A GdW10@PDA-CAT Sensitizer with High-Z Effect and Self-Supplied Oxygen for Hypoxic-Tumor Radiotherapy
Abstract
:1. Introduction
2. Results and Discussion
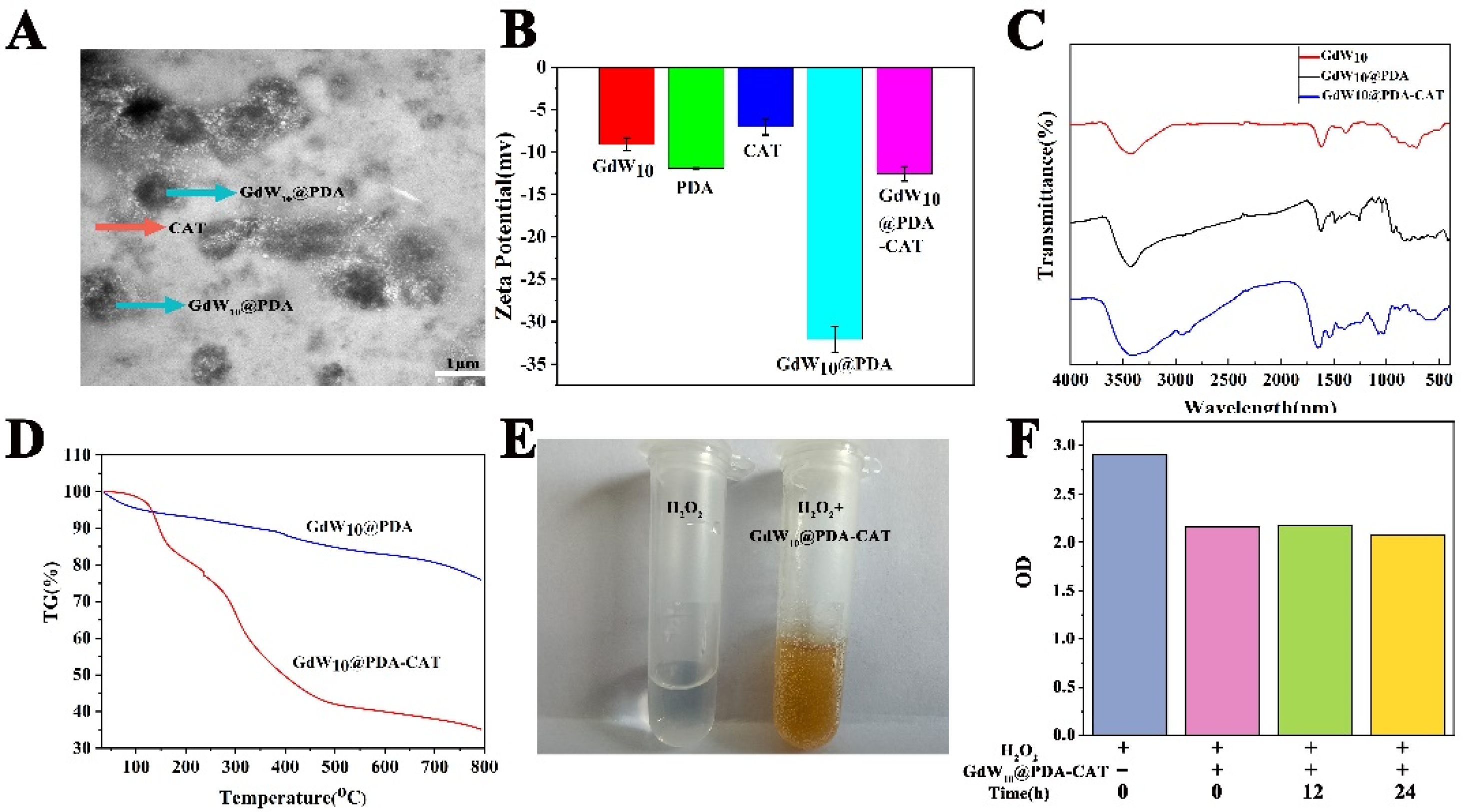
2.1. Fabrication and Characterization of GdW10@PDA-CAT
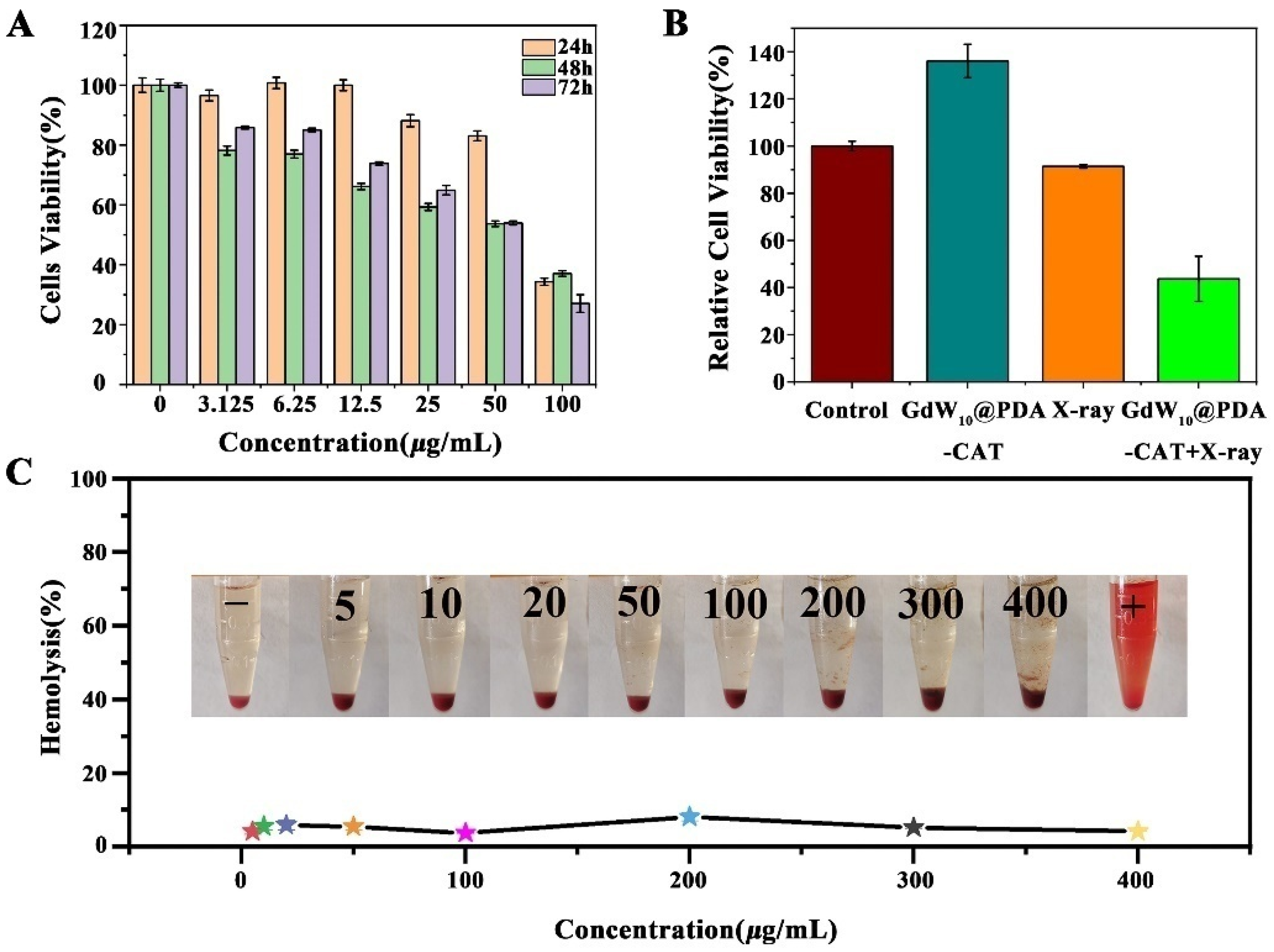
2.2. The Cytotoxicity of GdW10@PDA-CAT
2.3. Hemolysis Test of GdW10@PDA-CAT
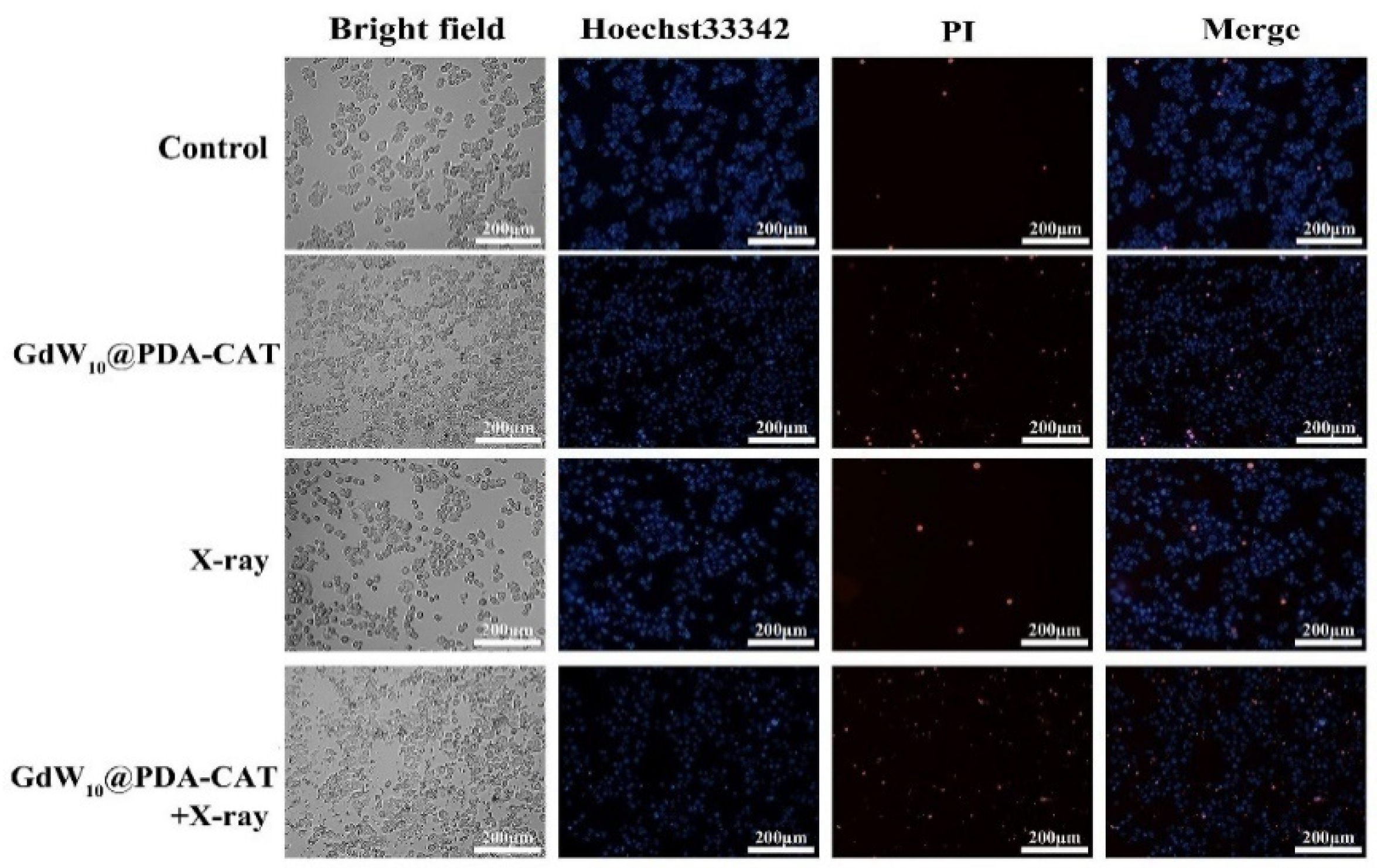
2.4. Live/Dead Cell Staining
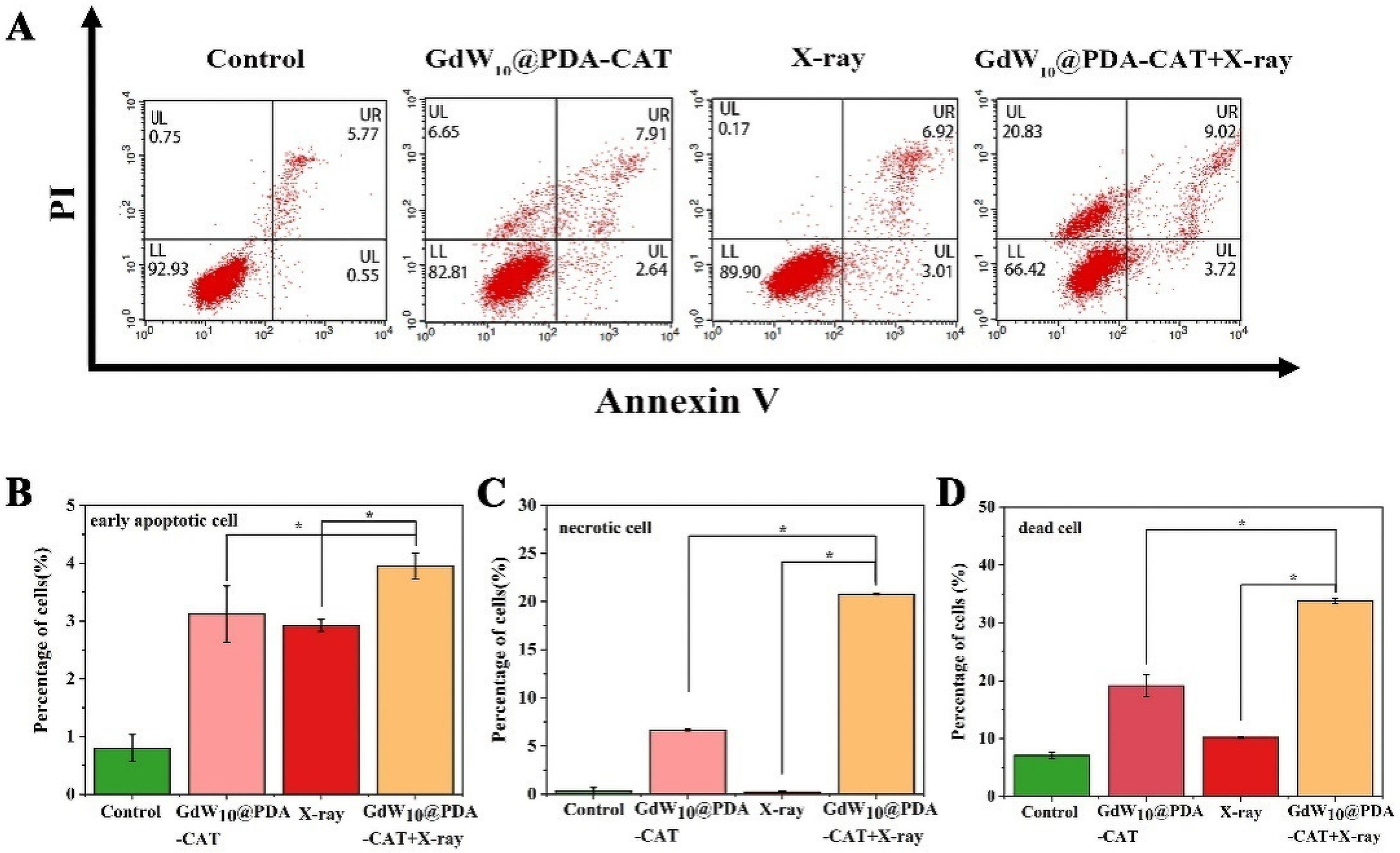
2.5. GdW10@PDA-CAT Particles Induced Death of HT29 Cells by Flow Cytometry
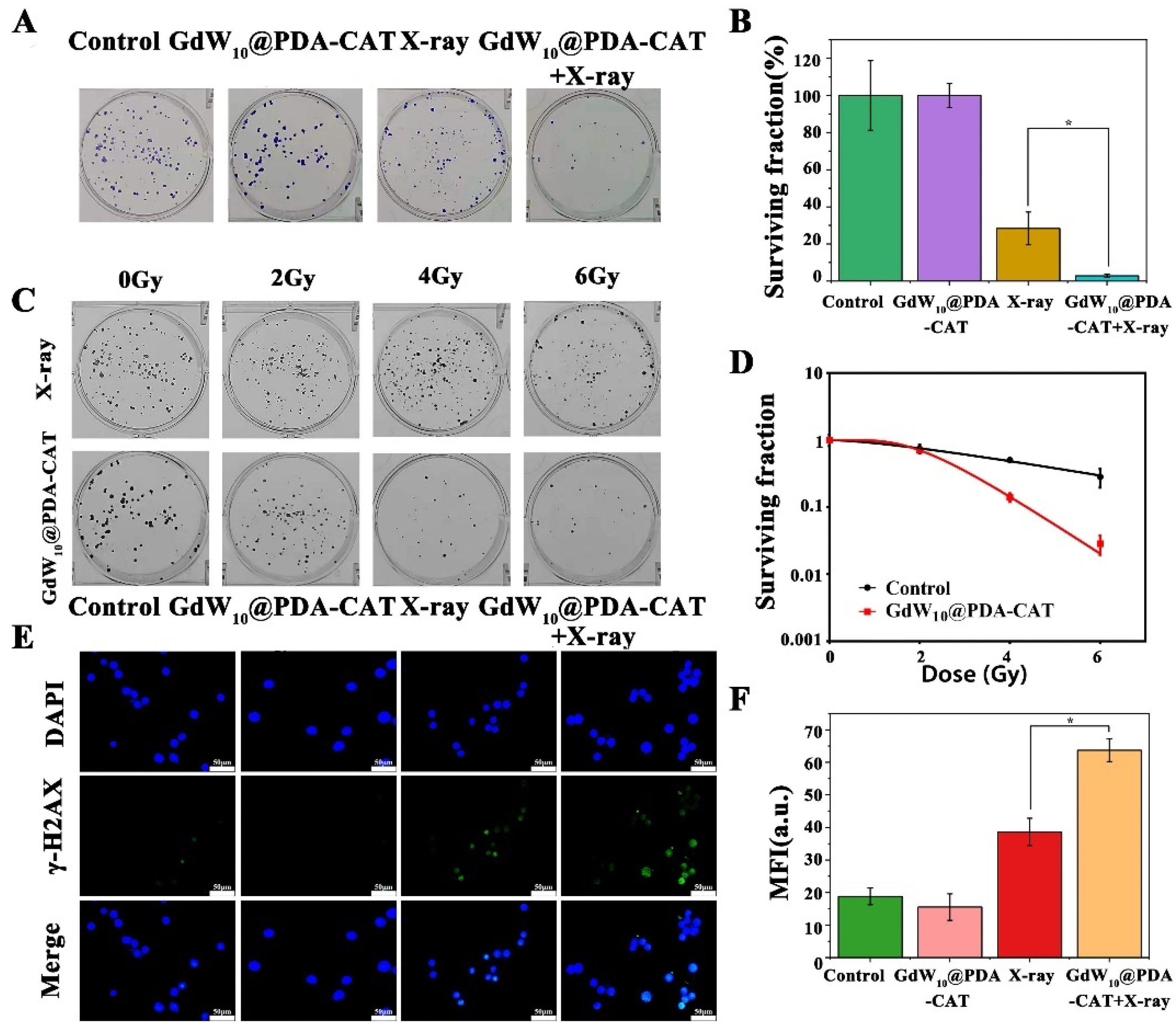
2.6. Radiosensitizing Efficiencies of GdW10@PDA-CAT
2.7. Detection of DNA Double Strand Breaks In Vitro
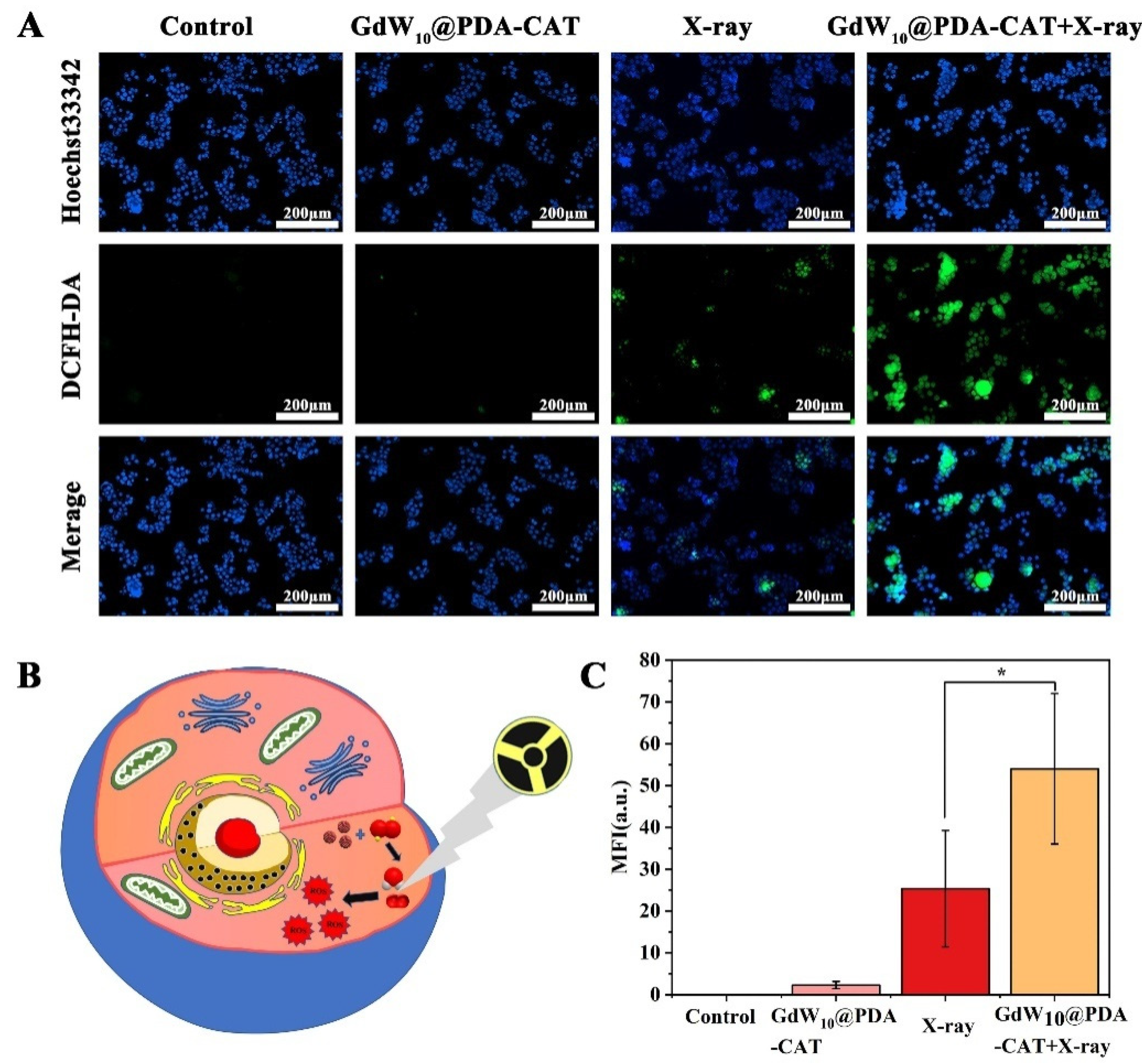
2.8. ROS in HT29 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Characterization
4.3. Synthesis of K3Na4H2 (GdW10O36)·2H2O
4.4. Preparation of GdW10@PDA Nanocomposites
4.5. Preparation of GdW10@PDA-CAT Composites
4.6. Catalase Activity Detection
4.7. In Vitro Drug-Release Study
4.8. Cell Culture
4.9. In Vitro Cytotoxicity of GdW10@PDA-CAT Composites
4.10. Hemolysis Test of GdW10@PDA-CAT Composites
4.11. Colony Survival Assay
4.12. Cell Morphological Observation
4.13. Flow Cytometry Assay
