Spotlight on the Life Cycle of Acrylamide-Based Polymers Supporting Reductions in Environmental Footprint: Review and Recent Advances
Abstract
:1. Introduction
- Prevention: Prevent waste formation instead of treating or cleaning waste after it has been created. Roger Sheldon described the concept of the E-factor as a dimensionless number which measures the weight of waste coproduced with the weight of desired product [14].
- Atom economy: This concept was developed by Barry Trost in a way to shift initial paradigms focusing on measuring reaction efficiency by calculating the yield to the efficiency of the incorporation of atoms from reactants to desired product [15].
- Less hazardous chemical syntheses: Synthetic methods should be designed to use and generate substances that possess little to no toxicity to human health and environment.
- Catalysis: Catalytic reagents (as selective as possible) are superior to stoichiometric reagents. It lies in the prevention, atom economy and design for energy efficiency principles previously described where the catalyst can increase the selectivity and kinetic energy of a reaction while minimizing the waste generation and reaction temperature. The most exquisite catalysts are the enzymes which are particularly effective at enhancing selectivity with simple or complex substrates under mild conditions.
- Safer solvents and auxiliaries: The use of auxiliary substances (e.g., solvents, separation agents, etc.) should be made unnecessary wherever possible and innocuous when used.
- Design for energy efficiency: Energy requirements should be recognized for their environmental and economic impacts and should be minimized. Synthetic methods should be conducted at ambient temperature and pressure.
- Use of renewable feedstocks: A raw material or feedstock should be renewable rather than depleting whenever technically and economically practicable.
2. A Review of Raw Material Sourcing Options
2.1. Raw Materials
- Acrylamide
- Acrylic acid and derivatives
2.2. Emerging Technologies
2.3. Circular Economy and Biobased Mass Balance Approach
3. Sustainable Manufacturing Processes and Polymers
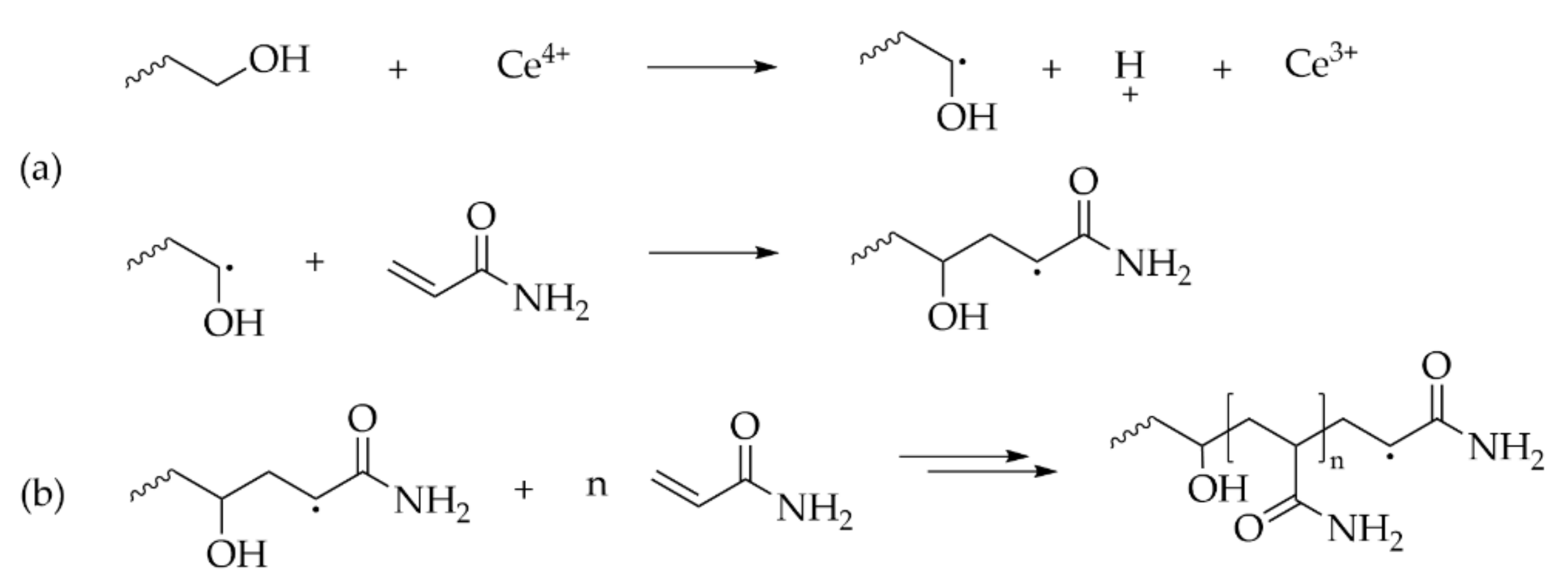
3.1. Radical (Co)Polymerization of Acrylamide
3.2. Homogeneous Water Polymerization
3.3. Gel Polymerization
3.4. Heterogeneous Inverse Emulsion Polymerization
4. Reduction of Water Consumption and CO2 Emissions: Considering end User Benefits
4.1. Introduction: Handprint
4.2. Water Treatment
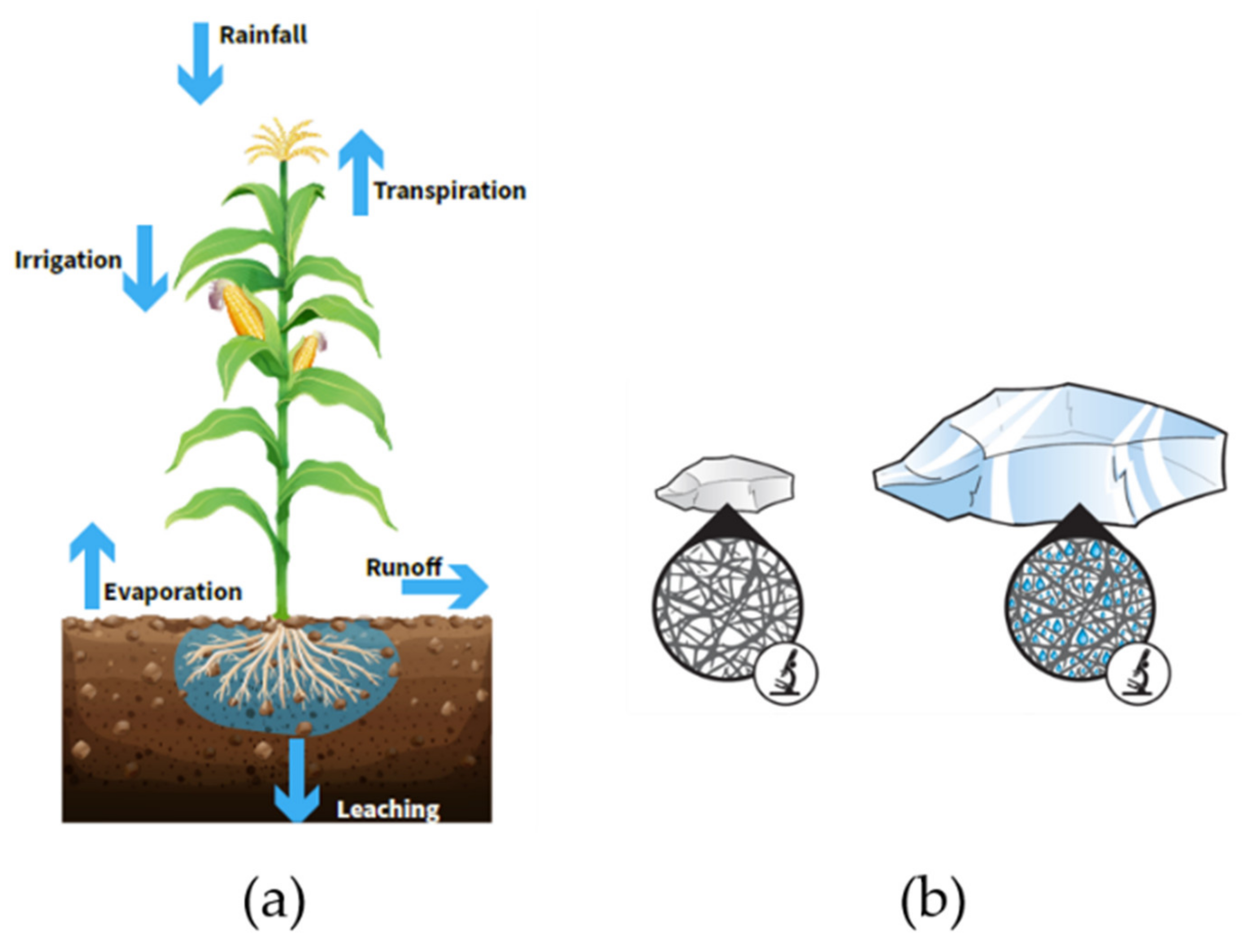
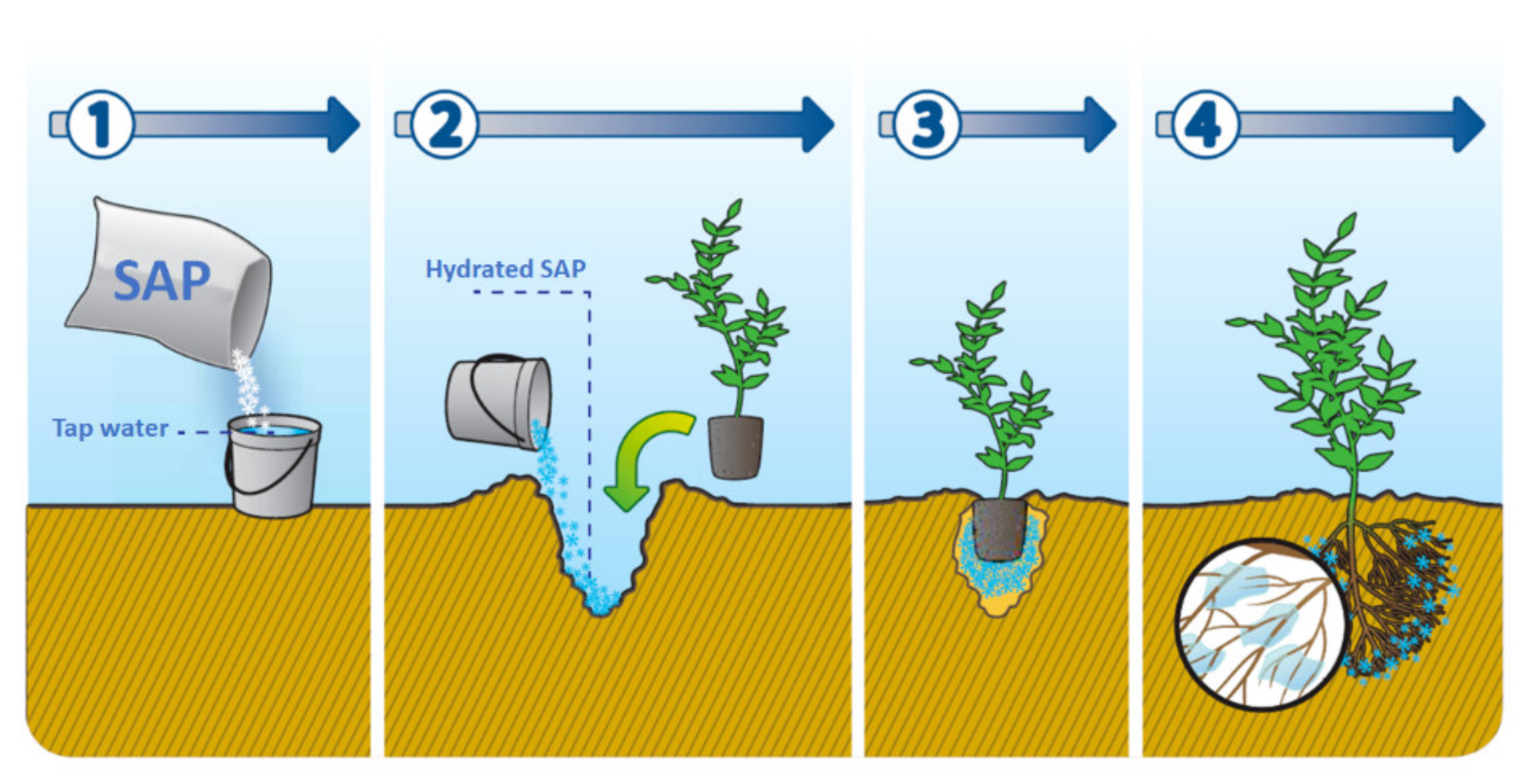
4.3. Agriculture
4.4. Improved Oil and Gas Recovery
5. Environmental Fate of Polymers
5.1. Current Framework and Procedures for the Evaluation of Polymer Biodegradation and Challenges
5.2. State-of-the-Art and Discussion
6. Macromolecular Engineering to Favor Biodegradability
- Copolymerization with biodegradable moieties
- Blending natural and synthetic polymers
- Introducing cleavable units within the backbone of the polymer
6.1. Copolymerization with Degradable Monomers
6.2. Blend of Natural and Synthetic Polymers
6.2.1. Interpenetrated Network (IPN)
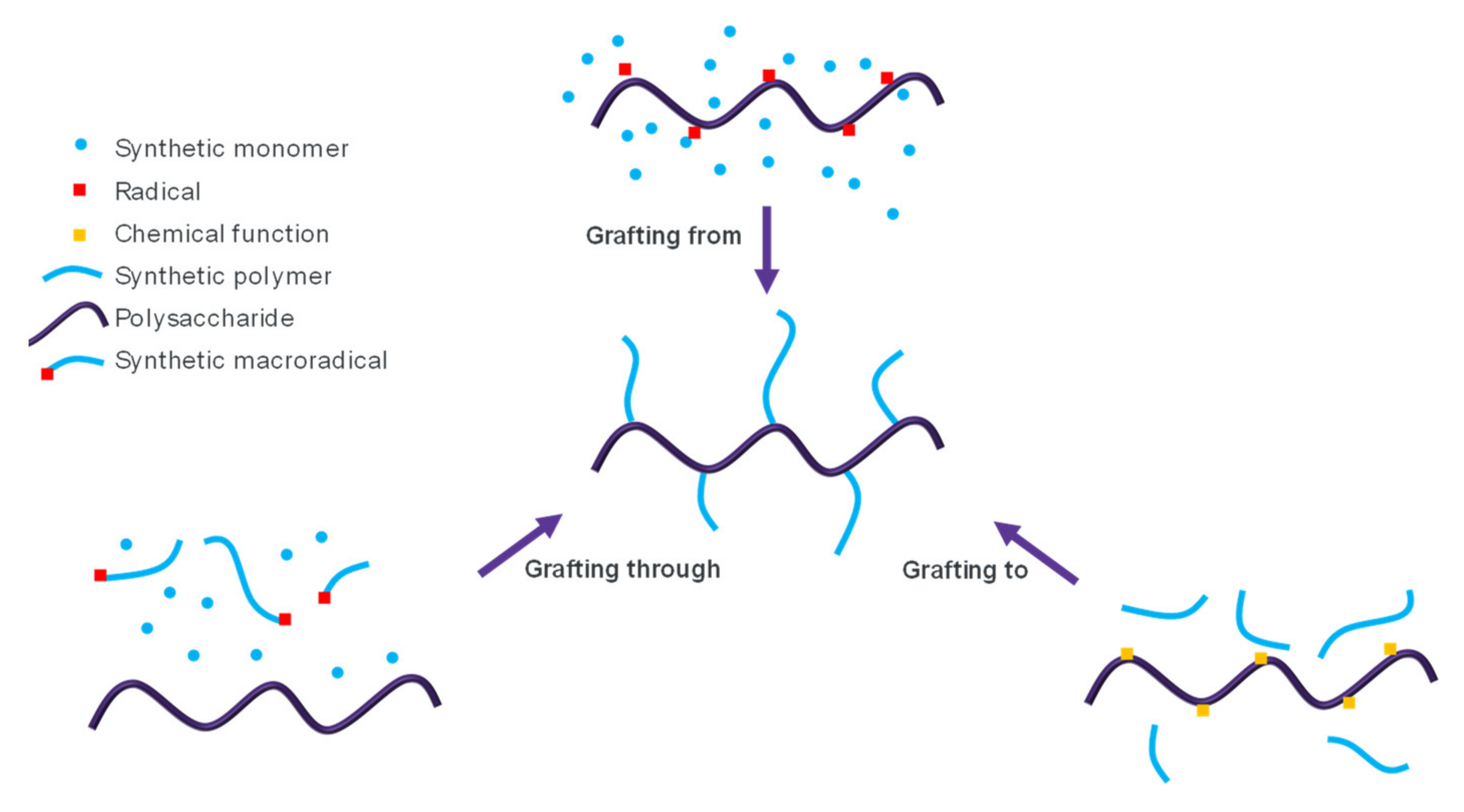
6.2.2. Grafting Material
6.3. Cleavable Polymers
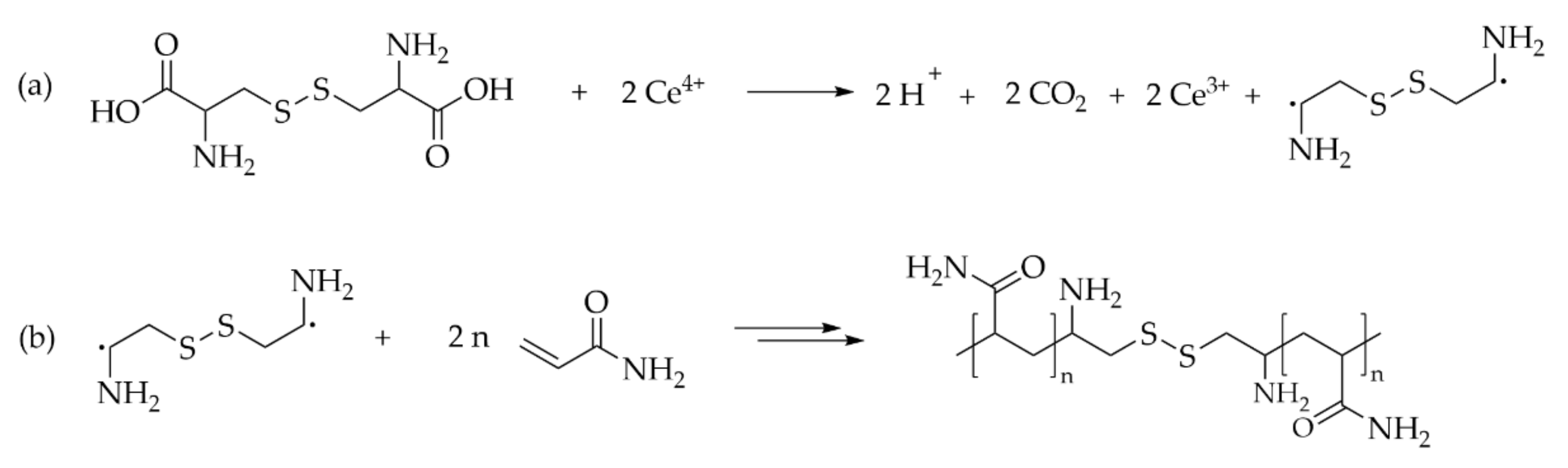
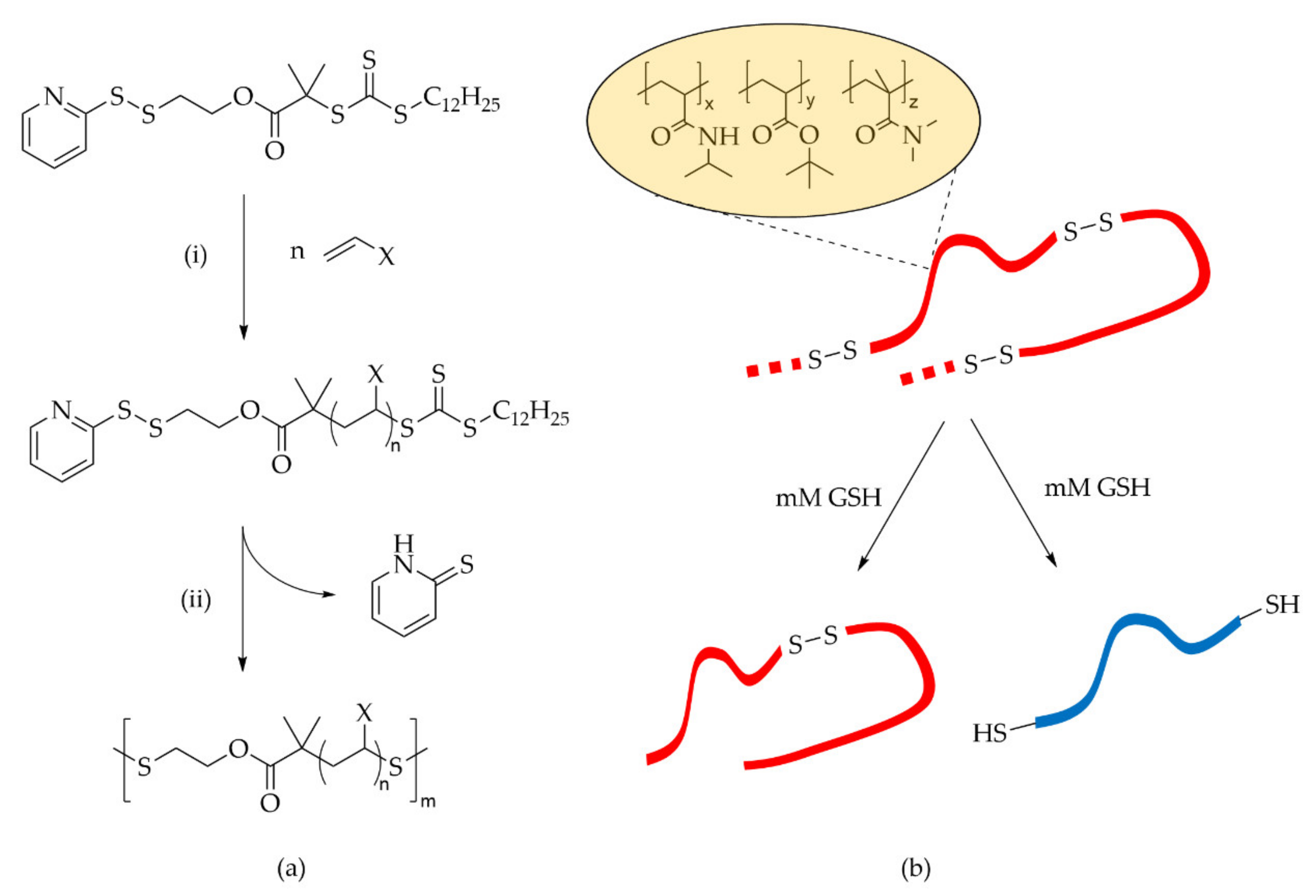
6.3.1. Disulfide
6.3.2. Polydisulfide
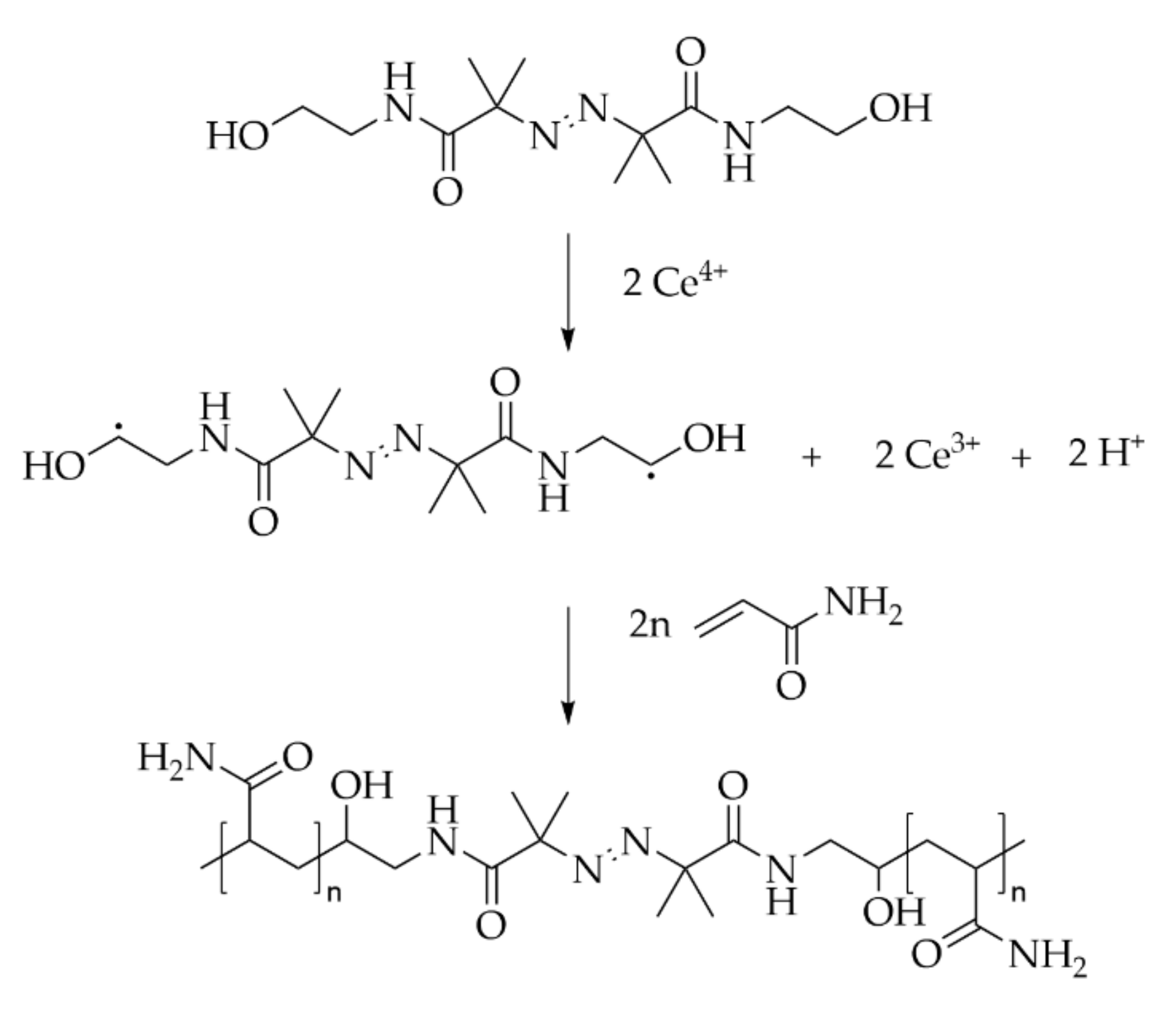
6.3.3. Azo-Bis
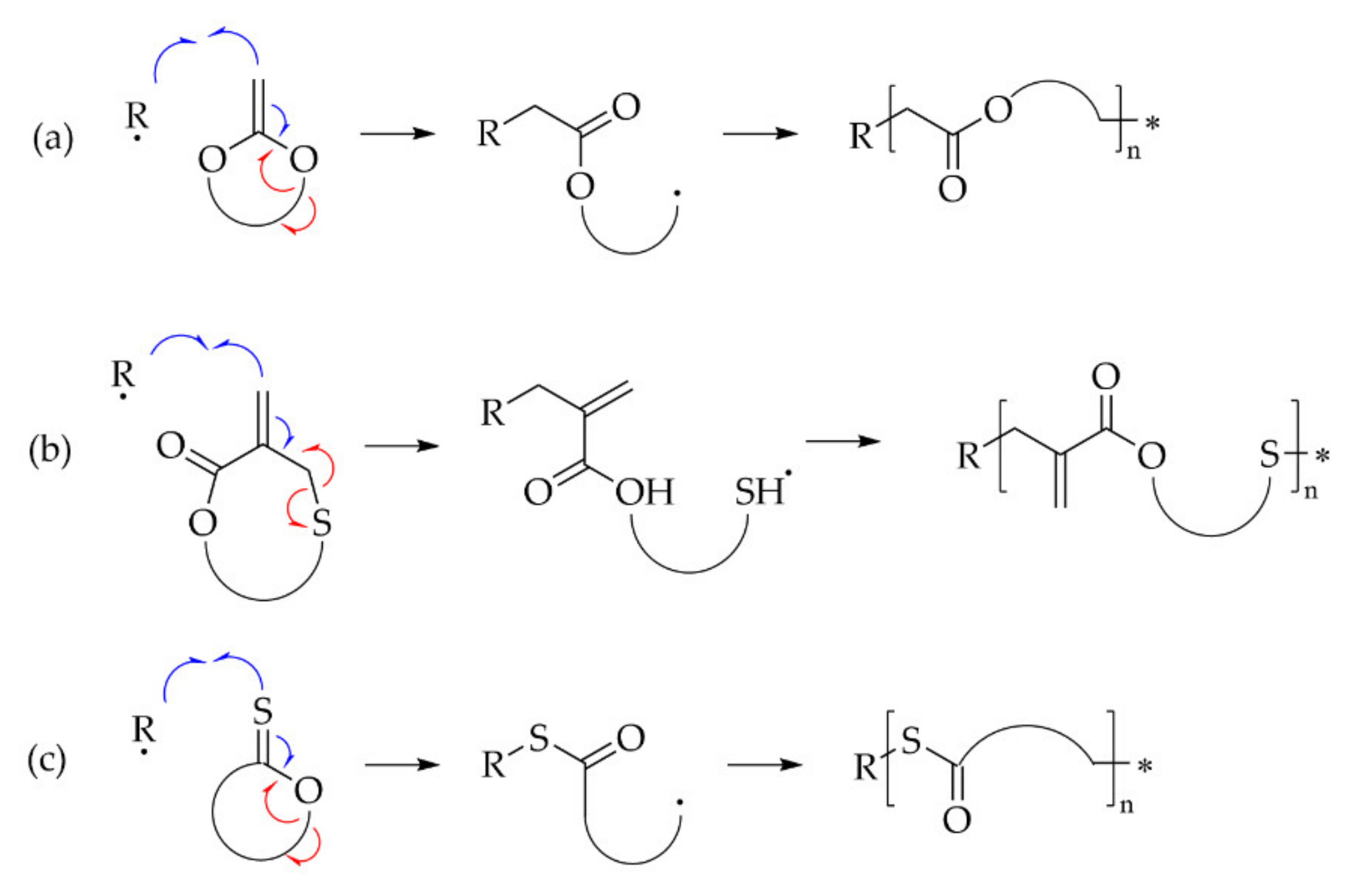
6.3.4. r-ROP
- -
- -
- -
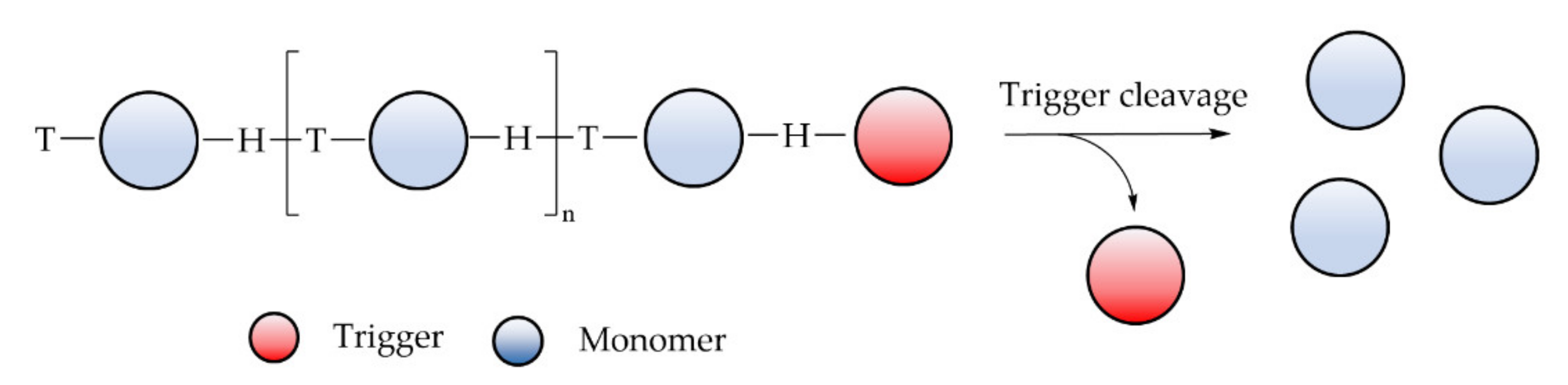
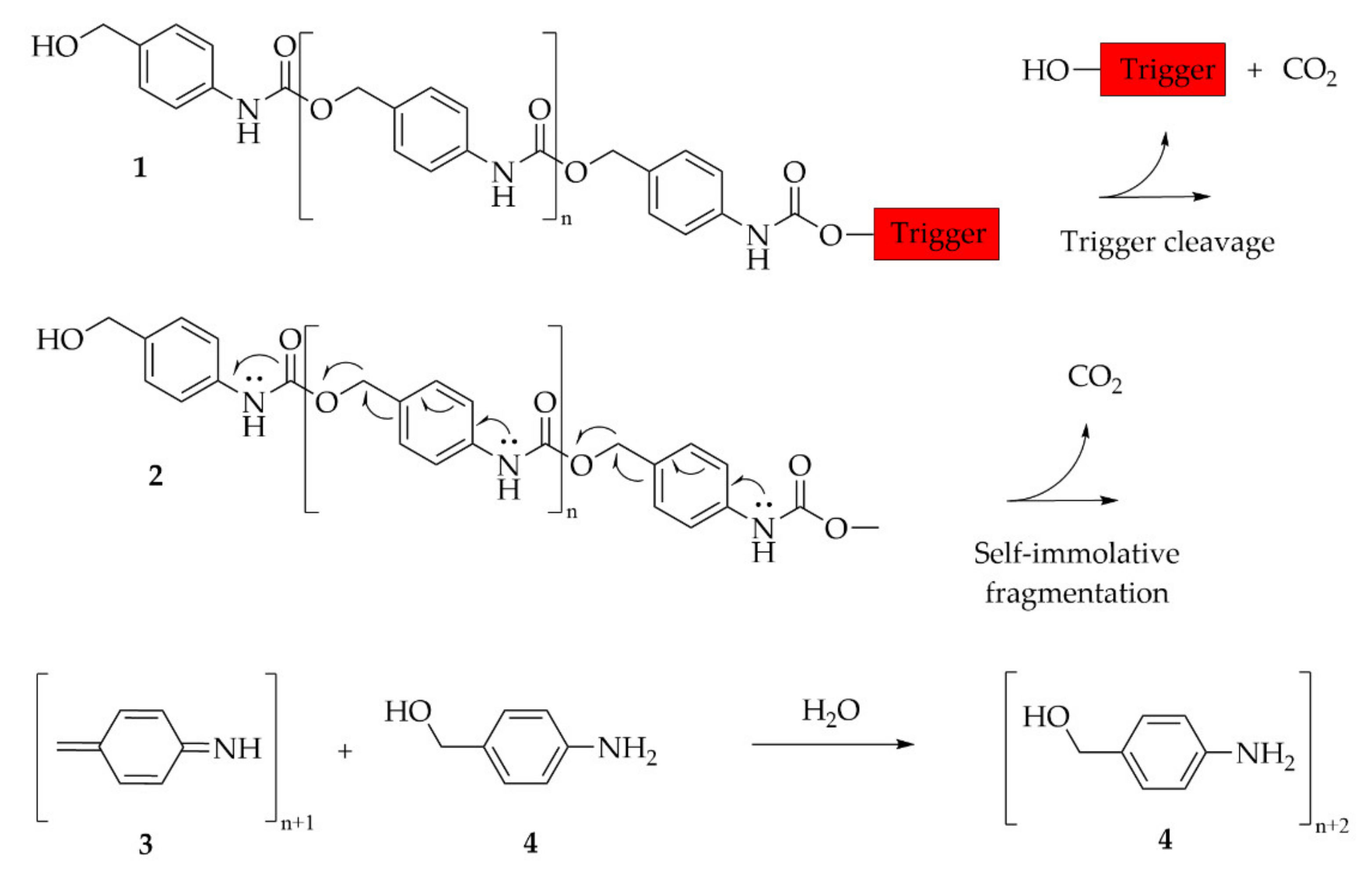
6.3.5. Self-Immolative Polymer
6.4. Conclusion on Macromolecular Engineering
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIBN | Azo-Bis-IsobutyroNitrile |
| ATRP | Atom Transfer Radical Polymerization |
| BFR | Bundesinstitut für Risikobewertung |
| CAGR | Compound Annual Growth Rate |
| CAN | Ceric Ammonium Nitrate |
| CCS | Carbon Capture and Storage |
| CEFAS | Centre for Environment, Fisheries and Aquaculture Science |
| CKA | Cyclic Ketene Acetal |
| COVID19 | Corona Virus Disease 2019 |
| ECHA | European Chemical Agency |
| EOR | Enhanced Oil Recovery |
| ICCA | International Congress and Convention Association |
| IPN | Interpenetrated Network |
| ISCC+ | International Sustainability and Carbon Certification |
| KPS | Potassium Persulfate |
