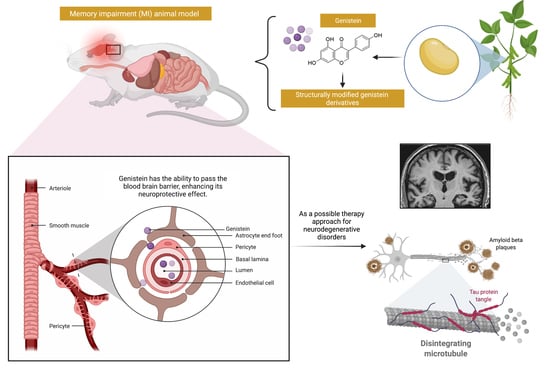
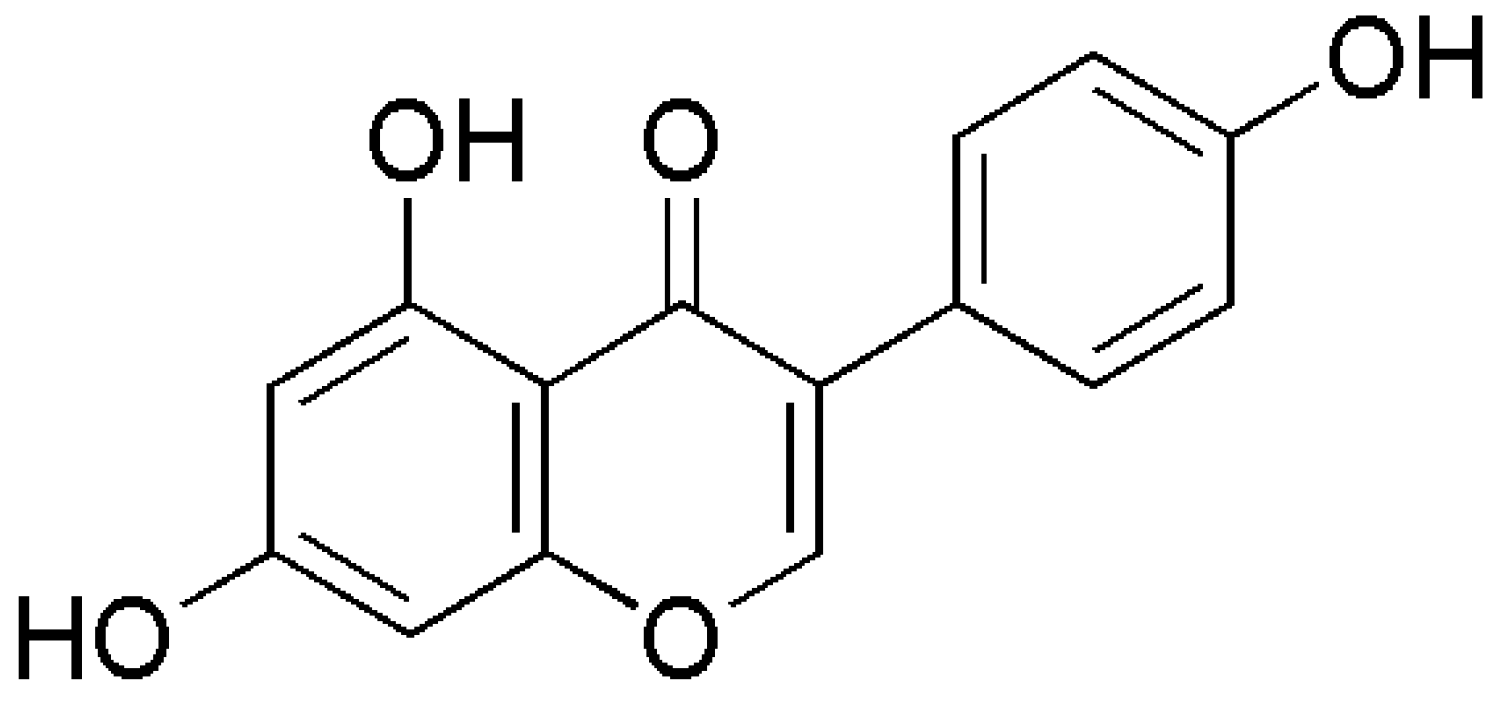
Genistein: A Potential Natural Lead Molecule for New Drug Design and Development for Treating Memory Impairment
Abstract
1. Introduction
2. Description of Study Design
2.1. Animals
2.2. MI Models
2.3. Genistein Dose
2.4. Toxicity Profile of Genistein
2.5. Memory Testing Procedure
2.5.1. Morris Water Maze (MWM)
2.5.2. Passive Avoidance Task (PAT)
2.5.3. Novel Object Recognition (NOR)
2.5.4. Object Location Recognition (OLR)
2.5.5. Novel Object Discrimination (NOD)
2.5.6. Elevated Plus Maze (EPM)
2.5.7. Delayed Spatial Alternation (DSA)/Differential Reinforcement of Low Rates of Responding (DRL)
2.5.8. RAM Task
2.5.9. Y-Maze
3. Effectiveness of Genistein
3.1. Hypoxia
3.2. Chronic Sleep Deprivation (CSD)
3.3. Streptozotocin (STZ)
3.4. Scopolamine
3.5. Lipopolysaccharides (LPS)
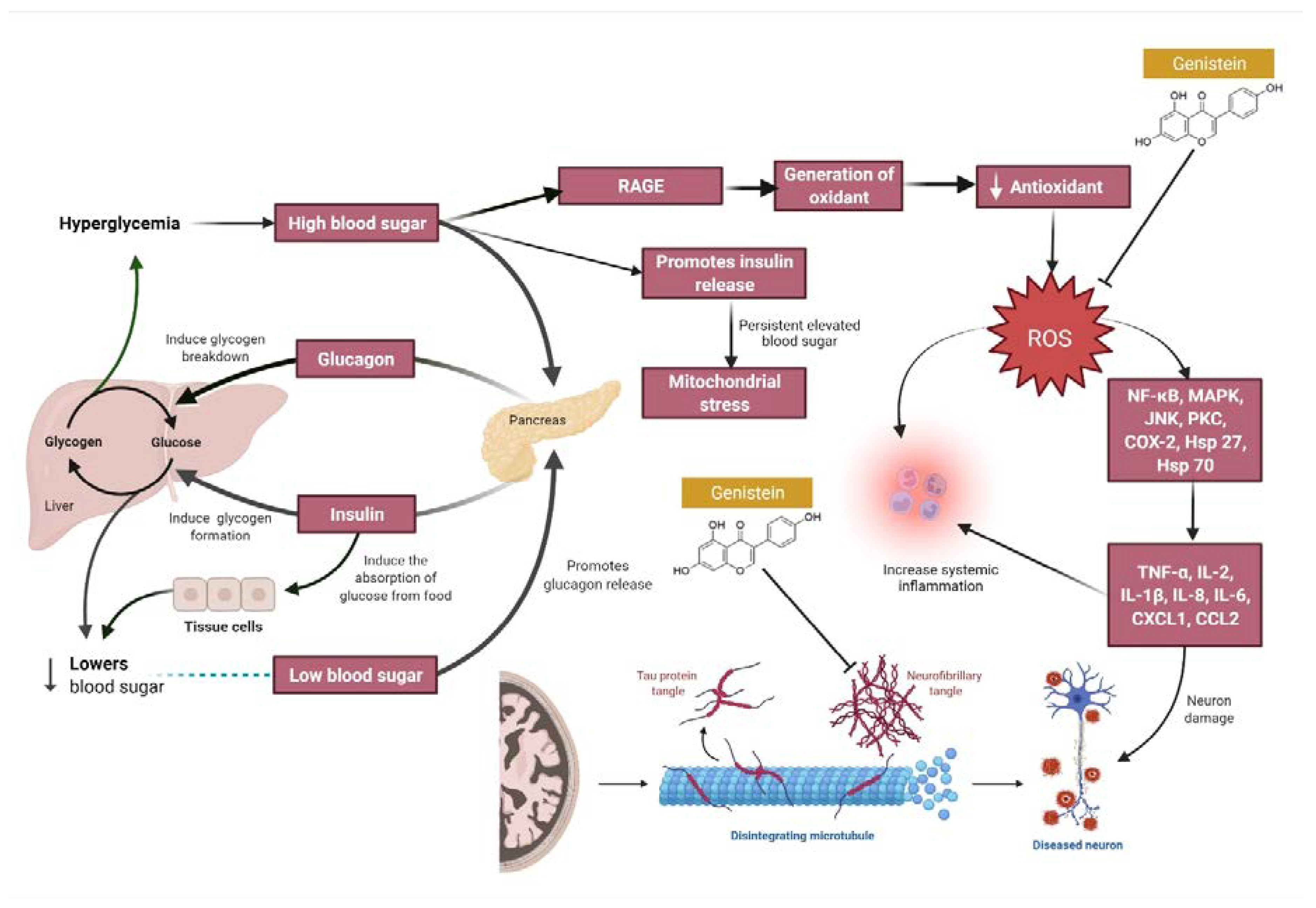
3.6. Streptozotocin (STZ)-Induced Diabetes
3.7. Lead
3.8. Kainic Acid (KA)-Induced Seizure
3.9. Aging
3.10. β-. Amyloid
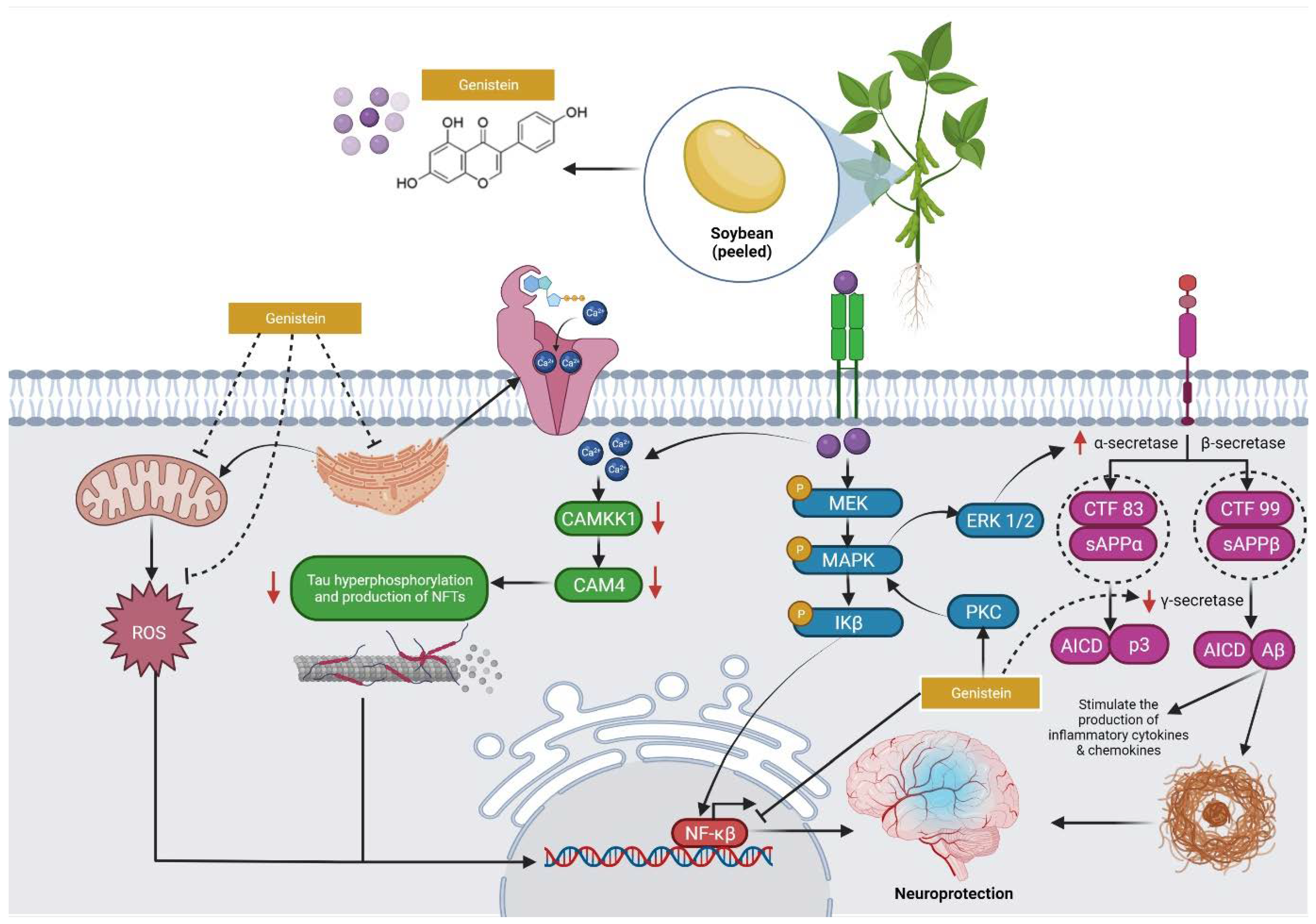
4. Overview of the Mechanisms of Action of Genistein against MI
5. Pharmacokinetics and Bioavailability of Genistein
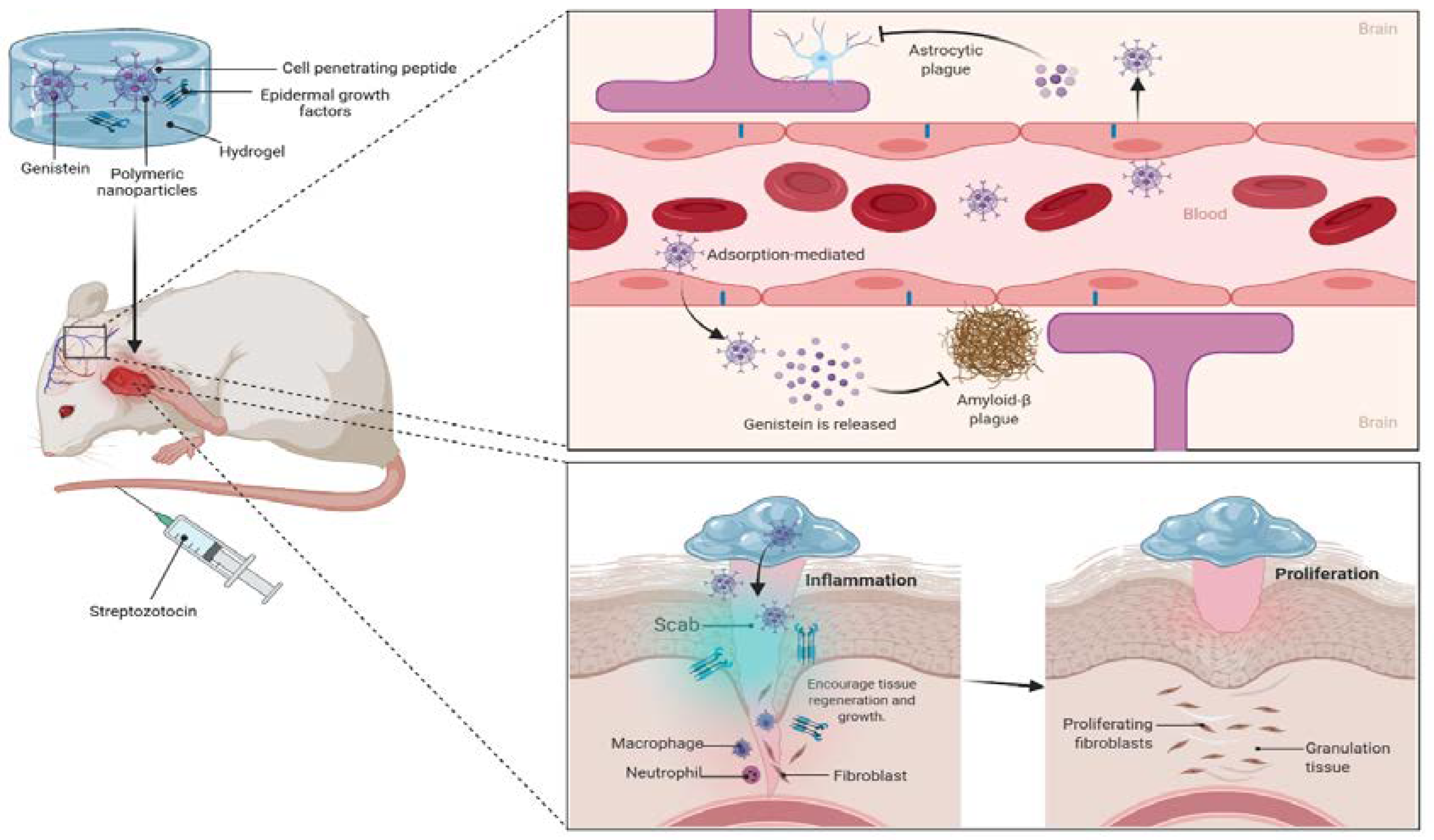
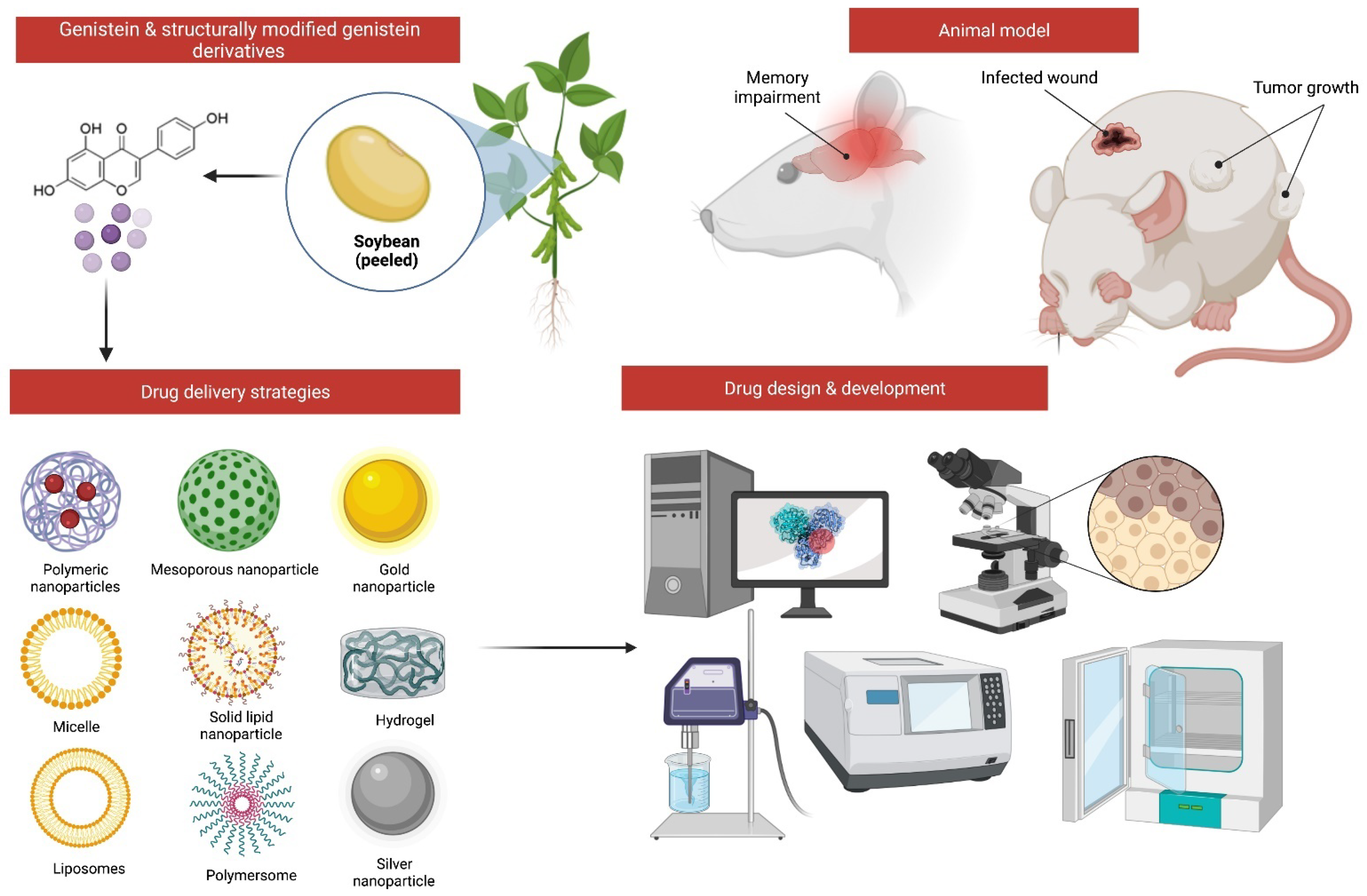
6. Challenges and Opportunities to Improve the Drug Delivery of Genistein for MI
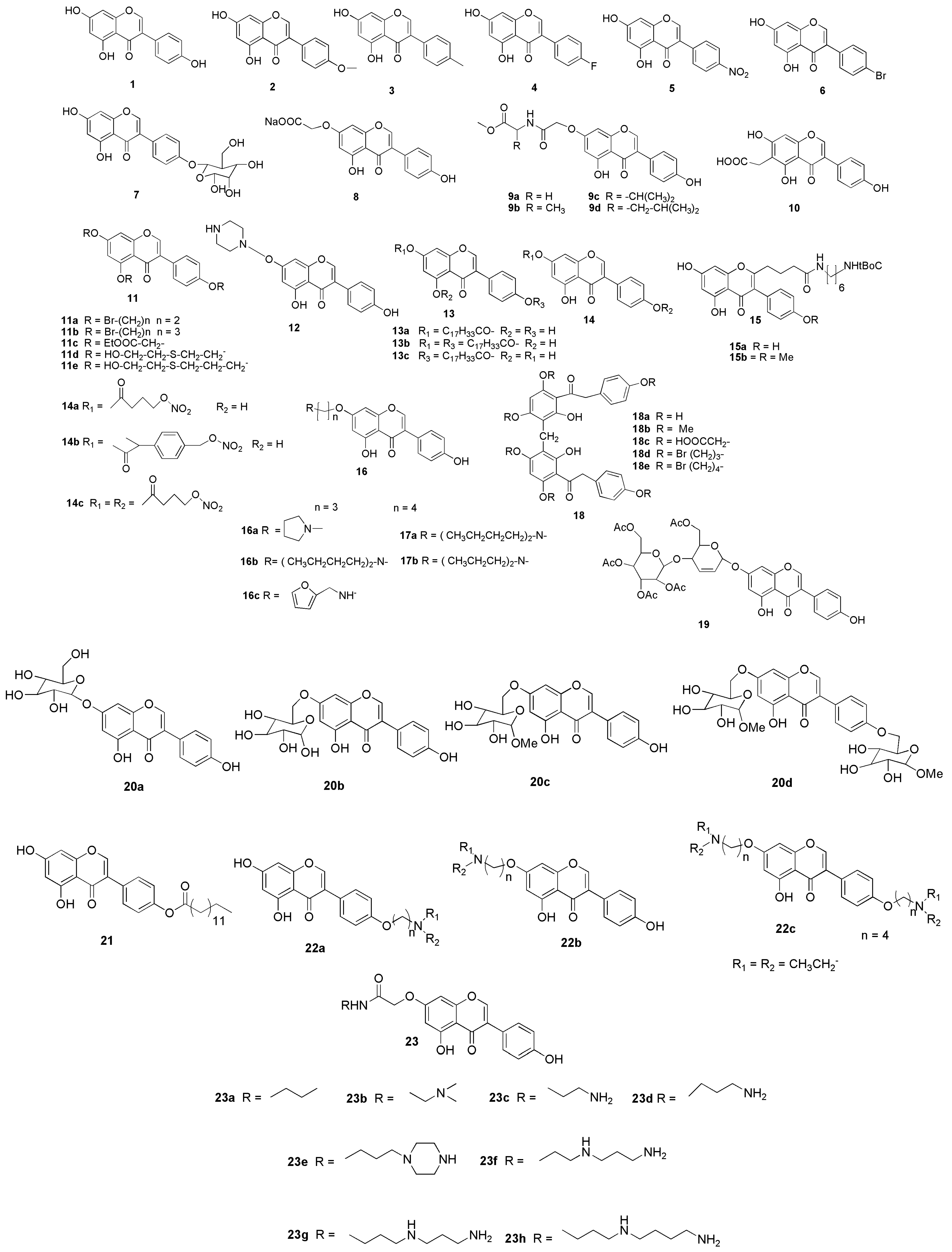
7. Possible Structural Modifications and Derivatives of Genistein
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brewer, J.B.; Gabrieli, J.D.; Preston, A.R.; Vaidya, C.J.; Rosen, A.C. Chpater 5–Memory. In Textbook of Clinical Neurology; Goetz, C.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 71–86. [Google Scholar]
- Caselli, R.J.; Boeve, B.F. Chapter 33–The degenerative Dementias. In Textbook of Clinical Neurology; Goetz, C.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 687–717. [Google Scholar]
- Jankovic, J. Chapter 34–Movement disorders. In Textbook of Clinical Neurology; Goetz, C.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 722–750. [Google Scholar]
- Kinsella, L.J.; Riley, D.E. Chapter 40–Nutritional deficiencies and syndromes associated with alcoholism. In Textbook of Clinical Neurology; Goetz, C.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 882–902. [Google Scholar]