4.14. Immunofluorescence of DNA Damage in HT29 Cells
4.15. Detection of ROS Generation in HT29 Cells
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for multimodal synergistic cancer therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef]
- Liang, H.; Deng, L.; Hou, Y.; Meng, X.; Huang, X.; Rao, E.; Zheng, W.; Mauceri, H.; Mack, M.; Xu, M.; et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat. Commun. 2017, 8, 1736. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Liang, Y.; Chen, Y.; Qian, X.; Luo, W.; Chen, S.; Zhang, S.; Dan, Q.; Zhang, L.; Li, M.; et al. Hollow mesoporous tantalum oxide based nanospheres for triple Sensitization of radiotherapy. ACS Appl. Mater. Interfaces 2020, 12, 5520–5530. [Google Scholar] [CrossRef]
- Chen, Y.; Zhong, H.; Wang, J.; Wan, X.; Li, Y.; Pan, W.; Li, N.; Tang, B. Catalase-like metal-organic framework nanoparticles to enhance radiotherapy in hypoxic cancer and prevent cancer recurrence. Chem. Sci. 2019, 10, 5773–5778. [Google Scholar] [CrossRef] [Green Version]
- Barker, H.E.; Paget, J.T.; Khan, A.A.; Harrington, K.J. The tumor microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Bristow, R.G.; Berlin, A.; Dal Pra, A. An arranged marriage for precision medicine: Hypoxia and genomic assays in localized prostate cancer radiotherapy. Br. J. Radiol. 2014, 87, 20130753. [Google Scholar] [CrossRef] [Green Version]
