UPLC-G2Si-HDMS Untargeted Metabolomics for Identification of Yunnan Baiyao’s Metabolic Target in Promoting Blood Circulation and Removing Blood Stasis
Abstract
1. Introduction
2. Materials and Method
2.1. Drugs and Chemical Reagents
2.2. Animals and Experimental Design
2.3. Hemorheology, Coagulation Function, and Histopathology Detection Methods
2.4. Urine Collection and Preparation
2.5. Metabolomics Analysis
2.6. Multivariate Statistical Analysis
2.7. Metabolite Identification
2.8. Construction and Analysis of Biological Network of Key Biomarkers
2.9. Statistical Analysis
3. Results
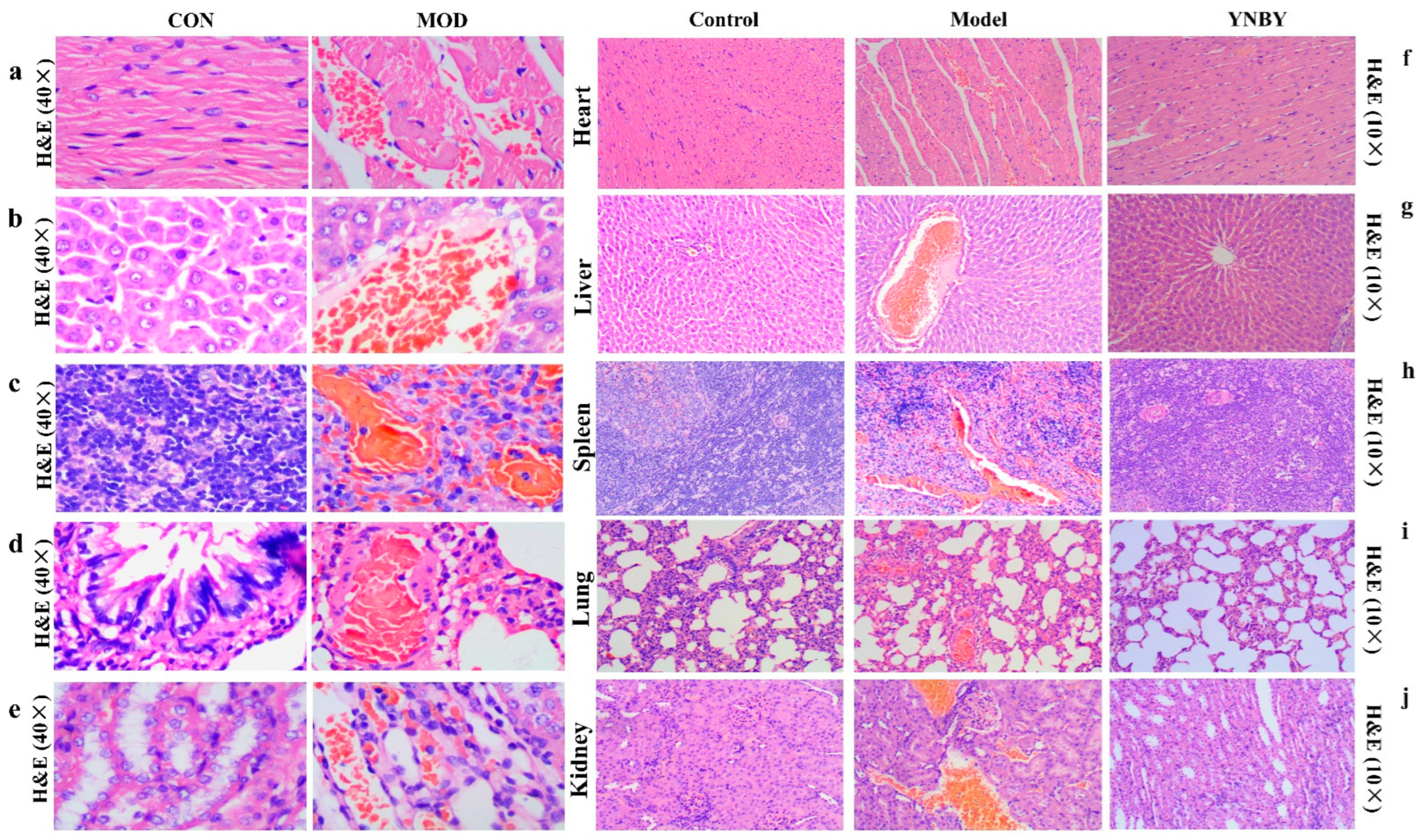
3.1. Hemorheology, Blood Coagulation Function Test, and Histopathology Test
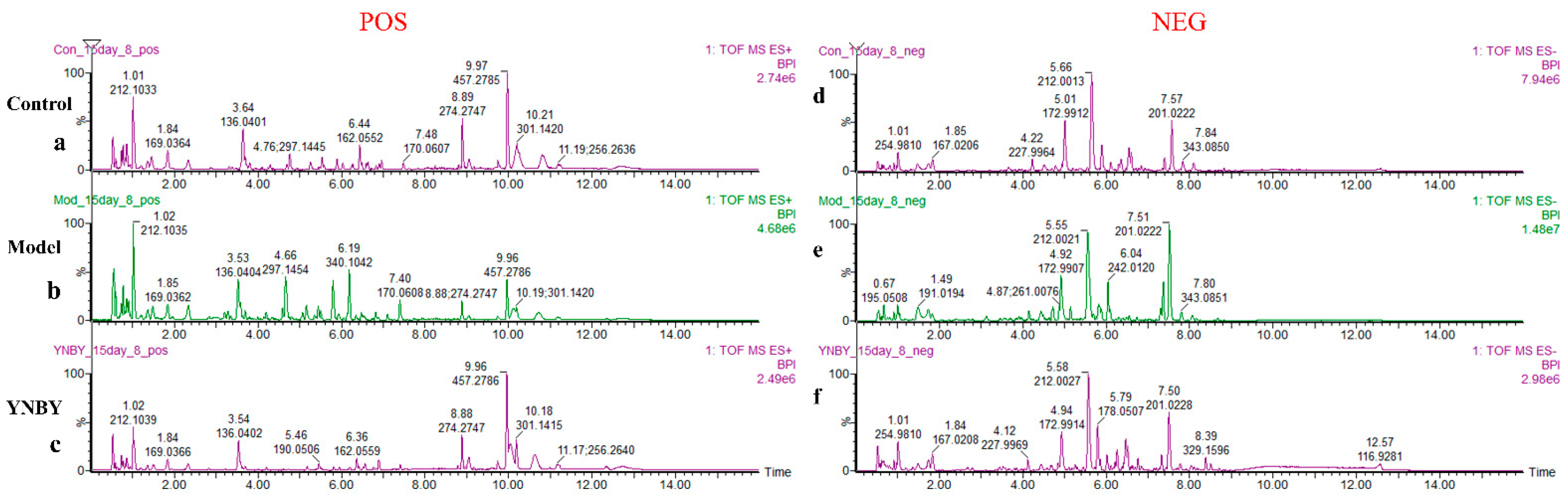
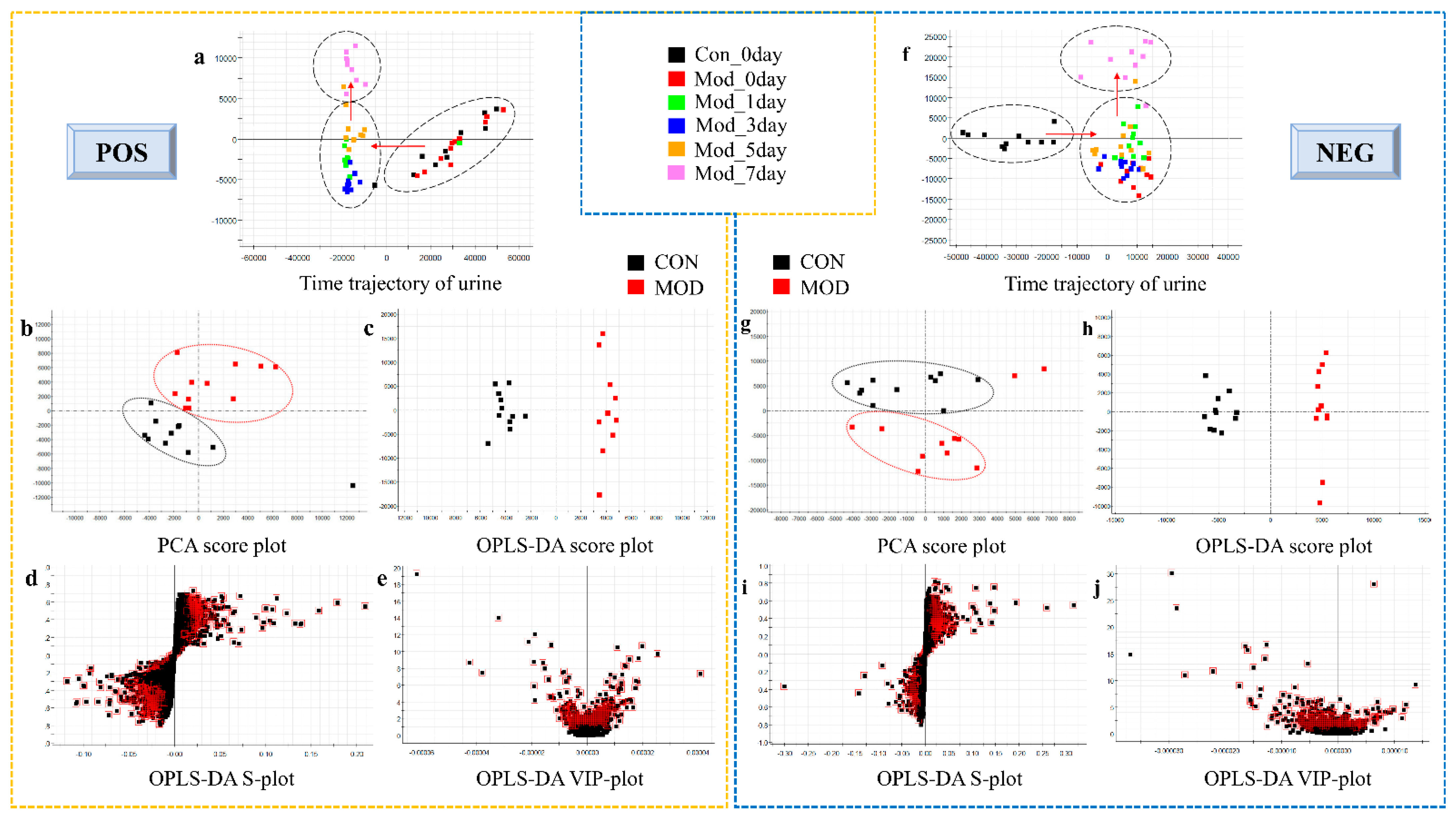
3.2. Metabolite Profile Analysis and Biomarker Identification
3.3. Analysis of Related Metabolic Pathways of Serum Biomarkers
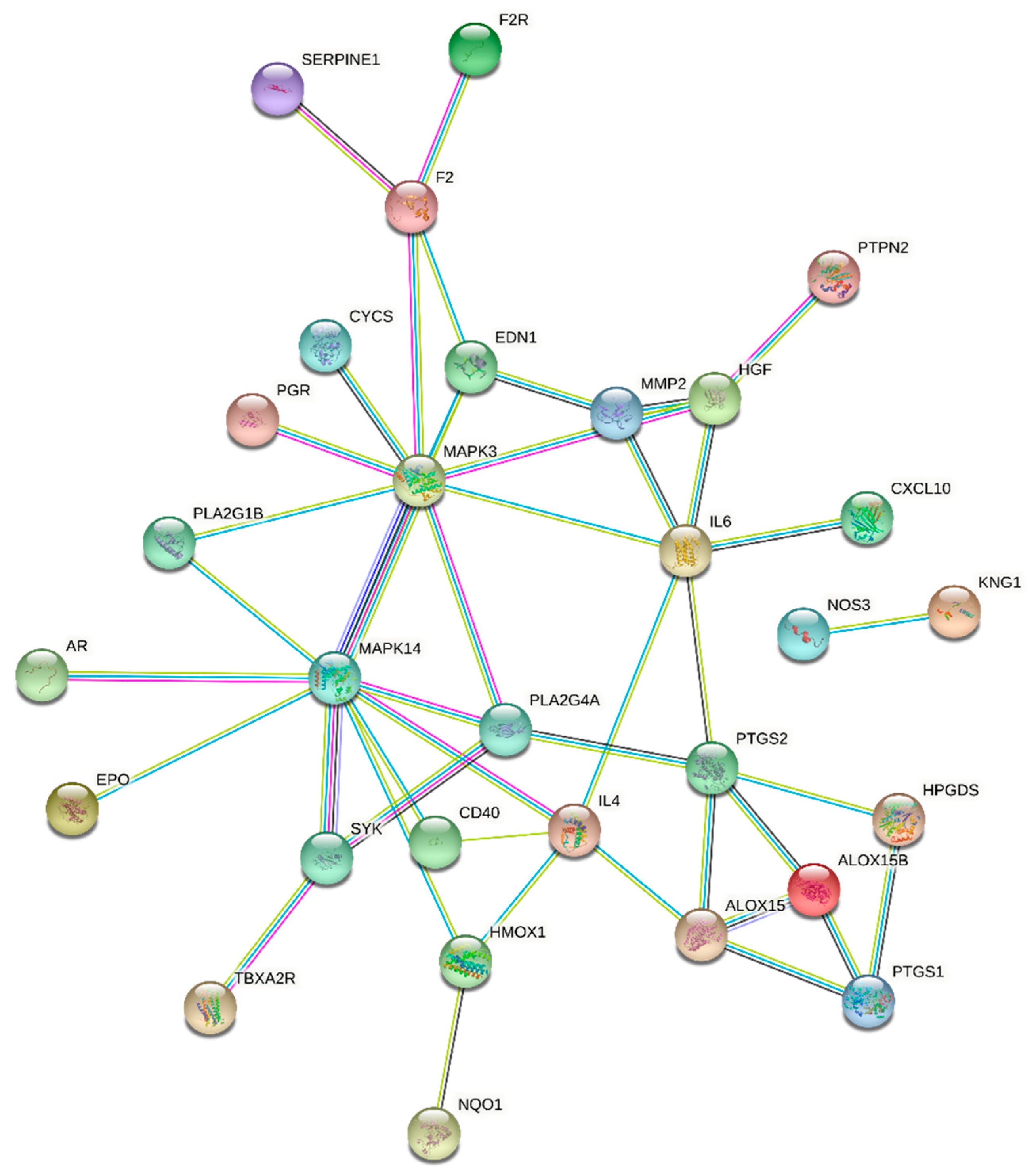
3.4. Construction and Analysis of Biological Network of Key Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Patlogar, J.E.; Tansey, C.; Wiebe, M.; Hybki, G.C.; Trostel, T.; Murphy, L.A.; Nakamura, R.K. A prospective evaluation of oral yunnan baiyao therapy on thromboelastographic parameters in apparently healthy cats. J. Vet. Emerg. Crit. Care 2019, 29, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Tansey, C.; Wiebe, M.L.; Hybki, G.C.; Patlogar, J.E.; Murphy, L.A.; Bianco, D.; Nakamura, R.K. A prospective evaluation of oral yunnan baiyao therapy on thromboleastographic parameters in apparently healthy dogs. J. Vet. Emerg. Crit. Care 2018, 28, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Ness, S.L.; Frye, A.H.; Divers, T.J.; Rishniw, M.; Erb, H.N.; Brooks, M.B. Randomized placebo-controlled study of the effects of yunnan baiyao on hemostasis in horses. Am. J. Vet. Res. 2017, 78, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Ladas, E.; Karlik, J.; Rooney, D.; Taromina, K.; Ndao, D.; Granowetter, L.; Kelly, K. Topical yunnan baiyao administration as an adjunctive therapy for bleeding complications in adolescents with advanced cancer. Support. Care Cancer 2012, 20, 3379–3383. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, H.; Xing, G.; Guan, B.; Yang, Y.; Ahmed, A.; Ma, X. Effects of yunanan baiyao adjunct therapy on postoperative recovery and clinical prognosis of patients with traumatic brain injury: A randomized controlled trial. Phytomedicine 2021, 89, 153593. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, K.X.; Zhao, Y.L.; Qin, X.J.; Yang, X.W.; Liu, L.; Liu, Y.P.; Luo, X.D. Potent anti-inflammatory and analgesic steroidal alkaloids from veratrum taliense. J. Ethnopharmacol. 2016, 179, 274–279. [Google Scholar] [CrossRef]