- Brown, P. Chapter 43–Transmissible spongiform encephalopathy. In Textbook of Clinical Neurology; Goetz, C.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 953–965. [Google Scholar]
- Menze, E.T.; Esmat, A.; Tadros, M.G.; Abdel-Naim, A.B.; Khalifa, A.E. Genistein improves 3-NPA-induced memory impairment in ovariectomized rats: Impact of its antioxidant, anti-inflammatory and acetylcholinesterase modulatory properties. PLoS ONE 2015, 10, e0117223. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Baroni, L. Soy, soy foods and their role in vegetarian diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Han, B.-Z.; Rombouts, F.M.; Nout, M.R. A Chinese fermented soybean food. Int. J. Food Microbiol. 2001, 65, 1–10. [Google Scholar] [CrossRef]
- Nazari-Khanamiri, F.; Ghasemnejad-Berenji, M. Cellular and molecular mechanisms of genistein in prevention and treatment of diseases: An overview. J. Food Biochem. 2021, 45, e13972. [Google Scholar] [CrossRef]
- Qiang, X.; Sang, Z.; Yuan, W.; Li, Y.; Liu, Q.; Bai, P.; Shi, Y.; Ang, W.; Tan, Z.; Deng, Y. Design, synthesis and evaluation of genistein-O-alkylbenzylamines as potential multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 76, 314–331. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Gaffke, L.; Cyske, Z.; Węgrzyn, G. Genistein induces degradation of mutant huntingtin in fibroblasts from Huntington’s disease patients. Metab. Brain Dis. 2019, 34, 715–720. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Podlacha, M.; Gaffke, L.; Majkutewicz, I.; Mantej, J.; Węgrzyn, A.; Osiadły, M.; Myślińska, D.; Węgrzyn, G. Autophagy-dependent mechanism of genistein-mediated elimination of behavioral and biochemical defects in the rat model of sporadic Alzheimer’s disease. Neuropharmacology 2019, 148, 332–346. [Google Scholar] [CrossRef]
- Yu, H.-L.; Li, L.; Zhang, X.-H.; Xiang, L.; Zhang, J.; Feng, J.-F.; Xiao, R. Neuroprotective effects of genistein and folic acid on apoptosis of rat cultured cortical neurons induced by β-amyloid 31–35. Br. J. Nutr. 2009, 102, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yuan, L.; Zhao, X.; Hou, C.; Ma, W.; Yu, H.; Xiao, R. Genistein antagonizes inflammatory damage induced by β-amyloid peptide in microglia through TLR4 and NF-κB. Nutrition 2014, 30, 90–95. [Google Scholar] [CrossRef]
