Abstract
Plant polyphenols offer several benefits for the prevention of diverse illnesses. Fruit’s edible and inedible parts (pulp, seeds, peels, stems, flowers) are important sources of polyphenols. Different industrial processes for fruit treatment and commercialization affect the total polyphenol content (TPC), and probably the biological activity. The purpose of the present work was to determine the TPC and antioxidant activity (by DPPH) of polyphenols extracted from the pulp and seeds of Mauritia flexuosa (aguaje), in fresh and dehydrated forms, in order to determine the possible connection with the quantity of polyphenols and their specific antioxidant activity. The highest phenolic content for M. flexuosa seeds in fresh form (non-dehydrated) was 270.75 mg GAE/100 g with a 96-h extraction. With respect to the dehydrated samples, the best yield was quantified in the 96-h dehydrated seed sample. For all pulp and seeds, dehydrated for 24, 48, and 96 h, TPC showed a slightly decreasing pattern. The DPPH results were the highest in the 96-h dehydrated samples and the differences among all dehydrated pulp and seed samples were minimal. More studies testing the presence of other antioxidant components could help in understanding the detailed antioxidant activity, and related more to the specific action, rather than only total polyphenol content.
1. Introduction
The antioxidant benefit of fruit consumption is mainly connected to the polyphenol content; these types of phytochemicals display several other functions [1,2]. Multiple publications have reported improved lipid metabolism in overweight and obese humans due to a regular diet inclusion of plant polyphenols [3,4,5]. These phytochemicals offer a protective activity, leading to health benefits; several studies relate oxidative stress with the development of diseases such as cardiovascular diseases, neurodegenerative disorders, and cancer [6,7]. With respect to ovarian cancer, there is histological evidence of aortas showing promising anti-proliferative and anti-inflammatory effects and leading to a reduction of cancer cell viability [8,9]. Given the multiple health benefits of polyphenols, it is of general interest to know the phenolic content and major phenolic compounds, such as flavonoids, found in regularly harvested and consumed fruits [10,11,12].
The oxido-reducing activity of compounds containing phenolic rings is the most studied biological property in plant polyphenols. This type of chemical reaction is representative of a misbalance in oxidizing and reducing compounds; which at the cellular level can lead to molecular damage [13]. Plants and fruits can contribute to the reduction of negative side effects in different pathologies.
Commonly known as buriti plant, Mauritia flexuosa (Arecaceae) is a palm broadly cultivated in Colombia, Venezuela, the Guianas, Trinidad, Ecuador, Peru, Brazil, and Bolivia [2,14]. The fresh form of M. flexuosa fruits (Figure 1) has an orange, soft, water-soluble, and edible pulp and numerous small circular dark red and brown flat seeds. Several South American aboriginal groups use this Amazonic fruit for medicinal purposes [15,16,17,18,19]. The mesocarp oil is used to treat respiratory symptoms, pneumonia, influenza, snake bites, and heart problems [20]. In Colombia, M. flexuosa is also named “aguaje” [2].

Figure 1.
(a) Mauritia flexuosa (aguaje) palm and fruit (seedless pulp) [2]. (b) Dehydrated aguaje samples, seeds and pulp.
Different variables can affect the total polyphenol content (TPC) and the antioxidant capacity of fruits, such as passing from a fresh to a dehydrated long-shelf-life state. This is still an area of active research, while conflicting results have been reported in the literature [21,22,23]. Information is lacking with respect to the specific activity and bio-accessibility of polyphenols, especially due to structural alterations, such as ring modifications, and polyphenol interaction with other food matrixes based on high or low water contents [24,25]. Multiple studies have focused on testing total phenolic content and antioxidant activity in M. flexuosa, but a comparison of the influence in dehydration states in pulp or seeds and its connection to antioxidant activity is still lacking in information and research [26,27,28,29]. The contradicting results show that some studies highlight decreasing TPC and higher antioxidant activity, while others report the opposite situations tested in a different food matrix, such as grapes, onions, rice, and other vegetables [30,31,32,33]. The goal of the present study was to determine a comparative approach to polyphenols from M. flexuosa, with high and low water content (fresh and de-hydrated samples), under active interconversion, and their relation to effective antioxidant activity.
