Effect of Atmospheres on Transformation of Heavy Metals during Thermal Treatment of MSWI Fly Ash: By Thermodynamic Equilibrium Calculation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Properties of MSWI Fly Ash
2.2. Thermodynamic Equilibrium Calculation
2.3. Experimental Apparatus and Procedure
3. Results and Discussion
3.1. Effect of Atmosphere on the Fusibility of MSW Fly Ash
3.2. Transformation of Heavy Metals in Different Atmospheres
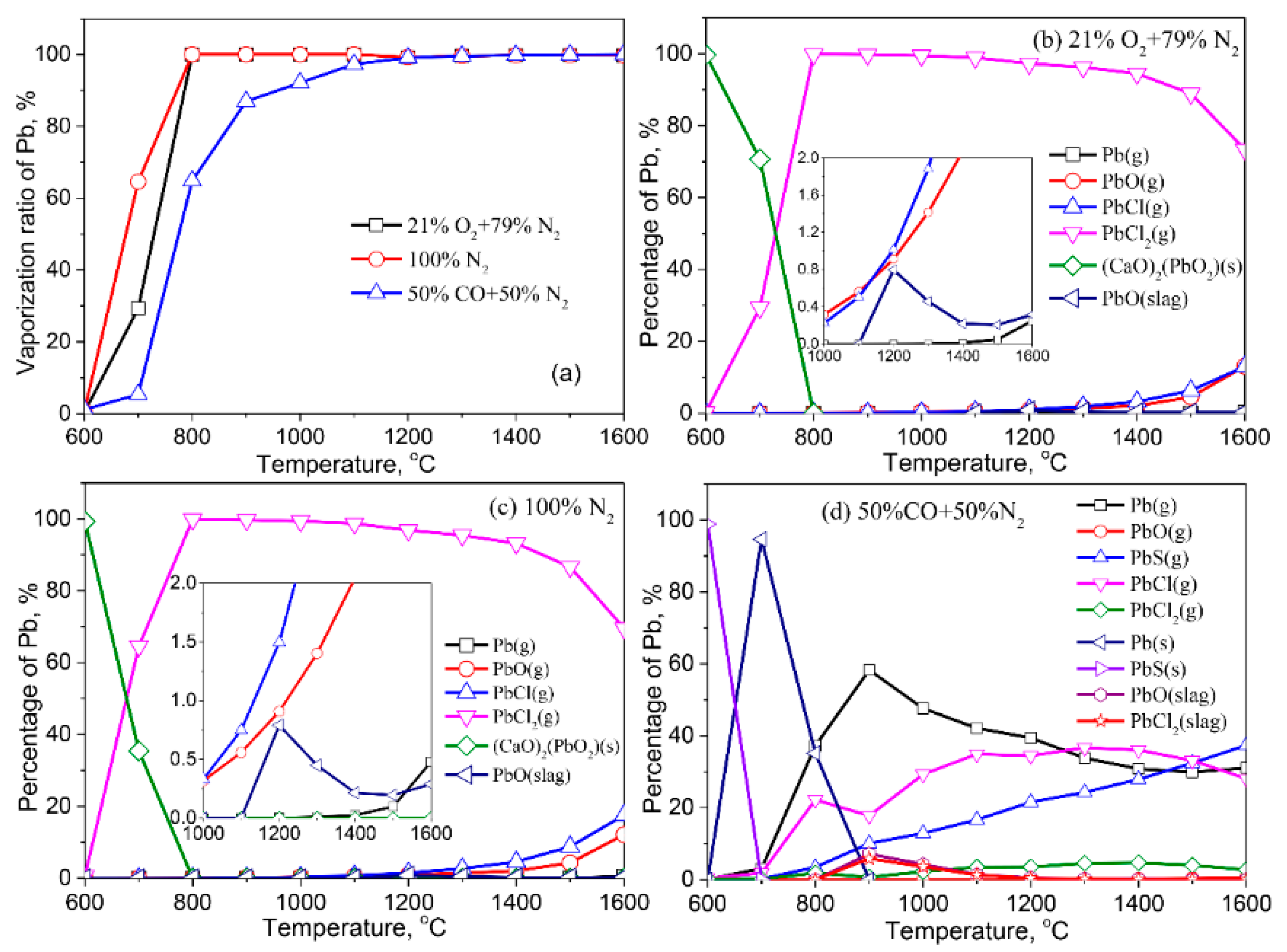