- Ren, X.; Zhu, Y.; Xie, L.; Zhang, M.; Gao, L.; He, H. Yunnan baiyao diminishes lipopolysaccharide-induced inflammation in osteoclasts. J. Food Biochem. 2020, 44, e13182. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, M.; Chen, L.; Zhang, W.; Huang, Y.; Luo, H.; Li, L.; He, H. The anti-inflammatory effects of yunnan baiyao are involved in regulation of the phospholipase a2/arachidonic acid metabolites pathways in acute inflammation rat model. Mol. Med. Rep. 2017, 16, 4045–4053. [Google Scholar] [CrossRef]
- Yang, B.; Xu, Z.Q.; Zhang, H.; Xu, F.Y.; Shi, X.Y.; Zou, Z.; Ling, C.Q.; Tang, L. The efficacy of yunnan baiyao on haemostasis and antiulcer: A systematic review and meta-analysis of randomized controlled trials. Int. J. Clin. Exp. Med. 2014, 7, 461. [Google Scholar]
- Harikrishnan, H.; Jantan, I.; Haque, M.A.; Kumolosasi, E. Anti-inflammatory effects of Phyllanthus amarus schum. & thonn. through inhibition of nf-κb, mapk, and pi3k-akt signaling pathways in lps-induced human macrophages. BMC Complement. Altern. Med. 2018, 18, 224. [Google Scholar]
- Li, W.; Guo, H.; Wang, X. Relationship between endogenous hydrogen sulfide and blood stasis syndrome based on the qi-blood theory of chinese medicine. Chin. J. Integr. Med. 2013, 19, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wang, Y.; Feng, T.; Wei, S. Differential proteomics analysis of endometriosis in blood stasis syndrome. Chin. J. Integr. Med. 2018, 24, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Pan, Y.; Fu, W.; Huang, S.; Xu, B.; Dou, L.; Hou, Q.; Li, C.; Yu, L. Investigation of invigorating qi and activating blood circulation prescriptions in treating qi deficiency and blood stasis syndrome of ischemic stroke patients: Study protocol for a randomized controlled trial. Front. Pharmacol. 2020, 11, 892. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yin, H.; Chen, K. Platelet proteomics and its advanced application for research of blood stasis syndrome and activated blood circulation herbs of chinese medicine. Sci. China Life Sci. 2013, 56, 1000–1006. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, A.; Kong, L.; Yu, J.; Gao, H.; Liu, Z.; Sun, H. Rapid discovery of quality-markers from kaixin san using chinmedomics analysis approach. Phytomedicine 2019, 54, 371–381. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, A.; Yang, L.; Li, M.; Fang, H.; Xie, J.; Wang, X. High-throughput chinmedomics strategy for discovering the quality-markers and potential targets for yinchenhao decoction. Phytomedicine 2019, 54, 328–338. [Google Scholar] [CrossRef]
- Bentley, P.; Peck, G.; Smeeth, L.; Whittaker, J.; Sharma, P. Causal Relationship of Susceptibility Genes to Ischemic Stroke: Comparison to Ischemic Heart Disease and Biochemical Determinants. PLoS ONE 2010, 5, e9136. [Google Scholar] [CrossRef]