2. Results and Discussion
2.1. Food Matrix Dehydration and Polyphenol Extraction
Samples of M. flexuosa pulp and seeds were treated by extraction and a controlled dehydration process at four different times (24, 48, 72, and 96 h). The biggest weight difference was recorded for the first 24 h of dehydration. The water content in pulp and seeds was approximately 85% and 44%, respectively. Dehydrated weights were stable at 96 h at 50 °C. Considering the high difference in water content for pulp and seeds, the dehydration pattern (Figure 2) yielded similar values.
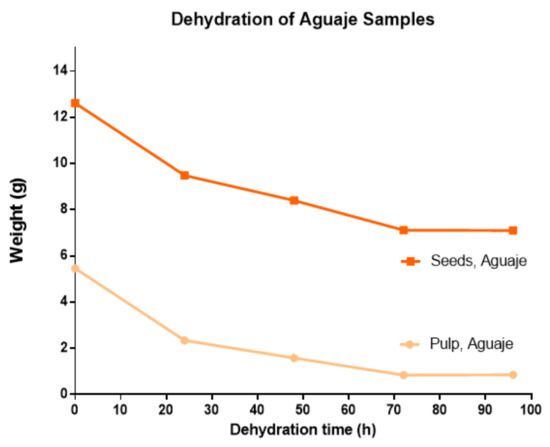
Figure 2.
Dehydration curve for M. flexuosa, pulp and seeds at a constant 50 °C from 0 to 96 h.
Polyphenols from fresh pulp and seeds were extracted in ethanol (80% w/v) and more polyphenol extractions were done with seeds and pulp at three dehydration intervals (24, 48, and 96 h). The comparative extraction looked for differences in polyphenol content, based on conflicting studies that reported higher phenolics and antioxidant activity in fresh fruit, seeds, or peels [34,35]. Figure 3 shows the comparative values for total polyphenol content from pulp and seeds in fresh or dehydrated form.
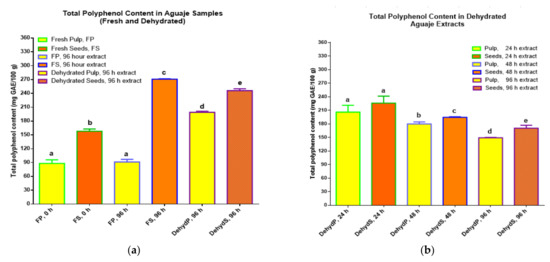
Figure 3.
TPC (mg of gallic acid equivalents per 100 g) of fresh and dehydrated pulp and seeds of (a) M. flexuosa (aguaje) fresh and dehydrated pulp and seeds for the initial experimental assay and (b) TPC in M. flexuosa dehydrated pulp and seeds in a second assay. Data are means (±standard deviation), and lower-case letters represent significant differences, for total polyphenol content in mg GAE/100 g of initial sample, based on ANOVA followed by Tukey test (p < 0.05).
2.2. Total Polyphenol Content (TPC)
Polyphenols that were initially extracted from the fresh pulp of aguaje at 0 h of dehydration and the samples of fresh pulp that continued with the extraction process for another 96 h, in the dark without stirring, registered the lowest TPC with statistical significance, with respect to the phenolic content in fresh and dehydrated (96 h) seeds, registering approximately 90 mg GAE/100 g. The highest TPC was detected in fresh and dehydrated aguaje seeds (dehydration at 96 h) yielding more than 270 mg GAE/100 g in fresh seeds, as shown in Figure 3a that represents the results for the first experimental assays. The best aqueous conditions for polyphenol detection and reactions (fresh pulp matrix) did not favor higher TPC. These results suggest, coinciding with other researchers, that higher phenolic presence is more connected to the food matrix and the solvent affinity due to polyphenol polarity [35]. Nevertheless, in comparison with other fruits, we registered a high TPC; other studies reported M. flexuosa, with TPC values of 435.08 ± 6.97 and 362.90 ± 7.98 mg GAE/100 g of the whole pulp in fresh form [29,36]. The results here differed with studies where the TPC in fresh fruit, after food processing, was lowered or lost due to temperature or processing changes [31,32].
For the second experimental assay, concerning the TPC results for dehydrated M. flexuosa pulp and seeds, as shown in Figure 3b, a regular decreasing TPC pattern from 24 to 96 h of dehydration was registered. TPC values at the three different dehydration points were higher than the TPC in fresh pulp and seeds; the highest TPC was quantified at 24 h of dehydrating pulp and seeds at a constant 50 °C. The results at 48 and 96 h yielded slightly lower TPC values with statistical significance. The lowest phenolic content was measured in dehydrated pulp at 96 h (149.28 ± 0.81 mg GAE/100 g). This decreasing trend in phenolic concentration is consistent with research done with the purpose of evaluating TPC values in fruits processed with long effective storage time for exportation purposes [31].
