Preparation of Cellulose Nanocrystals from Jujube Cores by Fractional Purification
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition and Purification
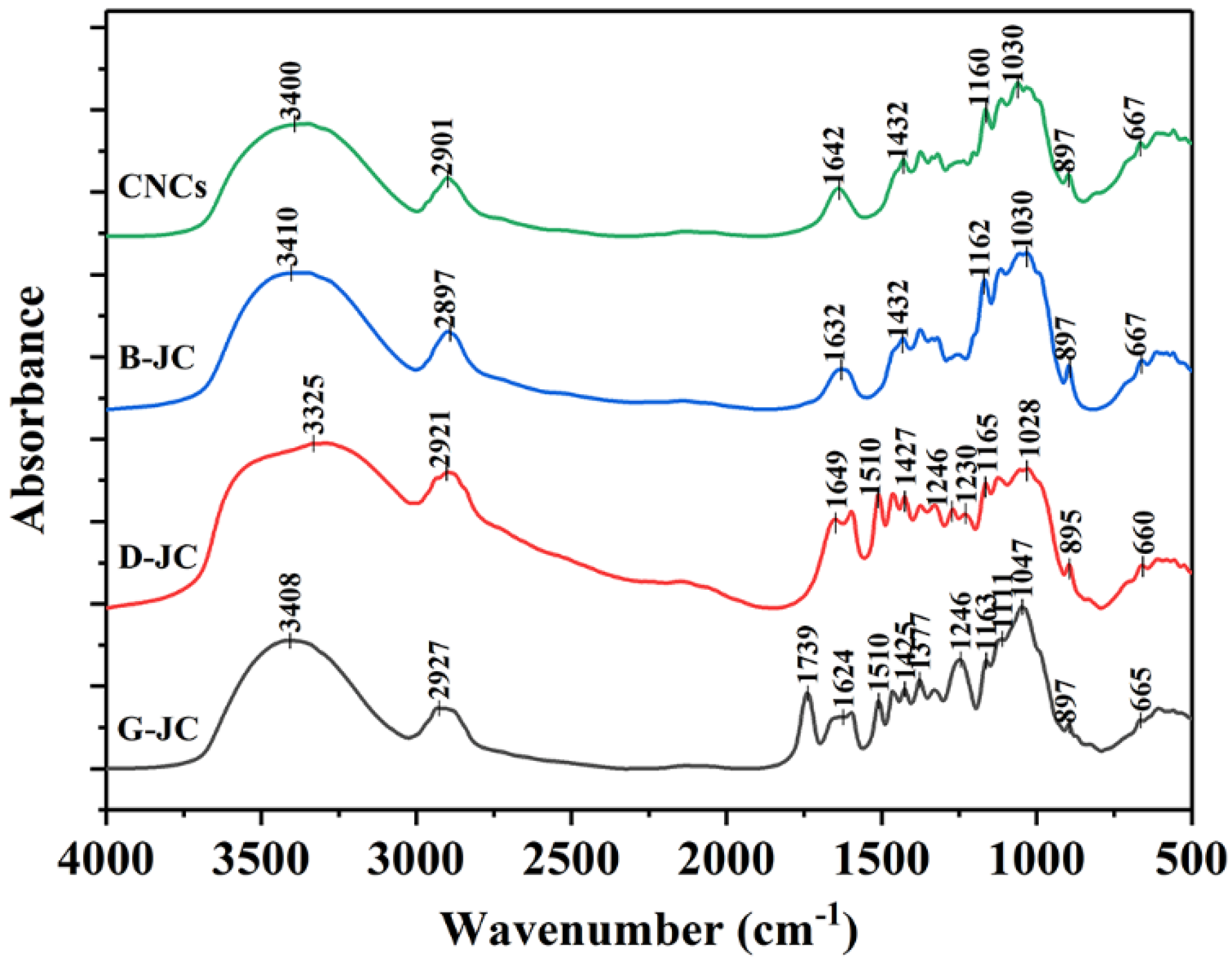
2.2. FT-IR Spectroscopic Analysis
| Band Range (cm−1) | Changes in Functional Components | References | |||
|---|---|---|---|---|---|
| G-JC | D-JC | B-JC | CNCs | ||
| 3408 | 3325 | 3410 | 3400 | Stretch vibration of O–H group in the cellulose molecule | [35,37,38] |
| 2927 | 2921 | 2897 | 2901 | Stretch vibration of C–H group in the cellulose molecule and asymmetry stretch vibration of CNCs | [5,35,36,39] |
| 1739 | - | - | - | Stretch vibration in the CC-’s in-plane of the aromatic ring in lignin, and the C=O vibration in the carboxylic acid bond of lignin or the carbonyl group in the ester group in hemicellulose | [33,34,35,39] |
| 1624 | 1649 | 1632 | 1642 | O–H bending vibrations of strong interaction between cellulose and water | [35,39,41] |
| 1510 | 1510 | - | - | C=C vibration of lignin | [5,36,39] |
| 1425 | 1427 | 1432 | 1432 | C–H vibrations in cellulose | [39,41] |
| 1246 | 1246 | - | - | C–O–C stretching vibration of acetyl linkage in lignin, hemicelluloses | [5,36,39] |
| 1163 | 1165 | 1162 | 1160 | C–O–C stretching vibration of β-glycosidic linkages between glucose units in cellulose | [38,39,42] |
| 1047 | 1028 | 1030 | 1030 | C–O–C pyranose ring in cellulose | [5,39,41] |
| 897 | 895 | 897 | 897 | C–H swing in the β-glycosidic linkages of glucose ring | [5,35,39,40] |
| 665 | 660 | 667 | 667 | ||
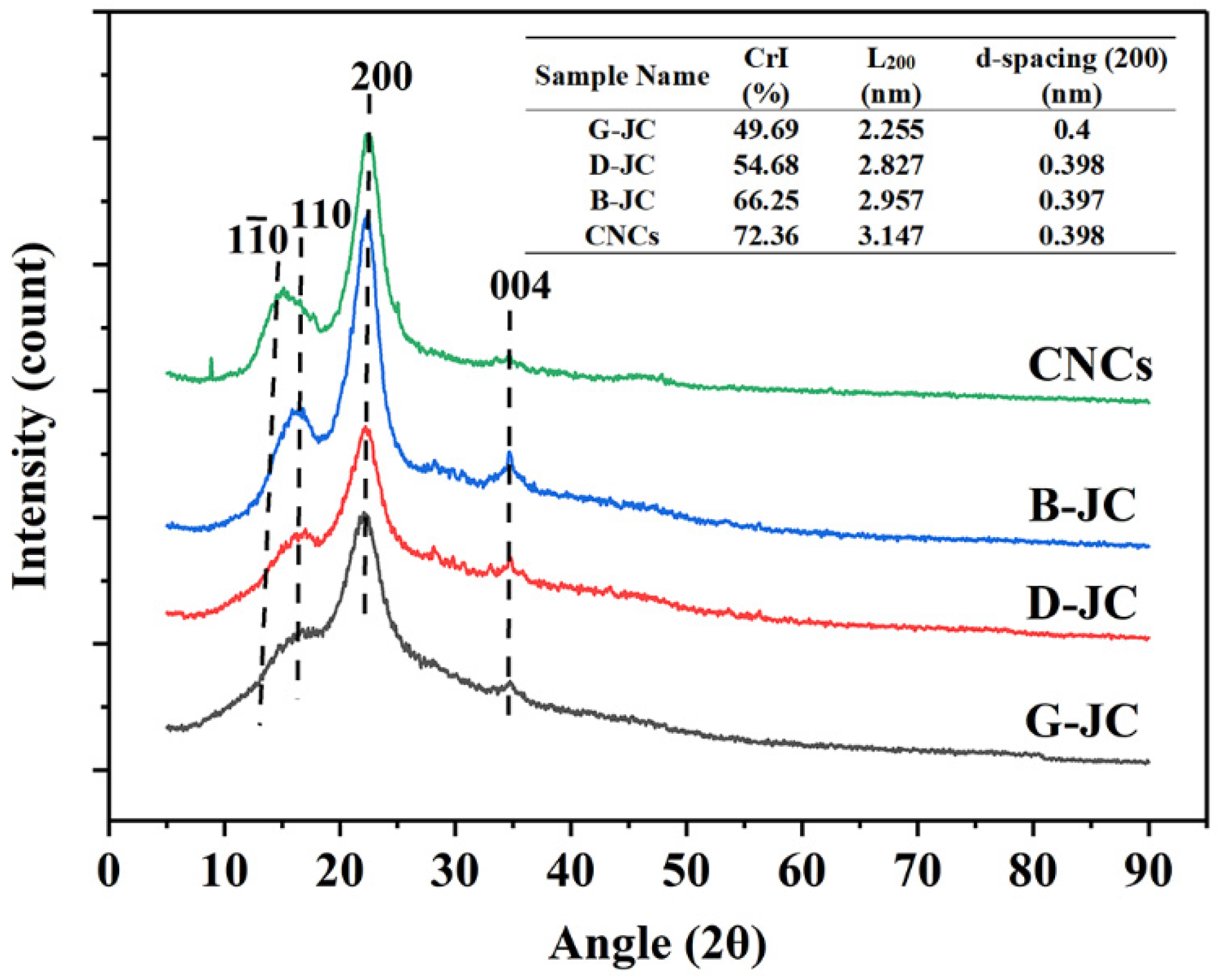
2.3. X-ray Diffraction Analysis
2.4. Thermogravimetric Analysis
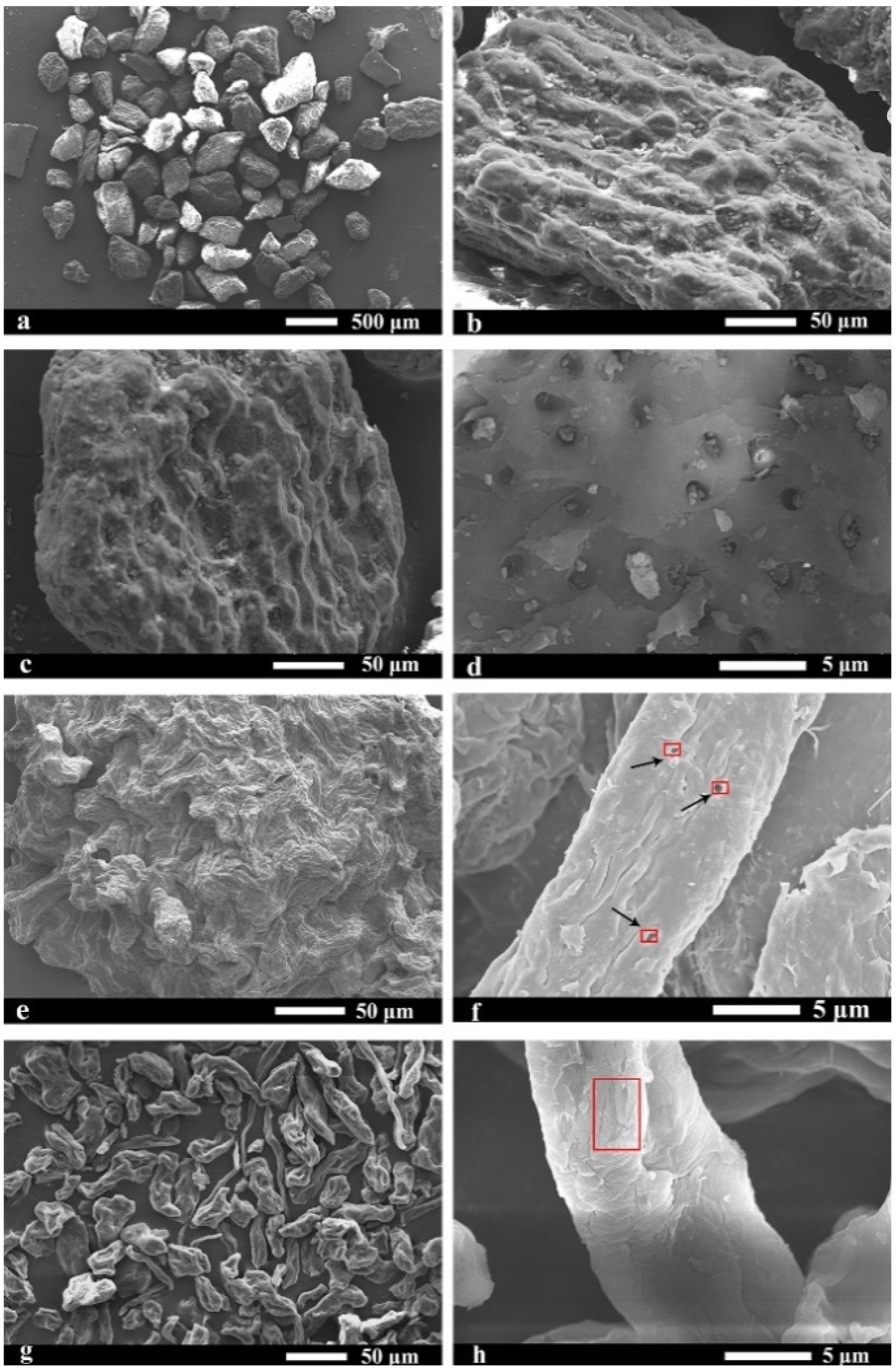
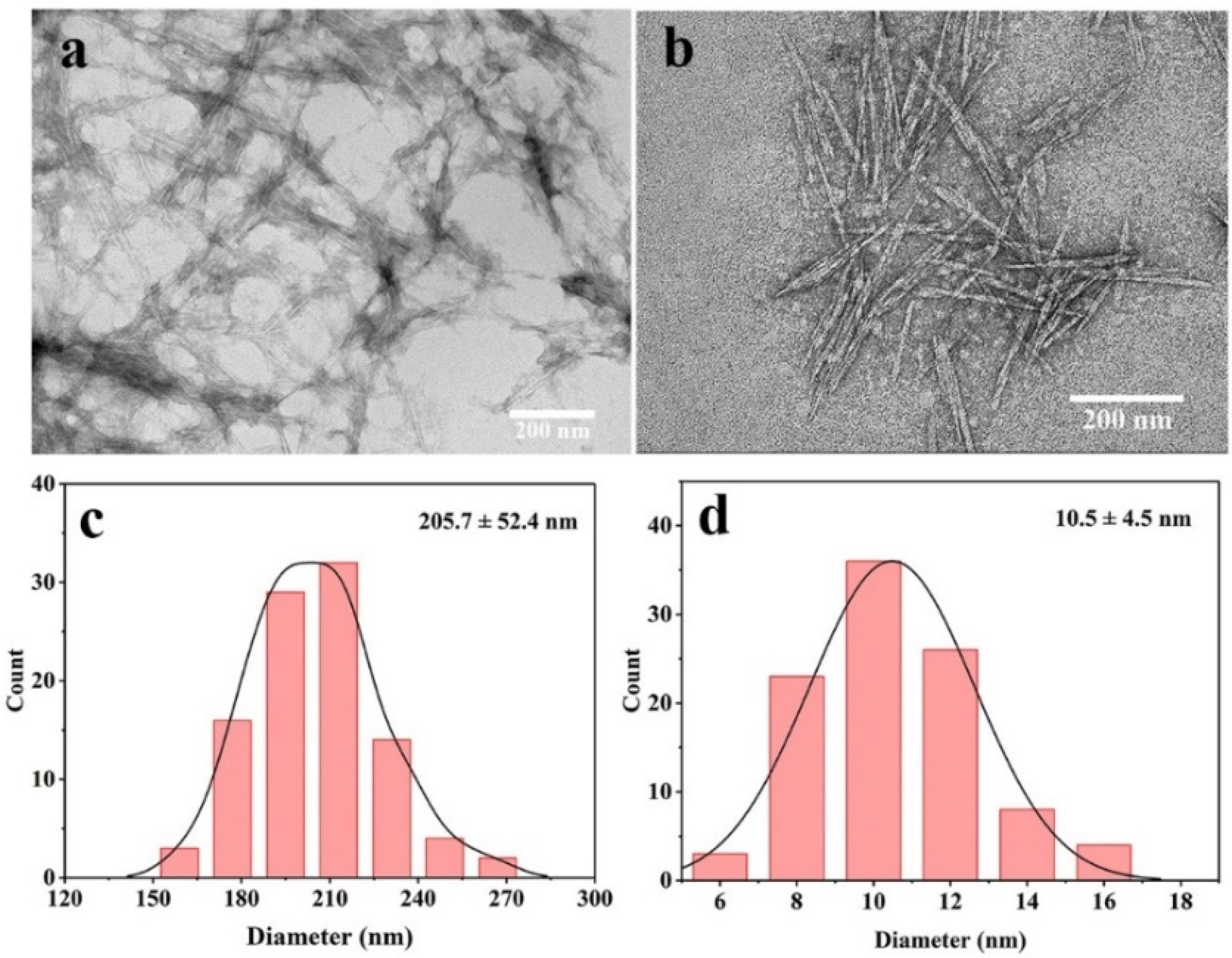
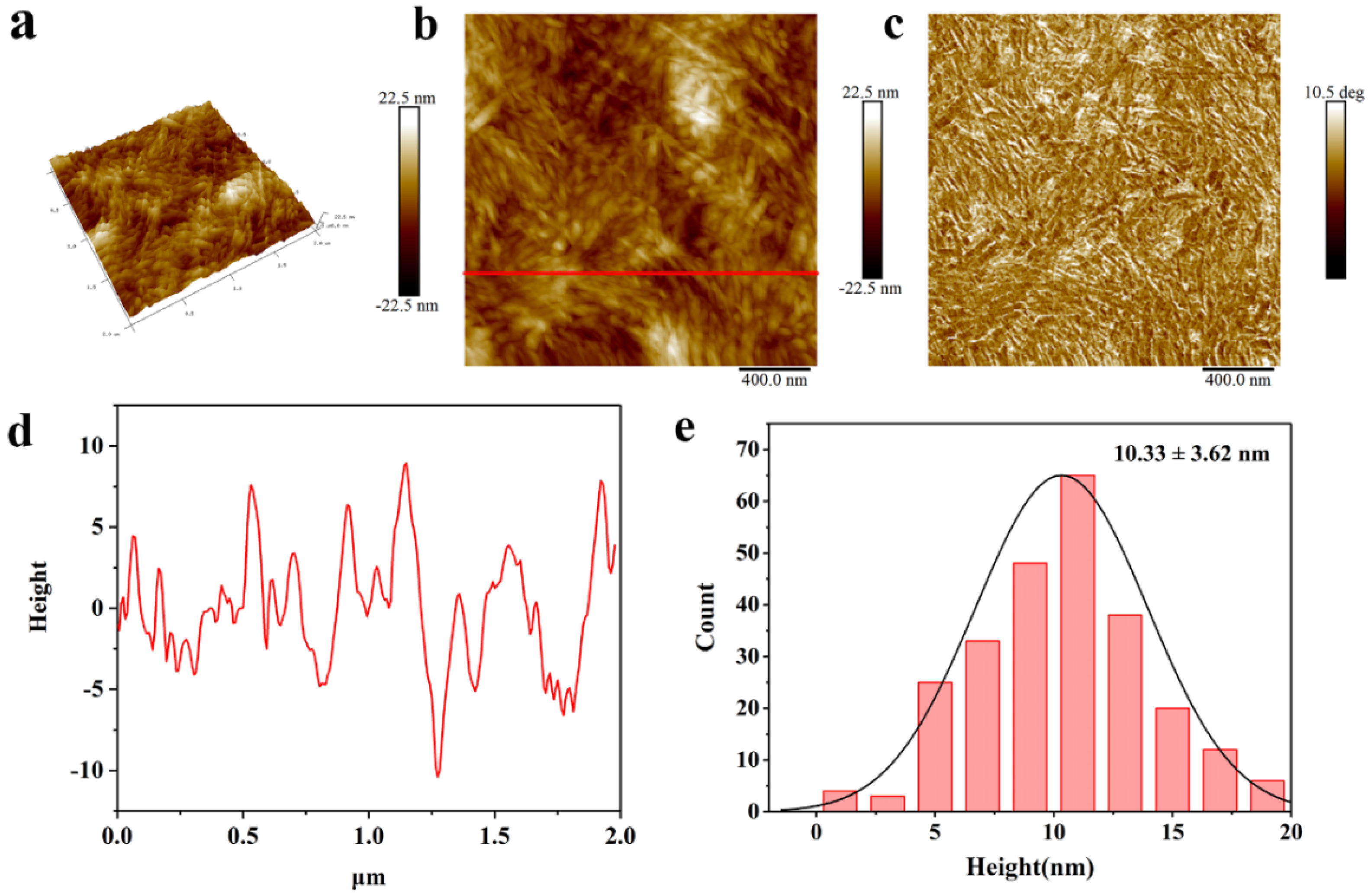
2.5. Morphological Analysis
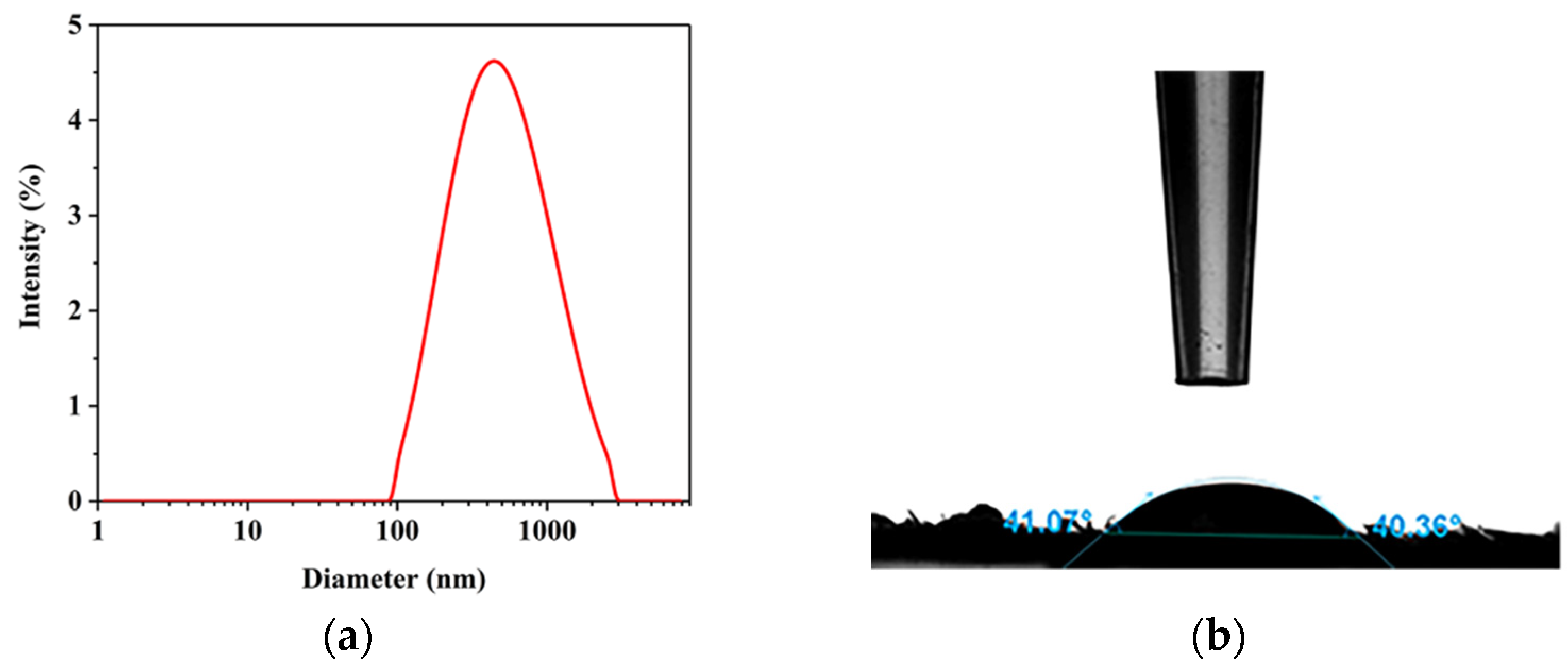
2.6. Particle Size and Zeta (ζ) Potential Measurements
2.7. Water Contact Angle (WCA)
3. Discussion
4. Materials and Methods
4.1. Chemicals and Raw Materials
4.2. Fractionation and Purification of Cellulose from Jujube Cores
4.3. Preparation of Cellulose Nanocrystals
4.4. Characterizations
4.4.1. Chemical Composition
4.4.2. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
4.4.3. X-ray Diffraction (XRD) Analysis
4.4.4. Thermogravimetric Analysis (TGA)