- Shi, D.-H.; Yan, Z.-Q.; Zhang, L.-N.; Wang, Y.-R.; Jiang, C.-P.; Wu, J.-H. A novel 7-O-modified genistein derivative with acetylcholinesterase inhibitory effect, estrogenic activity and neuroprotective effect. Arch. Pharmacal Res. 2012, 35, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Petry, F.; Hoppe, J.B.; Klein, C.P.; Dos Santos, B.G.; Hözer, R.M.; Bifi, F.; Matté, C.; Salbego, C.G.; Trindade, V.M.T. Genistein attenuates amyloid-beta-induced cognitive impairment in rats by modulation of hippocampal synaptotoxicity and hyperphosphorylation of Tau. J. Nutr. Biochem. 2021, 87, 108525. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-J.; Ko, J.W.; Jeon, S.; Kwon, Y.H. Protective effect of genistein against neuronal degeneration in ApoE−/− mice fed a high-fat diet. Nutrients 2016, 8, 692. [Google Scholar] [CrossRef]
- Rumman, M.; Pandey, S.; Singh, B.; Gupta, M.; Ubaid, S.; Mahdi, A.A. Genistein Prevents Hypoxia-Induced Cognitive Dysfunctions by Ameliorating Oxidative Stress and Inflammation in the Hippocampus. Neurotox. Res. 2021, 39, 1123–1133. [Google Scholar] [CrossRef]
- Lu, C.; Lv, J.; Jiang, N.; Wang, H.; Huang, H.; Zhang, L.; Li, S.; Zhang, N.; Fan, B.; Liu, X. Protective effects of Genistein on the cognitive deficits induced by chronic sleep deprivation. Phytother. Res. 2020, 34, 846–858. [Google Scholar] [CrossRef]
- Rajput, M.S.; Sarkar, P.D.; Nirmal, N.P. Inhibition of DPP-4 activity and neuronal atrophy with genistein attenuates neurological deficits induced by transient global cerebral ischemia and reperfusion in streptozotocin-induced diabetic mice. Inflammation 2017, 40, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, Y.; Xu, T.; Li, Q.; Wang, D.; Zhang, L.; Fan, B.; Wang, F.; Liu, X. Genistein ameliorates scopolamine-induced amnesia in mice through the regulation of the cholinergic neurotransmission, antioxidant system and the ERK/CREB/BDNF signaling. Front. Pharmacol. 2018, 9, 1153. [Google Scholar] [CrossRef] [PubMed]
- Shahmohammadi, A.; Rousta, A.-M.; Azadi, M.-R.; Fahanik-Babaei, J.; Baluchnejadmojarad, T.; Roghani, M. Soy isoflavone genistein attenuates lipopolysaccharide-induced cognitive impairments in the rat via exerting anti-oxidative and anti-inflammatory effects. Cytokine 2018, 104, 151–159. [Google Scholar]
- Su, P.; Zhang, J.; Wang, S.; Aschner, M.; Cao, Z.; Zhao, F.; Wang, D.; Chen, J.; Luo, W. Genistein alleviates lead-induced neurotoxicity in vitro and in vivo: Involvement of multiple signaling pathways. Neurotoxicology 2016, 53, 153–164. [Google Scholar] [CrossRef]
