Chemical Composition and Thermogravimetric Behaviors of Glanded and Glandless Cottonseed Kernels
Abstract
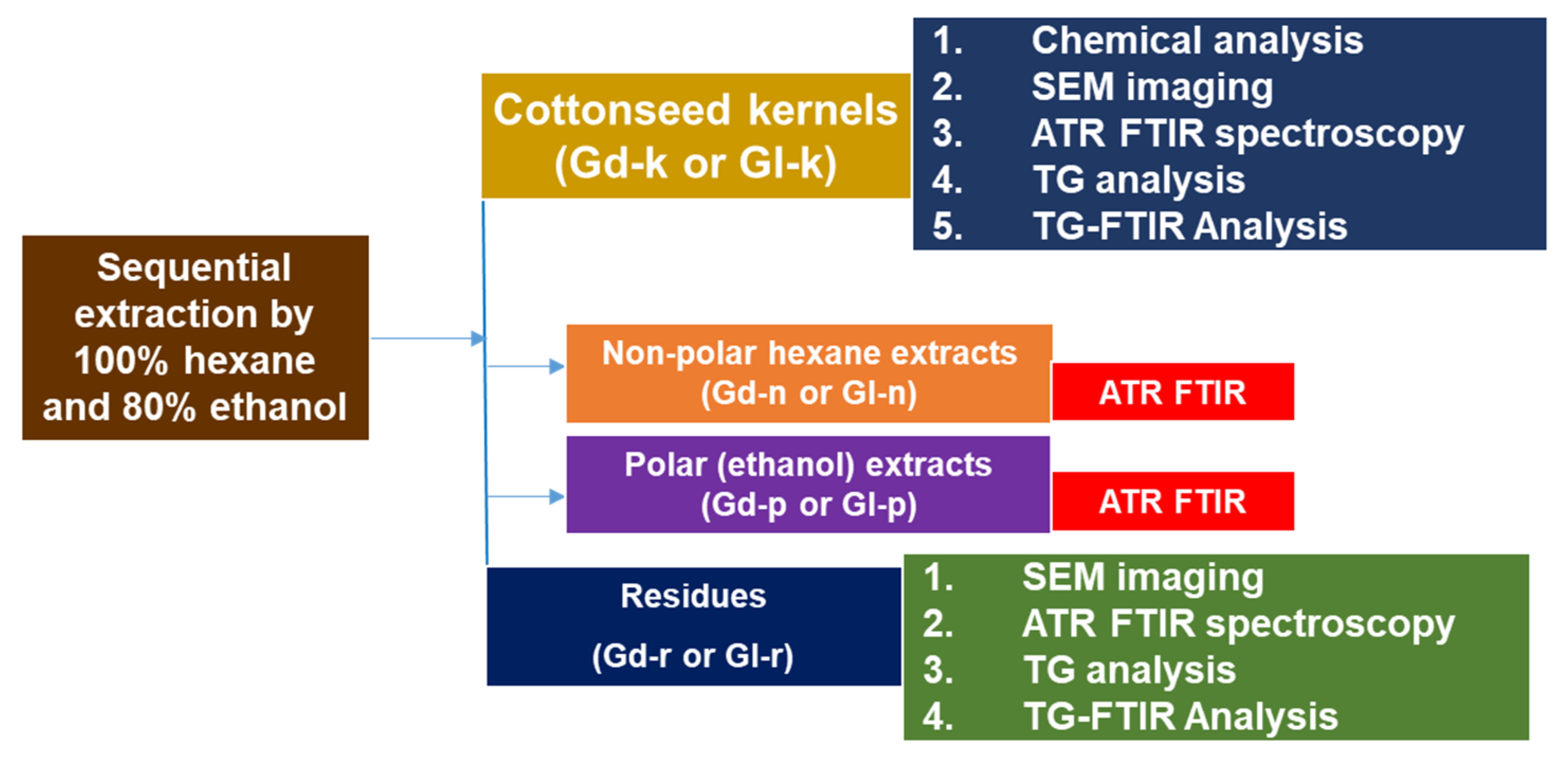
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Gd and Gl Kernels
2.2. Microstructure of Gd and Gl Cottonseed Kernels
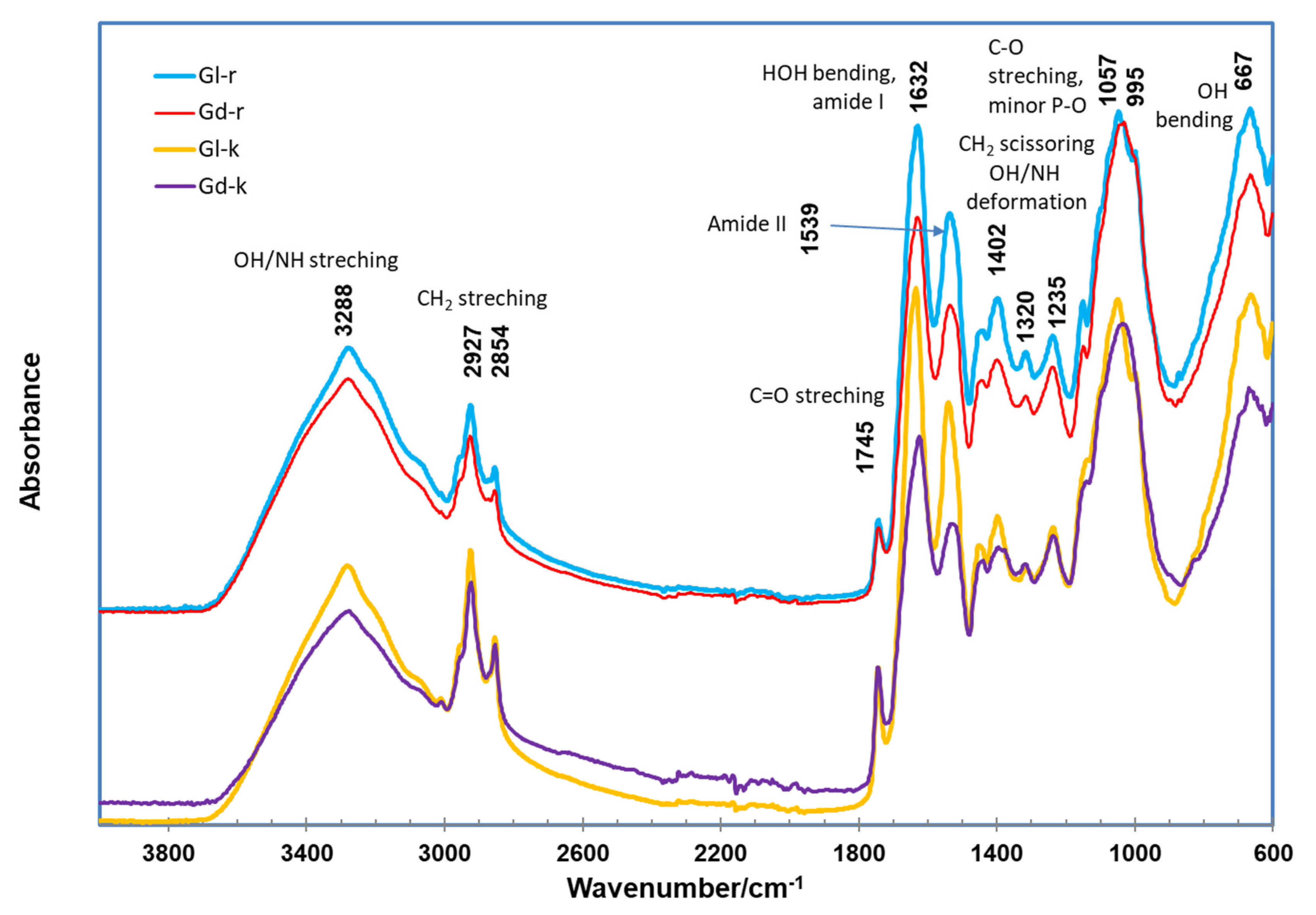
2.3. ATR FTIR Spectra of Gd and Gl Kernels and Their Extraction Residues
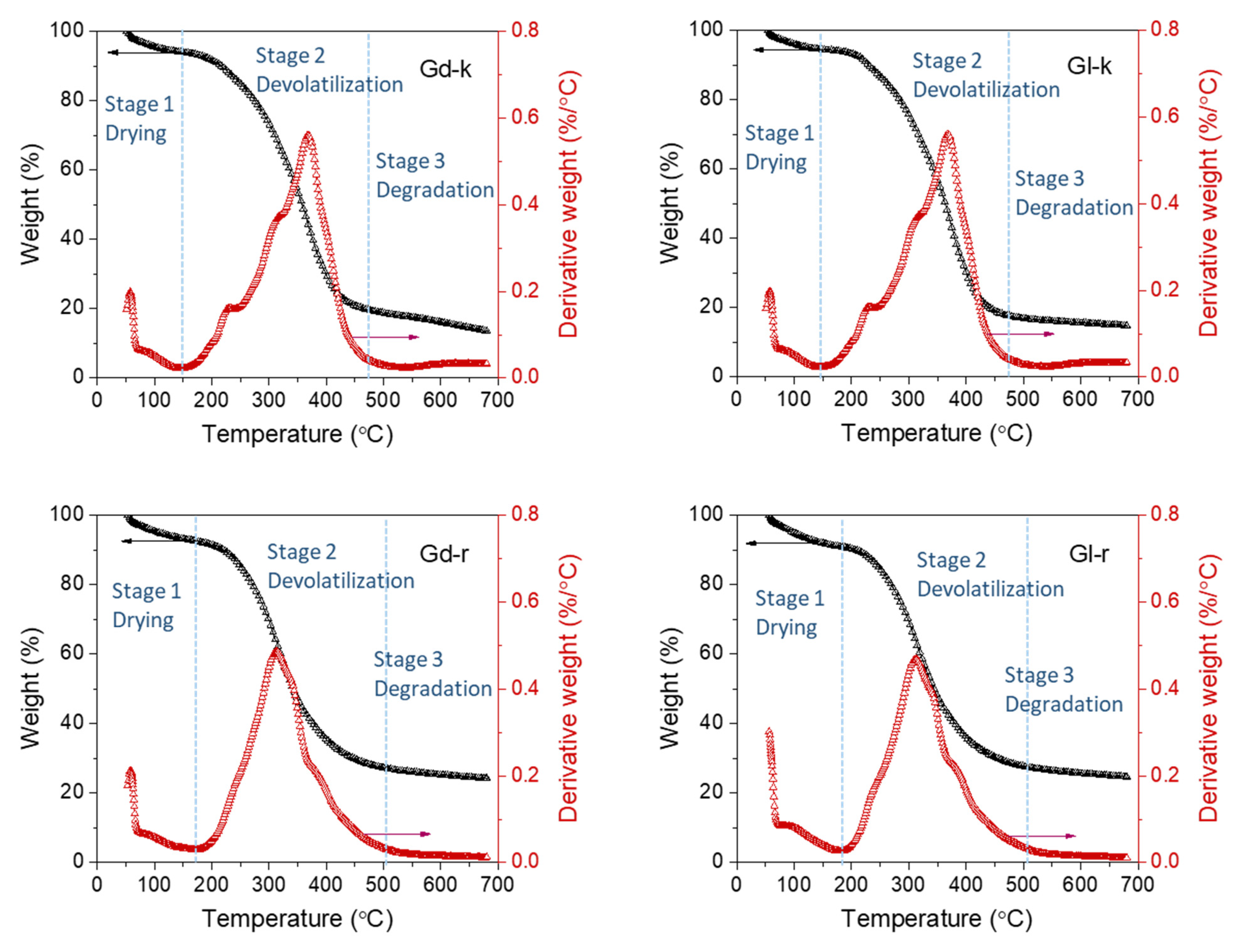
2.4. Thermogravimetric Observations
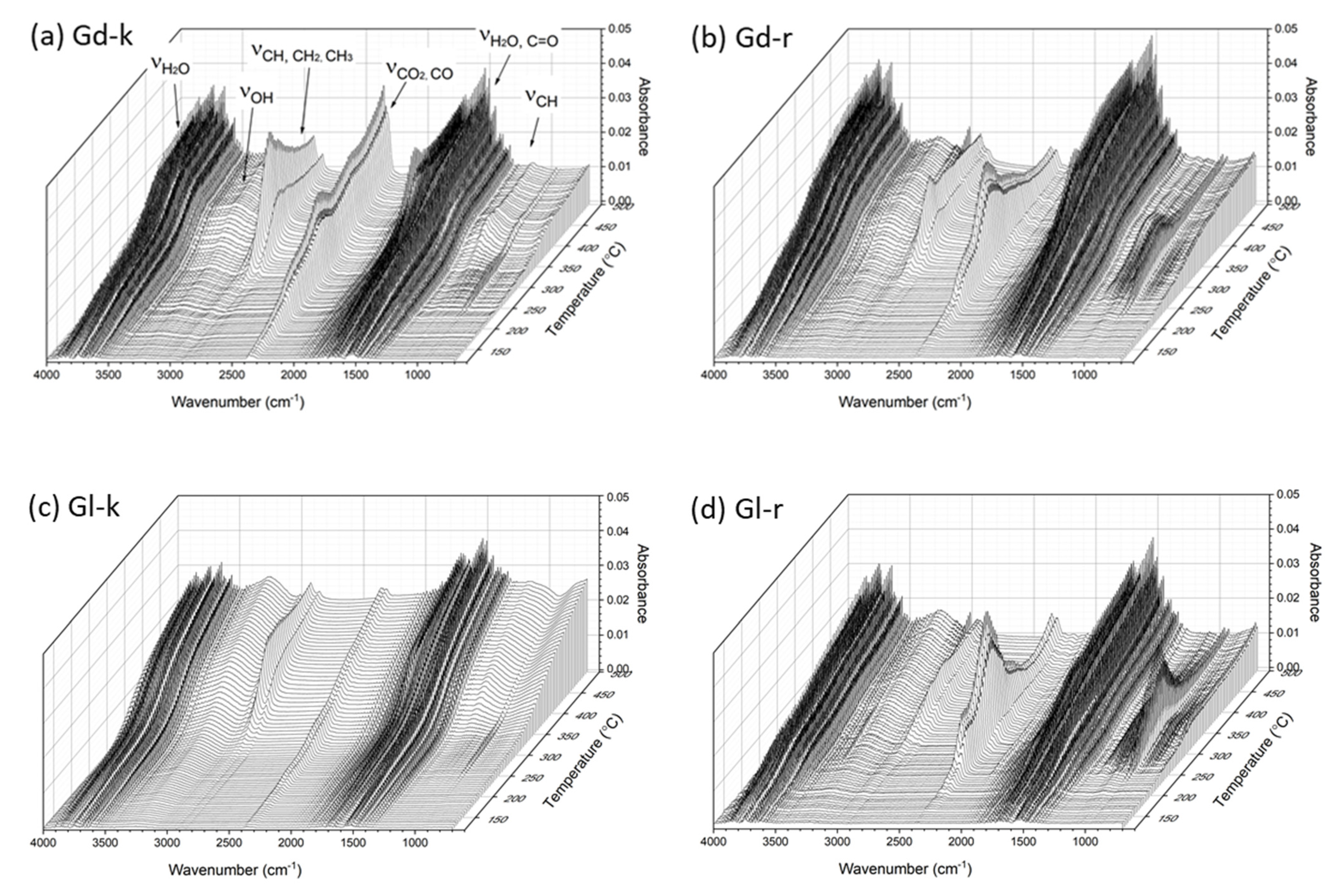
2.5. TG-FTIR Observations
3. Materials and Methods
3.1. Cottonseed Source and Treatment
