A New Extraction System Based on Isopropyl Salicylate and Trioctylphosphine Oxide for Separating Alkali Metals
Abstract
:1. Introduction
2. Results and Discussion
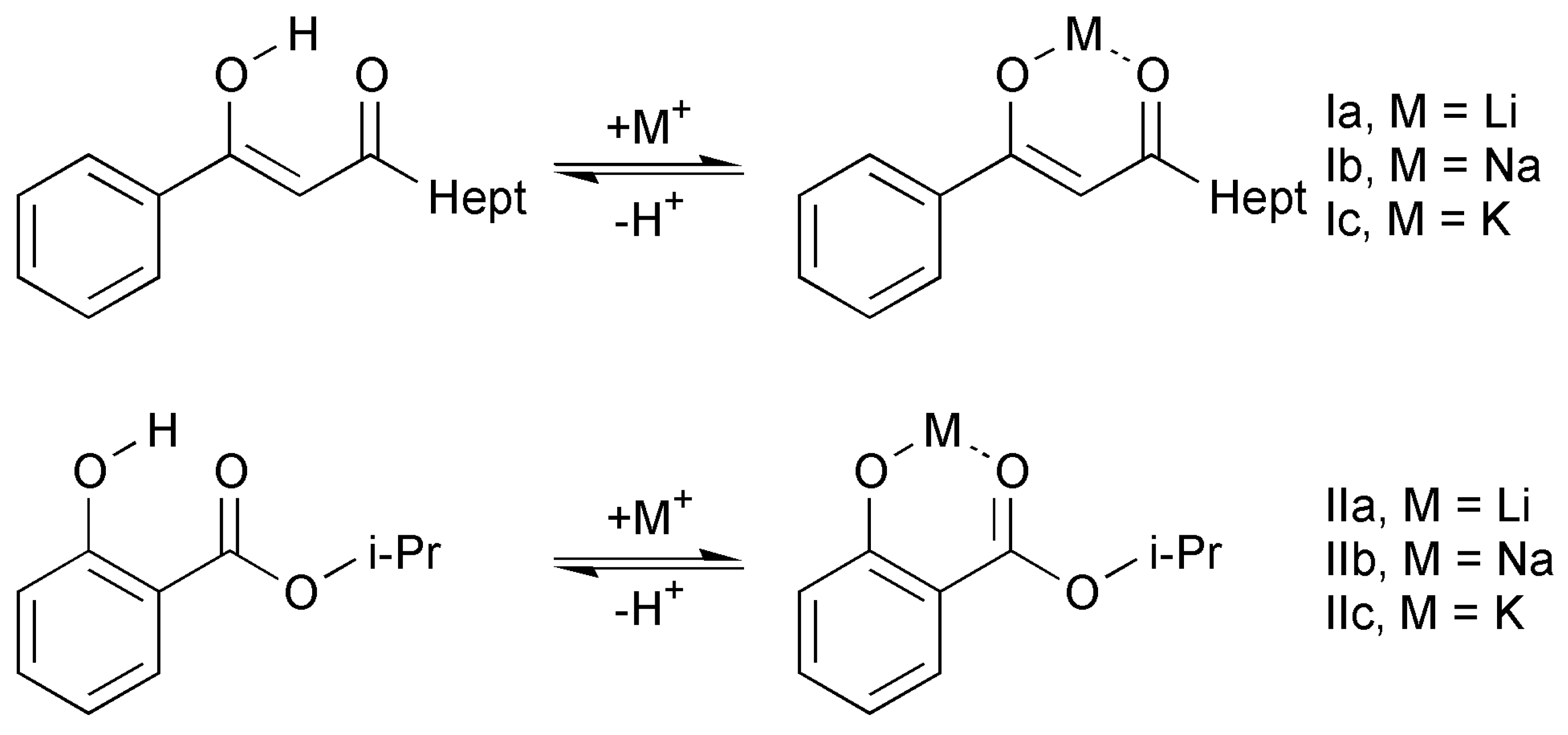
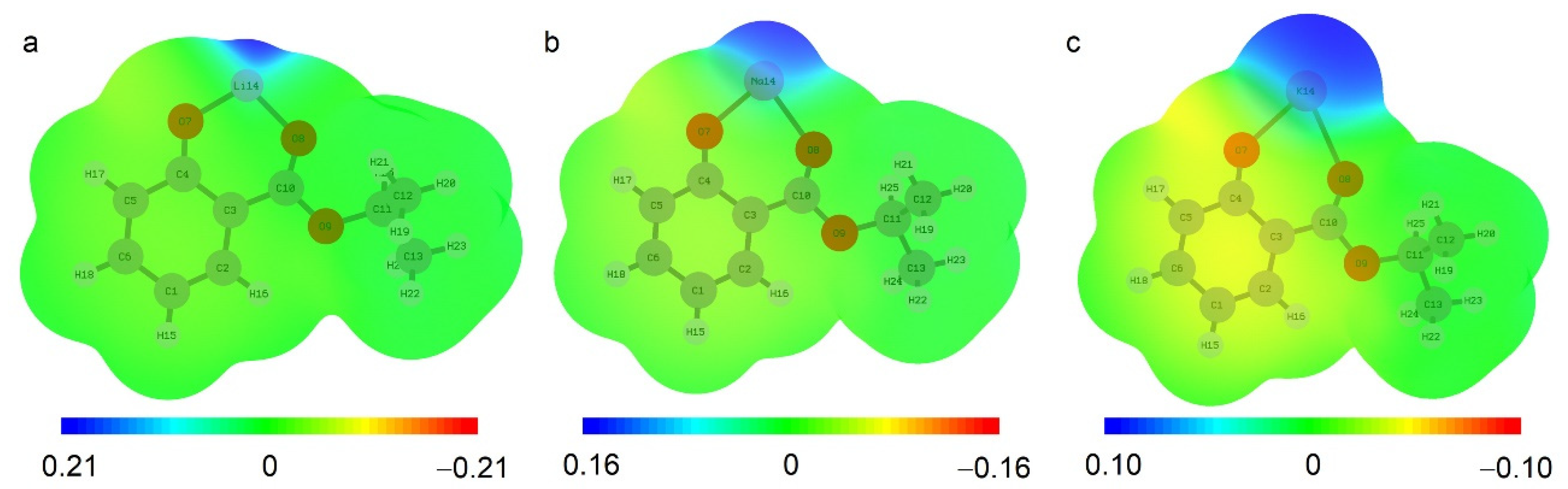