- Helbig, L.; Koi, L.; Bruchner, K.; Gurtner, K.; Hess-Stumpp, H.; Unterschemmann, K.; Pruschy, M.; Baumann, M.; Yaromina, A.; Zips, D. Hypoxia-inducible factor pathway inhibition resolves tumor hypoxia and improves local tumor control after single-dose irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 159–166. [Google Scholar] [CrossRef]
- Sadri, N.; Zhang, P.J. Hypoxia-inducible factors: Mediators of cancer progression; prognostic and therapeutic targets in soft tissue sarcomas. Cancers 2013, 5, 320–333. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Mu, X.; He, H.; Zhang, X.D. Cancer radiosensitizers. Trends Pharmacol. Sci. 2018, 39, 24–48. [Google Scholar] [CrossRef]
- Adams, G.E.D. Chemical radiosensitization of hypoxic cells. Br. Med. Bull. 1973, 29, 48–53. [Google Scholar] [CrossRef]
- Ahmed, B.S.; Rao, A.G.; Sankarshan, B.M.; Vicas, C.; Namratha, K.; Umesh, T.; Somashekar, R.; Byrappa, K. Evaluation of gold, silver and silver–gold (bimetallic) nanoparticles as radiosensitizers for radiation therapy in cancer treatment. Cancer Oncol. Res. 2016, 4, 42–51. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, X.H.; Zhang, W.; Lou, Z.C.; Xie, L.H.; Hu, X.D.; Peidang, L.; Zhang, H.Q. Enhancement of radiotherapy by ceria nanoparticles modified with neogambogic acid in breast cancer cells. Int. J. Nanomed. 2015, 10, 4957–4969. [Google Scholar] [CrossRef] [Green Version]
- Goswami, N.; Luo, Z.; Yuan, X.; Leong, D.T.; Xie, J. Engineering gold-based radiosensitizers for cancer radiotherapy. Mater. Horizons 2017, 4, 817–831. [Google Scholar] [CrossRef]
- Subiel, A.; Ashmore, R.; Schettino, G. Standards and methodologies for characterizing radiobiological impact of high-Z nanoparticles. Theranostics 2016, 6, 1651–1671. [Google Scholar] [CrossRef]
- Zhang, X.D.; Chen, J.; Luo, Z.; Wu, D.; Shen, X.; Song, S.S.; Sun, Y.M.; Liu, P.X.; Zhao, J.; Huo, S.; et al. Enhanced tumor accumulation of sub-2 nm gold nanoclusters for cancer radiation therapy. Adv. Healthc. Mater. 2014, 3, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Butterworth, K.T.; McMahon, S.J.; Currell, F.J.; Prise, K.M. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012, 4, 4830–4838. [Google Scholar] [CrossRef]