3.2. Non-Polar and Polar Solvent Extraction
3.3. Microstructural Imaging Analysis
3.4. Chemical Analysis
3.5. Instrumental Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rojo-Gutiérrez, E.; Buenrostro-Figueroa, J.; López-Martínez, L.; Sepúlveda, D.; Baeza-Jiménez, R. Biotechnological potential of cottonseed, a by-product of cotton production. In Valorisation of Agro-Industrial Residues–Volume II: Non-Biological Approaches, Zainul Akmar Zakaria; Aguilar, C.N., Kusumaningtyas, R.D., Binod, P., Eds.; Springer: Amsterdam, The Netherlands, 2020; pp. 63–82. [Google Scholar]
- Kumar, M.; Tomar, M.; Punia, S.; Grasso, S.; Arrutia, F.; Choudhary, J.; Singh, S.; Verma, P.; Mahapatra, A.; Patil, S. Cottonseed: A sustainable contributor to global protein requirements. Trends Food Sci. Technol. 2021, 111, 100–113. [Google Scholar] [CrossRef]
- Statista. Global Cottonseed Production from 2013/2014 to 2020/2021. Available online: Https://www.Statista.Com/statistics/259489/worldwide-production-of-cottonseed/ (accessed on 1 January 2022).
- NCPA. National Cottonseed Products Association—The Products. Available online: Https://www.Cottonseed.Com/products/ (accessed on 1 January 2022).
