.Z.; Kong, D.Y.; He, J.; Kong, X.J.; Shan, Z.J. Degradation of Foramsulfuron in the Simulated Environment. Environ. Chem. 2016, 35, 439–444. [Google Scholar]
- Brigante, M.; Emmelin, C.; Previtera, L.; Baudot, R.; Chovelon, J.M. Abiotic Degradation of Iodosulfuron-Methyl-Ester in Aqueous Solution. J. Agric. Food Chem. 2005, 53, 5347–5352. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, E.O.; Markovic, S.; Gavrilovic, Z.; Dakic, P. The Broadleaf Weeds in Corn of Crop on Area Southwestern Banat and Their Control. Banat. J. Biotechnol. 2010, 1, 52–55. [Google Scholar]
- Nurse, R.E.; Hamill, A.S.; Swanton, C.J.; Tardif, F.J.; Sikkema, P.H. Weed Control and Yield Response to Foramsulfuron in Corn. Weed Technol. 2007, 21, 453–458. [Google Scholar] [CrossRef]


| Soils | Soil Texture | pH | Cation Exchange Capacity (cmol+·kg−1) | Organic Matter (g·kg−1) | Soil Separation (mm)/Mechanical Composition (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkaline soils | Loam | 8.39 | 7.30 | 19.4 | 1–2 | 0.5–1 | 0.025–0.5 | 0.05–0.02 | 0.02–0.002 | <0.002 | 0.25–0.05 | 2.0–0.05 | 0.05–0.002 |
| 0.795 | 2.46 | 2.33 | 7.90 | 28.6 | 28.2 | 29.7 | 35.3 | 36.5 | |||||
| Compound | HPLC Analysis Condition (Retention Time, Flow Rate, Mobile Phase (V:V)) | Extraction Solvent (V:V) | Additive Concentration (mg·kg−1) | Average Recovery Rate (%) | Coefficient of Variation RSD (%) |
|---|---|---|---|---|---|
| L101 | 12.32 min, 0.70 mL·min−1, CH3OH: H3PO4 (aq) (pH 3.0) = 64:36 | CH3COCH3:CH2Cl2:THF: MeOH:H3PO4 (aq) (pH 1.5) = 30:10:20:20:5 | 5 | 82.19 | 1.04 |
| 2 | 83.05 | 2.49 | |||
| 0.5 | 81.06 | 1.81 | |||
| L102 | 13.01 min, 0.70 mL·min−1, CH3OH: H3PO4 (aq) (pH 3.0) = 64:36 | CH3COCH3:CH2Cl2:THF: MeOH:H3PO4 (aq) (pH 1.5) = 30:10:10:20:10 | 5 | 86.56 | 1.10 |
| 2 | 84.17 | 0.72 | |||
| 0.5 | 91.33 | 1.80 | |||
| L103 | 12.06 min, 0.80 mL·min−1, CH3OH: H3PO4 (aq) (pH 3.0) = 75:25 | CH3COCH3:CH2Cl2:THF: MeOH:H3PO4 (aq) (pH 1.5) = 30:10:10:20:10 | 5 | 82.21 | 2.21 |
| 2 | 82.67 | 2.14 | |||
| 0.5 | 90.01 | 2.01 | |||
| L104 | 11.93 min, 0.80 mL·min−1, CH3OH:H3PO4 (aq) (pH 3.0) = 75:25 | CH3COCH3:CH2Cl2:THF: H3PO4 (aq) (pH 1.5) = 30:10:10:10 | 5 | 92.30 | 2.11 |
| 2 | 85.51 | 0.92 | |||
| 0.5 | 79.95 | 2.65 | |||
| L105 | 15.27 min, 0.80 mL·min−1, CH3OH: H3PO4 (aq) (pH 3.0) = 62:38 | CH3COCH3:CH2Cl2: H3PO4 (aq) (pH 1.5) = 40:5:5 | 5 | 85.10 | 1.74 |
| 2 | 86.91 | 0.68 | |||
| 0.5 | 90.14 | 1.59 | |||
| L106 | 14.48 min, 0.75 mL·min−1, CH3OH: H3PO4 (aq) (pH 3.0) = 75:25 | CH3COCH3:CH2Cl2:MeOH: H3PO4 (aq) (pH 1.5) = 30:10:30:10 | 5 | 82.40 | 1.37 |
| 2 | 82.62 | 0.99 | |||
| 0.5 | 74.46 | 2.99 | |||
| L107 | 12.32 min, 0.80 mL·min−1, CH3OH: H3PO4 (aq) (pH 3.0) = 62:38 | CH3COCH3:CH2Cl2:THF: MeOH:H3PO4 (aq) (pH 1.5) = 30:10:10:10:10 | 5 | 87.03 | 1.21 |
| 2 | 88.33 | 0.68 | |||
| 0.5 | 78.70 | 1.13 | |||