| LAMs | Less Active Monomers |
| LCA | Life Cycle Assessment |
| MAMs | More Active Monomers |
| MBA | Methylene-Bis-Acrylamide |
| NFS | National Sanitation Foundation |
| Nhase | Nitrile hydratase |
| NMP | Nitroxide-Mediated Polymerization |
| OECD | Organization for Economic Co-operation and Development |
| PAA | Poly(acrylic acid) |
| PAM | Poly(acrylamide) |
| PVA | Poly(vinyl alcohol) |
| RAFT | Reversible Addition–Fragmentation chain-Transfer polymerization |
| r-ROP | Radical Ring Opening Polymerization |
| RSB | Roundtable on Sustainable Biomaterials |
| RSPO | Roundtable on Sustainable Palm Oil |
| SAP | Super Absorbent Polymer |
| TARO | Thiocarbonyl Addition Ring Opening |
| UNESCO | United Nations Educational, Scientific, and Cultural Organization |
| VA-086 | 2,2′-Azobis[2 -methyl-N-(2-hydroxyethyl)propionamide] |
| WBCSD | World Business Council for Sustainable Development |
References
- Matlin, S.A.; Mehta, G.; Hopf, H.; Krief, A. The role of chemistry in inventing a sustainable future. Nat. Chem. 2015, 7, 941–943. [Google Scholar] [CrossRef]
- Mildner, S.-A.; Richter, S.; Lauster, G. Resource Scarcity—A Global Security Threat? SWP German Institute for International and Security Affairs: Berlin, Germany, 2011. [Google Scholar]
- International Energy Agency (IEA). World Energy Outlook 2021; International Energy Agency: Paris, France, October 2021. [Google Scholar]
- Pedro-Monzonís, M.; Solera, A.; Ferrer, J.; Estrela, T.; Paredes-Arquiola, J. A review of water scarcity and drought indexes in water resources planning and management. J. Hydrol. 2015, 527, 482–493. [Google Scholar] [CrossRef]
- Global Polyacrylamide Market Size & Share|Industry Report 2019–2025. In Market Analysis Report; Grand View Research: San Francisco, CA, USA, April 2019.
- European Chemicals Agency (ECHA). Acrylamide—Brief Profile; European Chemicals Agency: Brussels, Belgium, 16 November 2021. [Google Scholar]
- Lindeman, B.; Johansson, Y.; Andreassen, M.; Husøy, T.; Dirven, H.; Hofer, T.; Knutsen, H.K.; Caspersen, I.H.; Vejrup, K.; Paulsen, R.E.; et al. Does the food processing contaminant acrylamide cause developmental neurotoxicity? A review and identification of knowledge gaps. Reprod. Toxicol. 2021, 101, 93–114. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Acrylamide; Public Health Service, U.S. Department of Health and Human Services: Atlanta, GA, USA, December 2012.
- Minnesota Department of Health (MDH). Acrylamide and Drinking Water; Minnesota Department of Health: St. Paul, MN, USA, August 2014.
- Nawaz, M.S.; Billedeau, S.M.; Cerniglia, C.E. Influence of selected physical parameters on the biodegradation of acrylamide by immobilized cells of Rhodococcus sp. Biodegradation 1998, 9, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Gafar, A.A.; Khayat, M.E.; Rahim, M.B.H.A.; Shukor, M.Y. Acrylamide toxicity and its biodegradation. Bioremediat. Sci. Technol. Res. 2017, 5, 8–12. [Google Scholar]
- World Health Organization (WHO). Acrylamide in drinking-water. In Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011; ISBN 978-92-4-154995-0. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; ISBN 0-19-850234-6. [Google Scholar]
- Sheldon, R.A. The E factor: Fifteen years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Trost, B.M. The atom economy—A search for synthetic efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Kiikka, O.A. Process for Preparation of Acrylamide. U.S. Patent 3,546,289A, 8 December 1970. [Google Scholar]
- Vanderkooi, W.N.; Jewett, G.L. Hydration of Acrylonitrile to Acrylamide. U.S. Patent 4,177,210A, 4 December 1979. [Google Scholar]
- Banba, H.; Morooka, N. Process for Producing Acrylamide Using a Microbial Catalyst Having Been Washed with Aqueous Acrylic Acid Solution. U.S. Patent 7,205,133, B2, 17 April 2007. [Google Scholar]
- Kariya, T.; Banba, H.; Kano, M. Method for Producing Acrylamide Using Microbial Catalyst. U.S. Patent 8,980,588 B2, 7 July 2015. [Google Scholar]
- Yamada, H.; Nagasawa, T.; Beppu, T.; Horinouch, S.; Nishiyama, M. DNA Fragment Encoding a Polypeptide Having Nitrile Hydratase Activity, a Transformant Containing the Gene and a Process for the Production of Amides Using the Transformant. U.S. Patent 5,753,472A, 15 July 1998. [Google Scholar]