- He, Z.; Cheng, H.N. Preparation and utilization of water washed cottonseed meal as wood adhesives. In Bio-Based Wood Adhesives: Preparation, Characterization, and Testing; He, Z., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 156–178. [Google Scholar]
- Dowd, M.K. Seed. In Cotton: Agronomy Monograph, 2nd ed.; Fang, D.D., Percy, R.G., Eds.; ASA, CSSA, and SSSA: Madison, WI, USA, 2015; Volume 57, pp. 745–781. [Google Scholar]
- Risco, C.; Chase, C. Gossypol. In Handbook of Plant and Fungal Toxicants; D′Mello, J.P.F., Ed.; CRC Press: Boca Raton, FL, USA, 1997; pp. 87–98. [Google Scholar]
- He, Z.; Waldrip, H.M.; Wang, Y. Application of capillary electrophoresis in agricultural and soil chemistry research. In Capillary Electrophoresis: Fundamentals, Techniques and Applications; He, Z., Ed.; Nova Science Publishers: New York, NY, USA, 2012; pp. 131–151. [Google Scholar]
- He, Z.; Mattison, C.P.; Zhang, D.; Grimm, C.C. Vicilin and legumin storage proteins are abundant in water and alkali soluble protein fractions of glandless cottonseed. Sci. Rep. 2021, 11, 9209. [Google Scholar] [CrossRef]
- Xie, S.; Zhou, Q.; Zhang, X.; Zhu, T.; Guo, C.; Yang, Z.; Luo, J.; Yuan, Y.; Hu, X.; Jiao, L. Effect of dietary replacement of fish meal with low-gossypol cottonseed protein concentrate on growth performance and expressions of genes related to protein metabolism for swimming crab (Portunus trituberculatus). Aquaculture 2022, 549, 737820. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Li, C.; Zhang, F.; Mei, L.; Chindudzi, E.; Chen, J.; Zhu, S. Genome-wide analysis of genetic variations between dominant and recessive nils of glanded and glandless cottons. Sci. Rep. 2019, 9, 9226. [Google Scholar] [CrossRef] [PubMed]
- Rathore, K.S.; Pandeya, D.; Campbell, L.M.; Wedegaertner, T.C.; Puckhaber, L.; Stipanovic, R.D.; Thenell, J.S.; Hague, S.; Hake, K. Ultra-low gossypol cottonseed: Selective gene silencing opens up a vast resource of plant-based protein to improve human nutrition. Critic. Revi. Plant Sci. 2020, 39, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Bellaloui, N.; Saha, S.; Tonos, J.; Scheffler, J.; Jenkins, J.; McCarty, J.; Stelly, D.M. Effect of chromosome substitution from alien tetraploid cotton species in upland cotton on (+) and (−) gossypol enantiomer levels in cottonseed. J. Cotton Sci. 2021, 25, 7–20. [Google Scholar]
- Cheng, H.N.; He, Z.; Ford, C.; Wyckoff, W.; Wu, Q. A review of cottonseed protein chemistry and non-food applications. Sustain. Chem. 2020, 1, 256–274. [Google Scholar] [CrossRef]
- He, Z.; Zhang, D.; Cheng, H.N. Modeling and thermodynamic analysis of the water sorption isotherms of cottonseed products. Foundations 2021, 1, 32–44. [Google Scholar] [CrossRef]
- He, Z.; Cheng, H.N.; Chapital, D.C.; Dowd, M.K. Sequential fractionation of cottonseed meal to improve its wood adhesive properties. J. Am. Oil Chem. Soc. 2014, 91, 151–158. [Google Scholar] [CrossRef]
- Cao, H.; Sethumadhavan, K.; Bland, J.M. Isolation of cottonseed extracts that affect human cancer cell growth. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Sethumadhavan, K.; Wu, X.; Zeng, X. Cottonseed-derived gossypol and ethanol extracts differentially regulate cell viability and vegf gene expression in mouse macrophages. Sci. Rep. 2021, 11, 15700. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, D.; Olanya, O.M. Antioxidant activities of the water-soluble fractions of glandless and glanded cottonseed protein. Food Chem. 2020, 325, 126907. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, D.; Cao, H. Protein profiling of water and alkali soluble cottonseed protein isolates. Sci. Rep. 2018, 8, 9306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, E.; Valverde-Quiroz, L.; Lopez, D.; Cooke, P.; Valles-Rosales, D.; Flores, N. Characterization of soluble glandless cottonseed meal proteins based on electrophoresis, functional properties, and microscopic structure. J. Food Sci. 2019, 84, 2820–2830. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, J.J.; Gardner, H.K. Food uses for cottonseed protein. J. Am. Oil Chem. Soc. 1979, 56, 422–424. [Google Scholar] [CrossRef]
- Lusas, E.; Jividen, G. Glandless cottonseed: A review of the first 25 years of processing and utilization research. J. Am. Oil Chem. Soc. 1987, 64, 839–854. [Google Scholar] [CrossRef]
- Reyes-Jáquez, D.; Casillas, F.; Flores, N.; Andrade-González, I.; Solís-Soto, A.; Medrano-Roldán, H.; Carrete, F.; Delgado, E. The effect of glandless cottonseed meal content and process parameters on the functional properties of snacks during extrusion cooking. Food Nutrit. Sci. 2012, 3, 1716–1725. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Jáquez, D.; Casillas, F.; Flores, N.; Cooke, P.; Licon, E.D.; Soto, A.S.; González, I.A.; Carreón, F.O.C.; Roldán, H.M. Effect of glandless cottonseed meal content on the microstructure of extruded corn-based snacks. Adv. Food Sci. 2014, 36, 125–130. [Google Scholar]