- Rubin-Asher, D.; Zeilig, G.; Ratner, A.; Asher, I.; Zivelin, A.; Seligsohn, U.; Lubetsky, A. Risk factors for failure of heparin thromboprophylaxis in patients with acute traumatic spinal cord injury. Thromb. Res. 2010, 125, 501–504. [Google Scholar] [CrossRef]
- Pihusch, R.; Hiller, E.; Buchholz, T.; Rogenhofer, N.; Hasbargen, U.; Thaler, C.J.; Lohse, P.; Rübsamen, H. Thrombophilic gene mutations and recurrent spontaneous abortion: Prothrombin mutation increases the risk in the first trimester. Am. J. Reprod. Immunol. 2001, 46, 124–131. [Google Scholar] [CrossRef]
- Sloane, D.L.; Leung, R.; Craik, C.S.; Sigal, E. A primary determinant for lipoxygenase positional specificity. Nature 1991, 354, 149–152. [Google Scholar] [CrossRef]
- Horn, T.; Kakularam, K.R.; Anton, M.; Richter, C.; Reddanna, P.; Kuhn, H. Functional characterization of genetic enzyme variations in human lipoxygenases. Redox Biol. 2013, 1, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Teder, T.; Boeglin, W.E.; Brash, A.R. Lipoxygenase-catalyzed transformation of epoxy fatty acids to hydroxy-endoperoxides: A potential P450 and lipoxygenase interaction. J. Lipid Res. 2014, 55, 2587–2596. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.C.; Kalyanaraman, C.; Tourdot, B.E.; Conrad, W.S.; Akinkugbe, O.; Freedman, J.C.; Holinstat, M.; Jacobson, M.P.; Holman, T.R. 15-Lipoxygenase-1 biosynthesis of 7S,14S-diHDHA implicates 15-lipoxygenase-2 in biosynthesis of resolvin D5—ScienceDirect. J. Lipid Res. 2020, 61, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; O’Donnell, V.B.; Balzar, S.; Croix, C.M.; Trudeau, J.B.; Wenzel, S.E. 15-Lipoxygenase 1 interacts with phosphatidylethanolamine-binding protein to regulate MAPK signaling in human airway epithelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 14246–14251. [Google Scholar] [CrossRef] [PubMed]
- Rufanova, V.A.; Alexanian, A.; Wakatsuki, T.; Lerner, A.; Sorokin, A. Pyk2 mediates endothelin-1 signaling via p130Cas/BCAR3 cascade and regulates human glomerular mesangial cell adhesion and spreading. J. Cell. Physiol. 2010, 219, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Zhang, A.; Guo, Y.; Zhou, X.; Sun, H.; Yang, L.; Fang, H.; Yan, G.; Wang, X. A clinical and animal experiment integrated platform for small-molecule screening reveals potential targets of bioactive compounds from a herbal prescription based on the therapeutic efficacy of yinchenhao tang for jaundice syndrome. Engineering 2021, 7, 1293–1305. [Google Scholar] [CrossRef]