2.3. Antioxidant Activity, DPPH (2,2-Diphenyl-1-picrylhydrazyl) Radical Scavenging Assays
The results for the antioxidant action of phenolic compounds, extracted in ethanol 80%, in a fresh and dehydrated matrix at different times for M. flexuosa (aguaje) samples are shown in Figure 4, showing the comparative results for two different experimental assays. The total inhibitory concentration, IC 50%, that yielded the best output was due to phenolics in dehydrated seeds at 96 h (78.28 ± 0.67 mg AAE/100 g of dehydrated sample). All DPPH results were higher in the extractions from fresh or dehydrated seeds. In both assays, Figure 4a,b, the antioxidant activity registered an increasing tendency. The best DPPH results, with statistical significance, were detected in M. flexuosa pulp and seeds dehydrated for 96 h.
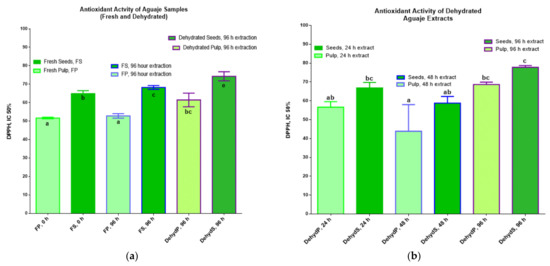
Figure 4.
Antioxidant activity (mg AAE/100 g FP) of extracts in two different experimental assays. (a) fresh and dehydrated M. flexuosa) and (b) dehydrated pulp and seeds at different times. Data are means (±standard deviation) and lower-case letters represent significant differences, for antioxidant activity of aguaje extracts according to DPPH (IC 50%), based on ANOVA followed by a Tukey test (p < 0.05).
These results for antioxidant activity coincide with the assays, in the same fruit, where other parts of the plant were evaluated [26,37]. The dehydration approaches in the present work led to improved DPPH values, with respect to the previously cited studies. The results are comparable and coincide with studies applying more complex dehydration processes [38,39]. Figure 5 represents a summary display of the dehydration process with respect to TPC and antioxidant activity seen in M. flexuosa pulp and seed extracts, as a comparative base for the various other studies.
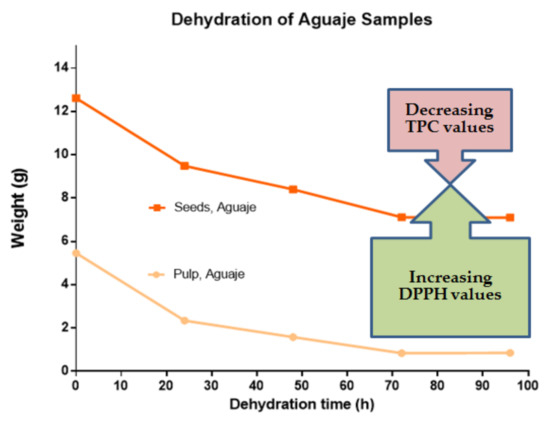
Figure 5.
TPC (mg of gallic acid equivalents) and DPPH (mg ascorbic acid equivalents) in M. flexuosa (aguaje) pulp and seeds with respect to a constant dehydration pattern from 0 to 96 h. The stable flattening dehydration pattern is connected to lower TPC values and higher redox activity. From 72 to 96 h, the weight differences were stable, and statistical significance was registered at different points in the dehydration process.
Studies evaluating the biological activity of polyphenols in red raspberries showed that dehydration led to a lowering polyphenol content and less antioxidant activity, while the rate of this reduction was connected to the dehydration method, and this showed that a high dehydration temperature was linked to polyphenol content loss [40,41]. Increasing temperature promotes higher solubility and diffusion coefficient of polyphenolic compounds into the extracting solvents, and higher temperatures also enhance the penetration of solvents into the cell matrix; hence, increasing the TPC of the extracts [41].