- Delgado, E.; Valles-Rosales, D.; Pámanes-Carrasco, G.; Cooke, P.; Flores, N. Structural, rheological and calorimetric properties of an extruded shrimp feed using glandless cottonseed meal as a protein source. J. Aquac. Res. Develop. 2021, 12, 637. [Google Scholar]
- Delgado, E.; Valles-Rosales, D.J.; Flores, N.C.; Reyes-Jáquez, D. Evaluation of fish oil content and cottonseed meal with ultralow gossypol content on the functional properties of an extruded shrimp feed. Aquac. Rep. 2021, 19, 100588. [Google Scholar] [CrossRef]
- Alam, M.S.; Watanabe, W.O.; Carroll, P.M.; Gabel, J.E.; Corum, M.A.; Seaton, P.; Wedegaertner, T.C.; Rathore, K.S.; Dowd, M.K. Evaluation of genetically-improved (glandless) and genetically-modified low-gossypol cottonseed meal as alternative protein sources in the diet of juvenile southern flounder paralichthys lethostigma reared in a recirculating aquaculture system. Aquaculture 2018, 489, 36–45. [Google Scholar] [CrossRef]
- Abidi, N.; Manike, M. X-ray diffraction and ftir investigations of cellulose deposition during cotton fiber development. Text. Res. J. 2018, 88, 719–730. [Google Scholar] [CrossRef]
- He, Z.; Nam, S.; Fang, D.D.; Cheng, H.N.; He, J. Surface and thermal characterization of cotton fibers of phenotypes differing in fiber length. Polymers 2021, 13, 994. [Google Scholar] [CrossRef]
- Liu, Y.; He, Z.; Shankle, M.; Tewolde, H. Compositional features of cotton plant biomass fractions characterized by attenuated total reflection fourier transform infrared spectroscopy. Ind. Crop. Prod. 2016, 79, 283–286. [Google Scholar] [CrossRef]
- He, Z.; Liu, Y. Fourier transform infrared spectroscopic analysis in applied cotton fiber and cottonseed research: A review. J. Cotton Sci. 2021, 25, 167–183. [Google Scholar]
- Kaur, A.; Singh, B.; Kaur, A.; Singh, N. Chemical, thermal, rheological and ftir studies of vegetable oils and their effect on eggless muffin characteristics. J. Food Process. Preser. 2019, 43, e13978. [Google Scholar] [CrossRef]
- Mahesar, S.A.; Shah, S.N.; Mahesar, A.W.; Kandhro, A.A.; Khaskheli, A.R.; Menghwar, P.; Sherazi, S.T.H. A chemometric approach for the quantification of free fatty acids in cottonseed oil by fourier transform infrared spectroscopy. Inter. J. Food Propert. 2017, 20, 1913–1920. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.N.; Kilgore, K.; Ford, C.; Fortier, C.; Dowd, M.K.; He, Z. Cottonseed protein-based wood adhesive reinforced with nanocellulose. J. Adhes. Sci. Technol. 2019, 33, 1357–1368. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Wu, Y.; He, Z.; Wan, H. “Greener” adhesives composed of urea-formaldehyde resin and cottonseed meal for wood-based composites. J. Clean. Prod. 2018, 187, 361–371. [Google Scholar] [CrossRef]
- Liu, Y.; He, Z.; Uchimiya, M. Comparison of biochar formation from various agricultural by-products using ftir spectroscopy. Modern Appl. Sci. 2015, 9, 246–253. [Google Scholar] [CrossRef]
- He, Z.; Cao, H.; Cheng, H.N.; Zou, H.; Hunt, J.F. Effects of vigorous blending on yield and quality of protein isolates extracted from cottonseed and soy flours. Mod. Appl. Sci. 2013, 7, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Prodyawong, S.; He, Z.; Sun, X.S.; Wang, D. Effect of drying methods on the physicochemical properties and adhesion performance of water-washed cottonseed meal. Ind. Crop. Prod. 2017, 109, 281–287. [Google Scholar] [CrossRef]
- Silwal, D.K.; Phambu, N.; Pokharel, B.; Aziz, A.N. Identification of hydrated and dehydrated lipids and protein secondary structures in seeds of cotton (Gossypium hirsutum) line. Pure App. Biol. 2017, 6, 965–975. [Google Scholar]
- Sun, C.; Wu, X.; Wang, L.; Wang, Y.; Zhang, Y.; Chen, L.; Wu, Z. Comparison of chemical composition of different transgenic insect-resistant cotton seeds using fourier transform infrared spectroscopy (ftir). Afric. J. Agric. Res. 2012, 7, 2918–2925. [Google Scholar]
- Ong, H.C.; Chen, W.-H.; Singh, Y.; Gan, Y.Y.; Chen, C.-Y.; Show, P.L. A state-of-the-art review on thermochemical conversion of biomass for biofuel production: A tg-ftir approach. Energy Conver. Manag. 2020, 209, 112634. [Google Scholar] [CrossRef]
- Fischer, M.; Wohlfahrt, S.; Varga, J.; Matuschek, G.; Saraji-Bozorgzad, M.R.; Walte, A.; Denner, T.; Zimmermann, R. Evolution of volatile flavor compounds during roasting of nut seeds by thermogravimetry coupled to fast-cycling optical heating gas chromatography-mass spectrometry with electron and photoionization. Food Anal. Met. 2017, 10, 49–62. [Google Scholar] [CrossRef]