3.2.1. Lead (Pb)
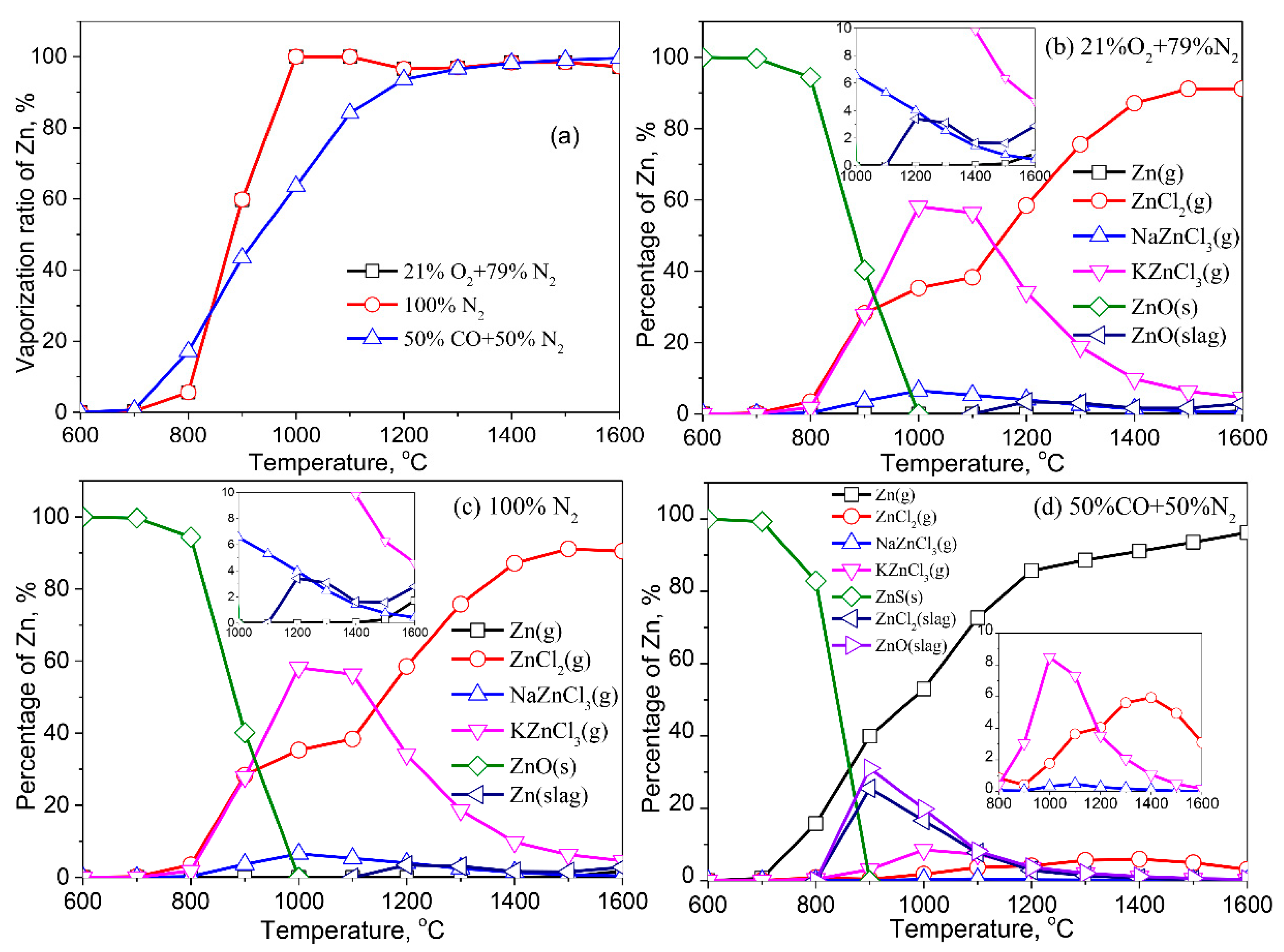
3.2.2. Zinc (Zn)
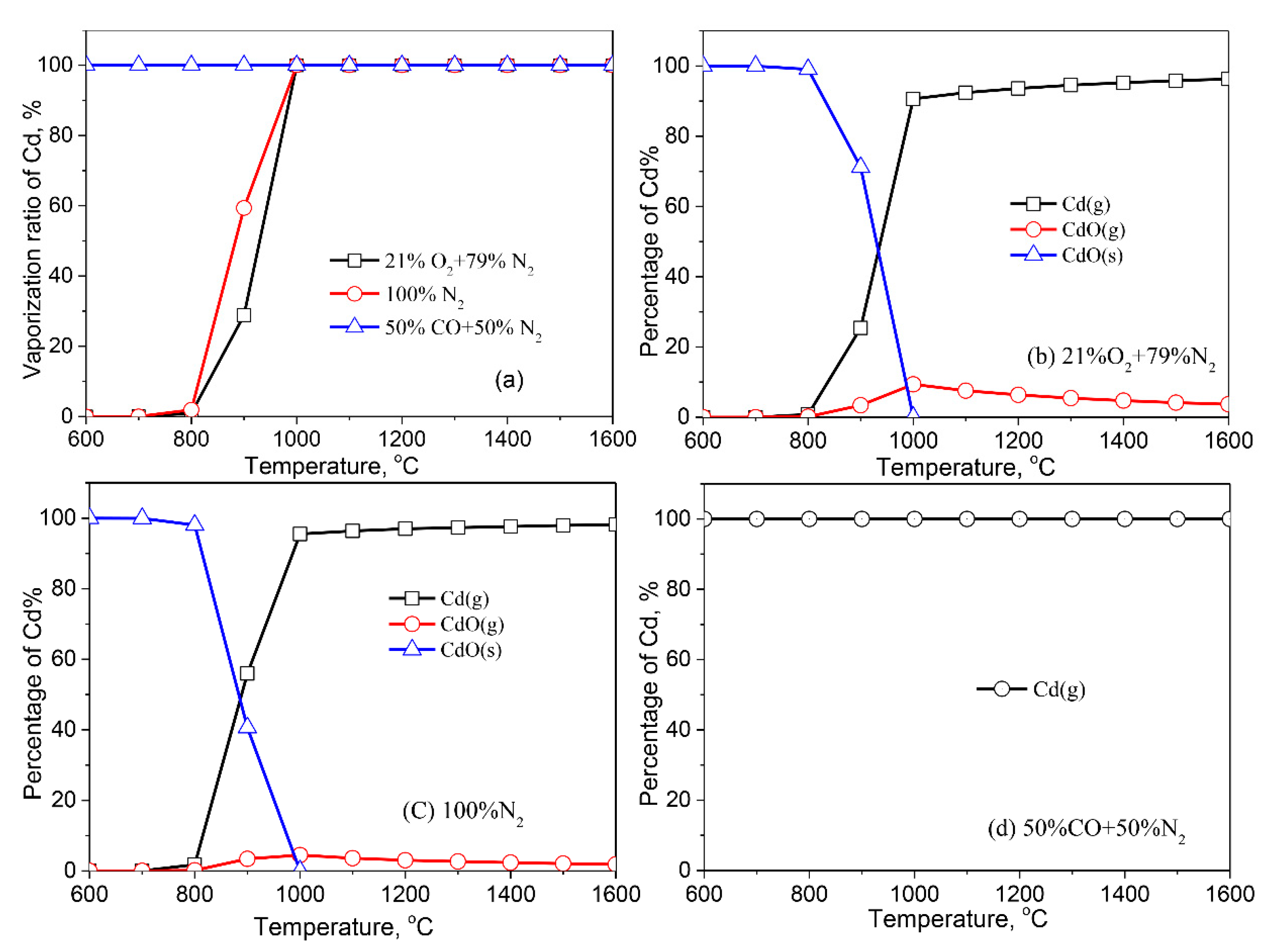
3.2.3. Cadmium (Cd)
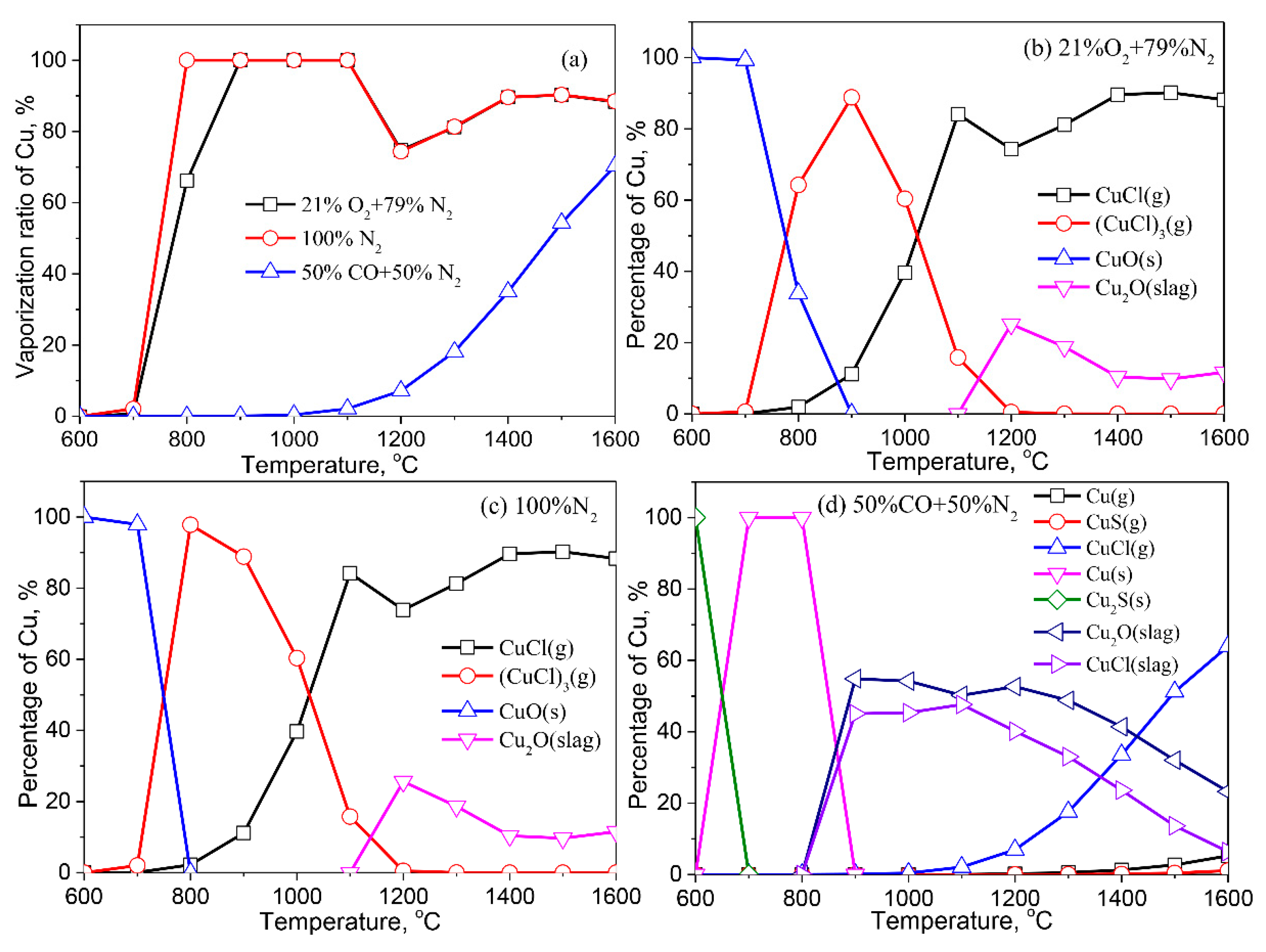
3.2.4. Copper (Cu)
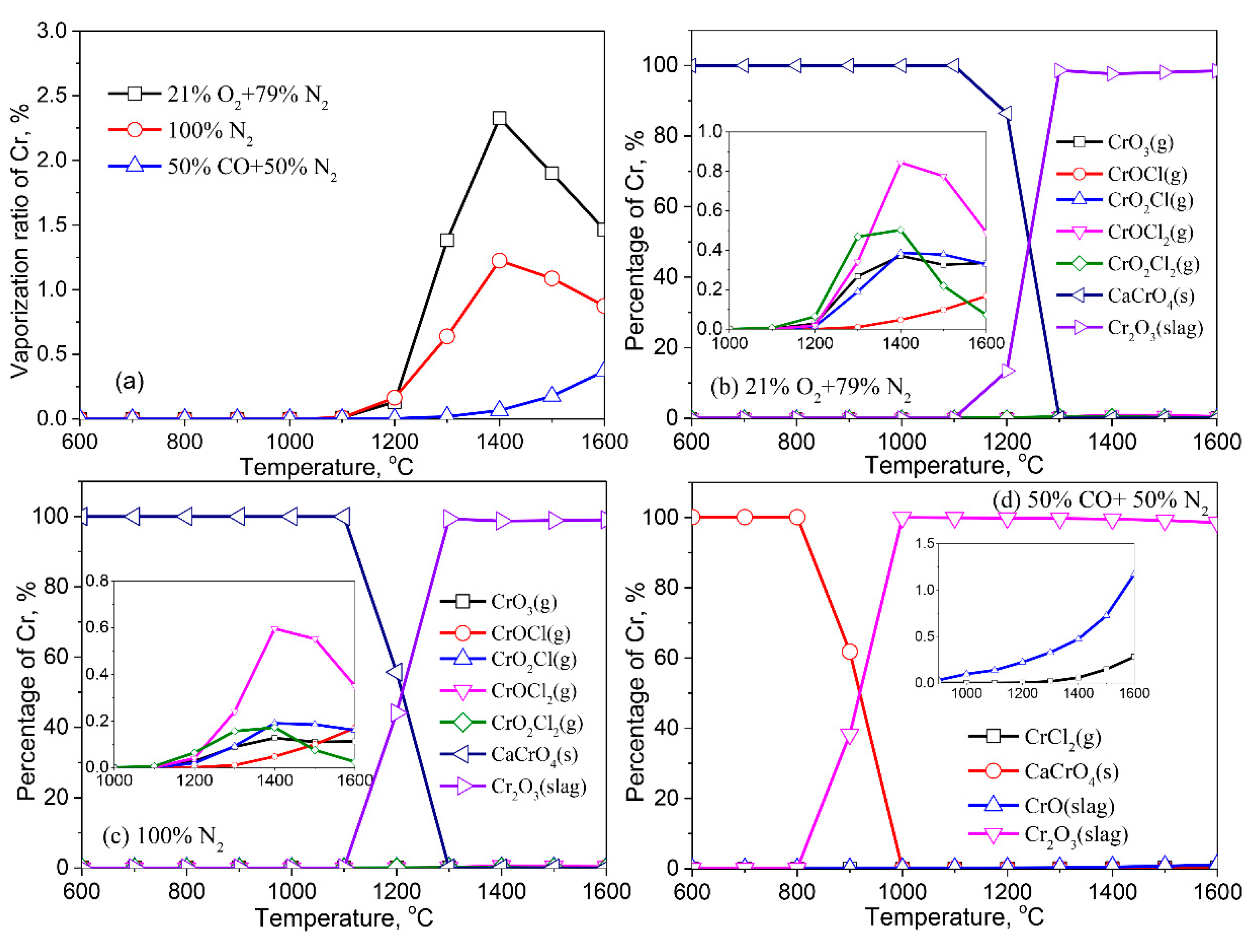