2.1. Quantum-Chemical Calculations of Alkali Metals Salicylates
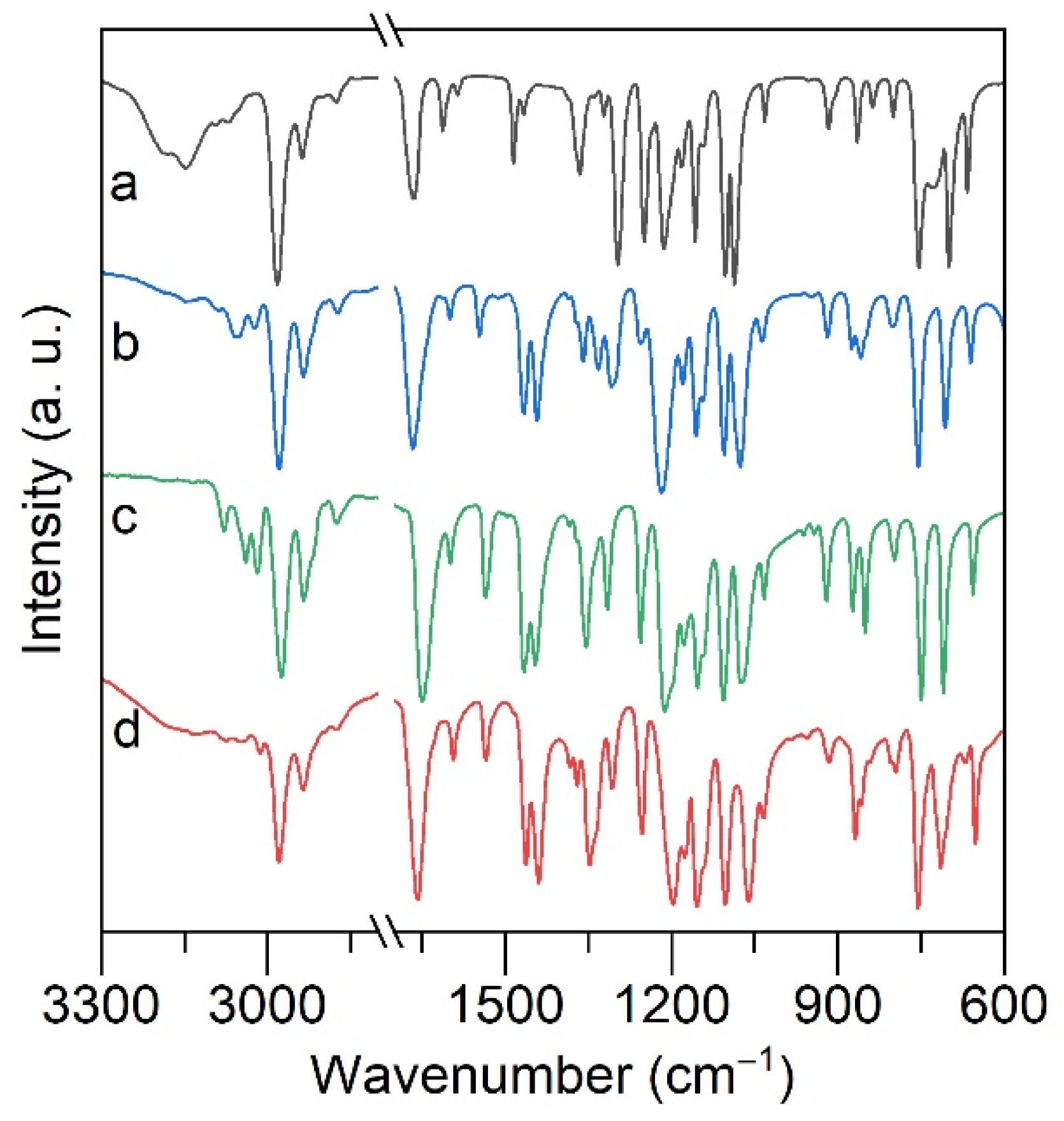
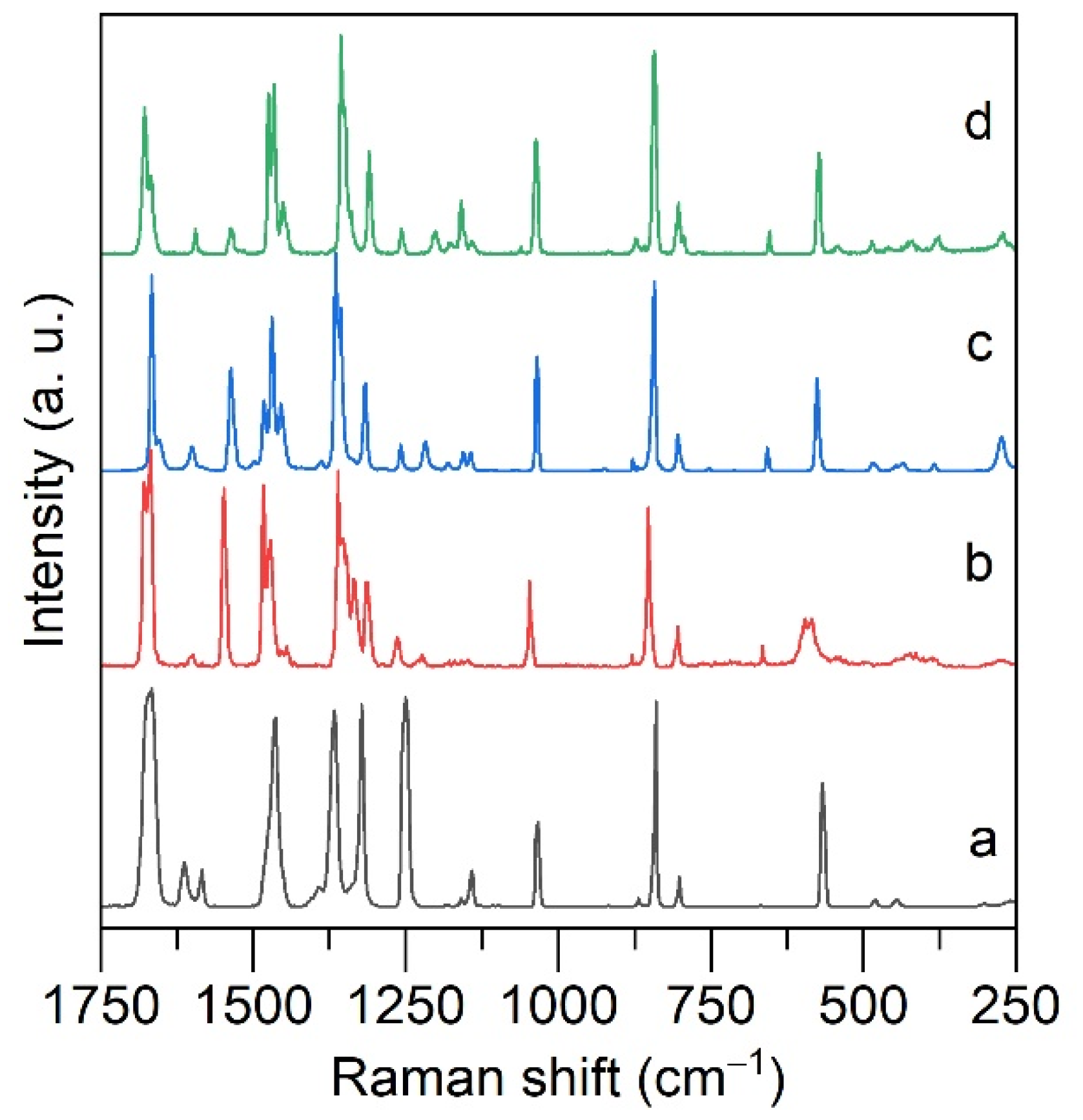
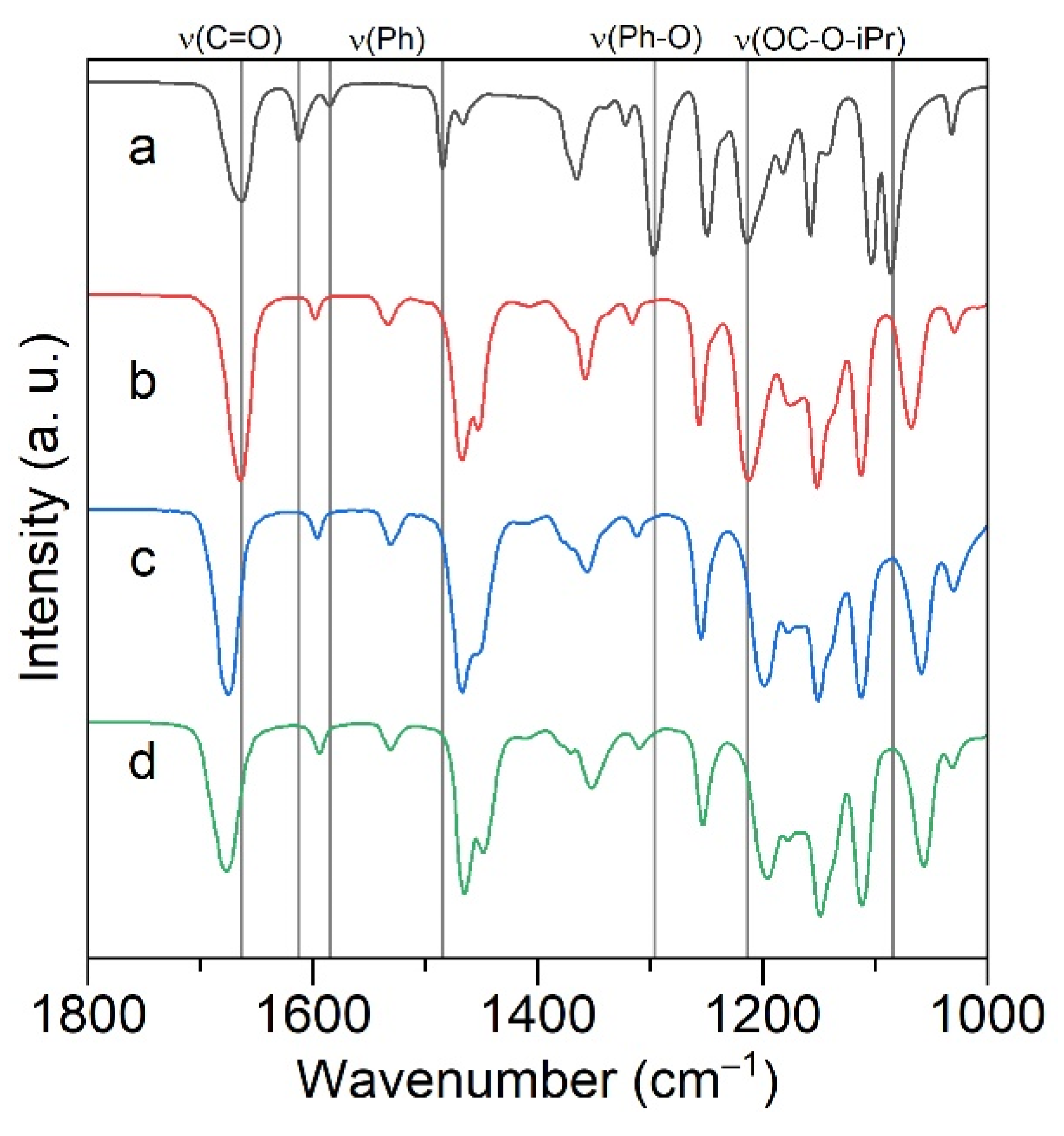
2.2. ATR FTIR and Raman Spectroscopy of Alkali Metal Salicylates