4.4.5. Field Emission Scanning Electron Microscopy (FE-SEM)
4.4.6. Transmission Electron Microscopy (TEM)
4.4.7. Atomic Force Microscopy (AFM)
4.4.8. Particle Size and Zeta Potential Measurements
4.4.9. Water Contact Angle (WCA)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez-Jiménez, J.; Viuda-Martos, M. Introduction to the special issue Byproducts from agri-food industry: New strategies for their revalorization. Food Res. Int. 2015, 73, 1–2. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Barber, X.; Fernandez-Lopez, J.; Perez-Alvarez, J.A.; Viuda-Martos, M. Bioaccessibility, changes in the antioxidant potential and colonic fermentation of date pits and apple bagasse flours obtained from co-products during simulated in vitro gastrointestinal digestion. Food Res. Int. 2015, 78, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Schieber, A. Editorial. Food Res. Int. 2014, 65, 299–300. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Karim, N.; Shishir, M.R.I.; Bao, T.; Lu, Y.; Chen, W. Jujube fruit: A potential nutritious fruit for the development of functional food products. J. Funct. Foods 2020, 75, 104205. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y.; Judeh, A.A.; Hakeem, A.S.; Ul-Hamid, A.; Umar, Y.; Ahmad, A. Isolation and characterization of microcrystalline cellulose from date seeds (Phoenix dactylifera L.). Int. J. Biol. Macromol. 2020, 155, 730–739. [Google Scholar] [CrossRef]
- Reddy, M.C.; Sivaramakrishna, L.; Reddy, A.V. The use of an agricultural waste material, Jujuba seeds for the removal of anionic dye (Congo red) from aqueous medium. J. Hazard. Mater. 2012, 203–204, 118–127. [Google Scholar] [CrossRef]
- Abu-Jrai, A.M.; Jamil, F.; Al-Muhtaseb, A.a.H.; Baawain, M.; Al-Haj, L.; Al-Hinai, M.; Al-Abri, M.; Rafiq, S. Valorization of waste Date pits biomass for biodiesel production in presence of green carbon catalyst. Energy Convers. Manag. 2017, 135, 236–243. [Google Scholar] [CrossRef]
- Al-Khalili, M.; Al-Habsi, N.; Al-Alawi, A.; Al-Subhi, L.; Zar Myint, M.T.; Al-Abri, M.; Waly, M.I.; Al-Harthi, S.; Al-Mamun, A.; Rahman, M.S. Structural characteristics of alkaline treated fibers from date-pits: Residual and precipitated fibers at different pH. Bioact. Carbohydr. Diet. Fibre 2021, 25, 100251. [Google Scholar] [CrossRef]