3.2.5. Chromium (Cr)
3.2.6. Cobalt (Co)
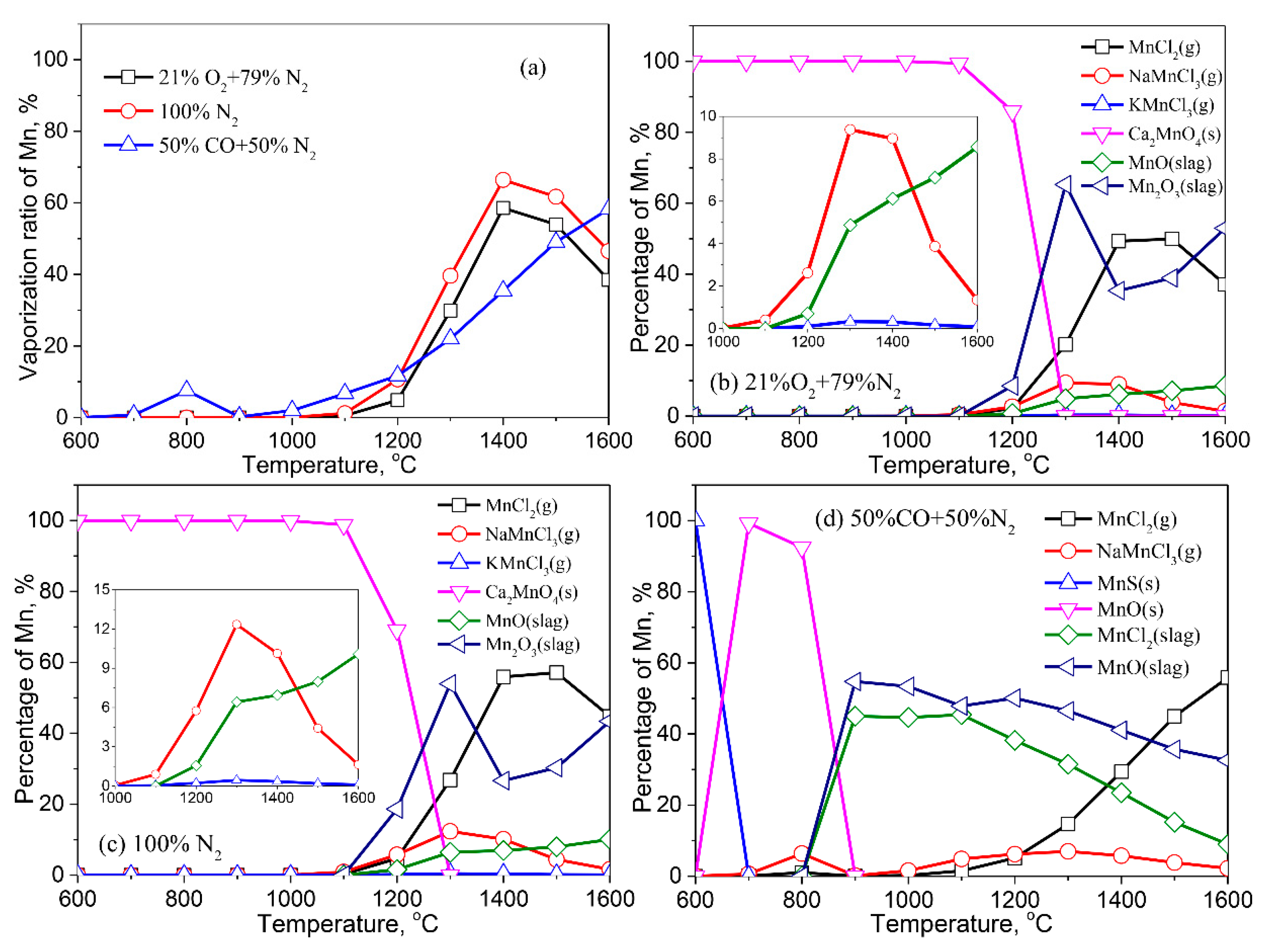
3.2.7. Manganese (Mn)
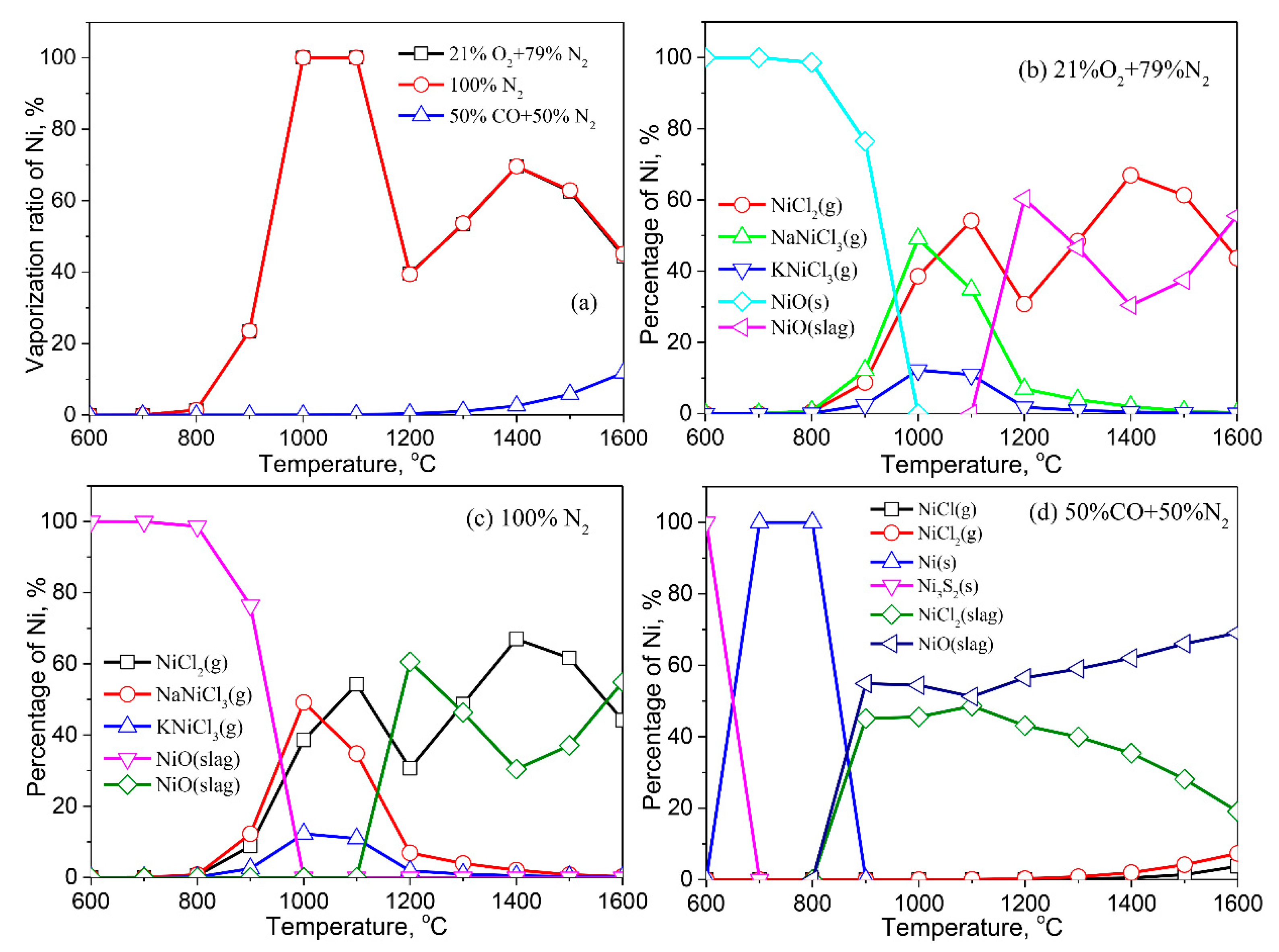
3.2.8. Nickle (Ni)
3.3. Validation of the Vaporization of Heavy Metals
4. Conclusions
- (1)
- Except for Cd, the formation of liquid slag in the treated ash dissolves the heavy metals, lowering their vaporization ratio. With the increase of the reaction temperature, the dissolved heavy metals in the liquid slag are released into the gaseous phase.