Furthermore, the results confirmed a positive correlation between the total polyphenol content and the antioxidant activity for the aglycone compounds undergoing the drying process (74.7%). These lowering TPC values may be due to oxidative and thermal degradation of the phenolic compounds [42,43]. The major phenolic content drop, during fruit processing at high temperatures in enzymatic reactions, could be related to the action of oxidative enzymes such as polyphenol oxidases (PODs) and polyphenol peroxidases (PPOs) [43,44]. PODs can enhance the degradation of phenols when coexisting with PPOs; both the PPO and POD enzymatic activities play a key role in determining the phenolic profile of olive oil. In contrast, low temperatures during the dehydration process decrease the oxidation of volatile compounds [45].
When Cabernet Sauvignon and Merlot grapes were dried using a constant low temperature (7 °C) for several weeks, there was an increase in TPC and antioxidant activity [46]. This could be due to the effect of the concentration of the phenolic compounds as a result of water loss caused by the dehydration, even if the constant temperature (40 °C) was higher [47]. The results in these two previous studies partially coincide with the findings in the present work, with respect to the higher antioxidant activity of polyphenols in dehydrated aguaje fruit samples, but differ with respect to the decreasing phenolic content during d the dehydration process.
In a study evaluating the redox activity of phenolics from goldenberry, the levels of TPC and antioxidant activity (determined with the ferric reducing antioxidant power FRAP method) increased in dehydrated samples [20]. Furthermore, the highest TPC was registered in samples that were dried at 90 °C, coinciding with the results in this study, where samples were dehydrated at lower temperature (50 °C). The interconversion of phenolic compounds at high temperatures might be caused by the availability of phenolic precursor molecules through the non-enzymatic rearrangement between phenolic molecules [48]. This higher phenolic content may originate from the disruption of cell walls during processing or the breakdown of insoluble phenolic compounds. Therefore, this could lead to a better extractability for these particular types of phenolic compounds [49].
These comparative studies suggest that the antioxidant activity could be due to the combined reactions of total phenolics rather that certain individual components or the action of polyphenols as a whole group. The results in this study, using fresh and dehydrated aguaje seeds and pulp, coincide with the findings of Gupta et al. (2021), testing different parts of pomelo fruit (Citrus grandis (L.) Osbeck) in similar experiments for antioxidant activity. They found that TPC was highest in the membrane of the fruit, and DPPH registered the highest activity in the pomelo juice [50]. Considering the total polyphenols in the present study, the improvement in TPC could be a result of the destruction of the covering structure and the release of more phenolic compounds, facilitating and increasing the extraction yield [48]. Moreover, the dehydration process could induce metabolic pathways that can generate, and increase the number of, precursors for different categories of phenolic compounds [49].
3. Materials and Methods
3.1. Reagents and Chemicals
Ethanol, sodium carbonate, and Folin–Ciocalteu reagent were purchased from PanReac AppliChem, ITW Reagents, (Darmstadt, Germany), and methanol, from Sigma Aldrich (St. Louis, MO, USA). Ascorbic acid (Sebion) and DPPH (2,2-Diphenyl-1-picrylhydrazyl, Sigma-Aldrich) were purchased from Merck KGaA (Darmstadt, Germany).
3.2. Plant Material, Sample Preparation, and Polyphenol Extractions
M. flexuosa pulp with seeds was obtained in the Colombian city of Leticia. All samples were refrigerated before laboratory analyses. The extraction of total polyphenol compounds was performed following a previous method from this research group [2,51]. Different amounts of fresh and dehydrated pulp and seeds of M. flexuosa were placed in ethanol (80% w/v) at proportions of 1:5 per volume, stirred for 15 min at 500 rpm, homogenized, and stored at room temperature, in the dark, for 24 h without stirring. Two different extractions times (—al fresco—0 h and 96 h of extraction) for the first experimental assay, as an explorative comparison, and three different dehydration times (24, 48, and 96 h) at a constant of 50 °C, including only samples in dry state as a second assay, were considered in this experimental process. The extracts were centrifuged for 10 min at 3500 rpm, and the supernatant was recovered for polyphenol quantitation and antioxidant activity evaluation.
3.3. Total Polyphenol Content (TPC)
The total polyphenol content in M. flexuosa pulp and seeds (fresh and dehydrated matrix) was quantified following the Folin–Ciocalteu (F–C) assay [51]. Samples of 1 mL of each extract were mixed with 1 mL F–C reagent (10% w/v), allowed to react for 2 min, and mixed with 2 mL sodium carbonate, Na2CO3, (3.5% w/v). Reactants were kept in the dark at room temperature for 90 min. All runs were performed in triplicate. Absorbance was read at 655 nm in a UV-Vis spectrophotometer (Mecasys Optizen POP, Daejeon, Korea). All data were calculated based on a gallic acid standard calibration curve, with a range of 0–4.0 mg/L and r2 of 0.9982). TPC is expressed as milligrams gallic acid equivalent (GAE) per 100 g of fresh or dehydrated sample (mg GAE/100 g).