- Khodamoradi, M.; Asadi-Shekaari, M.; Esmaeili-Mahani, S.; Esmaeilpour, K.; Sheibani, V. Effects of genistein on cognitive dysfunction and hippocampal synaptic plasticity impairment in an ovariectomized rat kainic acid model of seizure. Eur. J. Pharmacol. 2016, 786, 1–9. [Google Scholar] [CrossRef]
- Neese, S.L.; Bandara, S.B.; Doerge, D.R.; Helferich, W.G.; Korol, D.L.; Schantz, S.L. Effects of multiple daily genistein treatments on delayed alternation and a differential reinforcement of low rates of responding task in middle-aged rats. Neurotoxicology Teratol. 2012, 34, 187–195. [Google Scholar] [CrossRef]
- Bagheri, M.; Joghataei, M.-T.; Mohseni, S.; Roghani, M. Genistein ameliorates learning and memory deficits in amyloid β (1–40) rat model of Alzheimer’s disease. Neurobiol. Learn. Mem. 2011, 95, 270–276. [Google Scholar] [CrossRef]
- Ek, O.; Yanishevskic, Y.; Zeren, T.; Waurzyniak, B.; Gunther, R.; Chelstrom, L.; Chandan-Langlie, M.; Schneider, E.; Myers, D.E.; Evans, W. In Vivo., Toxicity and Pharmacokinetic Features of B43 (Anti-CD19)-Genistein Immunoconjugate. Leuk. Lymphoma 1998, 30, 389–394. [Google Scholar] [CrossRef]
- Nasri, A.; Pohjanvirta, R. In vitro estrogenic, cytotoxic, and genotoxic profiles of the xenoestrogens 8-prenylnaringenine, genistein and tartrazine. Environ. Sci. Pollut. Res. 2021, 28, 27988–27997. [Google Scholar] [CrossRef]
- Levin, E.D.; Buccafusco, J.J. Introduction. In Animal Models of Cognitive Impairment; Levin, E.D., Buccafusco, J.J., Eds.; Taylor & Francis: Abingdon, UK, 2006; pp. 20–23. [Google Scholar]
- Fahn, S. Chapter 16–Hypokinesia and Hyperkinesia. In Textbook of Clinical Neurology; Goetz, C.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 292–309. [Google Scholar]
- Terry, A.V. Muscarinic receptor antagonists in rats. In Animal Models of Cognitive Impairment; Levin, E.D., Buccafusco, J.J., Eds.; Taylor & Francis: Abingdon, UK, 2006; pp. 24–39. [Google Scholar]
- Roegge, C.S.; Levin, E.D. Nicotinic receptor antagonist in rats. In Animal Models of Cognitive Impairment; Levin, E.D., Buccafusco, J.J., Eds.; Taylor & Francis: Abingdon, UK, 2006; pp. 40–55. [Google Scholar]
- Rezvani, A.H. Involvement of the NMDA System in Learning and Memory. In Animal Models of Cognitive Impairment; Levin, E.D., Buccafusco, J.J., Eds.; Taylor & Francis: Abingdon, UK, 2006; pp. 56–67. [Google Scholar]