- Ren, J.; Dong, H.; Han, Y.; Yang, L.; Zhang, A.; Sun, H.; Li, Y.; Yan, G.; Wang, X. Network pharmacology combined with metabolomics approach to investigate the protective role and detoxification mechanism of yunnan baiyao formulation. Phytomedicine 2020, 77, 153266. [Google Scholar] [CrossRef]
- Han, Y.; Sun, H.; Zhang, A.; Yan, G.; Wang, X. Chinmedomics, a new strategy for evaluating the therapeutic efficacy of herbal medicines. Pharmacol. Ther. 2020, 216, 107680. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, A.; Kong, L.; Han, Y.; Yan, G.; Sun, H.; Wang, X. Analytical strategies for the discovery and validation of quality-markers of traditional chinese medicine. Phytomedicine 2020, 67, 153165. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Q.; Zhao, H.; Zhou, X.; Sun, H.; Nan, Y.; Zou, S.; Ma, C.W.; Wang, X. Phenotypic characterization of nanshi oral liquid alters metabolic signatures during disease prevention. Sci. Rep. 2016, 6, 19333. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, X. Urinary metabolic profiling of rat models revealed protective function of scoparone against alcohol induced hepatotoxicity. Sci. Rep. 2014, 4, 6768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.H.; Yu, J.B.; Sun, H.; Kong, L.; Wang, X.Q.; Zhang, Q.Y.; Wang, X.J. Identifying quality-markers from shengmai san protects against transgenic mouse model of alzheimer’s disease using chinmedomics approach. Phytomedicine 2018, 45, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, A.; Wang, P.; Sun, H.; Wu, G.; Sun, W.; Lv, H.; Jiao, G.; Xu, H.; Yuan, Y. Metabolomics coupled with proteomics advancing drug discovery toward more agile development of targeted combination therapies. Mol. Cell. Proteom. 2013, 12, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lu, S.; Sun, H.; Zhang, A.; Wang, H.; Wei, W.; Han, J.; Guo, Y.; Wang, X. Deciphering the q-markers of nourishing kidney-yin of cortex phellodendri amurense from zhibaidihuang pill based on chinmedomics strategy. Phytomedicine 2021, 91, 153690. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zhang, A.; Zhou, X.; Yu, J.; Song, Q.; Wang, X. High-throughput metabolomics reveals the perturbed metabolic pathways and biomarkers of yang huang syndrome as potential targets for evaluating the therapeutic effects and mechanism of geniposide. Front. Med. 2020, 14, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Using metaboanalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinform. 2016, 55, 14.10.11–14.10.91. [Google Scholar] [CrossRef]
- Li, Y.; Ju, L.; Hou, Z.; Deng, H.; Zhang, Z.; Wang, L.; Yang, Z.; Yin, J.; Zhang, Y. Screening, verification, and optimization of biomarkers for early prediction of cardiotoxicity based on metabolomics. J. Proteome Res. 2015, 14, 2437–2445. [Google Scholar] [CrossRef]