- Engelbach, H.; Krabetz, R.; Duembgen, G.; Willersinn, C.-H.; Lebert, U.; Thiessen, F. Manufacture of Acrylic Acid by Oxidation of Propylene with Oxygen-Containing Gases in Two Separate Catalyst Stages. U.S. Patent 4,031,135A, 21 June 1977. [Google Scholar]
- Kadowaki, K.; Sarumaru, K.; Shibano, T. Production of Acrylic Acid. U.S. Patent 4,365,087A, 21 December 1982. [Google Scholar]
- Yunoki, H. Process for Producing Acrylic Acid. U.S. Patent 6,657,080, B2, 14 August 2003. [Google Scholar]
- Favero, C.; Kieffer, J.; Ling, J. Method for Producing 2-Dimethylaminoethyl (Meth)Acrylate. U.S. Patent 2021/0,114,969A1, 15 February 2021. [Google Scholar]
- Liu, L.Z. Process for Making a (Meth)Acrylamide Monomer. U.S. Patent 2008/0,234,515, A1, 19 March 2008. [Google Scholar]
- Geisendoerfer, M.; Dams, A.; Nestler, G. Method for Producing (Meth) Acrylic Acid Esters. U.S. Patent 7,268,251, B2, 18 November 2007. [Google Scholar]
- Dubois, J.-L.; Duquenne, C.; Holderich, W. Process for Dehydrating Glycerol to Acrolein. U.S. Patent 7,396,962, B1, 8 July 2008. [Google Scholar]
- Warnecke-Lipscomb, T.; Lynch, M.; Gill, R. Methods, Systems and Compositions for Increased Microorganism Tolerance to and Production of 3-Hydroxypropionic Acid (3-Hp). U.S. Patent 9,273,953,A1, 29 August 2010. [Google Scholar]
- Tengler, R.; Decoster, D. Purification of 3-Hydroxypropionic Acid from Crude Cell Broth and Production of Acrylamide. WO Patent 2013/192,450,A1, 23 September 2013. [Google Scholar]
- Decoster, D.; Hoyt, S.; Roach, S. Dehydration of 3-Hydroxypropionic Acid to Acrylic Acid. WO Patent 2013/192,451,A1, 15 March 2013. [Google Scholar]
- Chu, H.S.; Ahn, J.-H.; Yun, J.; Choi, I.S.; Nam, T.-W.; Cho, K.M. Direct Fermentation route for the production of acrylic acid. Metab. Eng. 2015, 32, 23–29. [Google Scholar] [CrossRef]
- Burk, M.J.; Burgard, A.P.; Pharkya, P. Microorganisms and Methods for the Biosynthesis of Fumarate, Malate, and Acrylate. U.S. Patent 9,062,330,B2, 21 August 2015. [Google Scholar]
- Sawicki, R.A. Catalyst for Dehydration of Lactic Acid to Acrylic Acid. U.S. Patent 4,729,978A, 8 March 1988. [Google Scholar]
- Albert, J.; Wasserscheid, P.; Taccardi, N.; Nagengast, J.; Kehrer, M.; Kadar, J.; Collias, D.I. Methods of Making Acrylic Acid from Lactic Acid or Its Derivatives in Liquid Phase. WO Patent 2018/022,826,A1, 1 February 2018. [Google Scholar]
- Farmer, J.J.; Galebach, P.; Sherry, K.; Sookraj, S.H. Process for Production of Acrylic Acid. U.S. Patent 2020/0247,742,A1, 6 August 2020. [Google Scholar]
- Sookraj, S.H. Systems and Processes for Polyacrylic Acid Production. U.S. Patent 2020/002,446,A1, 2020. [Google Scholar]
- Sanders, J.P.M.; Haveren, J.V.; Scott, E.; Es, D.S.V.; Nôtre, J.L.; Spekreijse, J. Bio-Derived Olefin Synthesis. U.S. Patent 2012/0,178,961,A1, 23 March 2012. [Google Scholar]
- Spekreijse, J.; Nôtre, J.L.; van Haveren, J.; Scott, E.L.; Sanders, J.P.M. Simultaneous production of biobased styrene and acrylates using ethenolysis. Green Chem. 2012, 14, 2747–2751. [Google Scholar] [CrossRef]
- Schofer, S.; Safir, A.; Vazquez, R. Synthesis of Olefins. WO Patent 2013/082,264,A1, 25 April 2013. [Google Scholar]
- Goyal, A.; Samad, J. Compositions and Methods Related to the Production of Acrylonitrile. U.S. Patent 9,708,249,B1, 18 July 2017. [Google Scholar]
- Hughes, J.; Symes, K.C. Biocatalytic Manufacturing of (Meth) Acrylylcholine or 2-(N,N-Dimethylamino) Ethyl (Meth) Acrylate. U.S. Patent 7,754,455,B2, 7 February 2010. [Google Scholar]
- Winkler, C.K.; Schrittwieser, J.H.; Kroutil, W. Power of biocatalysis for organic synthesis. ACS Cent. Sci. 2021, 7, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Nordblad, M.; Adlercreutz, P. Effects of acid concentration and solvent choice on enzymatic acrylation by candida antarctica lipase B. J. Biotechnol. 2008, 133, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Haring, D.; Wagner, E.; Bruchmann, B.; Beck, E.; Hauer, B. Enzymatic Production of (Meth) Acrylic Acid Esters. U.S. Patent 2006/0,141,593,A1, 12 May 2006. [Google Scholar]
- Haering, D.; Meisenburg, U.; Hauer, B. Enzymatic Preparation of (Meth)Acrylic Esters. U.S. Patent 8,344,072,B2, 1 January 2013. [Google Scholar]
- Haring, D.; Meisenburg, U.; Hauer, B.; Dietsche, F. Enzymatic Production of (Meth)Acrylic Acid Esters. U.S. Patent 1,415,3A1, 26 August 2008. [Google Scholar]