- Rajput, M.S.; Sarkar, P.D. Modulation of neuro-inflammatory condition, acetylcholinesterase and antioxidant levels by genistein attenuates diabetes associated cognitive decline in mice. Chem.-Biol. Interact. 2017, 268, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Komada, M.; Takao, K.; Miyakawa, T. Elevated plus maze for mice. JoVE 2008, e1088. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Salim, S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Schain, M.; Kreisl, W.C. Neuroinflammation in neurodegenerative disorders—A review. Curr. Neurol. Neurosci. Rep. 2017, 17, 25. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef]
- Moghaddam, H.S.; Zare-Shahabadi, A.; Rahmani, F.; Rezaei, N. Neurotransmission systems in Parkinson’s disease. Rev. Neurosci. 2017, 28, 509–536. [Google Scholar] [CrossRef]
- Duan, X.; Li, Y.; Xu, F.; Ding, H. Study on the neuroprotective effects of Genistein on Alzheimer’s disease. Brain Behav. 2021, 11, e02100. [Google Scholar] [CrossRef]
- Yang, Z.; Kulkarni, K.; Zhu, W.; Hu, M. Bioavailability and pharmacokinetics of genistein: Mechanistic studies on its ADME. Anti-Cancer Agents Med. Chem. 2012, 12, 1264–1280. [Google Scholar] [CrossRef]
- Jaiswal, N.; Akhtar, J.; Singh, S.P.; Ahsan, F. An overview on genistein and its various formulations. Drug Res. 2019, 69, 305–313. [Google Scholar] [CrossRef]
- Kwon, S.H.; Kang, M.J.; Huh, J.S.; Ha, K.W.; Lee, J.R.; Lee, S.K.; Lee, B.S.; Han, I.H.; Lee, M.S.; Lee, M.W. Comparison of oral bioavailability of genistein and genistin in rats. Int. J. Pharm. 2007, 337, 148–154. [Google Scholar] [CrossRef]
- NIPER. Drug Likeness Tool (DruLiTo). Available online: http://www.niper.gov.in/pi_dev_tools/DruLiToWeb/DruLiTo_Reference.html (accessed on 20 December 2021).
- Sherif, S.H.; Gebreyohannes, B. Synthesis, characterization, and antioxidant activities of genistein, biochanin A, and their analogues. J. Chem. 2018, 2018, 4032105. [Google Scholar]
- Cao, Z.; Wu, Q.; Cheng, J.; Zhu, D.; Teng, W.; Liu, W.; Sun, X.; Yao, G. Synthesis of water-soluble 7-O-carboxymethyl-genistein. J. Chem. Res. 2017, 41, 183–185. [Google Scholar] [CrossRef]
- Long, X.; Zeng, Y.-F.; Liu, Y.; Liu, Y.; Li, T.; Liao, L.; Guo, Y. Synthesis of novel genistein amino acid derivatives and investigation on their interactions with bovine serum albumin by spectroscopy and molecular docking. RSC Adv. 2018, 8, 31201–31212. [Google Scholar] [CrossRef]