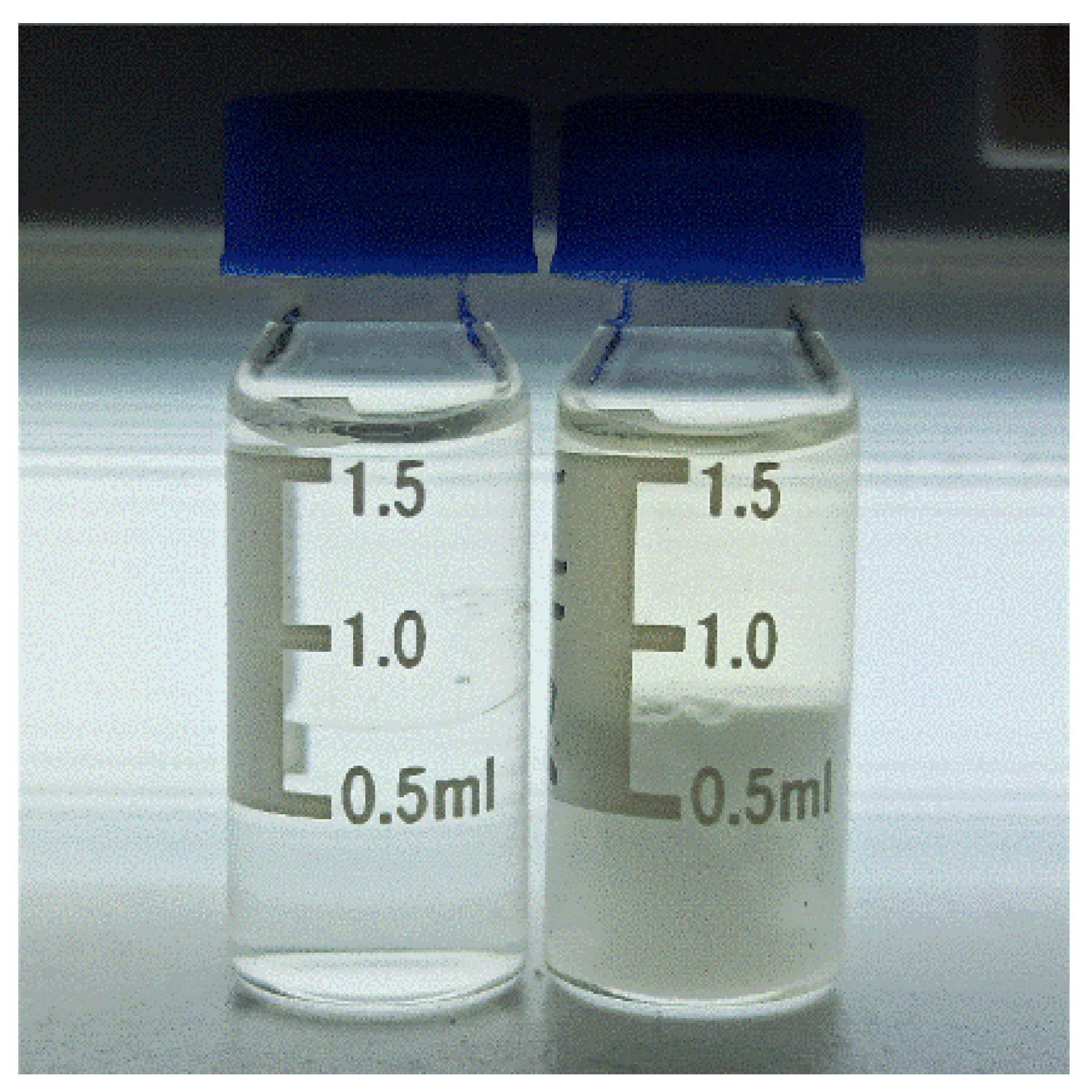
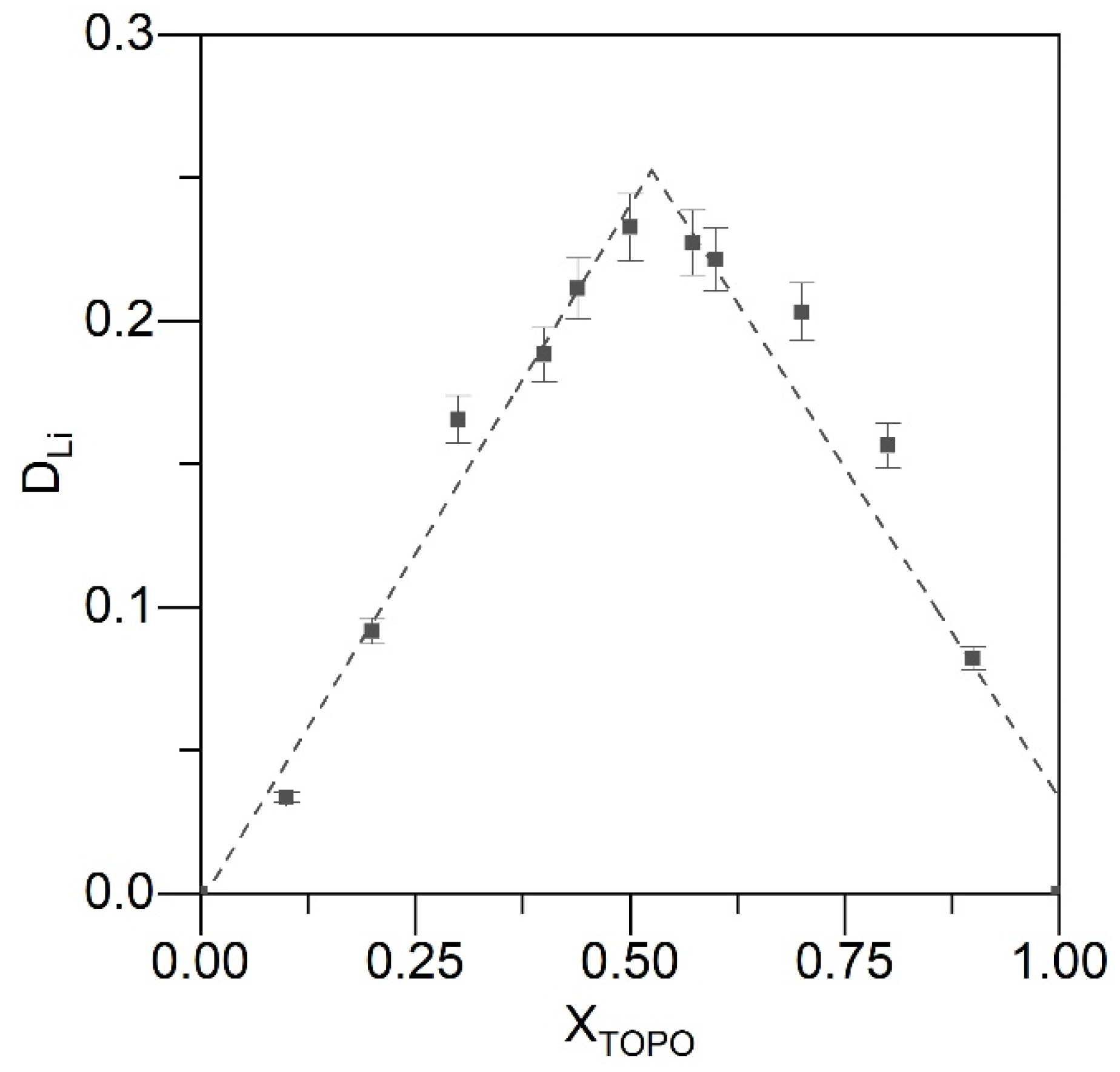
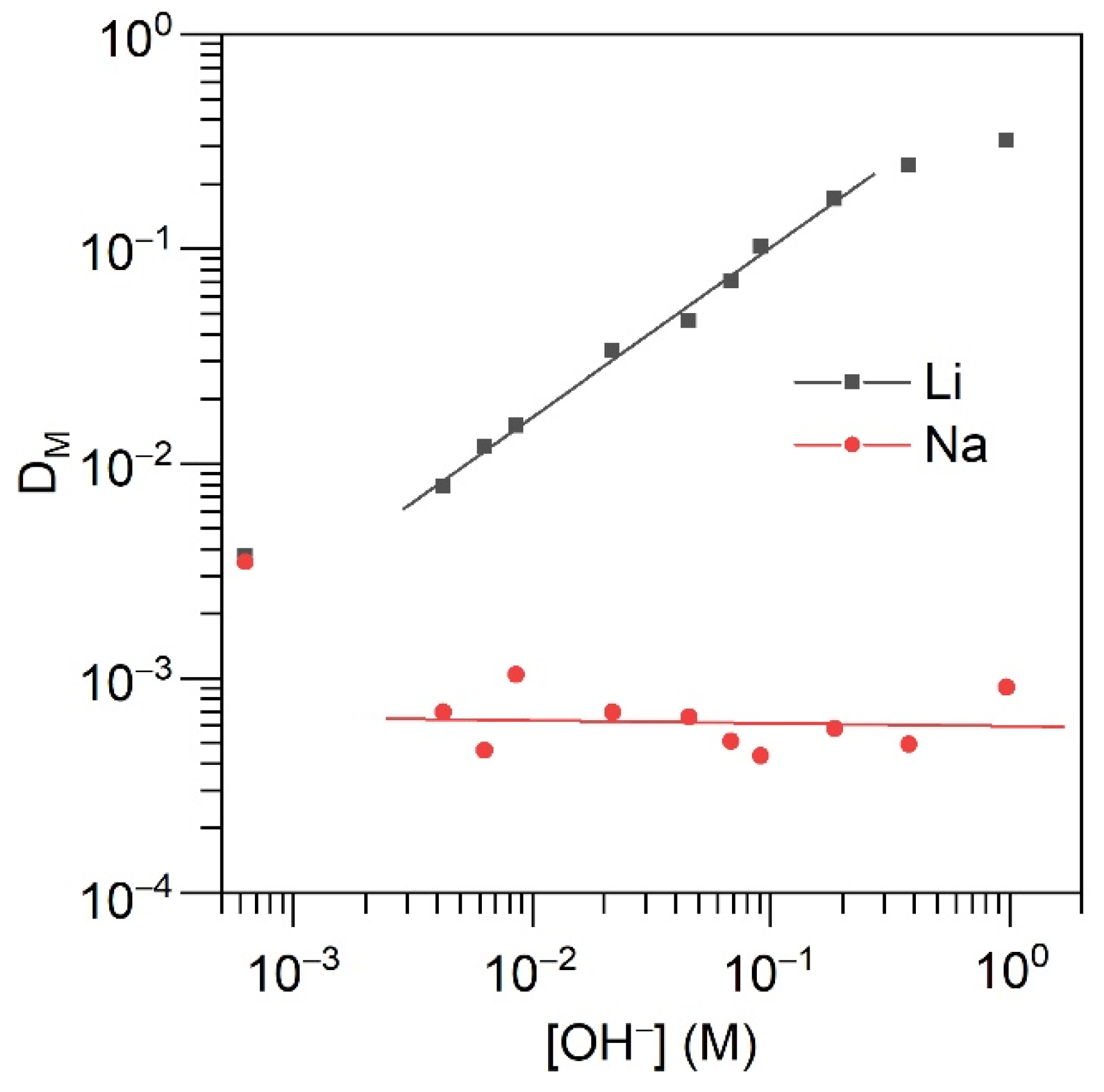
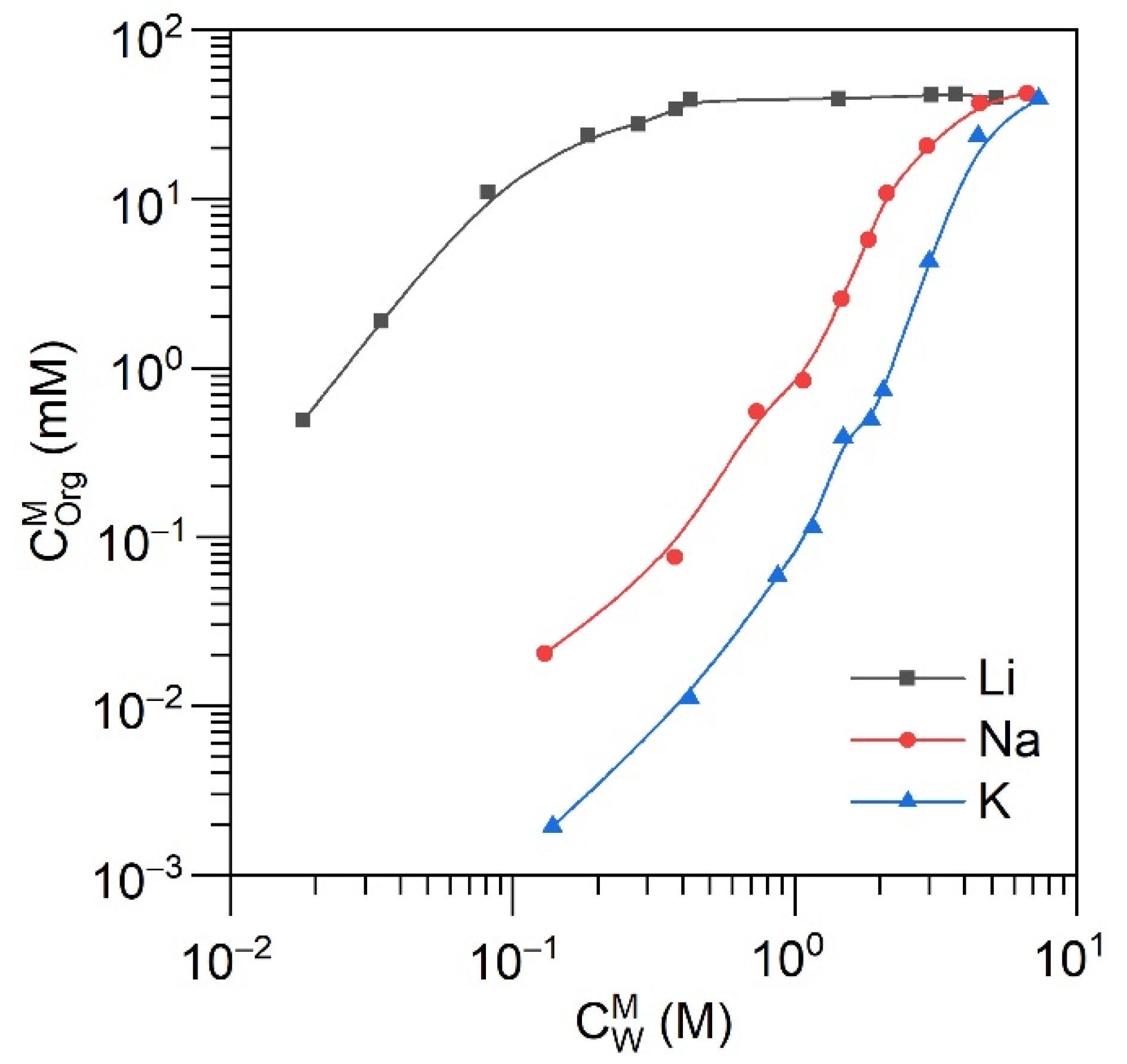
2.3. Solvent Extraction
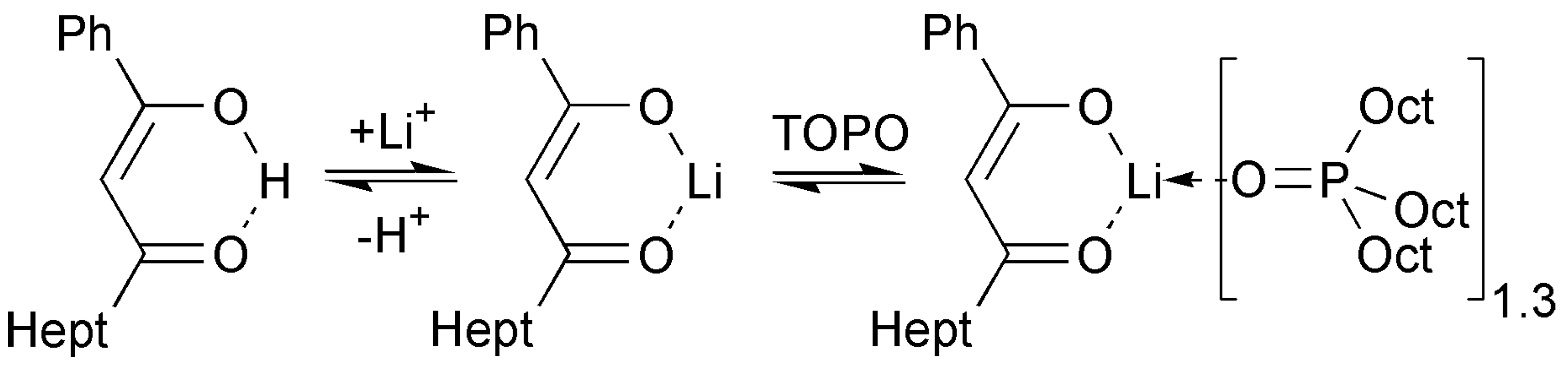