- Bartel, J.; Krumsiek, J.; Theis, F.J. Statistical methods for the analysis of high-throughput metabolomics data. Comput. Struct. Biotechnol. J. 2013, 4, e201301009. [Google Scholar] [CrossRef]
- Heller, J.I.; Crowley, J.R.; Hazen, S.L.; Salvay, D.M.; Wagner, P.; Pennathur, S.; Heinecke, J.W. P-hydroxyphenylacetaldehyde, an aldehyde generated by myeloperoxidase, modifies phospholipid amino groups of low-density lipoprotein in human atherosclerotic intima. J. Biol. Chem. 2000, 275, 9957–9962. [Google Scholar] [CrossRef]
- Abe, I.; Islam, F.; Lam, A.K.Y. Glucose intolerance on phaeochromocytoma and paraganglioma—The current understanding and clinical perspectives. Front. Endocrinol. 2020, 11, 593780. [Google Scholar] [CrossRef]
- Shigemura, K.; Tanaka, K.; Arakawa, S.; Hara, I.; Kawabata, G.; Fujisawa, M. Malignant pheochromocytoma with ivc thrombus. Int. Urol. Nephrol. 2007, 39, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Takeno, K.; Mita, T.; Nakayama, S.; Goto, H.; Komiya, K.; Abe, H.; Ikeda, F.; Shimizu, T.; Kanazawa, A.; Hirose, T. Masked hypertension, endothelial dysfunction, and arterial stiffness in type 2 diabetes mellitus: A pilot study. Am. J. Hypertens. 2012, 25, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Morita, A.; Namiki, T.; Nakaguchi, T.; Murai, K.; Watanabe, Y.; Nakamura, M.; Kawasaki, Y.; Shiko, Y.; Tamura, Y.; Suganami, A. Role of blood stasis syndrome of kampo medicine in the early pathogenic stage of atherosclerosis: A retrospective cross-sectional study, Evid. Based Complement. Alternat. Med. 2021, 2021, 5557392. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.; Win, T.; Gupta, R. Γ-l-glutamyltaurine. Amino Acids 2005, 28, 343–356. [Google Scholar] [CrossRef]
- He, H.; Ren, X.; Wang, X.; Shi, X.; Wang, X.; Ding, Z.; Gao, P.; Xu, G. Therapeutic effect of yunnan baiyao on rheumatoid arthritis was partially due to regulating arachidonic acid metabolism in osteoblasts. J. Pharm. Biomed. Anal. 2012, 59, 130–137. [Google Scholar] [CrossRef]
- Vezza, R.; Roberti, R.; Nenci, G.G.; Gresele, P. Prostaglandin e2 potentiates platelet aggregation by priming protein kinase c. Blood 1993, 82, 2704–2713. [Google Scholar] [CrossRef]
- Akiba, S.; Ohno, S.; Chiba, M.; Kume, K.; Hayama, M.; Sato, T. Protein kinase cα-dependent increase in Ca2+-independent phospholipase a2 in membranes and arachidonic acid liberation in zymosan-stimulated macrophage-like p388d1 cells. Biochem. Pharmacol. 2002, 63, 1969–1977. [Google Scholar] [CrossRef]
- Kuriyama, S.; Kashiwagi, H.; Yuhki, K.; Kojima, F.; Yamada, T.; Fujino, T.; Hara, A.; Takayama, K.; Maruyama, T.; Yoshida, A. Selective activation of the prostaglandin e2 receptor subtype ep2 or ep4 leads to inhibition of platelet aggregation. Thromb. Haemost. 2010, 104, 796–803. [Google Scholar]