- Banat, F.; Al-Asheh, S.; Al-Makhadmeh, L. Evaluation of the use of raw and activated date pits as potential adsorbents for dye containing waters. Process Biochem. 2003, 39, 193–202. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Govindan, B.; Banat, F.; Sagadevan, V.; Purushothaman, M.; Show, P.L. Date pits activated carbon for divalent lead ions removal. J. Biosci. Bioeng. 2019, 128, 88–97. [Google Scholar] [CrossRef]
- Al-Muhtaseb, A.a.H.; Jamil, F.; Al-Haj, L.; Al-Hinai, M.A.; Baawain, M.; Myint, M.T.Z.; Rooney, D. Efficient utilization of waste date pits for the synthesis of green diesel and jet fuel fractions. Energy Convers. Manag. 2016, 127, 226–232. [Google Scholar] [CrossRef]
- Al-Farsi, M.A.; Lee, C.Y. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008, 108, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Akram, M.; Al-Zuhair, S.; Elnajjar, E.; Munir, M.T. Subcritical water extraction of phenolics, antioxidants and dietary fibres from waste date pits. J. Environ. Chem. Eng. 2020, 8, 104490. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, Z.; Lin, X.; Ren, Z.; Li, B.; Zhang, Y. Preparation and characterization of microcrystalline cellulose (MCC) from tea waste. Carbohydr. Polym. 2018, 184, 164–170. [Google Scholar] [CrossRef]
- Lu, H.; Gui, Y.; Zheng, L.; Liu, X. Morphological, crystalline, thermal and physicochemical properties of cellulose nanocrystals obtained from sweet potato residue. Food Res. Int. 2013, 50, 121–128. [Google Scholar] [CrossRef]
- Dalli, S.S.; Uprety, B.K.; Samavi, M.; Singh, R.; Rakshit, S.K. Nanocrystalline Cellulose: Production and Applications. In Exploring the Realms of Nature for Nanosynthesis; Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2018; pp. 385–405. [Google Scholar]
- He, C.; Huang, J.; Li, S.; Meng, K.; Zhang, L.; Chen, Z.; Lai, Y. Mechanically Resistant and Sustainable Cellulose-Based Composite Aerogels with Excellent Flame Retardant, Sound-Absorption, and Superantiwetting Ability for Advanced Engineering Materials. ACS Sustain. Chem. Eng. 2017, 6, 927–936. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Chen, G. A comparative study on the starch-based biocomposite films reinforced by nanocellulose prepared from different non-wood fibers. Cellulose 2019, 26, 2425–2435. [Google Scholar] [CrossRef]