3.4. DPPH Assay for Radical Scavenging Antioxidant Activity
The DPPH radical scavenging test is one of the most useful techniques to evaluate the antioxidant activity in polyphenols extracted from natural products. The DPPH compound is a stable free radical in methanol. The DPPH assay was performed following previous work from this research group [2]. Volumes of 1900 μL of DPPH (100 μM) prepared in pure methanol were mixed with 100 μL of each diluted (1:5) extract and left to react in the dark at room temperature for 30 min. The antioxidant activity from phenolics, in fresh and dehydrated pulp and seeds, of M. flexuosa was measured via spectrophotometry at 517 nm, comparing against a methanol blank. A positive control of ascorbic acid based on a calibration curve, and in triplicates for each colorimetric reaction, was applied in this methodology. The control curve was prepared with concentrations of comparable reference ascorbic acid (Merck KGaA, Darmstadt, Germany) in a concentration range from 50 to 600 μg/mL (r2 of 0.9945). All dilutions followed the same DPPH reaction conditions for the antioxidant activity evaluated in fruit extracts. The slope taken from the calibration curve served as the calculation of the inhibition concentration (IC 50%), when 50% of the antioxidant component was reduced. The results for IC 50% were determined based on the equation:
% scavenging DPPH free radical = (ABSControl − ABSExtracts/ABSControl) × 100%
The antioxidant activity of M. flexuosa extracted phenolics is expressed as mg of ascorbic acid equivalents per 100 g of fresh or dehydrated pulp or seeds (mg AAE/100 g).
3.5. Statistical Analysis
All analyses were carried out in triplicate, and TPC and DPPH values are expressed as mean ± standard deviation (SD). Means were tested for normality and homogeneity. Data were analyzed based on a ANOVA test followed by Tukey test (p < 0.05) with the IBM SPSS Statistics software version 20.0 (IBM Corp., Armonk, NY, USA).
4. Conclusions
The total polyphenol content and antioxidant activity of M. flexuosa pulp and seeds, in fresh and dehydrated form, were tested in this work. The water content in a food matrix allows for a specific polyphenol oriented chemical reaction, yielding better results in some cases where the water content is higher. A controlled dehydration process was considered in this experimental approach, with the purpose of evaluating the polyphenol availability and antioxidant action. TPC values were not directly proportional to antioxidant activity, suggesting that the polyphenol reactions for radical scavenging in pulp and seeds of M. flexuosa do not depend directly in the specific quantity of phenolic compounds, but rather on the specific chemical structure or on its re-accommodation or interconversion. More studies based on the specific polyphenol/flavonoid content and the presence of other antioxidants, such as C vitamin, in M. flexuosa could lead to understanding more of the specific antioxidant activity of this fruit with multiple processes of it edible parts.