- Philipose, S.; Konya, V.; Sreckovic, I.; Marsche, G.; Lippe, I.T.; Peskar, B.A.; Heinemann, A.; Schuligoi, R. The prostaglandin e2 receptor ep4 is expressed by human platelets and potently inhibits platelet aggregation and thrombus formation. Atertio. Thromb. Vasc. Biol. 2010, 30, 2416. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, J.Q.; Li, Y.B.; Liu, M.; Deng, H.M.; Luo, Y.C.; Tan, Y.F.; Hou, J.; Yao, G.W.; Guan, W.W. Effect of alpinia officinarum hance alcohol extracts on primary dysmenorrheal. Asian Pac. J. Trop. Med. 2016, 9, 882–886. [Google Scholar] [CrossRef]
- Linden, M.D.; Jackson, D.E. Platelets: Pleiotropic roles in atherogenesis and atherothrombosis. Int. J. Biochem. Cell Biol. 2010, 42, 1762–1766. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, L.; Gao, J.; Ma, J.; Yu, H.; Zhang, Y.; Wang, T.; Han, L. Multiomics integrative analysis for discovering the potential mechanism of dioscin against hyperuricemia mice. J. Proteome Res. 2020, 20, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Nyhan, W.L. Disorders of purine and pyrimidine metabolism. Mol. Genet. Metab. MGM 2005, 86, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, Q.; Jiang, Z.; Li, Z.; Zhang, M.; Yang, P.; Wang, X.; Wang, Y.; Qin, Y.; Li, T. Clinical research linking traditional chinese medicine constitution types with diseases: A literature review of 1639 observational studies. J. Tradit. Chin. Med. 2020, 40, 690–702. [Google Scholar] [PubMed]
- Steelman, S.M.; Hein, T.W.; Gorman, A.; Bix, G.J. Effects of histidine-rich glycoprotein on cerebral blood vessels. J. Cereb. Blood Flow Metab. 2013, 33, 1373–1375. [Google Scholar] [CrossRef]
- Liu, L.L. Urine Metabonomics Study on Chronic Heart Failure with Qi Deficiency and Blood Stasis Syndrome and Yang Deficiency and Water Stop Syndrome; China Academy of Chinese Medical Sciences: Beijing, China, 2014. [Google Scholar]
- Zhao, L.; Qiu, X.; Wang, W.; Li, R.; Wang, D. Nmr metabolomics and random forests models to identify potential plasma biomarkers of blood stasis syndrome with coronary heart disease patients. Front. Physiol. 2019, 10, 1109. [Google Scholar] [CrossRef]
- Li, X.N.; Zhang, A.; Wang, M.; Sun, H.; Liu, Z.; Qiu, S.; Zhang, T.; Wang, X. Screening the active compounds of phellodendri amurensis cortex for treating prostate cancer by high-throughput chinmedomics. Sci. Rep. 2017, 7, 46234. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, A.; Yu, J.; Wang, L.; Liu, C.; Zhou, X.; Sun, H.; Song, Q.; Wang, X. Insight into the metabolic mechanism of scoparone on biomarkers for inhibiting yanghuang syndrome. Sci. Rep. 2016, 6, 37519. [Google Scholar] [CrossRef]