- Mondal, S. Preparation, properties and applications of nanocellulosic materials. Carbohydr. Polym. 2017, 163, 301–316. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, S.; Ma, L.; Yu, Y.; Dai, H.; Zhang, Y. Extraction and comparison of cellulose nanocrystals from lemon (Citrus limon) seeds using sulfuric acid hydrolysis and oxidation methods. Carbohydr. Polym. 2020, 238, 116180. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R. Isolation and characterization of nanocrystalline cellulose from sugar palm fibres (Arenga pinnata). Carbohydr. Polym. 2018, 181, 1038–1051. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Neto, W.P.F.; Silverio, H.A.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue-Soy hulls. Ind. Crops Prod. 2013, 42, 480–488. [Google Scholar] [CrossRef]
- Siqueira, G.; Tapin-Lingua, S.; Bras, J.; Perez, D.D.; Dufresne, A. Morphological investigation of nanoparticles obtained from combined mechanical shearing, and enzymatic and acid hydrolysis of sisal fibers. Cellulose 2010, 17, 1147–1158. [Google Scholar] [CrossRef]
- Hemmati, F.; Jafari, S.M.; Kashaninejad, M.; Barani Motlagh, M. Synthesis and characterization of cellulose nanocrystals derived from walnut shell agricultural residues. Int. J. Biol. Macromol. 2018, 120, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Lawton, D.; Thompson, M.R.; Liu, Q. Biocomposites reinforced with cellulose nanocrystals derived from potato peel waste. Carbohydr. Polym. 2012, 90, 709–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Chiralt Boix, A. Isolation and characterisation of microcrystalline cellulose and cellulose nanocrystals from coffee husk and comparative study with rice husk. Carbohydr. Polym. 2018, 191, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Cherhal, F.; Cousin, F.; Capron, I. Structural Description of the Interface of Pickering Emulsions Stabilized by Cellulose Nanocrystals. Biomacromolecules 2016, 17, 496–502. [Google Scholar] [CrossRef]
- Tang, M.; Zhu, Z.; Yang, K.; Yang, P.; Dong, Y.; Wu, Y.; Chen, M.; Zhou, X. Cellulose nanocrystals concentration and oil-water ratio for solid-liquid controllable emulsion polymerization. Int. J. Biol. Macromol. 2021, 191, 414–421. [Google Scholar] [CrossRef]