- Dietsche, F.; Haring, D.; Wagner, E.; Schwalm, R.; Rink, H.-P.; Beck, E. Enzymatic Production of (Meth)Acrylic Esters That Contain Urethane Groups. U.S. Patent 2006/0,084,779,A1, 20 April 2006. [Google Scholar]
- Paulus, W.; Hauer, B.; Haring, D.; Dietsche, F. Enzymatic Synthesis of Polyol Acrylates. U.S. Patent 2006/0,030,013,A1, 16 February 2006. [Google Scholar]
- Kim, J.S.; Seo, K.S.; Choi, W.G. Method for Preparing (Meth)Acrylate Group-Containing Benzophenone for Optical Adhesive Use and Optical Adhesive Composition. U.S. Patent 10,407,701,B2, 11 August 2019. [Google Scholar]
- Haering, D.; Meisenburg, U.; Chabanas, M.; Lipowsky, G. Process for Producing of Epoxy-Containing (Meth) Acrylic Esters, Using Lipases. U.S. Patent 2010/0,048,927,A1, 9 September 2010. [Google Scholar]
- Tawaki, S.; Takeichi, M.; Mizogami, K. Production of Acrylic Acid or Methacrylic Acid Alkylaminoalkyl Ester. Japan Patent 0,479,889A, 27 March 1992. [Google Scholar]
- Banks, I.; Gravis, L. The Circular Economy 100. In Enabling a Circular Economy for Chemicals with the Mass Balance Approach; Banks, I., Gravis, L., Eds.; Ellen MacArthur Foundation: Cologne, Germany, 2019. [Google Scholar]
- REDcert. Available online: https://www.redcert.org/en (accessed on 28 October 2021).
- RSB. Available online: https://rsb.org (accessed on 28 October 2021).
- RSPO. Available online: https://rspo.org (accessed on 28 October 2021).
- Fairtrade International. Available online: https://www.fairtrade.net (accessed on 28 October 2021).
- ISCC System. Available online: https://www.iscc-system.org (accessed on 28 October 2021).
- Stigsson, L.; Naydenov, V.; Lundbäck, J. Biorefining of Crude Tall Oil. U.S. Patent 2014/098,763, 26 June 2014. [Google Scholar]
- Vermeiren, W.; Gyseghem, N.V. A Process for the Production of Bio-Naphtha from Complex Mixtures of Natural Occurring Fats & Oils. WO Patent 2011/012,439,A1, 13 July 2012. [Google Scholar]
- Matyjaszewski, K.; Davis, T. Handbook of Radical Polymerization, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2002; ISBN 978-0-471-22045-9. [Google Scholar]
- Destarac, M. Industrial development of reversible-deactivation radical polymerization: Is the induction period over? Polym. Chem. 2018, 9, 4947–4967. [Google Scholar] [CrossRef]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Vana, P.; Davis, T.P. The kinetics of free-radical polymerization. In Handbook of Radical Polymerization; Matyjaszewski, K., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; Volume 4, pp. 187–261. ISBN 978-0-471-22045-9. [Google Scholar]
- Lide, D.R.; Haynes, W.M. CRC Handbook of Chemistry and Physics, 90th ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Seidel, A.; Kroschwitz, J. Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; Kirk-Othmer, Ed.; Wiley: Hoboken, NJ, USA, 2004; Volume 1, ISBN 978-0-471-48522-3. [Google Scholar]
- Zheng, H.; Ma, J.; Ji, F.; Tang, X.; Chen, W.; Zhu, J.; Liao, Y.; Tan, M. Synthesis and application of anionic polyacrylamide in water treatment. Asian J. Chem. 2013, 25, 7071–7074. [Google Scholar] [CrossRef]
- Ducheyne, W.; Stevens, C.; Bonte, S.; Rousseau, S.; Van Der Pol, E. New Industrial Chemical Heat Pump from Qpinch. In Proceedings of the 12th IEA Heat Pump Conference, Rotterdam, The Netherlands, 15–18 May 2017. [Google Scholar]
- Capek, I. Inverse emulsion polymerization of acrylamide initiated by oil- and water-soluble initiators: Effect of emulsifier concentration. Polym. J. 2004, 36, 793–803. [Google Scholar] [CrossRef]
- Chern, C.S. Emulsion polymerization mechanisms and kinetics. Prog. Polym. Sci. 2006, 31, 443–486. [Google Scholar] [CrossRef]
- Čuček, L.; Varbanov, P.S.; Klemeš, J.J.; Kravanja, Z. Total footprints-based multi-criteria optimisation of regional biomass energy supply chains. Energy 2012, 44, 135–145. [Google Scholar] [CrossRef]
- Kravanja, Z.; Čuček, L. Multi-objective optimisation for generating sustainable solutions considering total effects on the environment. Appl. Energy 2013, 101, 67–80. [Google Scholar] [CrossRef]
- Cronin, J.J.; Smith, J.S.; Gleim, M.R.; Ramirez, E.; Martinez, J.D. Green marketing strategies: An examination of stakeholders and the opportunities they present. J. Acad. Mark. Sci. 2011, 39, 158–174. [Google Scholar] [CrossRef]