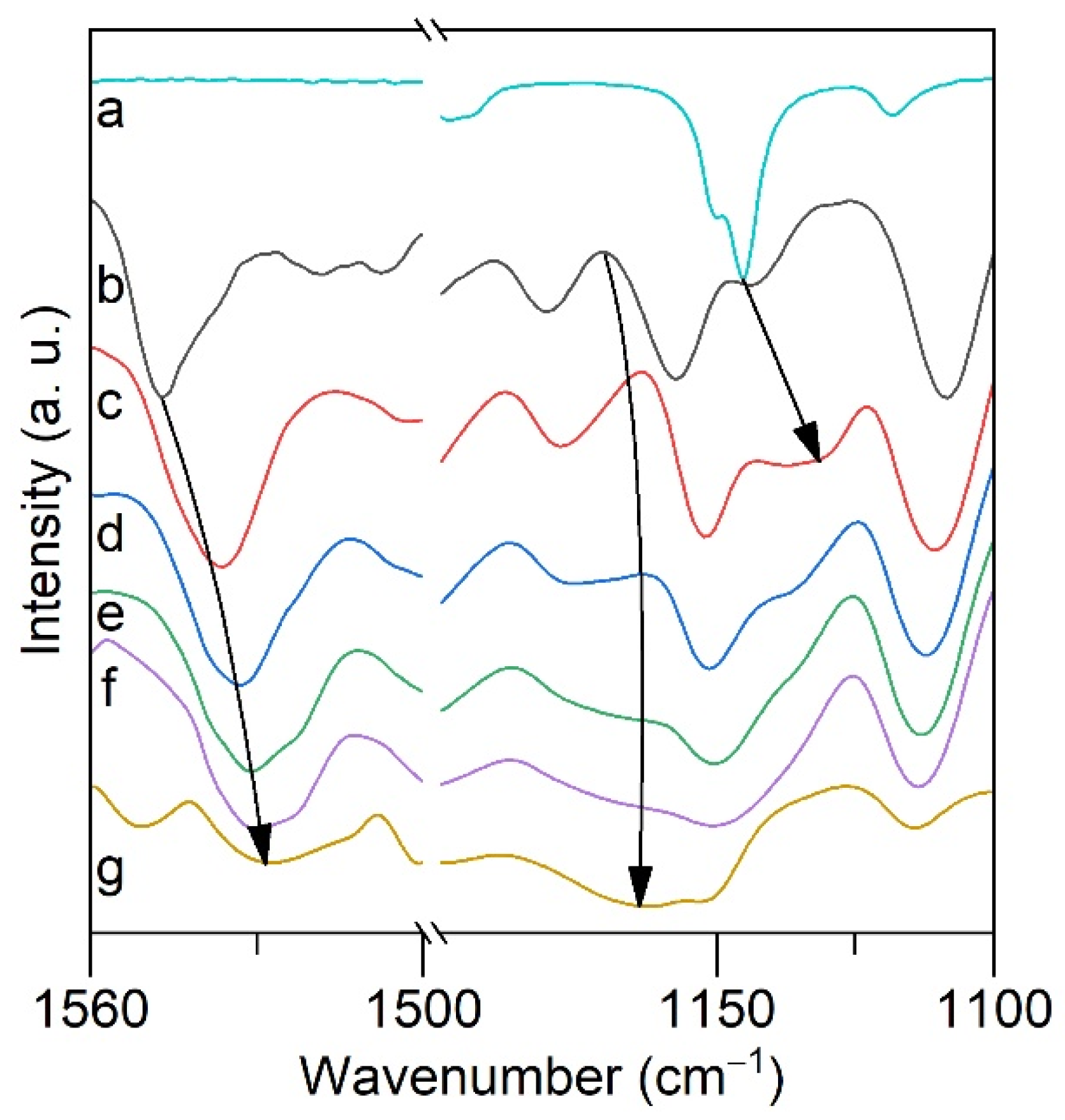
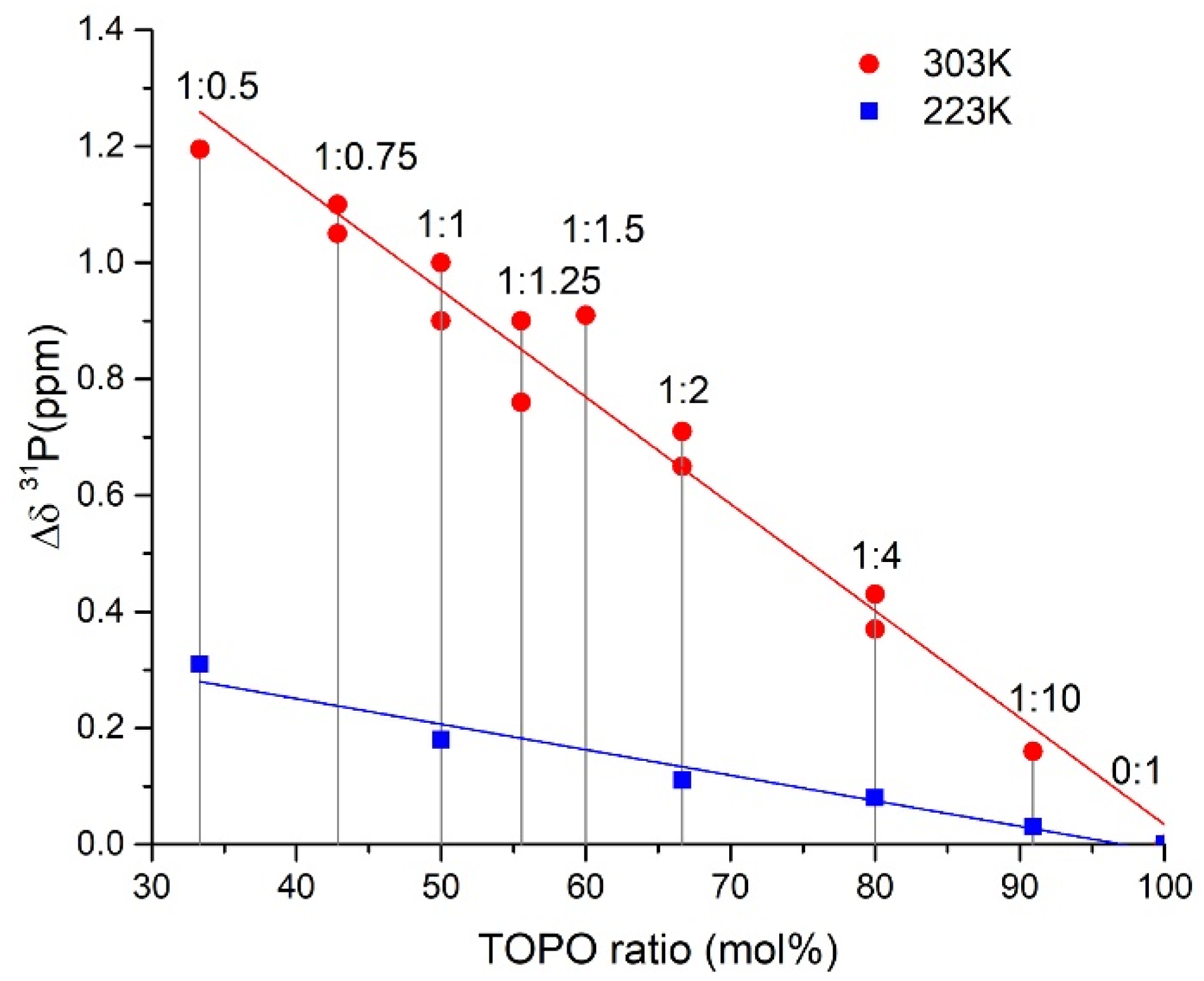
2.4. ATR FTIR- and NMR-Spectroscopy of Extracts, Quantum Chemical Calculations of Lithium Salicylate with TOPO
3. Materials and Methods
3.1. Quantum Chemical Calculations
3.2. Extraction
3.3. ATR FT-IR Spectra
3.4. Raman Spectra
3.5. NMR Spectra
3.6. Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Vikström, H.; Davidsson, S.; Höök, M. Lithium Availability and Future Production Outlooks. Appl. Energy 2013, 110, 252–266. [Google Scholar] [CrossRef] [Green Version]
- Lithium Market Size, Share & Growth|Industry Report, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/lithium-market (accessed on 8 April 2021).