- He, X.; Lu, W.; Sun, C.; Khalesi, H.; Mata, A.; Andaleeb, R.; Fang, Y. Cellulose and cellulose derivatives: Different colloidal states and food-related applications. Carbohydr. Polym. 2021, 255, 117334. [Google Scholar] [CrossRef]
- Ghori, W.; Saba, N.; Jawaid, M.; Asim, M. A review on date palm (Phoenix dactylifera) fibers and its polymer composites. IOP Conf. Ser. Mater. Sci. Eng. 2018, 368, 012009. [Google Scholar] [CrossRef] [Green Version]
- Wahib, S.A.; Da’na, D.A.; Al-Ghouti, M.A. Insight into the extraction and characterization of cellulose nanocrystals from date pits. Arab. J. Chem. 2022, 15, 103650. [Google Scholar] [CrossRef]
- Balaji, A.N.; Nagarajan, K.J. Characterization of alkali treated and untreated new cellulosic fiber from Saharan aloe vera cactus leaves. Carbohydr. Polym. 2017, 174, 200–208. [Google Scholar] [CrossRef]
- Sankhla, S.; Sardar, H.H.; Neogi, S. Greener extraction of highly crystalline and thermally stable cellulose micro-fibers from sugarcane bagasse for cellulose nano-fibrils preparation. Carbohydr. Polym. 2021, 251, 117030. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Ou, S.; Huang, Y.; Huang, H. Utilization of pineapple peel for production of nanocellulose and film application. Cellulose 2018, 25, 1743–1756. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Martins, J.T.; Vicente, A.A.; Menegalli, F.C. Cellulose nanofibers produced from banana peel by chemical and mechanical treatments: Characterization and cytotoxicity assessment. Food Hydrocoll. 2018, 75, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Rosa, S.M.L.; Rehman, N.; de Miranda, M.I.G.; Nachtigall, S.M.B.; Bica, C.I.D. Chlorine-free extraction of cellulose from rice husk and whisker isolation. Carbohydr. Polym. 2012, 87, 1131–1138. [Google Scholar] [CrossRef] [Green Version]
- Pandi, N.; Sonawane, S.H.; Anand Kishore, K. Synthesis of cellulose nanocrystals (CNCs) from cotton using ultrasound-assisted acid hydrolysis. Ultrason. Sonochem. 2021, 70, 105353. [Google Scholar] [CrossRef]
- Sai Prasanna, N.; Mitra, J. Isolation and characterization of cellulose nanocrystals from Cucumis sativus peels. Carbohydr. Polym. 2020, 247, 116706. [Google Scholar] [CrossRef]
- Feng, X.; Meng, X.; Zhao, J.; Miao, M.; Shi, L.; Zhang, S.; Fang, J. Extraction and preparation of cellulose nanocrystals from dealginate kelp residue: Structures and morphological characterization. Cellulose 2015, 22, 1763–1772. [Google Scholar] [CrossRef]