- Evolution of Handprint. Available online: https://www.handprint.in/handprint_legacy (accessed on 15 October 2021).
- Pajula, T.; Vatanen, S.; Behm, K.; Grönman, K.; Lakanen, L.; Kasurinen, H.; Soukka, R. Carbon Handprint Guide V. 2.0 Applicable for Environmental Handprint; VTT Technical Research Centre of Finland: Espoo, Finland, 2021. [Google Scholar]
- Grönman, K.; Pajula, T.; Sillman, J.; Leino, M.; Vatanen, S.; Kasurinen, H.; Soininen, A.; Soukka, R. Carbon handprint—An approach to assess the positive climate impacts of products demonstrated via renewable diesel case. J. Clean. Prod. 2019, 206, 1059–1072. [Google Scholar] [CrossRef]
- Geraghty, C.; Snook, S.J.; Alcorta, J.; Perou, N. ISO 14067; Greenhouse Gases—Carbon Footprint of Products—Requirements and Guidelines for Quantification. 1st ed. ISO: London, UK, 2018.
- Ismail, L.; Saling, P.; Alcorta, J.; Perou, N. ISO 14040; Environmental Management—Life Cycle Assessment—Principles and Framework. 2nd ed. ISO: London, UK, 2006.
- Ismail, L.; Saling, P.; Alcorta, J.; Perou, N. ISO 14044; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. 1st ed. ISO: London, UK, 2006.
- Haberkern, B.; Maier, W.; Schneider, U. Increasing Energy Efficiency at Municipal Wastewater Treatment Plants; Umweltbundesamt: Dessau-Roßlau, Germany, 2008. [Google Scholar]
- Capodaglio, A.G.; Olsson, G. Energy issues in sustainable urban wastewater management: Use, demand reduction and recovery in the urban water cycle. Sustainability 2020, 12, 10266. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Radoiu, M.T.; Martin, D.I.; Calinescu, I.; Iovu, H. Preparation of polyelectrolytes for wastewater treatment. J. Hazard. Mater. 2004, 106, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Tatsi, A.A.; Zouboulis, A.I.; Matis, K.A.; Samaras, P. Coagulation–flocculation pretreatment of sanitary landfill leachates. Chemosphere 2003, 53, 737–744. [Google Scholar] [CrossRef]
- Wong, S.S.; Teng, T.T.; Ahmad, A.L.; Zuhairi, A.; Najafpour, G. Treatment of Pulp and paper mill wastewater by polyacrylamide (PAM) in polymer induced flocculation. J. Hazard. Mater. 2006, 135, 378–388. [Google Scholar] [CrossRef]
- Zhong, J.; Sun, X.; Wang, C. Treatment of oily wastewater produced from refinery processes using flocculation and ceramic membrane filtration. Sep. Purif. Technol. 2003, 32, 93–98. [Google Scholar] [CrossRef]
- Renault, F.; Sancey, B.; Charles, J.; Morin-Crini, N.; Badot, P.-M.; Winterton, P.; Crini, G. Chitosan flocculation of cardboard-mill secondary biological wastewater. Chem. Eng. J. 2009, 155, 775–783. [Google Scholar] [CrossRef]
- Wang, H.; Min, X.; Chai, L.; Shu, Y. Biological preparation and application of poly-ferric sulfate flocculant. Trans. Nonferr. Metal. Soc. 2011, 21, 2542–2547. [Google Scholar] [CrossRef]
- Singh, R.P.; Karmakar, G.P.; Rath, S.K.; Karmakar, N.C.; Pandey, S.R.; Tripathy, T.; Panda, J.; Kanan, K.; Jain, S.K.; Lan, N.T. Biodegradable drag reducing agents and flocculants based on polysaccharides: Materials and applications. Polym. Eng. Sci. 2000, 40, 46–60. [Google Scholar] [CrossRef]
- Boráň, J.; Houdková, L.; Elsäßer, T. Processing of sewage sludge: Dependence of sludge dewatering efficiency on amount of flocculant. Resour. Conserv. Recycl. 2010, 54, 278–282. [Google Scholar] [CrossRef]
- Tillman, A.-M.; Svingby, M.; Lundström, H. Life cycle assessment of municipal waste water systems. Int. J. Life Cycle Assess. 1998, 3, 145–157. [Google Scholar] [CrossRef]
- Hospido, A.; Moreira, M.T.; Fernández-Couto, M.; Feijoo, G. Environmental performance of a municipal wastewater treatment plant. Int. J. Life Cycle Assess. 2004, 9, 261. [Google Scholar] [CrossRef]
- Wenzel, H.; Larsen, H.F.; Clauson-Kaas, J.; Høibye, L.; Jacobsen, B.N. Weighing environmental advantages and disadvantages of advanced wastewater treatment of micro-pollutants using environmental life cycle assessment. Water Sci. Technol. 2008, 57, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Remy, C.; Lesjean, B.; Waschnewski, J. Identifying energy and carbon footprint optimization potentials of a sludge treatment line with life cycle assessment. Water Sci. Technol. 2013, 67, 63–73. [Google Scholar] [CrossRef]
- ADEME-Bilans GES Site. Available online: https://www.bilans-ges.ademe.fr/en/accueil (accessed on 15 October 2021).