Author Contributions
Conceptualization, formal analysis, data curation and writing—review and editing, H.B., M.F.S.-G. and J.C.C.-H.; methodology, H.B., M.F.S.-G. and J.C.C.-H.; supervision, J.C.C.-H.; project administration, H.B. and J.C.C.-H.; funding acquisition, J.C.C.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank all laboratory members, especially Carmen Serna Hurtado, at Universidad de Manizales (Colombia) for their constant support and help.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Garzón, G.A.; Narváez, C.E.; Riedl, K.M.; Schwartz, S.J. Chemical composition, anthocyanins, non-anthocyanin phenolics and antioxidant activity of wild bilberry (Vaccinium meridionale Swartz) from Colombia. Food Chem. 2010, 122, 980–986. [Google Scholar] [CrossRef]
- Carmona-Hernandez, J.C.; Le, M.; Idárraga-Mejía, A.M.; González-Correa, C.H. Flavonoid/Polyphenol Ratio in Mauritia flexuosa and Theobroma grandiflorum as an Indicator of Effective Antioxidant Action. Molecules 2021, 26, 6431. [Google Scholar] [CrossRef] [PubMed]
- Solverson, P.M.; Rumpler, W.V.; Leger, J.L.; Redan, B.W.; Ferruzzi, M.G.; Baer, D.J.; Castonguay, T.W.; Novotny, J.A. Blackberry Feeding Increases Fat Oxidation and Improves Insulin Sensitivity in Overweight and Obese Males. Nutrients 2018, 10, 1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, S.A.; Shah, M.A.; Ganai, S.A.; Ahmad, T.; Gani, M. Understanding the role of active components from plant sources in obesity management. J. Saudi. Soc. Agric. Sci. 2019, 18, 168–176. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Cazarin, C.B.B.; Ruiz, A.L.T.G.; Paulino, B.N.; Molina, G.; Pastore, G.M. Targeting flavonoids on modulation of metabolic syndrome. J. Funct. Foods 2020, 73, 104132. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, R.; Verschuren, L.; Morrison, M.; Zadelaar, S.; van Erk, M.J.; Wielinga, P.Y.; Kooistra, T. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis 2011, 218, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Jiang, B.; Li, B.; Li, Z.; Jiang, B.-H.; Chen, Y.C. Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability. Int. J. Nanomed. 2012, 7, 3951–3959. [Google Scholar]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant Potential Overviews of Secondary Metabolites (Polyphenols) in Fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gryko, K.; Wróblewska, A.M.; Jabłońska-Trypuć, A.; Karpowicz, D. Phenolic content, chemical composition and anti-/pro-oxidant activity of Gold Milenium and Papierowka apple peel extracts. Sci. Rep. 2020, 10, 14951. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; Oliveira, H.; Fernandes, I.; Simal-Gandara, J.; Perez-Gregorio, R. Recent advances in extracting phenolic compounds from food and their use in disease prevention and as cosmetics. Crit. Rev. Food Sci. Nutr. 2021, 61, 1130–1151. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative stress and vascular disease. Arter. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Virapongse, A.; Endress, B.A.; Gilmore, M.P.; Horn, C.; Romulo, C. Ecology, livelihoods, and management of the Mauritia flexuosa palm in South America. Glob. Ecol. Conserv. 2017, 10, 70–92. [Google Scholar] [CrossRef]
- Trujillo-Gonzalez, J.M.; Torres-Mora, M.A.; Santana-Castañeda, E. The Moriche palm (Mauritia flexuosa L. f) represents astrategic ecosystem. Orinoquía 2011, 15, 62–70. [Google Scholar] [CrossRef]
- Gragson, T.L. Pumé Exploitation of Mauritia flexuosa (Palmae) in the Llanos of Venezuela. J. Ethnobiol. 1995, 15, 177–188. [Google Scholar]
- Case, C.; Lares, M.; Palma, A.; Brito, S.; Pérez, E.; Schroeder, M. Blood glucose and serum lipid levels in the Venezuelan Warao tribe: Possible relationship with moriche fruit (Mauritia flexuosa L.) intake. Nut. Metabol. Cardiovasc. Dis. 2007, 17, e1–e2. [Google Scholar] [CrossRef]
- Martins, R.C.; Filgueiras, T.S.; de Albuquerque, U.P. Ethnobotany of Mauritia flexuosa (Arecaceae) in a Maroon Community in Central Brazil. Econ. Bot. 2011, 66, 91–98. [Google Scholar] [CrossRef]