- Kohen, F.; Gayer, B.; Kulik, T.; Frydman, V.; Nevo, N.; Katzburg, S.; Limor, R.; Sharon, O.; Stern, N.; Somjen, D. Synthesis and evaluation of the antiproliferative activities of derivatives of carboxyalkyl isoflavones linked to Nt-Boc-hexylenediamine. J. Med. Chem. 2007, 50, 6405–6410. [Google Scholar] [CrossRef]
- Somjen, D.; Amir-Zaltsman, Y.; Gayer, B.; Kulik, T.; Knoll, E.; Stern, N.; Lu, L.; Toldo, L.; Kohen, F. 6-Carboxymethyl genistein: A novel selective oestrogen receptor modulator (SERM) with unique, differential effects on the vasculature, bone and uterus. J. Endocrinol. 2002, 173, 415–427. [Google Scholar] [CrossRef][Green Version]
- Wang, S.F.; Jiang, Q.; Yong, H.Y.; Li, Y.; Tan, R.X. Genistein derivatives as selective estrogen receptor modulators: Sonochemical synthesis and in vivo anti-osteoporotic action. Bioorganic Med. Chem. 2005, 13, 4880–4890. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Q.; Ge, H.M.; Chen, Y.X.; Xu, C.; Shi, L.; Ding, H.; Zhu, H.L.; Tan, R.X. Synthesis and cytotoxic evaluation of a series of genistein derivatives. Chem. Biodivers. 2006, 3, 463–472. [Google Scholar] [CrossRef]
- Mazurek, A.P.; Kozerski, L.; Sadlej, J.; Kawę, R.; Bednarek, E.; Sitkowski, J.; Dobrowolski, J.C.; Maurin, J.K.; Biniecki, K.; Witowska, J. Genistein complexes with amines: Structure and properties. J. Chem. Soc. Perkin Trans. 1998, 2, 1223–1230. [Google Scholar] [CrossRef]
- Meng, Q.-H.; Wähälä, K.; Adlercreutz, H.; Tikkanen, M.J. Antiproliferative efficacy of lipophilic soy isoflavone phytoestrogens delivered by low density lipoprotein particles into cultured U937 cells. Life Sci. 1999, 65, 1695–1705. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kobayashi, T.; Kikuchi, T.; Honda, T.; Kamata, K. Effects of dual-action genistein derivatives on relaxation in rat aorta. J. Smooth Muscle Res. 2005, 41, 23–33. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.-N.; Cao, P.; Tan, S.-H.; Gu, W.; Shi, L.; Zhu, H.-L. Synthesis and antimicrobial activities of 7-O-modified genistein derivatives. Eur. J. Med. Chem. 2008, 43, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Q.; Xue, J.-Y.; Shi, L.; Gui, S.-Y.; Zhu, H.-L. Synthesis, crystal structure and antimicrobial activity of deoxybenzoin derivatives from genistein. Eur. J. Med. Chem. 2008, 43, 662–667. [Google Scholar] [CrossRef]