| Chlorsulfuron | 12.66 min, 0.70 mL·min−1, CH3OH: H3PO4 (aq) (pH 3.0) = 62:38 | CH3COCH3:CH2Cl2:H3PO4 (aq) (pH 1.5): = 40:5:10:10 | 5 | 73.54 | 1.09 |
| 2 | 73.53 | 2.40 | |||
| 0.5 | 81.09 | 1.16 |
| Compound | Kinetic Equations of Soil Degradation | Correlation Coefficient (R2) | DT50 (Days) |
|---|---|---|---|
| L101 | Ct = 4.5066e−0.3406t | 0.9888 | 2.04 |
| L102 | Ct = 4.5113e−0.0444t | 0.9922 | 15.61 |
| L103 | Ct = 4.9924e−0.0249t | 0.9960 | 27.84 |
| L104 | Ct = 4.7036e−0.0143t | 0.9983 | 48.47 |
| L105 | Ct =4.2712e−0.0119t | 0.9977 | 58.25 |
| L106 | Ct = 3.7330e−0.0101t | 0.9926 | 68.63 |
| L107 | Ct = 4.3097e−0.0081t | 0.9964 | 85.57 |
| Chlorsulfuron | Ct= 4.3067e−0.0044t | 0.9884 | 157.53 |
| Compd. | DT50 (Days) | |
|---|---|---|
| Acidic Soil (pH = 5.41) | Alkaline Soil (pH = 8.39) | |
| L101 | 10.76 | 2.04 |
| L102 | 32.54 | 15.61 |
| L103 | 14.78 | 27.84 |
| L104 | 7.89 | 48.47 |
| L105 | 9.40 | 58.25 |
| L106 | 14.62 | 68.63 |
| L107 | 11.16 | 85.57 |
| Chlorsulfuron | 12.91 | 157.53 |
| Compound | Concentration (g·ha−1) | Wheat (Xinong 529) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Emergence (22 Days after Treatment) | Post-Emergence (28 Days after Treatment) | ||||||||
| Fresh Weight g/10 Strains | Analysis of Variance a | Inhibition (%) | Fresh Weight g/10 Strains | Analysis of Variance a | Inhibition (%) | ||||
| 5% | 1% | 5% | 1% | ||||||
| 0 | 2.423 | ab | A | 2.998 | a | A | |||
| Chlorsulfuron | 30 | 2.411 | ab | A | 0 | 2.576 | ab | AB | 14.1 |
| 60 | 2.519 | a | A | 0.5 | 2.538 | abc | AB | 15.3 | |
| L101 | 30 | 2.396 | ab | A | 1.1 | 2.133 | bcd | ABC | 28.8 |
| 60 | 2.300 | ab | A | 5.1 | 2.117 | bcde | ABC | 29.4 | |
| Compound | Concentration (g·ha−1) | Wheat (Xinong 529) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Emergence (22 Days after Treatment) | Post-Emergence (28 Days after Treatment) | ||||||||
| Fresh Weight g/10 Strains | Analysis of Variance a | Inhibition (%) | Fresh Weight g/10 Strains | Analysis of Variance a | Inhibition (%) | ||||
| 5% | 1% | 5% | 1% | ||||||
| 0 | 2.405 | a | A | 3.286 | abc | ABC | |||
| Chlorsulfuron | 30 | 2.421 | a | A | 0 | 3.691 | bcde | ABC | 13.9 |
| 60 | 2.438 | a | A | 0 | 3.661 | bcde | BC | 14.6 | |
| L102 | 30 | 2.560 | a | A | 0 | 3.201 | de | BCD | 25.3 |
| 60 | 2.224 | a | A | 7.5 | 3.140 | de | BCD | 26.7 | |
| L103 | 30 | 2.404 | a | A | 0 | 4.471 | ab | AB | 0 |
| 60 | 2.450 | a | A | 0 | 5.058 | a | A | 0 | |
| L104 | 30 | 2.422 | a | A | 0 | 3.585 | bcde | BC | 16.4 |
| 60 | 2.585 | a | A | 0 | 3.523 | bcde | BC | 17.8 | |
| L105 | 30 | 2.589 | a | A | 0 | 4.120 | abcd | ABC | 3.9 |
| 60 | 2.483 | a | A | 0 | 3.794 | bcde | ABC | 11.5 | |
| L106 | 30 | 2.464 | a | A | 0 | 4.127 | abcd | ABC | 3.7 |
| 60 | 2.472 | a | A | 0 | 3.893 | bcde | ABC | 9.2 | |
| L107 | 30 | 2.480 | a | A | 0 | 4.043 | bcd | ABC | 5.7 |
| 60 | 2.449 | a | A | 0 | 3.945 | bcde | ABC | 7.9 | |