- (2)
- The vaporization of Pb, Zn, Cu, Cr, Co, and Ni is inhibited by a reducing atmosphere when compared to air and inert atmospheres. The main reason is attributed to a large amount of liquid slag formed in the reducing atmosphere. As a result, the vaporization ratio of heavy metals is reduced.
- (3)
- The formation of metallic chlorides causes the vaporization of Pb, Zn, Cu, Co, Mn, and Ni during the thermal treatment of MSWI fly ash in the air and inert atmosphere. Cd vaporizes in the form of Cd or CdO. In the reducing atmosphere, Zn and Cd mainly vaporize in the form of Zn (g) and Cd (g). Concerning Pb, apart from its chloride, the formation of Pb (g) and PbS (s) are also contributed to the vaporization of Pb.
- (4)
- Cr has the lowest vaporization propensity among these heavy metals in the fly ash during thermal treatment. However, Cr readily reacts with Ca to form water-soluble CrCaO4 (s), potentially causing the leaching risk of Cr in the treated ash.
- (5)
- The calculated results for the vaporization of heavy metals as a function of reaction atmosphere matched the experimental observation. As a result, a reducing atmosphere is proposed as a method for preventing the vaporization of heavy metals during the thermal treatment of MSWI fly ash.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cheng, T.W.; Chen, Y.S. On formation of CaO-Al2O3-SiO2 glass-ceramics by vitrification of incinerator fly ash. Chemosphere 2003, 51, 817–824. [Google Scholar] [CrossRef]
- Hu, H.; Luo, G.; Liu, H.; Qiao, Y.; Xu, M.; Yao, H. Fate of chromium during thermal treatment of municipal solid waste incineration (MSWI) fly ash. Proc. Combust. Inst. 2013, 34, 2795–2801. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, S.; Wang, Q.; Huang, Q.; Yang, J. Melting characteristics during the vitrification of MSWI fly ash with a pilot-scale diesel oil furnace. J. Hazard. Mater. 2008, 160, 376–381. [Google Scholar] [CrossRef]
- Yu, J.; Qiao, Y.; Jin, L.; Ma, C.; Paterson, N.; Sun, L. Removal of toxic and alkali/alkaline earth metals during co-thermal treatment of two types of MSWI fly ashes in China. Waste Manag. 2015, 46, 287–297. [Google Scholar] [CrossRef]
- Wang, S.; He, P.; Xia, Y.; Lu, W.; Shao, L.; Zhang, H. Role of sodium chloride and mineral matrixes in the chlorination and volatilization of lead during waste thermal treatment. Fuel Process. Technol. 2016, 143, 130–139. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, S.; Mao, Q.; Buekens, A.; Chang, W.; Wang, X.; Yan, J. Suppressing heavy metal leaching through ball milling of fly ash. Energies 2016, 9, 524. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Li, J.; Liu, F.; Guan, J.; Dong, L.; Wang, Q. Behavior of heavy metals in the vitrification of MSWI fly ash with a pilot-scale diesel oil furnace. Procedia Environ. Sci. 2012, 16, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.C.Y.; Kirk, D.W. Behaviour of metals under the conditions of roasting MSW incinerator fly ash with chlorinating agents. J. Hazard. Mater. 1999, 64, 75–89. [Google Scholar] [CrossRef]