- Pan, Y.; Xue, P.; Liu, S.; Zhang, L.; Guan, Q.; Zhu, J.; Tian, X. Metal-based hybrid nanoparticles as radiosensitizers in cancer therapy. Colloid Interface Sci. Commun. 2018, 23, 45–51. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Alhadlaq, H.A.; Kumar, S.; Alrokayan, S.A.; Ahamed, M. Selective cancer-killing ability of metal-based nanoparticles: Implications for cancer therapy. Arch. Toxicol. 2015, 89, 1895–1907. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Zhang, C.; Zhu, S.; Li, L.; Gu, Z.; Zhao, Y. Ultrasmall BiOI Quantum dots with efficient renal clearance for enhanced radiotherapy of cancer. Adv. Sci. 2020, 7, 1902561. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.; Sommer, A.; Distel, L.V.; Neuhuber, W.; Kryschi, C. Superparamagnetic iron oxide nanoparticles as radiosensitizer via enhanced reactive oxygen species formation. Biochem. Biophys. Res. Commun. 2012, 425, 393–397. [Google Scholar] [CrossRef]
- Mirjolet, C.; Papa, A.; Créhange, G.; Raguin, O.; Seignez, C.; Paul, C.; Truc, G.; Maingon, P.; Millot, N. The radiosensitization effect of titanate nanotubes as a new tool in radiation therapy for glioblastoma: A proof-of-concept. Radiother. Oncol. 2013, 108, 136–142. [Google Scholar] [CrossRef]
- Takahashi, J.; Misawa, M. Analysis of potential radiosensitizing materials for X-ray-induced photodynamic therapy. NanoBiotechnology 2008, 3, 116–126. [Google Scholar] [CrossRef]