- Valdés García, A.; Beltrán, A.; Karabagias, I.; Badeka, A.; Kontominas, M.G.; Garrigós, M.C. Monitoring the oxidative stability and volatiles in blanched, roasted and fried almonds under normal and accelerated storage conditions by dsc, thermogravimetric analysis and atr-ftir. Eur. J. Lipid Sci. Technol. 2015, 117, 1199–1213. [Google Scholar] [CrossRef]
- Valdés García, A.; Beltrán Sanahuja, A.; Karabagias, I.K.; Badeka, A.; Kontominas, M.G.; Garrigós, M.C. Effect of frying and roasting processes on the oxidative stability of sunflower seeds (Helianthus annuus) under normal and accelerated storage conditions. Foods 2021, 10, 944. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.; Zaaboul, F.; Shoaib, M.; Ashraf, W.; Hussain, A.; Zhang, L. Physicochemical and structural characterization of microwave-roasted chickpea. J. Glob. Inno. Agri. Soci. Sci 2019, 7, 23–28. [Google Scholar] [CrossRef]
- Rojas, S.M.; Chejne, F.; Ciro, H.; Montoya, J. Roasting impact on the chemical and physical structure of criollo cocoa variety (Theobroma cacao L.). J. Food Proc. Engineer. 2020, 43, e13400. [Google Scholar] [CrossRef]
- Nam, S.; Condon, B.D.; Liu, Y.; He, Q. Natural resistance of raw cotton fiber to heat evidenced by the suppressed depolymerization of cellulose. Polymer Degrad. Stabili. 2017, 138, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Volli, V.; Gollakota, A.R.K.; Shu, C.-M. Thermal behavior of hazel sterculia seeds: A tg-ftir study. J. Innov. Technol. 2021, 3, 1–6. [Google Scholar]
- Tahir, M.H.; Mahmood, M.A.; Çakman, G.; Ceylan, S. Pyrolysis of oil extracted safflower seeds: Product evaluation, kinetic and thermodynamic studies. Bioresour. Technol. 2020, 314, 123699. [Google Scholar] [CrossRef]
- Zong, P.; Jiang, Y.; Tian, Y.; Li, J.; Yuan, M.; Ji, Y.; Chen, M.; Li, D.; Qiao, Y. Pyrolysis behavior and product distributions of biomass six group components: Starch, cellulose, hemicellulose, lignin, protein and oil. Energy Conver. Manag. 2020, 216, 112777. [Google Scholar] [CrossRef]
- González-Rivera, J.; Duce, C.; Falconieri, D.; Ferrari, C.; Ghezzi, L.; Piras, A.; Tine, M.R. Coaxial microwave assisted hydrodistillation of essential oils from five different herbs (lavender, rosemary, sage, fennel seeds and clove buds): Chemical composition and thermal analysis. Innov. Food Sci. Emerg. Technol. 2016, 33, 308–318. [Google Scholar] [CrossRef]
- Apaydin-Varol, E.; Uzun, B.B.; Onal, E.; Putun, A.E. Synthetic fuel production from cottonseed: Fast pyrolysis and a tga/ft-ir/ms study. J. Anal. Appl. Pyrol. 2014, 105, 83–90. [Google Scholar] [CrossRef]
- Seal, S.; Panda, A.K.; Kumar, S.; Singh, R. Production and characterization of bio oil from cotton seed. Environ. Progress Sustain. Energy 2015, 34, 542–547. [Google Scholar] [CrossRef]
- Primaz, C.T.; Ribes-Greus, A.; Jacques, R.A. Valorization of cotton residues for production of bio-oil and engineered biochar. Energy 2021, 235, 121363. [Google Scholar] [CrossRef]
- He, Z.; Shankle, M.; Zhang, H.; Way, T.R.; Tewolde, H.; Uchimiya, M. Mineral composition of cottonseed is affected by fertilization management practices. Agron. J. 2013, 105, 341–350. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Zhang, H.; Olk, D.C.; Shankle, M.; Way, T.R.; Tewolde, H. Protein and fiber profiles of cottonseed from upland cotton with different fertilizations. Mod. Appl. Sci. 2014, 8, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Bellaloui, N.; Turley, R.B.; Stetina, S.R. Cottonseed protein, oil, and minerals in cotton (Gossypium hirsutum L.) lines differing in curly leaf morphology. Plants 2021, 10, 525. [Google Scholar] [CrossRef]
- Bolek, Y.; Tekerek, H.; Hayat, K.; Bardak, A. Screening of cotton genotypes for protein content, oil and fatty acid composition. J. Agri. Sci. 2016, 8, 107–121. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Dai, Z.; Yang, J.; Snider, J.L.; Wang, S.; Meng, Y.; Wang, Y.; Chen, B.; Zhao, W.; Zhou, Z. Cultivar sensitivity of cotton seed yield to potassium availability is associated with differences in carbohydrate metabolism in the developing embryo. Field Crop. Res. 2017, 214, 301–309. [Google Scholar] [CrossRef]
- Han, Y.W. Removal of phytic acid from soybean and cottonseed meals. J. Agric. Food Chem. 1988, 36, 1181–1183. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Fang, D.D.; Zeng, L.; Jenkins, J.N.; McCarty, J.C. Effects of inter-species chromosome substitution on cottonseed mineral and protein nutrition profiles. Agron. J. 2020, 112, 3963–3974. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, C.; Clark, R.B. Micronutrients in crop production. Adv. Agron. 2002, 77, 185–268. [Google Scholar]