- Jiao, F.; Iwata, N.; Kinoshita, N.; Kawaguchi, M.; Asada, M.; Honda, M.; Sueki, K.; Ninomiya, Y. Vaporization mechanisms of water-insoluble Cs in Ash during thermal treatment with calcium chloride addition. Environ. Sci. Technol. 2016, 50, 13328–13334. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Y.; Liu, C.; Zhang, S.; Wang, Y.; Piao, G. Dynamic volatilization behavior of Pb and Cd during fixed bed waste incineration: Effect of chlorine and calcium oxide. Fuel 2017, 192, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Kasai, E. Effect of chlorine on the vaporization behavior of zinc and lead during high temperature treatment of dust and fly ash. ISIJ Int. 2004, 44, 1457–1468. [Google Scholar] [CrossRef] [Green Version]
- Nowak, B.; Frías Rocha, S.; Aschenbrenner, P.; Rechberger, H.; Winter, F. Heavy metal removal from MSW fly ash by means of chlorination and thermal treatment: Influence of the chloride type. Chem. Eng. J. 2012, 179, 178–185. [Google Scholar] [CrossRef]
- Nowak, B.; Pessl, A.; Aschenbrenner, P.; Szentannai, P.; Mattenberger, H.; Rechberger, H.; Hermann, L.; Winter, F. Heavy metal removal from municipal solid waste fly ash by chlorination and thermal treatment. J. Hazard. Mater. 2010, 179, 323–331. [Google Scholar] [CrossRef]
- Vogel, C.; Adam, C. Heavy metal removal from sewage sludge ash by thermochemical treatment with gaseous hydrochloric acid. Environ. Sci. Technol. 2011, 45, 7445–7450. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Xiang, J.; Hu, S.; Yao, H. Mechanism on heavy metals vaporization from municipal solid waste fly ash by MgCl2⋅6H2O. Waste Manag. 2016, 49, 124–130. [Google Scholar] [CrossRef]
- Kageyama, H.; Osada, S.; Nakata, H.; Kubota, H.; Matsuda, H. Effect of coexisting inorganic chlorides on lead volatilization from CaO–SiO2–Al2O3 molten slag under municipal solid waste gasification and melting conditions. Fuel 2013, 103, 94–100. [Google Scholar] [CrossRef]
- Xu, J.; Song, X.; Yu, G.; Du, C. Investigating the effect of flux on ash fusibility of high-calcium coal. ACS Omega 2020, 5, 11361–11368. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Bai, J.; Ilyushechkin, A.; Zhao, H.; Kong, L.; Li, H.; Bai, Z.; Guo, Z.; Li, W. Effect of chemical composition on the fusion behaviour of synthetic high-iron coal ash. Fuel 2019, 253, 1465–1472. [Google Scholar] [CrossRef]
- Shi, W.; Laabs, M.; Reinmöller, M.; Kong, L.; Vassilev, S.V.; Guhl, S.; Bai, J.; Meyer, B.; Li, W. The fusion mechanism of complex minerals mixture and prediction model for flow temperature of coal ash for gasification. Fuel 2021, 305, 121448. [Google Scholar] [CrossRef]
- Shi, W.; Bai, J.; Kong, L.; Li, H.; Bai, Z.; Vassilev, S.V.; Li, W. An overview of the coal ash transition process from solid to slag. Fuel 2021, 287, 119537. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Z.; Lin, X.; Wang, F.; Yan, J. Kinetics and fusion characteristics of municipal solid waste incineration fly ash during thermal treatment. Fuel 2020, 279, 118410. [Google Scholar] [CrossRef]
- Jiao, F.; Kinoshita, N.; Kawaguchi, M.; Asada, M.; Honda, M.; Sueki, K.; Ninomiya, Y. Enhancement of Cs vaporization from simulated granular ash through thermal treatment in N2 atmosphere with the addition of a mixture of CaCl2 and CaO. Fuel 2018, 214, 409–415. [Google Scholar] [CrossRef]
- Jiao, F.; Kinoshita, N.; Kawaguchi, M.; Asada, M.; Honda, M.; Sueki, K.; Ninomiya, Y. Role of CaCl2 and MgCl2 addition in the vaporization of water-insoluble cesium from incineration ash during thermal treatment. Chem. Eng. J. 2017, 323, 114–123. [Google Scholar] [CrossRef]