- Bano, S.; Negi, Y.S. Studies on cellulose nanocrystals isolated from groundnut shells. Carbohydr. Polym. 2017, 157, 1041–1049. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, H.J. Fourier Transform Infrared Spectroscopy (FT-IR) and Simple Algorithm Analysis for Rapid and Non-Destructive Assessment of Developmental Cotton Fibers. Sensors 2017, 17, 1469. [Google Scholar] [CrossRef] [PubMed]
- Deepa, B.; Abraham, E.; Cordeiro, N.; Mozetic, M.; Mathew, A.P.; Oksman, K.; Faria, M.; Thomas, S.; Pothan, L.A. Utilization of various lignocellulosic biomass for the production of nanocellulose: A comparative study. Cellulose 2015, 22, 1075–1090. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Ibeta and cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Benincasa, P.; Balestra, G.M.; Luzi, F.; Mazzaglia, A.; Del Buono, D.; Puglia, D.; Torre, L. Revalorization of barley straw and husk as precursors for cellulose nanocrystals extraction and their effect on PVA_CH nanocomposites. Ind. Crops Prod. 2016, 92, 201–217. [Google Scholar] [CrossRef]
- Perumal, A.B.; Nambiar, R.B.; Sellamuthu, P.S.; Sadiku, E.R.; Li, X.; He, Y. Extraction of cellulose nanocrystals from areca waste and its application in eco-friendly biocomposite film. Chemosphere 2021, 287, 132084. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.-J.; Eom, S.H.; Wada, M. Thermal decomposition of native cellulose: Influence on crystallite size. Polym. Degrad. Stab. 2010, 95, 778–781. [Google Scholar] [CrossRef]
- Kusmono; Listyanda, R.F.; Wildan, M.W.; Ilman, M.N. Preparation and characterization of cellulose nanocrystal extracted from ramie fibers by sulfuric acid hydrolysis. Heliyon 2020, 6, e05486. [Google Scholar] [CrossRef]
- Wijaya, C.J.; Saputra, S.N.; Soetaredjo, F.E.; Putro, J.N.; Lin, C.X.; Kurniawan, A.; Ju, Y.H.; Ismadji, S. Cellulose nanocrystals from passion fruit peels waste as antibiotic drug carrier. Carbohydr. Polym. 2017, 175, 370–376. [Google Scholar] [CrossRef] [Green Version]
- Evans, S.K.; Wesley, O.N.; Nathan, O.; Moloto, M.J. Chemically purified cellulose and its nanocrystals from sugarcane baggase: Isolation and characterization. Heliyon 2019, 5, e02635. [Google Scholar] [CrossRef] [Green Version]