- Swain, B. Recovery and Recycling of Lithium: A Review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Deng, Z.; Ma, Z.; Li, Y.; Li, Y.; Chen, L.; Yang, X.; Wang, H.E.; Su, B.L. Boosting Lithium-Ion Storage Capability in CuO Nanosheets via Synergistic Engineering of Defects and Pores. Front. Chem. 2018, 6, 428. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Lee, M.S. A Review on the Separation of Lithium Ion from Leach Liquors of Primary and Secondary Resources by Solvent Extraction with Commercial Extractants. Processes 2018, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Kalmykov, D.; Makaev, S.; Golubev, G.; Eremeev, I.; Vasilevsky, V.; Song, J.; He, T.; Volkov, A. Operation of Three-Stage Process of Lithium Recovery from Geothermal Brine: Simulation. Membranes 2021, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Ryabtsev, A.D.; Kotsupalo, N.P.; Menzheres, L.T. Prospective of Complex Processing of Lithium-Bearing Raw Brines of Sodium Chloride and Mixed Types. Theor. Found. Chem. Eng. 2015, 49, 471–480. [Google Scholar] [CrossRef]
- Torrejos, R.E.C.; Nisola, G.M.; Song, H.S.; Limjuco, L.A.; Lawagon, C.P.; Parohinog, K.J.; Koo, S.; Han, J.W.; Chung, W.J. Design of Lithium Selective Crown Ethers: Synthesis, Extraction and Theoretical Binding Studies. Chem. Eng. J. 2017, 326, 921–933. [Google Scholar] [CrossRef]
- Chen, W.; Tian, Y.; Hu, C.; Zhao, Z.; Xu, L.; Tong, B. Theoretical and Extraction Studies on the Selectivity of Lithium with 14C4 Derivatives. New J. Chem. 2020, 44, 20341–20350. [Google Scholar] [CrossRef]
- Li, Z.; Binnemans, K. Opposite Selectivities of Tri-n-butyl Phosphate and Cyanex 923 in Solvent Extraction of Lithium and Magnesium. AIChE J. 2021, 67, 17219. [Google Scholar] [CrossRef]
- Bai, R.; Wang, J.; Cui, L.; Yang, S.; Qian, W.; Cui, P.; Zhang, Y. Efficient Extraction of Lithium Ions from High Mg/Li Ratio Brine through the Synergy of TBP and Hydroxyl Functional Ionic Liquids. Chin. J. Chem. 2020, 38, 1743–1751. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Li, W.; Zhou, Y. The Key Factors and Mechanism Study on Lithium Extraction by TBP-FeCl3 Extraction System. Chem. Phys. Lett. 2020, 754, 137740. [Google Scholar] [CrossRef]
- Su, H.; Li, Z.; Zhang, J.; Liu, W.; Zhu, Z.; Wang, L.; Qi, T. Combining Selective Extraction and Easy Stripping of Lithium Using a Ternary Synergistic Solvent Extraction System through Regulation of Fe3+ Coordination. ACS Sustain. Chem. Eng. 2020, 8, 1971–1979. [Google Scholar] [CrossRef]
- Xiang, W.; Liang, S.; Zhou, Z.; Qin, W.; Fei, W. Lithium Recovery from Salt Lake Brine by Counter-Current Extraction Using Tributyl Phosphate/FeCl3 in Methyl Isobutyl Ketone. Hydrometallurgy 2017, 171, 27–32. [Google Scholar] [CrossRef]
- Pranolo, Y.; Zhu, Z.; Cheng, C.Y. Separation of Lithium from Sodium in Chloride Solutions Using SSX Systems with LIX 54 and Cyanex 923. Hydrometallurgy 2015, 154, 33–39. [Google Scholar] [CrossRef]
- Harvianto, G.R.; Kim, S.H.; Ju, C.S. Solvent Extraction and Stripping of Lithium Ion from Aqueous Solution and Its Application to Seawater. Rare Met. 2016, 35, 948–953. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Rui, H.; Shi, D.; Peng, X.; Ji, L.; Song, X. Lithium Recovery from Effluent of Spent Lithium Battery Recycling Process Using Solvent Extraction. J. Hazard. Mater. 2020, 398, 122840. [Google Scholar] [CrossRef] [PubMed]
- Masmoudi, A.; Zante, G.; Trébouet, D.; Barillon, R.; Boltoeva, M. Solvent Extraction of Lithium Ions Using Benzoyltrifluoroacetone in New Solvents. Sep. Purif. Technol. 2021, 255, 117653. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Shi, D.; Li, J.; Peng, X.; Nie, F. Selective Extraction of Lithium from Alkaline Brine Using HBTA-TOPO Synergistic Extraction System. Sep. Purif. Technol. 2017, 188, 167–173. [Google Scholar] [CrossRef]
- Hano, T.; Matsumoto, M.; Ohtake, T.; Egashira, N.; Hori, F. Recovery of Lithium from Geothermal Water by Solvent Extraction Technique. Solvent Extr. Ion Exch. 1992, 10, 195–206. [Google Scholar] [CrossRef]