- Fraissler, G.; Jöller, M.; Mattenberger, H.; Brunner, T.; Obernberger, I. Thermodynamic equilibrium calculations concerning the removal of heavy metals from sewage sludge ash by chlorination. Chem. Eng. Process. Process Intensif. 2009, 48, 152–164. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, Y.; Li, J.; Bao, Z.; Mu, L.; Yu, G. Prediction of coal ash fusibility based on metal ionic potential concentration. J. Energy Inst. 2021, 98, 29–34. [Google Scholar] [CrossRef]
- Ma, W.; Wenga, T.; Frandsen, F.J.; Yan, B.; Chen, G. The fate of chlorine during MSW incineration: Vaporization, transformation, deposition, corrosion and remedies. Prog. Energy Combust. Sci. 2020, 76, 100789. [Google Scholar] [CrossRef]
- Poole, D.; Argent, B.B.; Sharifi, V.N.; Swithenbank, J. Prediction of the distribution of alkali and trace elements between the condensed and gaseous phases in a municipal solid waste incinerator. Fuel 2008, 87, 1318–1333. [Google Scholar] [CrossRef]
- Hu, H.; Liu, H.; Shen, W.; Luo, G.; Li, A.; Lu, Z.; Yao, H. Comparison of CaO’s effect on the fate of heavy metals during thermal treatment of two typical types of MSWI fly ashes in China. Chemosphere 2013, 93, 590–596. [Google Scholar] [CrossRef]
- Dong, J.; Chi, Y.; Tang, Y.; Ni, M.; Nzihou, A.; Weiss-Hortala, E.; Huang, Q. Partitioning of heavy metals in municipal solid waste pyrolysis, gasification, and incineration. Energy Fuels 2015, 29, 7516–7525. [Google Scholar] [CrossRef]
- Wang, S.; He, P.; Lu, W.; Shao, L.; Zhang, H. Comparison of Pb, Cd, Zn, and Cu chlorination during pyrolysis and incineration. Fuel 2017, 194, 257–265. [Google Scholar] [CrossRef]
- Chen, J.; Jiao, F.; Zhang, L.; Yao, H.; Ninomiya, Y. Elucidating the mechanism of Cr(VI) formation upon the interaction with metal oxides during coal oxy-fuel combustion. J. Hazard. Mater. 2013, 261, 260–268. [Google Scholar] [CrossRef] [PubMed]
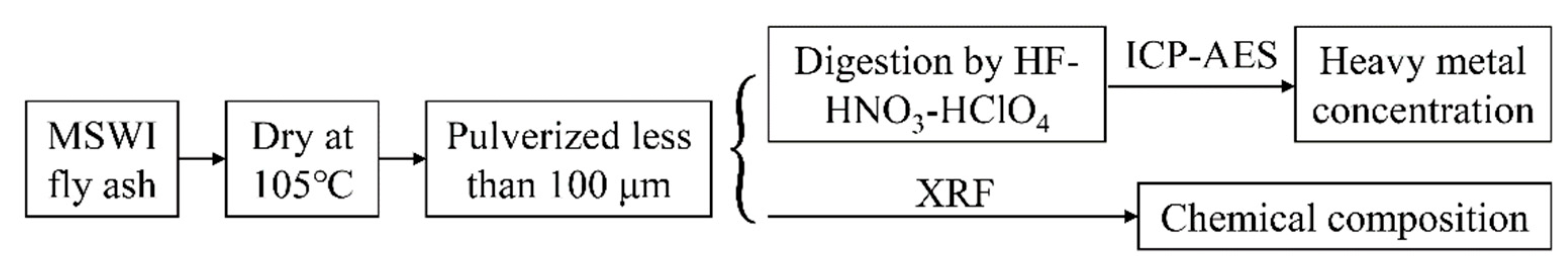
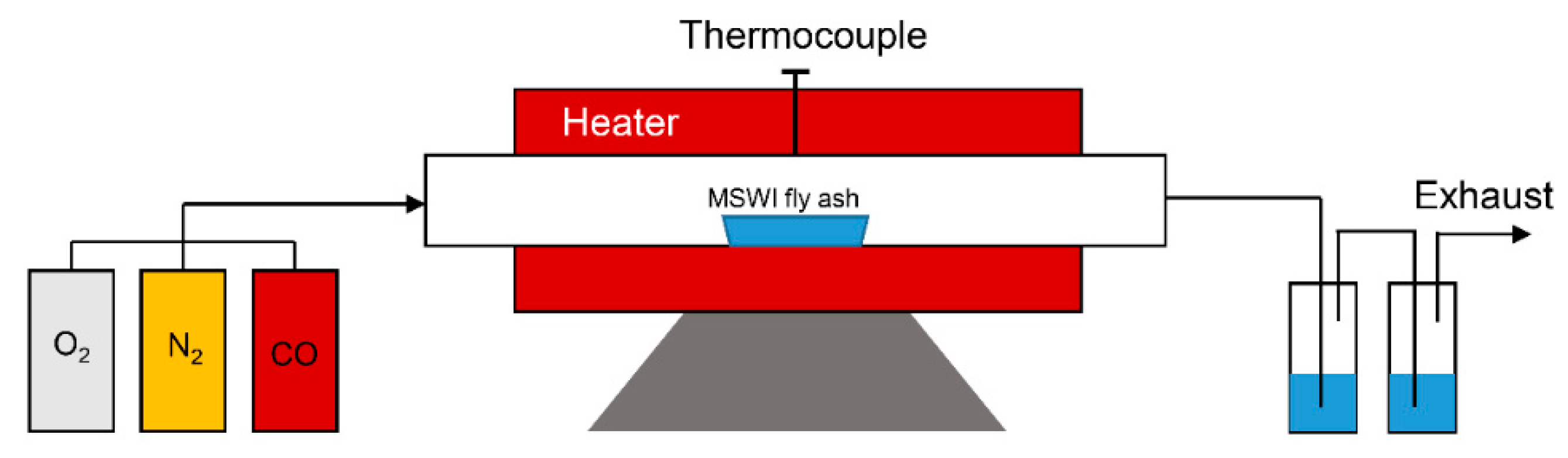



| Species | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 | SO3 | Cl |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 3.8 | 2.54 | 1.12 | 53.7 | 1.09 | 3.31 | 5.42 | 0.72 | 4.18 | 22 |
| Elements | Pb | Zn | Cu | Cd | Cr | Mn | Co | Ni |
|---|---|---|---|---|---|---|---|---|
| Concentration | 1623 | 6394 | 424 | 57 | 241 | 380 | 35 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, F.; Ma, X.; Liu, T.; Wu, C.; Li, H.; Dong, Z. Effect of Atmospheres on Transformation of Heavy Metals during Thermal Treatment of MSWI Fly Ash: By Thermodynamic Equilibrium Calculation. Molecules 2022, 27, 131. https://doi.org/10.3390/molecules27010131
Jiao F, Ma X, Liu T, Wu C, Li H, Dong Z. Effect of Atmospheres on Transformation of Heavy Metals during Thermal Treatment of MSWI Fly Ash: By Thermodynamic Equilibrium Calculation. Molecules. 2022; 27(1):131. https://doi.org/10.3390/molecules27010131
Chicago/Turabian StyleJiao, Facun, Xulong Ma, Tao Liu, Chengli Wu, Hanxu Li, and Zhongbing Dong. 2022. "Effect of Atmospheres on Transformation of Heavy Metals during Thermal Treatment of MSWI Fly Ash: By Thermodynamic Equilibrium Calculation" Molecules 27, no. 1: 131. https://doi.org/10.3390/molecules27010131
APA StyleJiao, F., Ma, X., Liu, T., Wu, C., Li, H., & Dong, Z. (2022). Effect of Atmospheres on Transformation of Heavy Metals during Thermal Treatment of MSWI Fly Ash: By Thermodynamic Equilibrium Calculation. Molecules, 27(1), 131. https://doi.org/10.3390/molecules27010131