- Wallace, A.; Wallace, G.A. Polyacrylamide (PAM) and Micronized PAM Soil Conditioners: 50 Years of Progress, 1st ed.; Wallace Laboratories: Los Angeles, CA, USA, 2003; ISBN 0-937892-15-7. [Google Scholar]
- Sojka, R.E.; Bjorneberg, D.L.; Entry, J.A.; Lentz, R.D.; Orts, W.J. Polyacrylamide in agriculture and environmental land management. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: San Diego, CA, USA, 2007; Volume 92, pp. 75–162. [Google Scholar]
- Behera, S.; Mahanwar, P.A. Superabsorbent polymers in agriculture and other applications: A review. Polym. Plast. Technol. Mater. 2020, 59, 341–356. [Google Scholar] [CrossRef]
- Stern, R.; Van Der Merwe, A.J.; Laker, M.C.; Shainberg, I. Effect of soil surface treatments on runoff and wheat yields under irrigation. Agron. J. 1992, 84, 114–119. [Google Scholar] [CrossRef]
- Aase, J.K.; Bjorneberg, D.L.; Sojka, R.E. Sprinkler irrigation runoff and erosion control with polyacrylamide-Laboratory tests. Soil. Sci. Soc. Am. J. 1998, 62, 1681–1687. [Google Scholar] [CrossRef]
- Lentz, R.D.; Sojka, R.E. Field results using polyacrylamide to manage furrow erosion and infiltration. Soil Sci. 1994, 158, 274–282. [Google Scholar] [CrossRef]
- Weston, D.P.; Lentz, R.D.; Cahn, M.D.; Ogle, R.S.; Rothert, A.K.; Lydy, M.J. Toxicity of anionic polyacrylamide formulations when used for erosion control in agriculture. J. Environ. Qual. 2009, 38, 238–247. [Google Scholar] [CrossRef]
- Zohourian Mehr, M.J.A.D.; Kabiri, K. Superabsorbent polymer materials: A review. Iran. Polym. J. 2008, 17, 451–477. [Google Scholar]
- Milani, P.; França, D.; Balieiro, A.G.; Faez, R. Polymers and its applications in agriculture. Polímeros 2017, 27, 256–266. [Google Scholar] [CrossRef]
- Islam, M.R.; Xue, X.; Mao, S.; Ren, C.; Eneji, A.E.; Hu, Y. Effects of water-saving superabsorbent polymer on antioxidant enzyme activities and lipid peroxidation in oat (Avena sativa L.) under drought stress. J. Sci. Food Agric. 2011, 91, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Liu, M.; Guo, M.; Wu, L. Preparation of superabsorbent polymer with slow-release phosphate fertilizer. J. Appl. Polym. Sci. 2004, 92, 3417–3421. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Annex XV Restriction Report Proposal for a Restriction of Intentionally Added Microplastics; European Chemicals Agency: Helsinki, Finland, 22 August 2019. [Google Scholar]
- Committee for Risk Assessment (RAC); Committee for Socio-Economic Analysis (SEAC). Opinion on an Annex XV Dossier Proposing Restrictions on Intentionally-Added Microplastics; European Chemicals Agency (ECHA): Brussels, Belgium, 11 June 2020. [Google Scholar]
- Morice, L.; Dupuis, G.; Al-Khoury, P.; Nieuwerf, J.; Favero, C. Using Polymer EOR to Reduce Carbon Intensity while Increasing Oil Recovery. In Proceedings of the IOR 2021, European Association of Geoscientists & Engineers, Online. 19 April 2021; Volume 2021, pp. 1–20. [Google Scholar]
- Gao, C. Viscosity of partially hydrolyzed polyacrylamide under shearing and heat. J. Pet. Explor. Prod. Technol. 2013, 3, 203–206. [Google Scholar] [CrossRef]
- Dupuis, G.; Nieuwerf, J. A cost-effective EOR technique to reduce carbon intensity with polymer flooding and modular skids. J. Pet. Technol. 2020. 25 August 2020. Available online: https://jpt.spe.org/cost-effective-eor-technique-reduce-carbon-intensity-polymer-flooding-and-modular-skids.
- Guo, H.; Dong, J.; Wang, Z.; Liu, H.; Ma, R.; Kong, D.; Wang, F.; Xin, X.; Li, Y.; She, H. 2018 EOR Survey in China—Part 1. In Proceedings of the SPE Improved Oil Recovery Conference, OnePetro, Tulsa, OK, USA, 14–18 April 2018. [Google Scholar]
- Dong, H.; Fang, S.; Wang, D.; Wang, J.; Liu, Z.L.; Hou, W. Review of Practical Experience & Management by Polymer Flooding at Daqing. In Proceedings of the SPE Symposium on Improved Oil Recovery, OnePetro, Tulsa, OK, USA, 20–23 April 2008. [Google Scholar]
- Liu, J.Z.; Adegbesan, K.; Bai, J.J. Suffield Area, Alberta, Canada–Caen Polymer Flood Pilot Project. In Proceedings of the SPE Heavy Oil Conference Canada, OnePetro, Calgary, AB, Canada, 12–14 June 2012. [Google Scholar]
- Delamaide, E. Comparison of Primary, Secondary and Tertiary Polymer Flood in Heavy Oil-Field Results. In Proceedings of the SPE Trinidad and Tobago Section Energy Resources Conference, OnePetro, Port of Spain, Trinidad and Tobago, 13–15 June 2016. [Google Scholar]
- Farajzadeh, R.; Kahrobaei, S.; Eftekhari, A.A.; Mjeni, R.A.; Boersma, D.; Bruining, J. Chemical enhanced oil recovery and the dilemma of more and cleaner energy. Sci. Rep. 2021, 11, 829. [Google Scholar] [CrossRef] [PubMed]
- McCollister, D.D.; Hake, C.L.; Sadek, S.E.; Rowe, V.K. Toxicologic investigations of polyacrylamides. Toxicol. Appl. Pharm. 1965, 7, 639–651. [Google Scholar] [CrossRef]
- Hansen, B.H.; Malzahn, A.; Hagemann, A.; Farkas, J.; Skancke, J.; Altin, D.; Nordtug, T. Acute and sub-lethal effects of an anionic polyacrylamide on sensitive early life stages of Atlantic Cod (Gadus morhua). Sci. Total Environ. 2019, 652, 1062–1070. [Google Scholar] [CrossRef]
- Farkas, J.; Altin, D.; Hansen, B.H.; Øverjordet, I.B.; Nordtug, T. Acute and long-term effects of anionic polyacrylamide (APAM) on different developmental stages of two marine copepod species. Chemosphere 2020, 257, 127259. [Google Scholar] [CrossRef]
- King, D.J.; Noss, R.R. Toxicity of polyacrylamide and acrylamide monome. Rev. Environ. Health 1989, 8, 3–16. [Google Scholar] [CrossRef]
- Smith, E.A.; Oehme, F.W. Acrylamide and polyacrylamide: A review of production, use, environmental fate and neurotoxicity. Rev. Environ. Health 1991, 9, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.S.; Castle, L. Tests for the depolymerization of polyacrylamides as a potential source of acrylamide in heated foods. J. Agric. Food Chem. 2003, 51, 6715–6718. [Google Scholar] [CrossRef] [PubMed]
- Ver Vers, L.M. Determination of acrylamide monomer in polyacrylamide degradation studies by high-performance liquid chromatography. J. Chromatogr. Sci. 1999, 37, 486–494. [Google Scholar] [CrossRef]
- Reber, A.C.; Khanna, S.N.; Ottenbrite, R. Thermodynamic stability of polyacrylamide and poly(N,N-Dimethyl Acrylamide). Polym. Adv. Technol. 2007, 18, 978–985. [Google Scholar] [CrossRef]
- Tabari, M.; Osterwalder, U.; Cook, P.; Casantosan, M. ISO 16128-1; Guidelines on Technical Definitions and Criteria for Natural and Organic Cosmetic Ingredients and Products—Part 1: Definitions for Ingredients. 1st ed. ISO: London, UK, 2016.
- Tabari, M.; Osterwalder, U.; Cook, P.; Casantosan, M. ISO 16128-2; Cosmetics—Guidelines on Technical Definitions and Criteria for Natural and Organic Cosmetic Ingredients—Part 2: Criteria for Ingredients and Products. 1st ed. ISO: London, UK, 2017.