- He, Z.; Cheng, H.N.; Olanya, O.M.; Uknalis, J.; Zhang, X.; Koplitz, B.D.; He, J. Surface characterization of cottonseed meal products by sem, sem-eds, xrd and xps analysis. J. Mater. Sci. Res. 2018, 7, 28–40. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Kyriakopoulou, K.; Ndiaye, M.; Bianeis, M.; Keppler, J.; van der Goot, A. Characteristics of soy protein prepared using an aqueous ethanol washing process. Foods 2021, 10, 2222. [Google Scholar] [CrossRef]
- Yatsu, L.; Jacks, T.J.; Kircher, H.W.; Godshall, M.A. Chemical and microscopic studies of the matrix substance in pigment glands of cotton (Gossypium hirsutum L.) seeds. J. Am. Oil Chem. Soc. 1986, 63, 534–537. [Google Scholar] [CrossRef]
- Shah, S.; Mahesar, S.A.; Abro, K.A.; Sherazi, S.T.H.; Nizamani, S.M. Ftir characterization and physicochemical evaluation of cottonseed oil. Pak. J. Anal. Environ. Chem. 2017, 18, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahman, S.A.; Yassin, A.A.; Mirghani, M.E.S.; Bashir, N.H. Determination of gossypol in hamid and bt (seeni 1) cottonseed oil using fourier transform infrared spectroscopy. Borneo J. Pharmacy 2020, 3, 227–234. [Google Scholar] [CrossRef]
- Riaz, T.; Iqbal, M.W.; Mahmood, S.; Yasmin, I.; Leghari, A.A.; Rehman, A.; Mushtaq, A.; Ali, K.; Azam, M.; Bilal, M. Cottonseed oil: A review of extraction techniques, physicochemical, functional, and nutritional properties. Crit. Rev. Food Sci. Nutrit. 2021. [Google Scholar] [CrossRef]
- Arslan, F.N.; Akin, G.; Elmas, Ş.N.K.; Yilmaz, I.; Janssen, H.-G.; Kenar, A. Rapid detection of authenticity and adulteration of cold pressed black cumin seed oil: A comparative study of atr–ftir spectroscopy and synchronous fluorescence with multivariate data analysis. Food Control 2019, 98, 323–332. [Google Scholar] [CrossRef]
- He, Z.; Chapital, D.C.; Cheng, H.N.; Dowd, M.K. Comparison of adhesive properties of water- and phosphate buffer-washed cottonseed meals with cottonseed protein isolate on maple and poplar veneers. Int. J. Adhes. Adhes. 2014, 50, 102–106. [Google Scholar] [CrossRef]
- He, Z.; Sleighter, R.L.; Hatcher, P.G.; Liu, S.; Wu, F.; Zou, H.; Olanya, O.M. Molecular level comparison of water extractives of maple and oak with negative and positive ion esi ft-icr mass spectrometry. J. Mass Spectrom. 2019, 54, 655–666. [Google Scholar] [CrossRef]
- Ling, T.-R.; Chang, J.-S.; Chiou, Y.-J.; Chern, J.-M.; Chou, T.-C. Characterization of high acid value waste cottonseed oil by temperature programmed pyrolysis in a batch reactor. J. Anal. Appl. Pyrol. 2016, 120, 222–230. [Google Scholar] [CrossRef]
- Villalpando, A.; Easson, M.; Cheng, H.; Condon, B. Use of cottonseed protein as a strength additive for nonwoven cotton. Textile Res. J. 2019, 89, 1725–1733. [Google Scholar] [CrossRef]
- Swiatkiewicz, S.; Arczewska-Wlosek, A.; Jozefia, D. The use of cottonseed meal as a protein source for poultry: An updated review. World Poultry Sci. J. 2016, 72, 473–484. [Google Scholar] [CrossRef]
- Kumar, M. Paruthi paal, a nutrient-rich healthy drink from cottonseed: An indian delicacy. J. Ethnic Foods 2019, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Potkule, J.; Patil, S.; Mageshwaran, V.; Radha; Satankar, V.; Berwal, M.K.; Mahapatra, A.; Saxena, S.; Ashtaputre, N.; et al. Evaluation of detoxified cottonseed protein isolate for application as food supplement. Toxin Rev. 2021, 1–8. [Google Scholar] [CrossRef]
- Kumar, M.; Potkule, J.; Patil, S.; Saxena, S.; Patil, P.G.; Mageshwaran, V.; Punia, S.; Varghese, E.; Mahapatra, A.; Ashtaputre, N.; et al. Extraction of ultra-low gossypol protein from cottonseed: Characterization based on antioxidant activity, structural morphology and functional group analysis. LWT 2021, 140, 110692. [Google Scholar] [CrossRef]
- Venkatesan, H.; Sivamani, S. Cotton seed biodiesel as alternative fuel: Production and its characterization analysis using spectroscopic studies. Inter. J. Renew. Energy Res. 2017, 7, 1333–1339. [Google Scholar]
- He, Z.; Guo, M.; Sleighter, R.L.; Zhang, H.; Fortier, C.A.; Hatcher, P.G. Characterization of defatted cottonseed meal-derived pyrolysis bio-oil by ultrahigh resolution electrospray ionization fourier transform ion cyclotron resonance mass spectrometry. J. Anal. Appl. Pyrol. 2018, 136, 96–106. [Google Scholar] [CrossRef]
- He, Z.; Uchimiya, S.M.; Guo, M. Production and characterization of biochar from agricultural by-products: Overview and use of cotton biomass residues. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Guo, M., He, Z., Uchimiya, S.M., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 2016; pp. 63–86. [Google Scholar]
- de Freitas Floriano, R.; Gräbin, K.; Rossi, R.C.; Ferreira, C.D.; Ziegler, V. Impact of roasting conditions on the quality and acceptance of the peanut paste. J. Texture Stud. 2020, 51, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Marzocchi, S.; Pasini, F.; Verardo, V.; Ciemniewska-Żytkiewicz, H.; Caboni, M.F.; Romani, S. Effects of different roasting conditions on physical-chemical properties of polish hazelnuts (Corylus avellana L. Var. Kataloński). LWT 2017, 77, 440–448. [Google Scholar] [CrossRef]