- Townley, H.E.; Kim, J.; Dobson, P.J. In vivo demonstration of enhanced radiotherapy using rare earth doped titania nanoparticles. Nanoscale 2012, 4, 5043–5050. [Google Scholar] [CrossRef]
- Yang, W.; Read, P.W.; Mi, J.; Baisden, J.; Reardon, K.A.; Larner, J.M.; Helmke, B.P.; Sheng, K. Semiconductor nanoparticles as energy mediators for photosensitizer-enhanced radiotherapy. Int. J. Radiat. Oncol. 2008, 72, 633–635. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of radiosensitizers in cancer radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Zhang, C.; Gu, Z.; Du, J.; Guo, Z.; Dong, X.; Xie, J.; Zhang, G.; Liu, X.; Zhao, Y. Polyoxometalate-based radiosensitization platform for treating hypoxic tumors by attenuating radioresistance and enhancing radiation response. ACS Nano 2017, 11, 7164–7176. [Google Scholar] [CrossRef]
- Li, C.; Cao, H.; Sun, J.; Tian, R.; Li, D.; Qi, Y.; Yang, W.; Li, J. Antileukemic activity of an arsenomolybdate in the human HL-60 and U937 leukemia cells. J. Inorg. Biochem. 2017, 168, 67–75. [Google Scholar] [CrossRef]
- Rhule, J.T.; Hill, C.L.; Judd, D.A.; Schinazi, R.F. Polyoxometalates in medicine. Chem. Rev. 1998, 98, 327–357. [Google Scholar] [CrossRef]