- Matsson, P.; Kihlberg, J. How big is too big for cell permeability? J. Med. Chem. 2017, 60, 1662–1664. [Google Scholar] [CrossRef] [PubMed]
- Brillet, F.; Cregut, M.; Durand, M.J.; Sweetlove, C.; Chenèble, J.C.; L’Haridon, J.; Thouand, G. Biodegradability assessment of complex chemical mixtures using a carbon balance approach. Green Chem. 2018, 20, 1031–1041. [Google Scholar] [CrossRef]
- Joshi, S.J.; Abed, R.M.M. Biodegradation of polyacrylamide and its derivatives. Environ. Process. 2017, 4, 463–476. [Google Scholar] [CrossRef]
- Guezennec, A.G.; Michel, C.; Bru, K.; Touze, S.; Desroche, N.; Mnif, I.; Motelica-Heino, M. Transfer and degradation of polyacrylamide-based flocculants in hydrosystems: A review. Environ. Sci. Pollut. Res. 2015, 22, 6390–6406. [Google Scholar] [CrossRef] [PubMed]
- Nyyssölä, A.; Ahlgren, J. Microbial degradation of polyacrylamide and the deamination product polyacrylate. Int. Biodeterior. Biodegrad. 2019, 139, 24–33. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. NPJ Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Seybold, C.A. Polyacrylamide review: Soil conditioning and environmental fate. Commun. Soil Sci. Plan. 1994, 25, 2171–2185. [Google Scholar] [CrossRef]
- Kawai, F.; Hayashi, T. Biodegradation of polyacrylate. In Biopolymers Online; Steinbüchel, A., Ed.; American Cancer Society: Atlanta, GA, USA, 2005. [Google Scholar]
- Caulfield, M.J.; Qiao, G.G.; Solomon, D.H. Some aspects of the properties and degradation of polyacrylamides. Chem. Rev. 2002, 102, 3067–3084. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.D.; Aust, S.D. Degradation of Chemicals by reactive radicals produced by cellobiose dehydrogenase from phanerochaete chrysosporium. Arch. Biochem. Biophys. 1999, 367, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Vinu, R.; Madras, G. Photocatalytic degradation of poly(Acrylamide-Co-Acrylic acid). J. Phys. Chem. B 2008, 112, 8928–8935. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.L.; Raudenbush, D.L.; Dentel, S.K. Aerobic and anaerobic biodegradability of a flocculant polymer. Water Sci. Technol. 2001, 44, 461–468. [Google Scholar] [CrossRef] [PubMed]
- McGaugh, M.C.; Kottle, S. The thermal degradation of poly(acrylic acid). J. Polym. Sci. Pol. Lett. 1967, 5, 817–820. [Google Scholar] [CrossRef]
- Gröllmann, U.; Schnabel, W. Free radical-induced oxidative degradation of polyacrylamide in aqueous solution. Polym. Degrad. Stabil. 1982, 4, 203–212. [Google Scholar] [CrossRef]
- Suzuki, J.; Harada, H.; Suzuki, S. Ozone treatment of water-soluble polymers. V. Ultraviolet irradiation effects on the ozonization of polyacrylamide. J. Appl. Polym. Sci. 1979, 24, 999–1006. [Google Scholar] [CrossRef]
- El-Mamouni, R.; Frigon, J.-C.; Hawari, J.; Marroni, D.; Guiot, S.R. Combining photolysis and bioprocesses for mineralization of high molecular weight polyacrylamides. Biodegradation 2002, 13, 221–227. [Google Scholar] [CrossRef]
- Gilbert, W.J.R.; Johnson, S.J.; Tsau, J.-S.; Liang, J.-T.; Scurto, A.M. Enzymatic degradation of polyacrylamide in aqueous solution with peroxidase and H2O2. J. Appl. Polym. Sci. 2017, 134, 44560. [Google Scholar] [CrossRef]
- Song, T.; Li, S.; Yin, Z.; Bao, M.; Lu, J.; Li, Y. Hydrolyzed polyacrylamide-containing wastewater treatment using ozone reactor-upflow anaerobic sludge blanket reactor-aerobic biofilm reactor multistage treatment system. Environ. Pollut. 2021, 269, 116111. [Google Scholar] [CrossRef] [PubMed]
- Yong, R.P.; Zhong, H.Z.; Mu, T.B.; Yi, M.L.; Ying, Y.Z.; Guo, L.S. Treatment of partially hydrolyzed polyacrylamide wastewater by combined fenton oxidation and anaerobic biological processes. Chem. Eng. J. 2015, 273, 1–6. [Google Scholar]
- Liu, X.; Xu, Q.; Wang, D.; Yang, Q.; Wu, Y.; Li, Y.; Fu, Q.; Yang, F.; Liu, Y.; Ni, B.-J.; et al. Thermal-alkaline pretreatment of polyacrylamide flocculated waste activated sludge: Process optimization and effects on anaerobic digestion and polyacrylamide degradation. Bioresour. Technol. 2019, 281, 158–167. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Q.; Wang, D.; Wu, Y.; Fu, Q.; Li, Y.; Yang, Q.; Liu, Y.; Ni, B.-J.; Wang, Q.; et al. Microwave pretreatment of polyacrylamide flocculated waste activated sludge: Effect on anaerobic digestion and polyacrylamide degradation. Bioresour. Technol. 2019, 290, 121776. [Google Scholar] [CrossRef]
- Hennecke, D.; Bauer, A.; Herrchen, M.; Wischerhoff, E.; Gores, F. Cationic polyacrylamide copolymers (PAMs): Environmental half life determination in sludge-treated soil. Environ. Sci. Eur. 2018, 30, 16. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Li, S.; Ding, W.; Li, H.; Bao, M.; Li, Y. Biodegradation of hydrolyzed polyacrylamide by the combined expanded granular sludge bed reactor-aerobic biofilm reactor biosystem and key microorganisms involved in this bioprocess. Bioresour. Technol. 2018, 263, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, Y.; Gao, Y.; Chen, D.; Yang, M. Cleavage of the main carbon chain backbone of high molecular weight polyacrylamide by aerobic and anaerobic biological treatment. Chemosphere 2017, 189, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, Y.; Hamidian, A.H.; Yang, M. Biodegradation of low molecular weight polyacrylamide under aerobic and anaerobic conditions: Effect of the molecular weight. Water Sci. Technol. 2020, 81, 301–308. [Google Scholar] [CrossRef]
- Dong, L.; Su, F.; Wang, Y.-Z. Treatment of partially hydrolyzed polyacrylamide by mixed bacteria isolated from wastewater. Environ. Prog. Sustain. Energy 2020, 39, e13445. [Google Scholar] [CrossRef]
- Wallace, A.; Wallace, G.A.; Abouzamzam, A.M. Effects of excess levels of a polymer as a soil conditioner on yields and mineral nutrition of plants. [Triticum Aestivum, Lycopersicon Esculentum]. Soil Sci. 1986, 141, 377–380. [Google Scholar] [CrossRef]
- Kawai, F.; Igarashi, K.; Kasuya, F.; Fukui, M. Proposed mechanism for bacterial metabolism of polyacrylate. J. Environ. Polym. Degrad. 1994, 2, 59–65. [Google Scholar] [CrossRef]
- Dai, X.; Luo, F.; Zhang, D.; Dai, L.; Chen, Y.; Dong, B. Waste-activated sludge fermentation for polyacrylamide biodegradation improved by anaerobic hydrolysis and key microorganisms involved in biological polyacrylamide removal. Sci. Rep. 2015, 5, 11675. [Google Scholar] [CrossRef] [PubMed]
- ECETOC—European Centre for Ecotoxicology and Toxicology of Chemicals. Polycarboxylate Polymers as Used in Detergents; Joint Assessment of Commodity Chemicals: Brussels, Belgium, 1993; Volume 23, ISSN 0773-6339-23. [Google Scholar]
- Hayashi, T.; Nishimura, H.; Sakano, K.; Tani, Y. Microbial degradation of poly(sodium acrylate). Biosci. Biotechnol. Biochem. 1994, 58, 444–446. [Google Scholar] [CrossRef]
- Kawai, F. Biodegradation of polyethers and polyacrylate. In Studies in Polymer Science; Doi, Y., Fukuda, K., Eds.; Biodegradable Plastics and Polymers; Elsevier: Amsterdam, The Netherlands, 1994; Volume 12, pp. 24–38. [Google Scholar]
- Andreoni, V.; Bernasconi, S.; Sorlini, C.; Villa, M. Microbial degradation of acrylic acid. Ann. Microbiol. Enzimol. 1990, 40, 279–286. [Google Scholar]
- Matsumura, S.; Maeda, S.; Takahashi, A.; Yoshikawa, S. Molecular design of biodegradable polyelectrolytes. I. Biodegradation of polyvinyl alcohol and poly [(Sodium Acrylate)-Co-(vinyl alcohol)]. Jpn. J. Polym. Sci. Technol. 1988, 45, 317–324. [Google Scholar]
- Liu, J.; Ren, J.; Xu, R.; Yu, B.; Wang, J. Biodegradation of partially hydrolyzed polyacrylamide by immobilized bacteria isolated from HPAM-containing wastewater. Environ. Prog. Sustain. Energy 2016, 35, 1344–1352. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, C.; Bao, M.; Lu, J. Advanced treatment for actual hydrolyzed polyacrylamide-containing wastewater in a biofilm/activated sludge membrane bioreactor system: Biodegradation and interception. Biochem. Eng. J. 2019, 141, 120–130. [Google Scholar] [CrossRef]
- Zhao, L.; Song, T.; Han, D.; Bao, M.; Lu, J. Hydrolyzed polyacrylamide biotransformation in an up-flow anaerobic sludge blanket reactor system: Key enzymes, functional microorganisms, and biodegradation mechanisms. Bioproc. Biosyst. Eng. 2019, 42, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Li, S.; Lu, Y.; Yan, D.; Sun, P.; Bao, M.; Li, Y. Biodegradation of hydrolyzed polyacrylamide by a Bacillus megaterium strain SZK-5: Functional enzymes and antioxidant defense mechanism. Chemosphere 2019, 231, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, L.; Bao, M.; Lu, J. Potential of hydrolyzed polyacrylamide biodegradation to final products through regulating its own nitrogen transformation in different dissolved oxygen systems. Bioresour. Technol. 2018, 256, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Al-Moqbali, W.; Joshi, S.J.; Al-Bahry, S.N.; Al-Wahaibi, Y.M.; Elshafie, A.E.; Al-Bemani, A.S.; Al-Hashmi, A.; Soundra Pandian, S.B. Biodegradation of Partially Hydrolyzed Polyacrylamide HPAM Using Bacteria Isolated from Omani Oil Fields. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 26–28 March 2018. [Google Scholar]
- Yan, M.; Zhao, L.; Bao, M.; Lu, J. Hydrolyzed polyacrylamide biodegradation and mechanism in sequencing batch biofilm reactor. Bioresour. Technol. 2016, 207, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Fu, R.; Xie, Y.; Chen, W. Isolation and characterization of polyacrylamide-degrading bacteria from dewatered sludge. Int. J. Environ. Res. Public Health 2015, 12, 4214–4230. [Google Scholar] [CrossRef] [PubMed]
- Bouchenafa, W.; Dewals, B.; Lefevre, A.; Mignot, E. Water soluble polymers as a means to increase flow capacity: Field experiment of drag reduction by polymer additives in an irrigation canal. J. Hydraul. Eng. 2021, 147, 05021003. [Google Scholar] [CrossRef]
- Brakstad, O.G.; Altin, D.; Aas, M.; Skancke, J.; Nordtug, T.; Farkas, J. Attachment of APAM to mineral particles in seawater. Sci. Total Environ. 2021, 758, 143888. [Google Scholar] [CrossRef] [PubMed]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Nord, F.F. Dehydrogenation activity of fusarium lini B. Naturwissenschaften 1936, 24, 763. [Google Scholar] [CrossRef]