- Rusin, A.; Gogler, A.; Głowala-Kosińska, M.; Bochenek, D.; Gruca, A.; Grynkiewicz, G.; Zawisza, J.; Szeja, W.; Krawczyk, Z. Unsaturated genistein disaccharide glycoside as a novel agent affecting microtubules. Bioorganic Med. Chem. Lett. 2009, 19, 4939–4943. [Google Scholar] [CrossRef]
- Rusin, A.; Krawczyk, Z.; Grynkiewicz, G.; Gogler, A.; Zawisza-Puchałka, J.; Szeja, W. Synthetic derivatives of genistein, their properties and possible applications. Acta Biochim. Pol. 2010, 57, 23–34. [Google Scholar] [CrossRef]
- Junior, C.O.; Castro, S.B.; Pereira, A.A.; Alves, C.C.; Oliveira, E.E.; Rêgo, R.T.; Ferreira, A.P.; de Almeida, M.V. Synthesis of genistein coupled with sugar derivatives and their inhibitory effect on nitric oxide production in macrophages. Eur. J. Med. Chem. 2014, 85, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Hong, C.; Luo, W.; Wang, C. Design, synthesis and evaluation of genistein-polyamine conjugates as multi-functional anti-Alzheimer agents. Acta Pharm. Sin. B 2015, 5, 67–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Animal, Sex | MI Model | Genistein Dose (mg/kg/day) | Route of Adminis-tration | Duration of Treatment (Days) | Memory Type | Memory Parameters | Remarks | References |
|---|---|---|---|---|---|---|---|---|
| Swiss albino mice, male | Hypoxia | 10, 20, 30 | p.o. | 28 | Spatial memory, retention memory, recognition memory | MWM, PAT, NOR | Cognitive dysfunction prevented | Rumman et al. [19] |
| ICR mice, male | CSD | 10, 20, 40 | p.o. | 23 | Spatial memory, recognition memory | MWM, OLR, NOR | Deleterious effects alleviated | Lu et al. [20] |
| Wistar rat, male | STZ | 150 | p.o. | 90 | Spatial memory | MWM | Degradation of pathological proteins increased | Pierzynowska et al. [13] |
| ICR mice, male | Scopolamine | 10, 20, 40 | p.o. | 24 | Spatial memory | OLR, MWM | Cognitive performance improved | Lu et al. [22] |
| Albino Wistar rat, male | LPS | 10, 50, 100 | p.o. | 7 | Spatial memory, recognition memory | Y-maze, NOD, PAT | Cognitive dysfunction alleviated | Shahmohammadi et al. [23] |
| Swiss albino mice, male | STZ-induced diabetes | 2.5, 5.0, 10.0 | i.p. | 14 | Spatial memory, retention memory | EPM | Cognitive deficits reduced | Rajput et al. [21] |
| Sprague-Dawley rat, male | Lead | 1 | p.o. | 56 | Spatial memory | MWM | Memory impairment reduced | Su et al. [24] |