- Horn, C.M.; Gilmore, M.P.; Endress, B.A. Ecological and socio-economic factors influencing aguaje (Mauritia flexuosa) resource management in two indigenous communities in the Peruvian Amazon. For. Ecol. Manag. 2012, 267, 93–103. [Google Scholar] [CrossRef]
- Lopez, J.; Vega-Gálvez, A.; Torres, M.J.; Lemus-Mondaca, R.; Quispe-Fuentes, I.; Di Scala, K. Effect of dehydration temperature on physico-chemical properties and antioxidant capacity of goldenberry (Physalis peruviana L.). Chil. J. Agric. Res. 2013, 73, 293–300. [Google Scholar] [CrossRef]
- Tolić, M.-T.; Jurčević, I.L.; Krbavčić, I.P.; Marković, K.; Vahčić, N. Phenolic Content, Antioxidant Capacity and Quality of Chokeberry (Aronia melanocarpa) Products. Food Technol. Biotechnol. 2015, 53, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Guo, Y.; Zhang, J.; Zhang, X.; Meng, Y. Effect of Different Drying Processes on the Physicochemical and Antioxidant Properties of Thinned Young Apple. Int. J. Food Eng. 2015, 11, 207–219. [Google Scholar] [CrossRef]
- Eran Nagar, E.; Okun, Z.; Shpigelman, A. Digestive fate of polyphenols: Updated view of the influence of chemical structure and the presence of cell wall material. Curr. Opin. Food Sci. 2020, 31, 38–46. [Google Scholar] [CrossRef]
- Starec, M.; Calabretti, A.; Berti, F.; Forzato, C. Oleocanthal Quantification Using 1H-NMR Spectroscopy and Polyphenols HPLC Analysis of Olive Oil from the Bianchera/Belica Cultivar. Molecules 2021, 26, 242. [Google Scholar] [CrossRef] [PubMed]
- Koolen, H.H.F.; da Silva, F.M.A.; Gozzo, F.C.; de Souza, A.Q.L.; de Souza, A.D.L. Antioxidant, antimicrobial activities and characterization of phenolic compounds from buriti (Mauritia flexuosa L. f.) by UPLC–ESI-MS/MS. Food Res. Int. 2013, 51, 467–473. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, D.M.; Siqueira, E.P.; Nunes, Y.R.F.; Cota, B.B. Flavonoids from leaves of Mauritia flexuosa. Rev. Bras. Farmacog. 2013, 23, 614–620. [Google Scholar] [CrossRef]
- Tauchen, J.; Bortl, L.; Huml, L.; Miksatkova, P.; Doskocil, I.; Marsik, P.; Villegas, P.P.P.; Flores, Y.B.; van Damme, P.; Lojka, B.; et al. Phenolic composition, antioxidant and anti-proliferative activities of edible and medicinal plants from the Peruvian Amazon. Rev. Bras. Farmacog. 2016, 26, 728–737. [Google Scholar] [CrossRef]
- Abreu-Naranjo, R.; Paredes-Moreta, J.G.; Granda-Albuja, G.; Iturralde, G.; González-Paramás, A.M.; Alvarez-Suarez, J.M. Bioactive compounds, phenolic profile, antioxidant capacity and effectiveness against lipid peroxidation of cell membranes of Mauritia flexuosa L. fruit extracts from three biomes in the Ecuadorian Amazon. Heliyon 2020, 6, e05211. [Google Scholar] [CrossRef]
- Mencarelli, F.; D’onofrio, C.; Bucci, S.; Baccelloni, S.; Cini, R.; Pica, G.; Bellincontro, A. Management of high-quality dehydrated grape in vinification to produce dry red wines. Food Chem. 2021, 338, 127623. [Google Scholar] [CrossRef]
- Lund, M.N. Reactions of plant polyphenols in foods: Impact of molecular structure. Trends Food Sci. Technol. 2021, 112, 241–251. [Google Scholar] [CrossRef]
- Nayeem, S.; Sundararajan, S.; Ashok, A.K.; Abusaliya, A.; Ramalingam, S. Effects of cooking on phytochemical and antioxidant properties of pigmented and non-pigmented rare Indian rice landraces. Biocatal. Agric. Biotechnol. 2021, 32, 101928. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costanzo, G.; Vitale, E.; Iesce, M.R.; Naviglio, D.; Amoresano, A.; Fontanarosa, C.; Spinelli, M.; Ciaravolo, M.; Arena, C. Antioxidant Properties of Pulp, Peel and Seeds of Phlegrean Mandarin (Citrus reticulata Blanco) at Different Stages of Fruit Ripening. Antioxidants 2022, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Wongnarat, C.; Srihanam, P. Phytochemical and Antioxidant Activity in Seeds and Pulp of Grape Cultivated in Thailand. Orient. J. Chem. 2017, 33, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Nakilcioğlu-Taş, E.; Ötleş, S. Influence of extraction solvents on the polyphenol contents, compositions, and antioxidant capacities of fig (Ficus carica L.) seeds. Acad. Bras. Cienc. 2021, 93, e20190526. [Google Scholar] [CrossRef]