- Tudorachi, N.; Lipsa, R. Copolymers based on poly(vinyl alcohol) and acrylamide. J. Optoelectron. Adv. Mater. 2006, 8, 659–662. [Google Scholar]
- Caneba, G.; Axland, J. Vinyl acetate-acrylic acid copolymer for enhanced oil recovery. J. Metall. Mater. Chem. Eng. 2002, 1, 97–109. [Google Scholar] [CrossRef]
- Pająk, J.; Ziemski, M.; Nowak, B. Poly(vinyl alcohol)–biodegradable vinyl material. Chemik 2010, 64, 523–530. [Google Scholar]
- Ahmad, S.; Ahmad, M.; Manzoor, K.; Purwar, R.; Ikram, S. A review on latest innovations in natural gums based hydrogels: Preparations & applications. Int. J. Biol. Macromol. 2019, 136, 870–890. [Google Scholar] [CrossRef]
- Sharma, K.; Kaith, B.S.; Kumar, V.; Kumar, V.; Som, S.; Kalia, S.; Swart, H.C. Synthesis and properties of poly(acrylamide-aniline)-grafted gum ghatti based nanospikes. RSC Adv. 2013, 3, 25830–25839. [Google Scholar] [CrossRef]
- Mahammed, N.; Deshpande, R.; Gowda, D.V. Modified polysaccharide as drug delivery: Review. Int. J. Pharm. Sci. Rev. Res. 2011, 11, 42–47. [Google Scholar]
- Sekhar, E.C.; Rao, K.S.V.K.; Raju, R.R. Chitosan/guargum-g-acrylamide semi IPN microspheres for controlled release studies of 5-fluorouracil. J. Appl. Pharm. Sci. 2011, 1, 199–204. [Google Scholar]
- Ma, M.; Mukerabigwi, J.F.; Huang, R.; Lei, S.; Huang, X.; Cao, Y. Eco-friendly superabsorbent synthesis based on polysaccharides. J. Polym. Environ. 2020, 28, 2801–2809. [Google Scholar] [CrossRef]
- Kalia, S.; Sabaa, M.W. Polysaccharide Based Graft Copolymers, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-36566-9. [Google Scholar]
- Deng, S.; Binauld, S.; Mangiante, G.; Frances, J.M.; Charlot, A.; Bernard, J.; Zhou, X.; Fleury, E. Microcrystalline cellulose as reinforcing agent in silicone elastomers. Carbohydr. Polym. 2016, 151, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Tizzotti, M.; Charlot, A.; Fleury, E.; Stenzel, M.; Bernard, J. Modification of polysaccharides through controlled/living radical polymerization grafting—Towards the generation of high performance hybrids. Macromol. Rapid Commun. 2010, 31, 1751–1772. [Google Scholar] [CrossRef]
- Khine, Y.Y.; Ganda, S.; Stenzel, M.H. Covalent tethering of temperature responsive PNIPAm onto TEMPO-oxidized cellulose nanofibrils via three-component passerini reaction. ACS Macro Lett. 2018, 7, 412–418. [Google Scholar] [CrossRef]
- Tizzotti, M.; Creuzet, C.; Labeau, M.-P.; Hamaide, T.; Boisson, F.; Drockenmuller, E.; Charlot, A.; Fleury, E. Synthesis of temperature responsive biohybrid guar-based grafted copolymers by click chemistry. Macromolecules 2010, 43, 6843–6852. [Google Scholar] [CrossRef]
- Kumar, D.; Pandey, J.; Raj, V.; Kumar, P. A review on the modification of polysaccharide through graft copolymerization for various potential applications. Open J. Med. Chem. 2017, 11, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Kot, E.M. Water Soluble Polymers Containing Weak Links. Ph.D. Thesis, Imperial College London, London, UK, 2011. [Google Scholar]
- Bismarck, A.; Kot, E.M.; Saini, R.K. Water-Soluble Degradable Synthetic Vinyl Polymers and Related Methods. U.S. Patent 8,772,205,B2, 10 May 2014. [Google Scholar]
- Phillips, D.J.; Gibson, M.I. Biodegradable poly(disulfide)s derived from RAFT Polymerization: Monomer scope, glutathione degradation, and tunable thermal responses. Biomacromolecules 2012, 13, 3200–3208. [Google Scholar] [CrossRef] [PubMed]
- Kot, E.; Bismarck, A. Polyacrylamide containing weak temperature labile azo links in the polymer backbone. Macromolecules 2010, 43, 6469–6475. [Google Scholar] [CrossRef]
- Delplace, V.; Nicolas, J. Degradable vinyl polymers for biomedical applications. Nat. Chem. 2015, 7, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Bailey, W.J.; Chou, J.L.; Feng, P.-Z.; Issari, B.; Kuruganti, V.; Zhou, L.-L. Recent advances in free-radical ring-opening polymerization. J. Macromol. Sci. A 1988, 25, 781–798. [Google Scholar] [CrossRef]
- Nuyken, O.; Pask, S.D. Ring-opening polymerization—An introductory review. Polymers 2013, 5, 361–403. [Google Scholar] [CrossRef]
- Pesenti, T.; Nicolas, J. 100th anniversary of macromolecular science viewpoint: Degradable polymers from radical ring-opening polymerization: Latest advances, new directions, and ongoing challenges. ACS Macro Lett. 2020, 9, 1812–1835. [Google Scholar] [CrossRef]
- Tardy, A.; Nicolas, J.; Gigmes, D.; Lefay, C.; Guillaneuf, Y. Radical ring-opening polymerization: Scope, limitations, and application to (bio)degradable materials. Chem. Rev. 2017, 117, 1319–1406. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Andrieu, J.; Üzgün, S.; Rudolph, C.; Agarwal, S. Biocompatible, thermoresponsive, and biodegradable: Simple preparation of “All-in-One” biorelevant polymers. Macromolecules 2007, 40, 8540–8543. [Google Scholar] [CrossRef]
- Hedir, G.G.; Bell, C.A.; Ieong, N.S.; Chapman, E.; Collins, I.R.; O’Reilly, R.K.; Dove, A.P. Functional degradable polymers by Xanthate-mediated polymerization. Macromolecules 2014, 47, 2847–2852. [Google Scholar] [CrossRef]
- Jackson, A.W. Reversible-deactivation radical polymerization of cyclic ketene acetals. Polym. Chem. 2020, 11, 3525–3545. [Google Scholar] [CrossRef]
- Carter, M.C.D.; Hejl, A.; Woodfin, S.; Einsla, B.; Janco, M.; DeFelippis, J.; Cooper, R.J.; Even, R.C. Backbone-degradable vinyl acetate latex: Coatings for single-use paper products. ACS Macro Lett. 2021, 10, 591–597. [Google Scholar] [CrossRef]
- Evans, R.A.; Rizzardo, E. Free-radical ring-opening polymerization of cyclic allylic sulfides. Macromolecules 1996, 29, 6983–6989. [Google Scholar] [CrossRef]
- Paulusse, J.M.J.; Amir, R.J.; Evans, R.A.; Hawker, C.J. Free radical polymers with tunable and selective bio- and chemical degradability. J. Am. Chem. Soc. 2009, 131, 9805–9812. [Google Scholar] [CrossRef] [PubMed]
- Ivanchenko, O.; Authesserre, U.; Coste, G.; Mazières, S.; Destarac, M.; Harrisson, S. ε-Thionocaprolactone: An accessible monomer for preparation of degradable poly(vinyl esters) by radical ring-opening polymerization. Polym. Chem. 2021, 12, 1931–1938. [Google Scholar] [CrossRef]
- Bingham, M.N.; Roth, J.P. Degradable vinyl copolymers through thiocarbonyl addition–ring-opening (TARO) polymerization. Chem. Comm. 2019, 55, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Fu, G.; McAteer, O.; Xu, M.; Gutekunst, W.R. Radical approach to thioester-containing polymers. J. Am. Chem. Soc. 2019, 141, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Sagi, A.; Weinstain, R.; Karton, N.; Shabat, D. Self-immolative polymers. J. Am. Chem. Soc. 2008, 130, 5434–5435. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, H. Über polymerisation. Ber. Deutsch. Chem. Ges. A/B 1920, 53, 1073–1085. [Google Scholar] [CrossRef]
| Emissions (in kg CO2e·ton−1 of Total Solid) | Heat with Natural Gas | Polymer Production | Polymer Dissolution | Total | Emissions Reduction (%) |
|---|---|---|---|---|---|
| Without polymer | 140 | / | / | 140 | / |
| With polymer | / | 17.5 | 0.4 | 17.9 | 87 |
| With ISCC+ polymer | / | 3.2 | 0.4 | 3.6 | 97 |
| Biodegradation Test | OECD Guideline | Pass Level | Incubation Conditions | Chemical Concentration | Inoculum Source | Test Duration |
|---|---|---|---|---|---|---|
| Ready biodegradability tests (screening tests) | OECD 301 A | 70% DOC removal | Aerobic | 10–40 mg DOC·L−1 | Activated sludge, sewage effluents, surface waters, soils or mixture of these | 28 days |
| OECD 301 B | 60% ThCO2 | 10–20 mg DOC·L−1 | ||||
| OECD 301 C | 60% ThOD | 100 mg·L−1 | Fresh samples from sewage treatment works, industrial WWTPs, soils, lakes orseas, mixed thoroughly together | |||
| OECD 301 D | 2–10 mg·L−1 or 5–10 mg ThOD·L−1 | Derived from secondary effluent of WWTP or laboratory-scale unit, predominantly domestic sewage, alternatively surface water, e.g., river or lake | ||||
| OECD 301 E | 70% DOC removal | 10–40 mg DOC·L−1 | Derived from secondary effluent of WWTP or laboratory-scale unit, predominantly domestic sewage | |||
| OECD 301 F | 60% ThOD | 100 mg·L−1 or 50–100 mg ThOD·L−1 | Activated sludge, sewage effluents, surface waters, soils or mixture of these | |||
| Inherent (potential) biodegradability tests | OECD 302 A | >20% ThBOD, ThDOC removal or ThCOD (primary biodegradation; >20% Th BOD, ThDOC removal or ThCOD (ultimate biodegradation) | Aerobic | 2 –10 mg·L−1 | Mixed settled sludges after two week aeration period | Not defined |
| OECD 302 B | 50–400 mg DOC·L−1 | Activated sludge | ||||
| OECD 302 C | 30 mg·kg−1 | Activated sludge | ||||
| Biodegradability in seawater | OECD 306 Shake flash method | 70% DOC removal | Aerobic | 50–40 mg DOC·L−1 | Natural seawater (after filtration) | 60 days (can be extended) |
| OECD 306 closed bottle method | 60% ThOD | 2–10 mg test substance·L−1 | 28 days (can be extended) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braun, O.; Coquery, C.; Kieffer, J.; Blondel, F.; Favero, C.; Besset, C.; Mesnager, J.; Voelker, F.; Delorme, C.; Matioszek, D. Spotlight on the Life Cycle of Acrylamide-Based Polymers Supporting Reductions in Environmental Footprint: Review and Recent Advances. Molecules 2022, 27, 42. https://doi.org/10.3390/molecules27010042
Braun O, Coquery C, Kieffer J, Blondel F, Favero C, Besset C, Mesnager J, Voelker F, Delorme C, Matioszek D. Spotlight on the Life Cycle of Acrylamide-Based Polymers Supporting Reductions in Environmental Footprint: Review and Recent Advances. Molecules. 2022; 27(1):42. https://doi.org/10.3390/molecules27010042
Chicago/Turabian StyleBraun, Olivier, Clément Coquery, Johann Kieffer, Frédéric Blondel, Cédrick Favero, Céline Besset, Julien Mesnager, François Voelker, Charlène Delorme, and Dimitri Matioszek. 2022. "Spotlight on the Life Cycle of Acrylamide-Based Polymers Supporting Reductions in Environmental Footprint: Review and Recent Advances" Molecules 27, no. 1: 42. https://doi.org/10.3390/molecules27010042
APA StyleBraun, O., Coquery, C., Kieffer, J., Blondel, F., Favero, C., Besset, C., Mesnager, J., Voelker, F., Delorme, C., & Matioszek, D. (2022). Spotlight on the Life Cycle of Acrylamide-Based Polymers Supporting Reductions in Environmental Footprint: Review and Recent Advances. Molecules, 27(1), 42. https://doi.org/10.3390/molecules27010042