| Compound | Concentration (g·ha−1) | Corn (Xindan 66) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Emergence (16 Days after Treatment) | Post-Emergence (23 Days after Treatment) | ||||||||
| Fresh Weight g/5 Strains | Analysis of Variance a | Inhibition (%) | Fresh Weight g/5 Strains | Analysis of Variance a | Inhibition (%) | ||||
| 5% | 1% | 5% | 1% | ||||||
| 0 | 11.599 | a | AB | 9.214 | a | A | |||
| Chlorsulfuron | 30 | 7.813 | b | BC | 32.6 | 5.928 | bc | BCD | 35.7 |
| 60 | 4.463 | c | C | 61.5 | 4.771 | bcde | BCD | 48.2 | |
| L101 | 30 | 11.548 | a | AB | 0.4 | 5.146 | bcde | BCD | 44.1 |
| 60 | 10.949 | ab | AB | 5.6 | 4.291 | cde | BCD | 53.4 | |
| Compound | Concentration (g·ha−1) | Corn (Xindan 66) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Emergence (16 Days after Treatment) | Post-Emergence (23 Days after Treatment) | ||||||||
| Fresh Weight g/5 Strains | Analysis of Variance a | Inhibition (%) | Fresh Weight g/5 Strains | Analysis of Variance a | Inhibition (%) | ||||
| 5% | 1% | 5% | 1% | ||||||
| 0 | 8.302 | ab | AB | 14.413 | a | A | |||
| Chlorsulfuron | 30 | 1.982 | e | D | 76.1 | 7.883 | cd | B | 45.3 |
| 60 | 1.713 | e | D | 79.4 | 7.796 | cd | B | 45.9 | |
| L102 | 30 | 9.117 | a | A | 0 | 12.213 | abc | AB | 15.3 |
| 60 | 8.311 | ab | AB | 0 | 10.515 | abcd | AB | 27.0 | |
| L103 | 30 | 8.109 | abc | AB | 2.3 | 10.883 | abcd | AB | 24.5 |
| 60 | 7.605 | abc | AB | 8.4 | 10.190 | abcd | AB | 29.3 | |
| L104 | 30 | 8.370 | ab | AB | 0 | 13.440 | ab | AB | 6.7 |
| 60 | 8.336 | ab | AB | 0 | 12.423 | abc | AB | 13.8 | |
| L105 | 30 | 7.963 | abc | AB | 4.1 | 13.708 | ab | AB | 4.9 |
| 60 | 7.923 | abc | AB | 4.6 | 13.500 | ab | AB | 6.3 | |
| L106 | 30 | 8.443 | ab | AB | 0 | 13.750 | ab | AB | 4.6 |
| 60 | 8.508 | ab | AB | 0 | 13.700 | ab | AB | 4.9 | |
| L107 | 30 | 8.335 | ab | AB | 0 | 14.250 | a | A | 1.1 |
| 60 | 8.327 | ab | AB | 0 | 12.668 | ab | AB | 12.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Hua, X.-W.; Li, Y.-H.; Wang, Z.-W.; Zhou, S.; Li, Z.-M. Alkaline Soil Degradation and Crop Safety of 5-Substituted Chlorsulfuron Derivatives. Molecules 2022, 27, 3318. https://doi.org/10.3390/molecules27103318
Wu L, Hua X-W, Li Y-H, Wang Z-W, Zhou S, Li Z-M. Alkaline Soil Degradation and Crop Safety of 5-Substituted Chlorsulfuron Derivatives. Molecules. 2022; 27(10):3318. https://doi.org/10.3390/molecules27103318
Chicago/Turabian StyleWu, Lei, Xue-Wen Hua, Yong-Hong Li, Zhong-Wen Wang, Sha Zhou, and Zheng-Ming Li. 2022. "Alkaline Soil Degradation and Crop Safety of 5-Substituted Chlorsulfuron Derivatives" Molecules 27, no. 10: 3318. https://doi.org/10.3390/molecules27103318
APA StyleWu, L., Hua, X.-W., Li, Y.-H., Wang, Z.-W., Zhou, S., & Li, Z.-M. (2022). Alkaline Soil Degradation and Crop Safety of 5-Substituted Chlorsulfuron Derivatives. Molecules, 27(10), 3318. https://doi.org/10.3390/molecules27103318