| Wistar rat, female | KA-induced seizure | 0.5, 5.0 | i.p. | 4 | Spatial memory | MWM | Memory impairment reduced | Khodamoradi et al. [25] |
| Long-Evans rat, female | Aging | 10.2 | p.o. | - | Working memory | DSA, DRL | Cognitive deficits are not affected | Neese et al. [26] |
| Wistar rat, male | β-amyloid | 10 | p.o. | - | Spatial memory, recognition memory, working memory, retention memory, reference memory | Y-maze, PAT, RAM | Memory impairment reduced | Bagheri et al. [27] |
| Property/Rule | Result |
|---|---|
| Molecular formula | C15H10O5 |
| Molecular weight | 270.24 |
| Hydrogen bond donors | 3 |
| Hydrogen bond acceptors | 5 |
| Rotatable bonds | 1 |
| Log P (Partition coefficient, predicted value) | 1.043 |
| Molar refractivity | 78.92 cm3 |
| Topological polar surface area | 86.99 Å2 |
| Lipinski’s rule of five | Passed |
| Ghose filter | Passed |
| Veber’s rule | Passed |
| BBB likeness rule | Passed |
| Unweighted QED | Passed |
| Weighted QED | Passed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuloria, S.; Yusri, M.A.A.; Sekar, M.; Gan, S.H.; Rani, N.N.I.M.; Lum, P.T.; Ravi, S.; Subramaniyan, V.; Azad, A.K.; Jeyabalan, S.; et al. Genistein: A Potential Natural Lead Molecule for New Drug Design and Development for Treating Memory Impairment. Molecules 2022, 27, 265. https://doi.org/10.3390/molecules27010265
Fuloria S, Yusri MAA, Sekar M, Gan SH, Rani NNIM, Lum PT, Ravi S, Subramaniyan V, Azad AK, Jeyabalan S, et al. Genistein: A Potential Natural Lead Molecule for New Drug Design and Development for Treating Memory Impairment. Molecules. 2022; 27(1):265. https://doi.org/10.3390/molecules27010265
Chicago/Turabian StyleFuloria, Shivkanya, Muhamad Azrul Amir Yusri, Mahendran Sekar, Siew Hua Gan, Nur Najihah Izzati Mat Rani, Pei Teng Lum, Subban Ravi, Vetriselvan Subramaniyan, Abul Kalam Azad, Srikanth Jeyabalan, and et al. 2022. "Genistein: A Potential Natural Lead Molecule for New Drug Design and Development for Treating Memory Impairment" Molecules 27, no. 1: 265. https://doi.org/10.3390/molecules27010265
APA StyleFuloria, S., Yusri, M. A. A., Sekar, M., Gan, S. H., Rani, N. N. I. M., Lum, P. T., Ravi, S., Subramaniyan, V., Azad, A. K., Jeyabalan, S., Wu, Y. S., Meenakshi, D. U., Sathasivam, K. V., & Fuloria, N. K. (2022). Genistein: A Potential Natural Lead Molecule for New Drug Design and Development for Treating Memory Impairment. Molecules, 27(1), 265. https://doi.org/10.3390/molecules27010265