- Kim, Y.S.; In, G.; Choi, J.M. Chemical Equilibrium and Synergism for Solvent Extraction of Trace Lithium with Thenoyltrifluoroacetone in the Presence of Trioctylphosphine Oxide. Bull. Korean Chem. Soc. 2003, 24, 1495–1500. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Binnemans, K. Selective Removal of Magnesium from Lithium-rich Brine for Lithium Purification by Synergic Solvent Extraction Using Β-diketones and Cyanex 923. AIChE J. 2020, 66, e16246. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Zhao, Z.; Ghahreman, A. Novel Approaches for Lithium Extraction from Salt-Lake Brines: A Review. Hydrometallurgy 2019, 187, 81–100. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Wang, W.; Cui, H.; Liu, W.; Liu, Y. Extraction Mechanism of Rare Earths from Chloride Acidic Solution with Ammonium-Bifunctionalized Ionic Liquid Extractants. Sci. China Chem. 2016, 59, 532–537. [Google Scholar] [CrossRef]
- Swain, B. Separation and Purification of Lithium by Solvent Extraction and Supported Liquid Membrane, Analysis of Their Mechanism: A Review. J. Chem. Technol. Biotechnol. 2016, 91, 2549–2562. [Google Scholar] [CrossRef]
- Pearson, R.G.; Songstad, J. Application of the Principle of Hard and Soft Acids and Bases to Organic Chemistry. J. Am. Chem. Soc. 1967, 89, 1827–1836. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infra-Red Spectra of Complex Molecules; Springer: Berlin/Heidelberg, Germany, 1975. [Google Scholar]
- Shuvaev, S.; Bushmarinov, I.S.; Sinev, I.; Dmitrienko, A.O.; Lyssenko, K.A.; Baulin, V.; Grünert, W.; Tsivadze, A.Y.; Kuzmina, N. Copper(II) Complexes with Aromatic o-Phosphorylated Phenols—Synthesis, Crystal Structures, and X-ray Photoelectron Spectroscopy. Eur. J. Inorg. Chem. 2013, 2013, 4823–4831. [Google Scholar] [CrossRef]
- Laurence, C.; Berthelot, M.; Graton, J. The Chemistry of Phenols, 2 Volume Set; John Wiley & Sons, Ltd.: Weinheim, Germany, 2004; Volume 2, ISBN 978-0-470-86945-1. [Google Scholar]
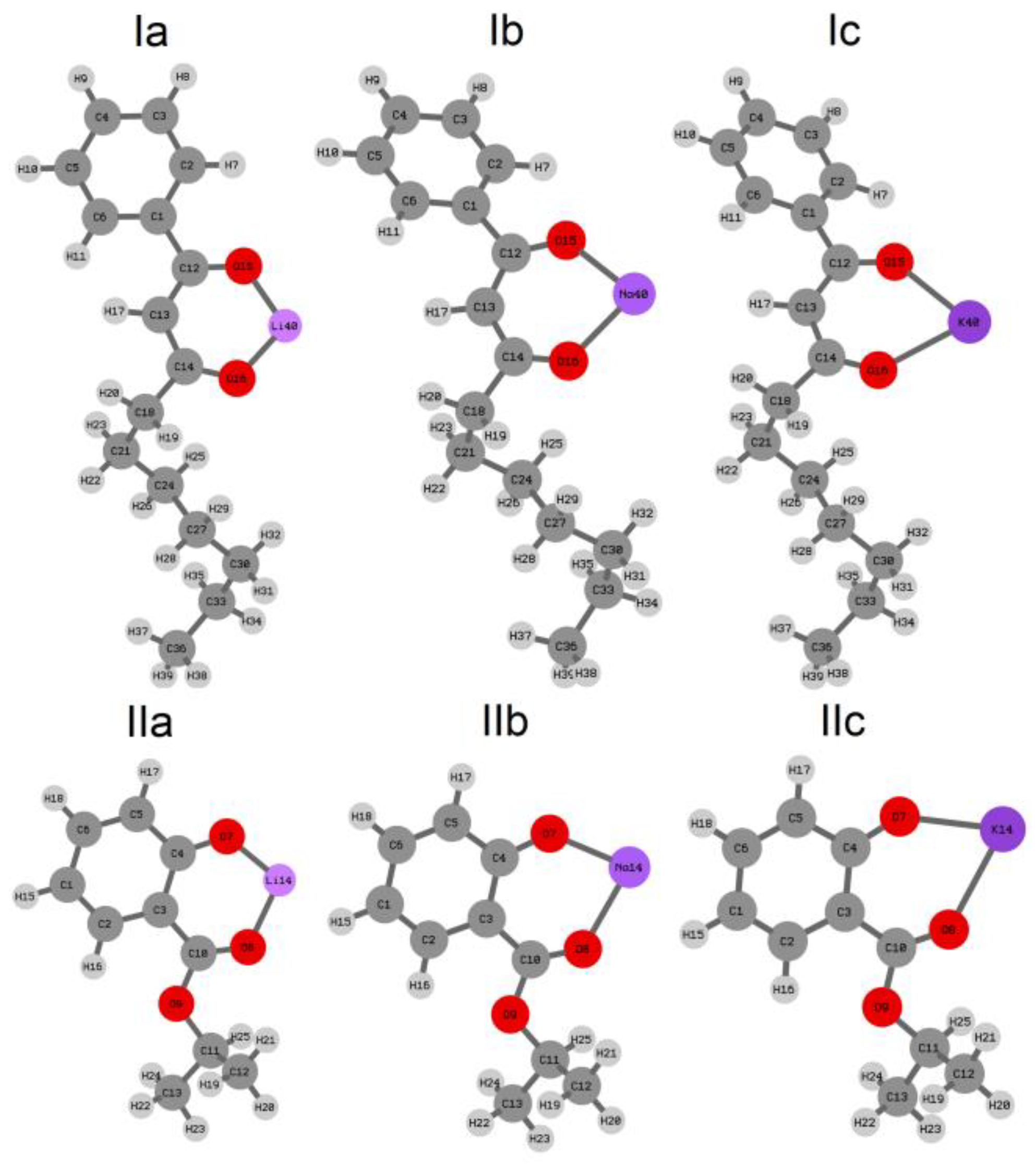
| M-O1 | M-O2 * | O1-M-O2 | |
|---|---|---|---|
| Ia | 1.775 | 1.781 | 106.55 |
| Ib | 2.134 | 2.140 | 88.83 |
| Ic | 2.446 | 2.454 | 74.26 |
| IIa | 1.733 | 1.805 | 103.99 |
| IIb | 2.088 | 2.163 | 84.86 |
| IIc | 2.374 | 2.505 | 71.06 |
| Li+ | Na+ | K+ |
|---|---|---|
| 0.455 | 37.9 | 107 |
| CHL+TOPO mol L−1 | DLi | DNa | DK | βLi/Na | βLi/K |
|---|---|---|---|---|---|
| 0.2 | 6.2500 | 0.0042 | 0.0008 | 1.5·103 | 7.6·103 |
| ν(C=O) | ν(Ph) | ν(Ph-O) | ν(OC-OiPr) | ν(OCO-iPr) | |
|---|---|---|---|---|---|
| HL | 1665 | 1613, 1585, 1485 | 1300 | 1213 | 1085 |
| LiL | 1668 | 1600, 1548, 1444 | 1309, 1332 | 1222 | 1078 |
| Li(L)TOPO | 1664 | 1598, 1533, 1453 | 1316 | 1212 | 1067 |
| NaL | 1651 | 1599, 1536, 1446 | 1315 | 1212 | 1075 |
| Na(L)TOPO | 1676 | 1596, 1531, 1452 | 1311 | 1199 | 1059 |
| KL | 1658 | 1600, 1547, 1443 | 1308 | 1200 | 1064 |
| K(L)TOPO | 1677 | 1595, 1531, 1448 | 1309 | 1196 | 1057 |
| Li-O1 | Li-O2 | Ph-O1 | C=O2 | |
|---|---|---|---|---|
| LiL | 1.745 | 1.804 | 1.289 | 1.248 |
| Li(L)TOPO | 1.817 | 1.864 | 1.285 | 1.238 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsivadze, A.Y.; Bezdomnikov, A.A.; Baulin, V.E.; Demina, L.I.; Birin, K.P.; Baulin, D.V.; Rogacheva, Y.I. A New Extraction System Based on Isopropyl Salicylate and Trioctylphosphine Oxide for Separating Alkali Metals. Molecules 2022, 27, 3051. https://doi.org/10.3390/molecules27103051
Tsivadze AY, Bezdomnikov AA, Baulin VE, Demina LI, Birin KP, Baulin DV, Rogacheva YI. A New Extraction System Based on Isopropyl Salicylate and Trioctylphosphine Oxide for Separating Alkali Metals. Molecules. 2022; 27(10):3051. https://doi.org/10.3390/molecules27103051
Chicago/Turabian StyleTsivadze, Aslan Yu., Alexey A. Bezdomnikov, Vladimir E. Baulin, Liudmila I. Demina, Kirill P. Birin, Dmitriy V. Baulin, and Yuliana I. Rogacheva. 2022. "A New Extraction System Based on Isopropyl Salicylate and Trioctylphosphine Oxide for Separating Alkali Metals" Molecules 27, no. 10: 3051. https://doi.org/10.3390/molecules27103051
APA StyleTsivadze, A. Y., Bezdomnikov, A. A., Baulin, V. E., Demina, L. I., Birin, K. P., Baulin, D. V., & Rogacheva, Y. I. (2022). A New Extraction System Based on Isopropyl Salicylate and Trioctylphosphine Oxide for Separating Alkali Metals. Molecules, 27(10), 3051. https://doi.org/10.3390/molecules27103051