- Ciemniewska-Żytkiewicz, H.; Bryś, J.; Sujka, K.; Koczoń, P. Assessment of the hazelnuts roasting process by pressure differential scanning calorimetry and mid-ft-ir spectroscopy. Food Anal. Methods 2015, 8, 2465–2473. [Google Scholar] [CrossRef] [Green Version]
- Siegmund, B.; Murkovic, M. Changes in chemical composition of pumpkin seeds during the roasting process for production of pumpkin seed oil (part 2: Volatile compounds). Food Chem. 2004, 84, 367–374. [Google Scholar] [CrossRef]
- Gorrepati, K.; Balasubramanian, S.; Chandra, P. Plant based butters. J. Food Sci. Technol. 2015, 52, 3965–3976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Guo, M.; Fortier, C.; Cao, X.; Schmidt-Rohr, K. Fourier transform infrared and solid state 13c nuclear magnetic resonance spectroscopic characterization of defatted cottonseed meal-based biochars. Mod. Appl. Sci. 2021, 15, 108–121. [Google Scholar] [CrossRef]
- Zhang, J.; Wedegaertner, T.; Idowu, O.J.; Flynn, R.; Hughs, S.E.; Jones, D.C. Registration of ‘numex cot 15 gls’glandless cotton. J. Plant Registr. 2016, 10, 223–227. [Google Scholar] [CrossRef]
- Chen, N.; Huang, J.; Li, K. Investigation of a formaldehyde-free cottonseed flour-based adhesive for interior plywood. BioResour. 2020, 15, 5546–5557. [Google Scholar] [CrossRef]
- Pettigrew, W.T.; Dowd, M.K. Nitrogen fertility and irrigation effects on cottonseed composition. J. Cotton Sci. 2014, 18, 410–419. [Google Scholar]
- McCall, E.R.; Jurgens, J.F. Chemical composition of cotton. Text. Res. J. 1951, 21, 19–21. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Olk, D.C. Chemical composition of defatted cottonseed and soy meal products. PLoS ONE 2015, 10, e0129933. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, W.T.; Dowd, M.K. Varying planting dates or irrigation regimes alters cottonseed composition. Crop Sci. 2011, 51, 2155–2164. [Google Scholar] [CrossRef]
- Novotny, L.; Fahey, G.; Layton, B.; Walter, M. Critical factors in determining fiber in feeds and forages. AAFCO’s Lab. Methods Serv. Comm. 2017, 1, 1–14. [Google Scholar]
- He, Z.; Klasson, K.T.; Wang, D.; Li, N.; Zhang, H.; Zhang, D.; Wedegaertner, T.C. Pilot-scale production of washed cottonseed meal and co-products. Mod. Appl. Sci. 2016, 10, 25–33. [Google Scholar] [CrossRef] [Green Version]
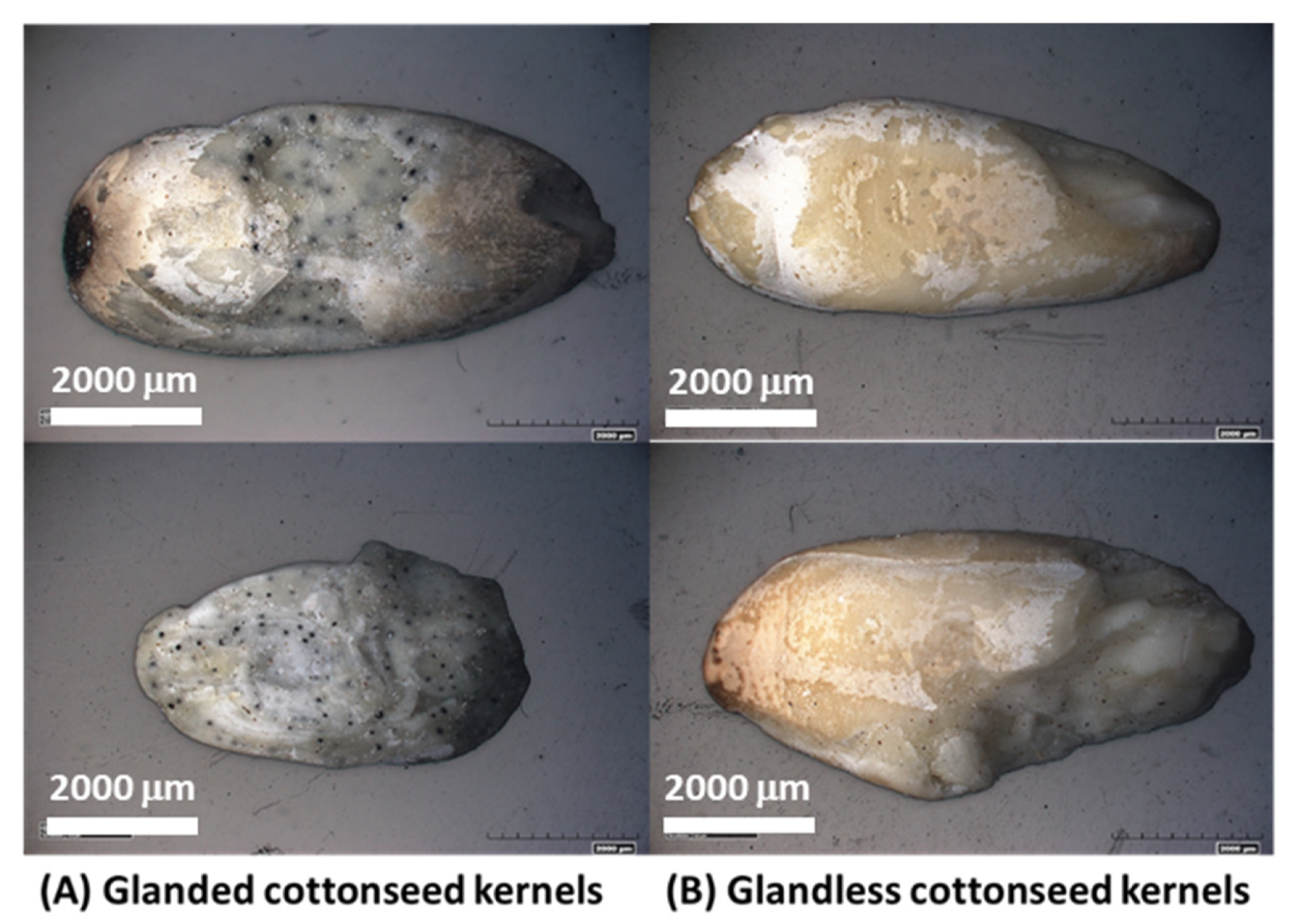


| (A) Major component (g kg−1) | |||||||
| Moisture | Gossypol *** 1 | Oil | Protein ** | ADF | ADL | Starch ** | |
| Gd | 67.9 ± 0.5 | 3.75 ± 0.02 | 387 ± 18 | 397 ± 8 | 100 ± 18 | 52.3 ± 10.1 | 12.2 ± 1.0 |
| Gl | 68.3 ± 0.2 | 0.06 ± 0.00 | 350 ± 14 | 421 ± 6 | 109 ± 23 | 67.8 ± 15.6 | 16.6 ± 0.4 |
| (B) Macro element and ash (g kg−1) | |||||||
| P * | Ca | K | Mg | Na | S | Ash | |
| Gd | 9.8 ± 0.8 | 2.0 ± 0.3 | 12.0 ± 0.7 | 5.4 ± 0.4 | 0.6 ± 0.0 | 4.5 ± 0.3 | 46.7 ± 0.8 |
| Gl | 11.5 ± 0.6 | 2.3 ± 0.2 | 12.8 ± 0.5 | 6.1 ± 0.3 | 0.6 ± 0.0 | 4.9 ± 0.2 | 47.9 ± 0.7 |
| (C) Trace element (mg kg−1) | |||||||
| Fe | Zn | Cu | Mn | B | Ni | Al | |
| Gd | 104 ± 43 | 70.5 ± 8.2 | 18.1 ± 1.1 | 12.9 ± 1.2 | 13.8 ± 1.1 | 1.9 ± 0.3 | 91.6 ± 61.2 |
| Gl | 111 ± 3 | 74.3 ± 2.5 | 19.0 ± 1.2 | 13.3 ± 0.7 | 14.2 ± 0.5 | 2.1 ± 0.1 | 109.8 ± 8.4 |
| To (°C) | WLo (%) | Tm (°C) | WLm (%) | Te (°C) | WLe (%) | Char (%) 1 | |
|---|---|---|---|---|---|---|---|
| Gd-k | 143.5 (1.2) | 5.9 (0.7) | 368.4 (0.8) | 55.9 (0.5) | 471.7 (1.2) | 80.3 (0.7) | 16.1 (1.5) |
| Gl-k | 147.1 * (1.3) | 5.5 ns (0.9) | 368.4 ns (0.7) | 52.9 * (0.7) | 468.7 * (1.5) | 82.2 * (1.1) | 15.6 ns (1.1) |
| Gd-r | 177.9 (1.2) | 7.6 (1.0) | 313.2 (0.9) | 36.8 (1.1) | 504.9 (1.2) | 72.9 (1.2) | 25.3 (1.2) |
| Gl-r | 183.6 * (1.0) | 9.1 ns (0.8) | 314.5 ns (0.9) | 37.4 ns (1.0) | 504.9 ns (1.0) | 72.4 ns (0.9) | 25.7 ns (1.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Nam, S.; Zhang, H.; Olanya, O.M. Chemical Composition and Thermogravimetric Behaviors of Glanded and Glandless Cottonseed Kernels. Molecules 2022, 27, 316. https://doi.org/10.3390/molecules27010316
He Z, Nam S, Zhang H, Olanya OM. Chemical Composition and Thermogravimetric Behaviors of Glanded and Glandless Cottonseed Kernels. Molecules. 2022; 27(1):316. https://doi.org/10.3390/molecules27010316
Chicago/Turabian StyleHe, Zhongqi, Sunghyun Nam, Hailin Zhang, and Ocen Modesto Olanya. 2022. "Chemical Composition and Thermogravimetric Behaviors of Glanded and Glandless Cottonseed Kernels" Molecules 27, no. 1: 316. https://doi.org/10.3390/molecules27010316
APA StyleHe, Z., Nam, S., Zhang, H., & Olanya, O. M. (2022). Chemical Composition and Thermogravimetric Behaviors of Glanded and Glandless Cottonseed Kernels. Molecules, 27(1), 316. https://doi.org/10.3390/molecules27010316