- Cândido, T.L.N.; Silva, M.R.; Agostini-Costa, T.S. Bioactive compounds and antioxidant capacity of buriti (Mauritia flexuosa L.f.) from the Cerrado and Amazon biomes. Food Chem. 2015, 177, 313–319. [Google Scholar] [CrossRef]
- da Rocha Romero, A.B.; de Carvalho e Martins, M.; Moreira Nunes, P.H.; Trindale Ferreira, N.R.; de Lima, A.; de Assis, R.C.; Araújo, E.M. La actividad antioxidante in vitro e in vivo de la fruta burití (Mauritia flexuosa L.f.). Nutr. Hosp. 2015, 32, 2153–2161. [Google Scholar]
- Chong, C.H.; Law, C.L.; Figiel, A.; Wojdyło, A.; Oziembłowski, M. Colour, phenolic content and antioxidant capacity of some fruits dehydrated by a combination of different methods. Food Chem. 2013, 141, 3889–3896. [Google Scholar] [CrossRef]
- Kidoń, M.; Grabowska, J. Bioactive compounds, antioxidant activity, and sensory qualities of red-fleshed apples dried by different methods. LWT 2021, 136, 110302. [Google Scholar] [CrossRef]
- Mejia-Meza, E.I.; Yáñez, J.A.; Remsberg, C.M.; Takemoto, J.K.; Davies, N.M.; Rasco, B.; Clary, C. Effect of dehydration on raspberries: Polyphenol and anthocyanin retention, antioxidant capacity, and antiadipogenic activity. J. Food Sci. 2010, 75, 5–12. [Google Scholar] [CrossRef]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol oxidases in crops: Biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 2017, 18, 377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liang, L.; Pan, X.; Lao, F.; Liao, X.; Wu, J. Alterations of phenolic compounds in red raspberry juice induced by high-hydrostatic-pressure and high-temperature short-time processing. Innov. Food Sci. Emerg. Technol. 2021, 67, 102569. [Google Scholar] [CrossRef]
- Alfaro, S.; Mutis, A.; Quiroz, A.; Seguel, I.; Scheuermann, E. Effects of Drying Techniques on Murtilla Fruit Polyphenols and Antioxidant Activity. J. Food Res. 2014, 3, 73. [Google Scholar] [CrossRef]
- Wan Mahmood, W.M.A.; Lorwirachsutee, A.; Theodoropoulos, C.; Gonzalez-Miquel, M. Polyol-Based Deep Eutectic Solvents for Extraction of Natural Polyphenolic Antioxidants from Chlorella vulgaris. ACS Sustain. Chem. Eng. 2019, 7, 5018–5026. [Google Scholar] [CrossRef]
- Cirilli, M.; Bellincontro, A.; De Santis, D.; Botondi, R.; Colao, M.C.; Muleo, R.; Mencarelli, F. Temperature and water loss affect ADH activity and gene expression in grape berry during postharvest dehydration. Food Chem. 2012, 132, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Panceri, C.P.; Gomes, T.M.; De Gois, J.S.; Borges, D.L.G.; Bordignon-Luiz, M.T. Effect of dehydration process on mineral content, phenolic compounds and antioxidant activity of Cabernet Sauvignon and Merlot grapes. Food Res. Int. 2013, 54, 1343–1350. [Google Scholar] [CrossRef]
- Marquez, A.; Serratosa, M.P.; Lopez-Toledano, A.; Merida, J. Colour and phenolic compounds in sweet red wines from Merlot and Tempranillo grapes chamber-dried under controlled conditions. Food Chem. 2012, 130, 111–120. [Google Scholar] [CrossRef]
- Tepe, F.; Tepe, T.; Ekinci, A. Impact of air temperature on drying characteristics and some bioactive properties of kiwi fruit slices. Chem. Ind. Chem. Eng. 2021, 7, 26. [Google Scholar] [CrossRef]
- Aguilera, Y.; Dueñas, M.; Estrella, I.; Hernández, T.; Benitez, V.; Esteban, R.M.; Martín-Cabrejas, M.A. Evaluation of phenolic profile and antioxidant properties of Pardina lentil as affected by industrial dehydration. J. Agric. Food Chem. 2010, 58, 10101–10108. [Google Scholar] [CrossRef]
- Gupta, A.K.; Dhua, S.; Sahu, P.P.; Abate, G.; Mishra, P.; Mastinu, A. Variation in Phytochemical, Antioxidant and Volatile Composition of Pomelo Fruit (Citrus grandis (L.) Osbeck) during Seasonal Growth and Development. Plants 2021, 10, 1941. [Google Scholar] [CrossRef]
- Carmona-Hernandez, J.C.; Taborda-Ocampo, G.; Gonzalez-Correa, C.H. Folin-Ciocalteu Reaction Alternatives for Higher Polyphenol Quantitation in Colombian Passion Fruits. Int. J. Food Sci. 2021, 2021, 8871301. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).