- Abraham, E.; Deepa, B.; Pothan, L.A.; Jacob, M.; Thomas, S.; Cvelbar, U.; Anandjiwala, R. Extraction of nanocellulose fibrils from lignocellulosic fibres: A novel approach. Carbohydr. Polym. 2011, 86, 1468–1475. [Google Scholar] [CrossRef]
- He, H.; An, F.; Wang, Y.; Wu, W.; Huang, Z.; Song, H. Effects of pretreatment, NaOH concentration, and extraction temperature on the cellulose from Lophatherum gracile Brongn. Int. J. Biol. Macromol. 2021, 190, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Kontturi, E.; Laaksonen, P.; Linder, M.B.; Nonappa; Groschel, A.H.; Rojas, O.J.; Ikkala, O. Advanced Materials through Assembly of Nanocelluloses. Adv. Mater. 2018, 30, e1703779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Achaby, M.; El Miri, N.; Aboulkas, A.; Zahouily, M.; Bilal, E.; Barakat, A.; Solhy, A. Processing and properties of eco-friendly bio-nanocomposite films filled with cellulose nanocrystals from sugarcane bagasse. Int. J. Biol. Macromol. 2017, 96, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Peresin, M.S.; Habibi, Y.; Zoppe, J.O.; Pawlak, J.J.; Rojas, O.J. Nanofiber Composites of Polyvinyl Alcohol and Cellulose Nanocrystals: Manufacture and Characterization. Biomacromolecules 2010, 11, 674–681. [Google Scholar] [CrossRef]
- Kassab, Z.; Abdellaoui, Y.; Salim, M.H.; Bouhfid, R.; Qaiss, A.E.K.; El Achaby, M. Micro- and nano-celluloses derived from hemp stalks and their effect as polymer reinforcing materials. Carbohydr. Polym. 2020, 245, 116506. [Google Scholar] [CrossRef]
- Naduparambath, S.; Jinitha, T.V.; Shaniba, V.; Sreejith, M.P.; Balan, A.K.; Purushothaman, E. Isolation and characterisation of cellulose nanocrystals from sago seed shells. Carbohydr. Polym. 2018, 180, 13–20. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, N.; Carlsson, D.O.; Hong, J.; Larsson, R.; Fellstrom, B.; Nyholm, L.; Stromme, M.; Mihranyan, A. Haemocompatibility and ion exchange capability of nanocellulose polypyrrole membranes intended for blood purification. J. R. Soc. Interface 2012, 9, 1943–1955. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, J.P.; Bruni, G.P.; El Halal, S.L.M.; Bertoldi, F.C.; Dias, A.R.G.; Zavareze, E.D. Cellulose nanocrystals from rice and oat husks and their application in aerogels for food packaging. Int. J. Biol. Macromol. 2019, 124, 175–184. [Google Scholar] [CrossRef]