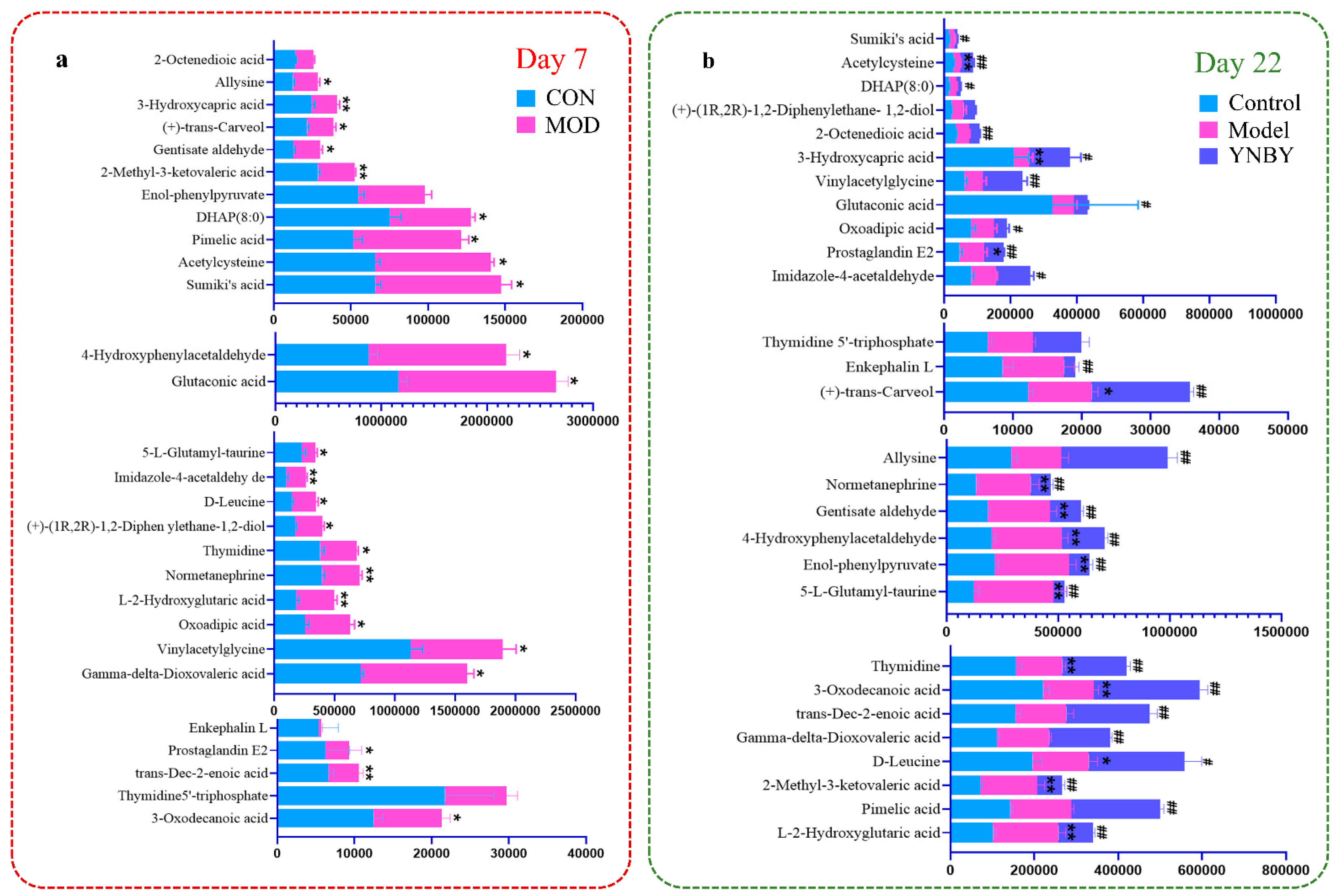
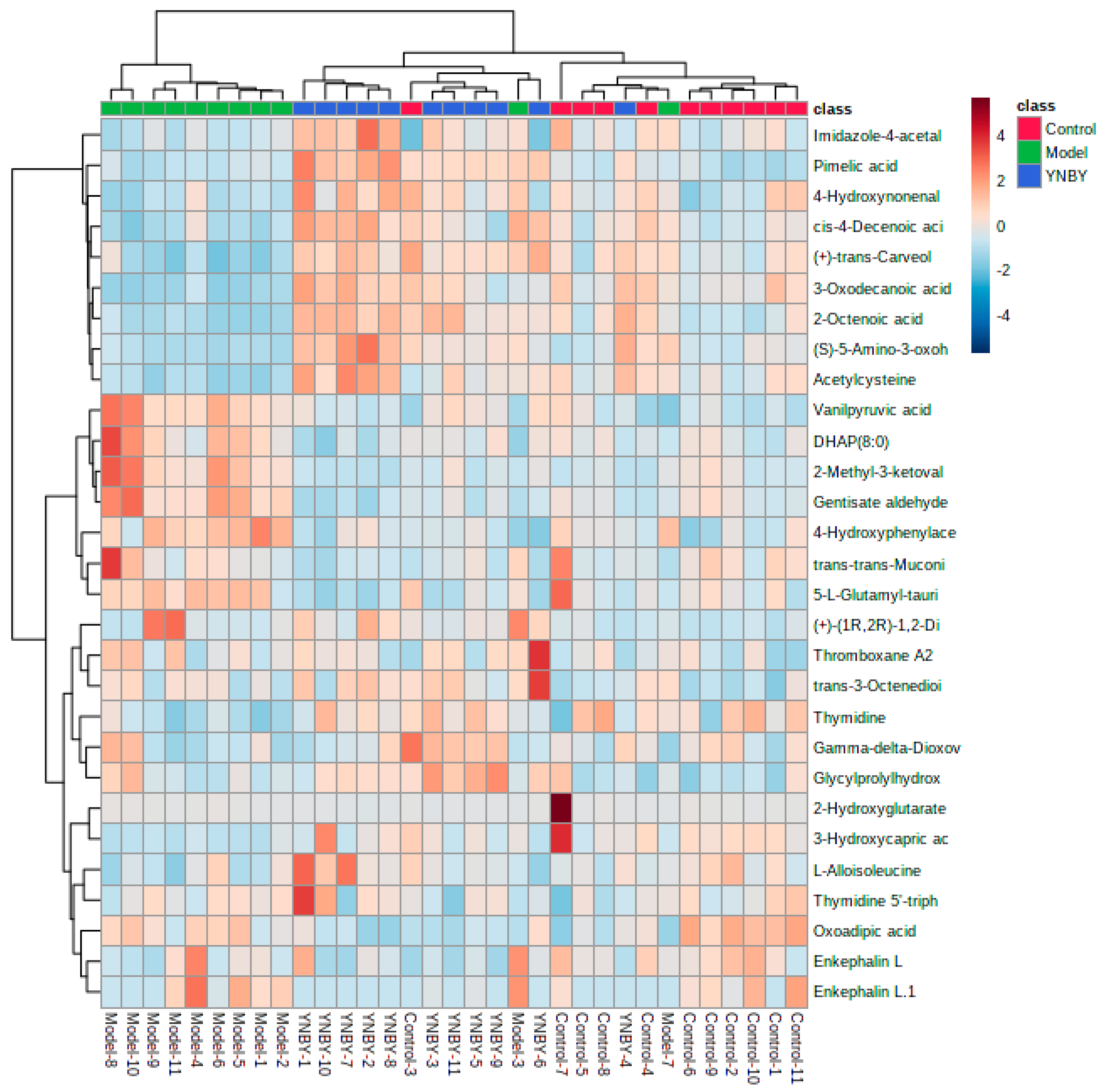
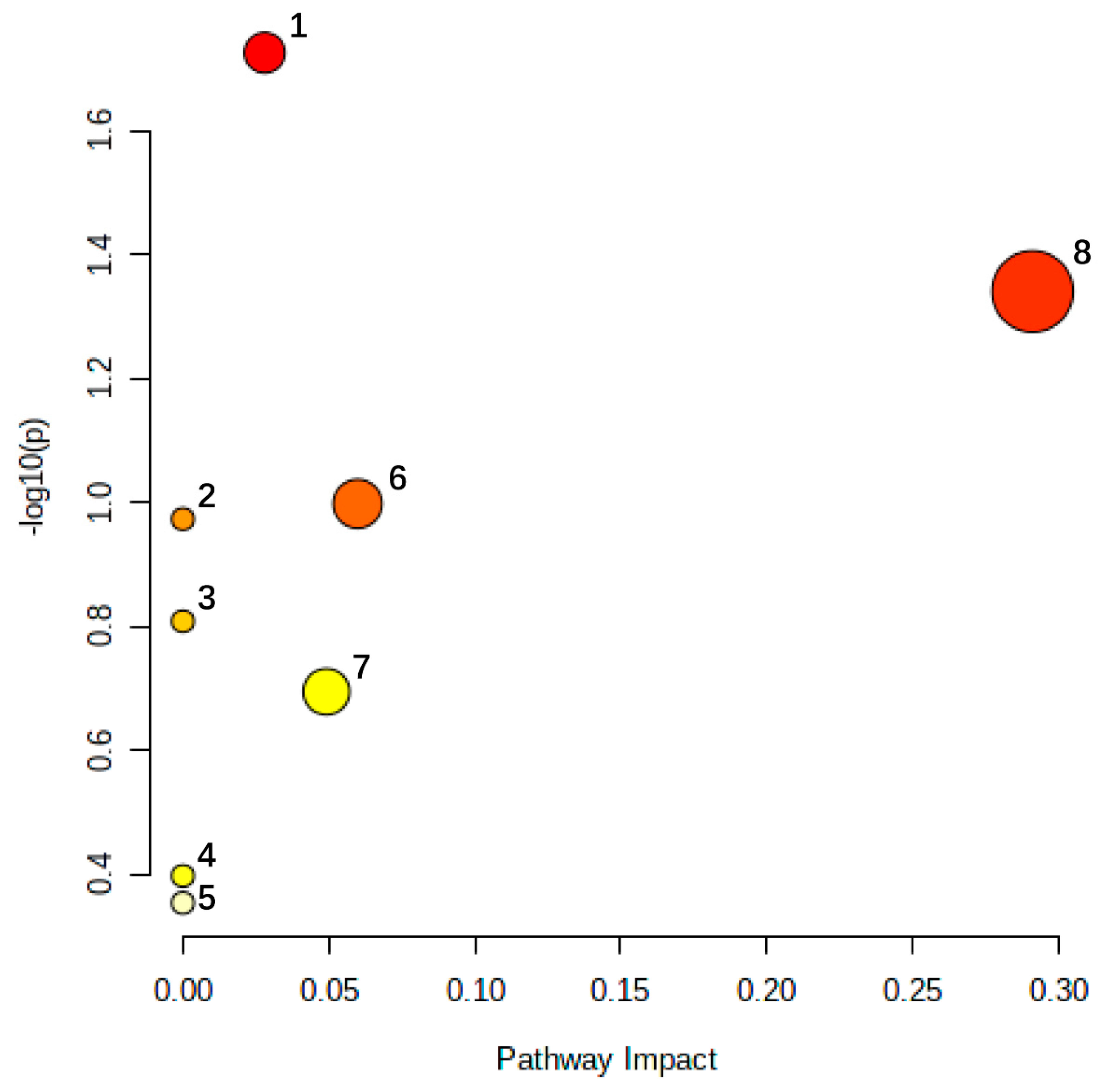
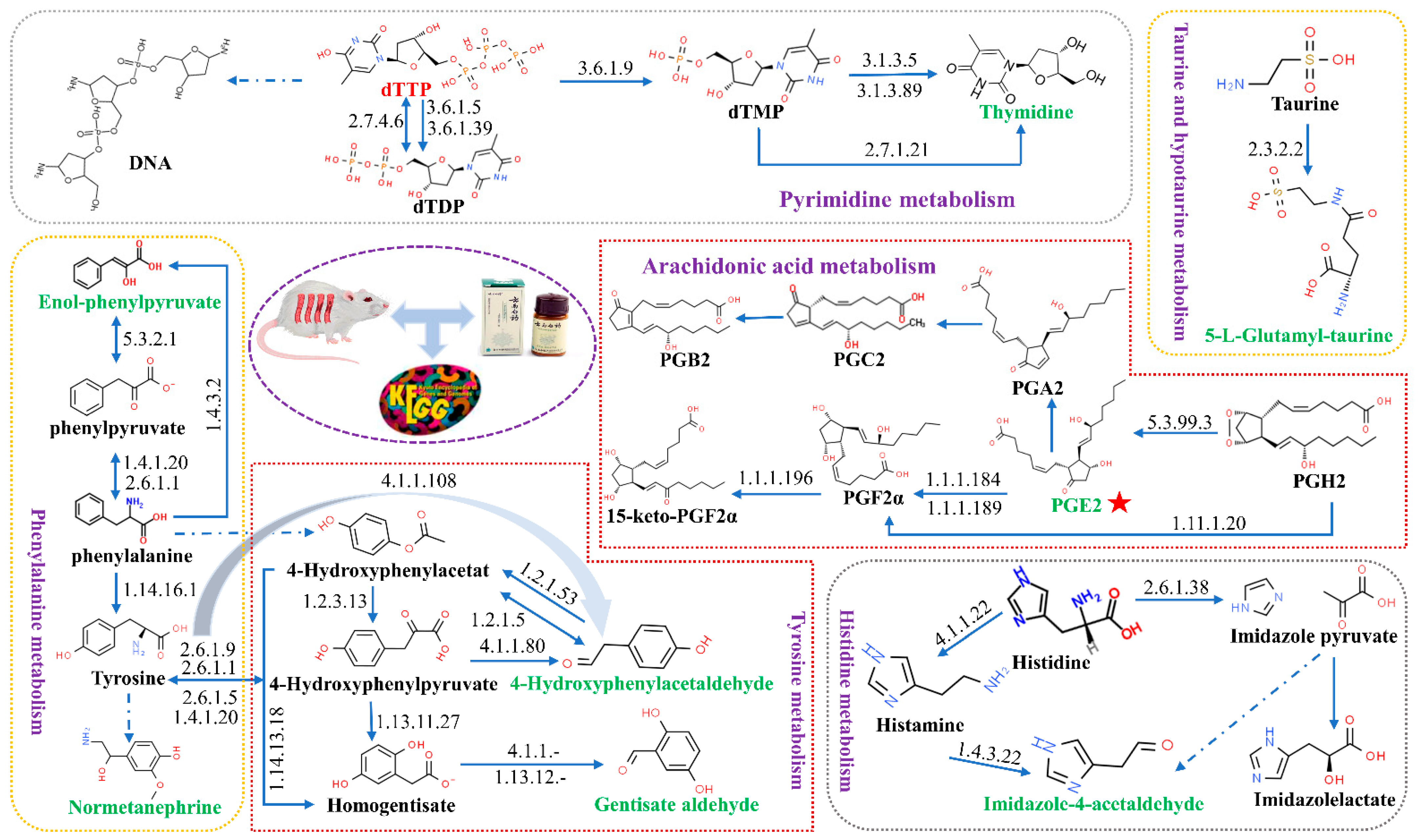
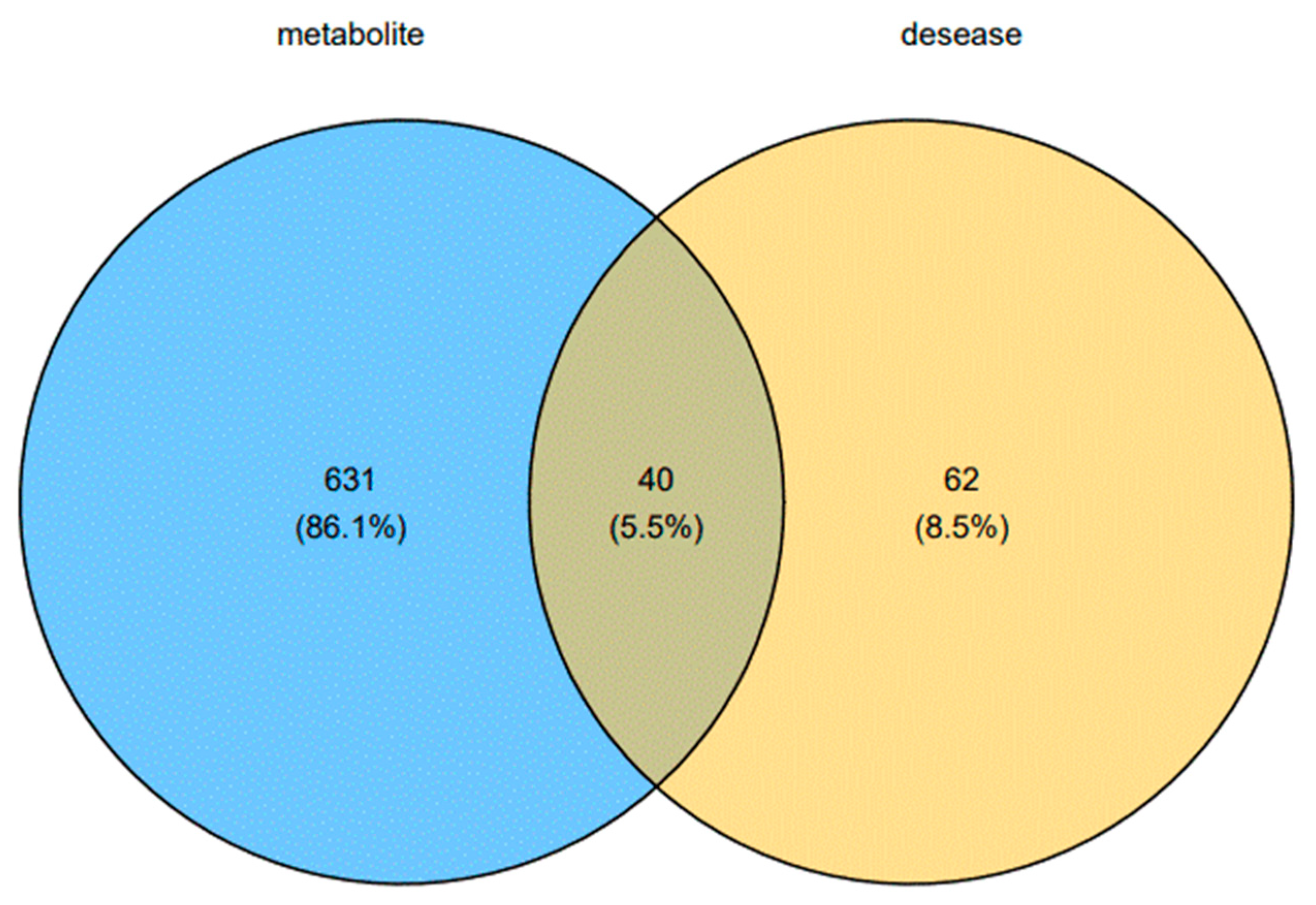
| NO. | Rt Min | m/z Determined | Adducts | HMDB | Proposed Composition | MS/MS Fragmentation (m/z) | Postulated Identity | Trend in Model |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.67 | 141.0190 | M-H | HMDB0002432 | C6H6O4 | 123.0335[M-H-H4O]− 105.9658[M-H-H5O2]− 80.9571[M-H-CH2O3]− | Sumiki’s acid | ↑ |
| 2 | 0.97 | 155.0458 | M + FA-H | HMDB0003905 | C5H6N2O | 93.0945[M-H-H2O]− 79.9555[M-H-CH3O]− | Imidazole-4-acetaldehyde | ↑ |
| 3 | 0.98 | 129.0188 | M-H | HMDB0013233 | C5H6O4 | 99.9185[M-H-CH3O]− 85.0256[M-H-CH2O2]− | Gamma-delta-Dioxovaleric acid | ↑ |
| 4 | 1.27 | 129.0189 | M-H | HMDB0000620 | C5H6O4 | 111.0621[M-H-H4O]− 83.0652[M-H-CH4O2]− | Glutaconic acid | ↑ |
| 5 | 2.12 | 159.0292 | M-H | HMDB0000225 | C6H8O5 | 115.0353[M-H-CH2O2]− 98.0222[M-H-CH3O3]− | Oxoadipic acid | ↑ |
| 6 | 2.37 | 147.0294 | M-H | HMDB0000694 | C5H8O5 | 103.0378[M-H-CH2O2] 87.0050[M-H-CH2O3]− 85.0239[M-H-CH4O3]− | L-2-Hydroxyglutaric acid | ↑ |
| 7 | 3.48 | 295.0947 | M-H | HMDB0011685 | C11H21O7P | 253.0503[M-H-C3H6]− 175.0756[M-H-C3H5O3P]− 159.0454[M-H-C4H9O3P]− | DHAP (8:0) | ↓ |
| 8 | 3.86 | 142.0503 | M-H | HMDB0000894 | C6H9NO3 | 124.0346[M-H-H4O]− 98.0528[M-H-CH2O2]− 83.0675[M-H-C2H5O2]− | Vinylacetylglycine | ↓ |
| 9 | 4.02 | 144.0660 | M-H | HMDB0001263 | C6H11NO3 | 87.0020[M-H-C2H5NO]− 85.0265[M-H- C2H7NO]− | Allysine | ↑ |
| 10 | 4.26 | 242.0893 | M-H | HMDB0000273 | C10H14N2O5 | 203.0009[M-H-C3H2]− 131.0824[M-H-C5H4NO2]− 108.0213[M-H-C5H11NO3]− | Thymidine | ↓ |
| 11 | 4.54 | 137.0238 | M-H | HMDB0004062 | C7H6O3 | 121.0161[M-H-H2O]− 109.0313[M-H-CH2O]− | Gentisate aldehyde | ↑ |
| 12 | 5.08 | 171.0655 | M-H | HMDB0000341 | C8H12O4 | 129.0916[M-H-CO2]− 111.0816[M-H-C2H4O2]− 86.9758[M-H-C4H4O2]− | 2-Octenedioic acid | ↓ |
| 13 | 5.29 | 554.2693 | M-H | HMDB0001045 | C28H37N5O7 | 236.1032[M-H-C17H22N2O4]− 169.0723[M-H-C22H29N2O4]− 130.0867[M-H-C24H28N2O5]− | Enkephalin L | ↓ |
| 14 | 4.27 | 184.0963 | M + H | HMDB0000819 | C9H13NO3 | 166.1053[M+H-H4N]+ 150.1259[M+H-H4NO]+ 137.9975[M+H-CH4NO]+ 131.0403[M+H-CH11NO]+ | Normetanephrine | ↓ |
| 15 | 5.43 | 175.0606 | M + FA-H | HMDB0000408 | C6H10O3 | 96.9583[M-H-CH6O]− 79.9693[M-H-C2H11O]− | 2-Methyl-3-ketovaleric acid | ↓ |
| 16 | 5.67 | 163.0397 | M-H | HMDB0012225 | C9H8O3 | 147.0202[M-H-H2O]− 119.0432[M-H-CH2O2]− 92.1066[M-H-C2HO3]− | Enol-phenylpyruvate | ↓ |
| 17 | 5.70 | 135.0445 | M-H | HMDB0003767 | C8H8O2 | 136.0288[M-H-H]− 108.0139[M-H-CHO]− 93.0328[M-H-C2H4O]− | 4-hydroxyphenylacetaldehyde | ↑ |
| 18 | 5.79 | 159.0656 | M-H | HMDB0000857 | C7H12O4 | 144.0050 [M-H-HO]− 125.0203[M-H-H4O2]− 96.0089[M-H-CH5O3]− | Pimelic acid | ↑ |
| 19 | 7.89 | 259.0985 | M + FA-H | HMDB0012111 | C14H14O2 | 137.0956[M-H-C6H6]− 135.0413[M-H-C6H8]− | (+)-(1R,2R)-1,2-Diphen ylethane-1,2-diol | ↑ |
| 20 | 6.39 | 253.0504 | M-H | HMDB0004195 | C7H14N2O6S | 209.0609[M-H-CO2]− 165.1283[M-H-C2H2NO3]− 143.0500[M-H-C2H8NO4]− | 5-L-Glutamyl-taurine | ↓ |
| 21 | 6.40 | 480.9840 | M-H | HMDB0001342 | C10H17N2O14P3 | 242.0126[M-H-H2O9P3]− 212.0024[M-H-CH4O10P3]− 162.0537[M-H-C3H14O11P3]− | Thymidine 5′-triphosphate | ↓ |
| 22 | 6.47 | 130.0867 | M-H | HMDB0013773 | C6H13NO2 | 89.0084[M-H-C3H7]− 72.9893[M-H-C3H7O]− | D-Leucine | ↑ |
| 23 | 7.51 | 185.1135 | M-H | HMDB0010724 | C10H18O3 | 141.0911[M-H-C3H8]− 125.1014[M-H-C3H8O]− 109.0276[M-H-C4H12O]− 79.9580[M-H-C5H13O2]− | 3-Oxodecanoic acid | ↓ |
| 24 | 8.06 | 187.1332 | M-H | HMDB0002203 | C10H20O3 | 151.0831[M-H-H6O2]− 145.0560[M-H-C2H4O]− 124.0039[M-H-C2H9O2]− | 3-Hydroxycapric acid | ↓ |
| 25 | 8.25 | 351.2160 | M-H | HMDB0001220 | C20H32O5 | 351.2107[M-H]− 162.0184[M-H-C10H21O3]− 115.0025[M-H-C14H20O3]− | Prostaglandin E2 | ↓ |
| 26 | 8.25 | 151.1121 | M-H | HMDB0059608 | C10H16O | 136.0390[M-H-HO]− 122.0321[M-H-CH3O]− 94.0446[M-H-C3H7O]− | (+)-trans-Carveol | ↓ |
| 27 | 8.57 | 215.1281 | M+FA-H | HMDB0010726 | C10H18O2 | 140.0370[M-H-C2H7]− 127.0250[M-H-C3H8]− 97.0009[M-H-C5H14]− 83.0496[M-H-C6H16]− | trans-Dec-2-enoic acid | ↓ |
| 28 | 8.75 | 162.0226 | M-H | HMDB0001890 | C5H9NO3S | 147.0233[M-H-HO]− 133.0462[M-H-CH3O]− 102.0408[M-H-C2H6O2]− | Acetylcysteine | ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Zhang, A.; Wu, F.; Wang, X. UPLC-G2Si-HDMS Untargeted Metabolomics for Identification of Yunnan Baiyao’s Metabolic Target in Promoting Blood Circulation and Removing Blood Stasis. Molecules 2022, 27, 3208. https://doi.org/10.3390/molecules27103208
Zhang Q, Zhang A, Wu F, Wang X. UPLC-G2Si-HDMS Untargeted Metabolomics for Identification of Yunnan Baiyao’s Metabolic Target in Promoting Blood Circulation and Removing Blood Stasis. Molecules. 2022; 27(10):3208. https://doi.org/10.3390/molecules27103208
Chicago/Turabian StyleZhang, Qingyu, Aihua Zhang, Fangfang Wu, and Xijun Wang. 2022. "UPLC-G2Si-HDMS Untargeted Metabolomics for Identification of Yunnan Baiyao’s Metabolic Target in Promoting Blood Circulation and Removing Blood Stasis" Molecules 27, no. 10: 3208. https://doi.org/10.3390/molecules27103208
APA StyleZhang, Q., Zhang, A., Wu, F., & Wang, X. (2022). UPLC-G2Si-HDMS Untargeted Metabolomics for Identification of Yunnan Baiyao’s Metabolic Target in Promoting Blood Circulation and Removing Blood Stasis. Molecules, 27(10), 3208. https://doi.org/10.3390/molecules27103208