- Pirich, C.L.; Picheth, G.F.; Machado, J.P.E.; Sakakibara, C.N.; Martin, A.A.; de Freitas, R.A.; Sierakowski, M.R. Influence of mechanical pretreatment to isolate cellulose nanocrystals by sulfuric acid hydrolysis. Int. J. Biol. Macromol. 2019, 130, 622–626. [Google Scholar] [CrossRef]
- Bruel, C.; Queffeulou, S.; Carreau, P.J.; Tavares, J.R.; Heuzey, M.C. Orienting Cellulose Nanocrystal Functionalities Tunes the Wettability of Water-Cast Films. Langmuir 2020, 36, 12179–12189. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, G.; Zhang, H.; Zhao, C.; Yang, F. Improvement of antifouling performances for modified PVDF ultrafiltration membrane with hydrophilic cellulose nanocrystal. Appl. Surf. Sci. 2018, 440, 1091–1100. [Google Scholar] [CrossRef]


| Sample | Step 1 (Evaporation of Water) | Step 2 (Degradation of Cellulose Chain) | Step 3 (Degradation of Carbonic Residue) | Char Yield (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TOn (°C) | Tmax (°C) | WL (%) | TOn (°C) | Tmax (°C) | WL (%) | TOn (°C) | Tmax (°C) | WL (%) | ||
| G-JC | 30 | 79 | 8.13 | 222 | 298 | 30.91 | 330 | 360 | 40.34 | 20.61 |
| D-JC | 30 | 80 | 8.48 | 221 | 328 | 62.25 | 352 | - | - | 29.27 |
| B-JC | 30 | 80 | 7.56 | 213 | 333 | 70.67 | 359 | - | - | 21.76 |
| CNCs | 30 | 72 | 8.03 | 209 | 275 | 72.84 | 365 | - | - | 18.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Le, H.; Guo, Y.; Zhao, Y.; Deng, X.; Zhang, J.; Zhang, L. Preparation of Cellulose Nanocrystals from Jujube Cores by Fractional Purification. Molecules 2022, 27, 3236. https://doi.org/10.3390/molecules27103236
Wang X, Le H, Guo Y, Zhao Y, Deng X, Zhang J, Zhang L. Preparation of Cellulose Nanocrystals from Jujube Cores by Fractional Purification. Molecules. 2022; 27(10):3236. https://doi.org/10.3390/molecules27103236
Chicago/Turabian StyleWang, Xiaorui, Hao Le, Yanmei Guo, Yunfeng Zhao, Xiaorong Deng, Jian Zhang, and Lianfu Zhang. 2022. "Preparation of Cellulose Nanocrystals from Jujube Cores by Fractional Purification" Molecules 27, no. 10: 3236. https://doi.org/10.3390/molecules27103236
APA StyleWang, X., Le, H., Guo, Y., Zhao, Y., Deng, X., Zhang, J., & Zhang, L. (2022). Preparation of Cellulose Nanocrystals from Jujube Cores by Fractional Purification. Molecules, 27(10), 3236. https://doi.org/10.3390/molecules27103236