- Aureliano, M.; Ohlin, C.A. Decavanadate in vitro and in vivo effects: Facts and opinions. J. Inorg. Biochem. 2014, 137, 123–130. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, M.; Zou, H.; Zhang, X.; Sheng, R.; Zhang, Y.; Zhang, B.; Li, C.; Qi, Y. An enhanced antibacterial nanoflowers AgPW@PDA@Nisin constructed from polyoxometalate and nisin. J. Inorg. Biochem. 2020, 212, 111212. [Google Scholar] [CrossRef]
- Yamase, T. Anti-tumor, -viral, and -bacterial activities of polyoxometalates for realizing an inorganic drug. J. Mater. Chem. 2005, 15, 4773–4782. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, L.; Gu, Z.; Zhao, Y. Strategies based on metal-based nanoparticles for hypoxic-tumor radiotherapy. Chem. Sci. 2019, 10, 6932–6943. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Liang, C.; Yi, X.; Zhao, Q.; Cheng, L.; Yang, K.; Liu, Z. Perfluorocarbon-loaded hollow Bi2Se3 nanoparticles for timely supply of oxygen under near-infrared light to enhance the radiotherapy of cancer. Adv. Mater. 2016, 28, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, R.; Dong, X.; Zhu, S.; Zhou, R.; Yan, L.; Zhang, C.; Wang, Q.; Gu, Z.; Zhao, Y. BiO2-x nanosheets as radiosensitizers with catalase-like activity for hypoxia alleviation and enhancement of the radiotherapy of tumors. Inorg. Chem. 2020, 59, 3482–3493. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zou, H.; Qi, Y.; Zhang, X.; Sheng, R.; Zhang, Y.; Sun, R.; Chen, L.; Lv, R. Assembly of polyoxometalates/polydopamine nanozymes as a multifunctional platform for glutathione and Escherichia coli O157:H7 detection. Microchem. J. 2021, 164, 106013. [Google Scholar] [CrossRef]
- Ball, V.; Haider, A.; Kortz, U. Composite films of poly(allylamine)-capped polydopamine nanoparticles and P8W48 polyoxometalates with electroactive properties. J. Colloid Interface Sci. 2016, 481, 125–130. [Google Scholar] [CrossRef]
- Long, D.-L.; Tsunashima, R.; Cronin, L. Polyoxometalates: Building blocks for functional nanoscale systems. Angew. Chem. Int. Ed. 2010, 49, 1736–1758. [Google Scholar] [CrossRef]
- Li, H.; Jia, Y.; Wang, A.; Cui, W.; Ma, H.; Feng, X.; Li, J. Self-Assembly of Hierarchical nanostructures from dopamine and polyoxometalate for oral drug delivery. Chemistry 2014, 20, 499–504. [Google Scholar] [CrossRef]
- Wang, S.S.; Yang, G.Y. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef]
- Cao, H.; Yang, Y.; Qi, Y.; Li, Y.; Sun, B.; Li, Y.; Cui, W.; Li, J.; Li, J. Intraparticle FRET for enhanced efficiency of two-photon activated photodynamic therapy. Adv. Heal. Mater. 2018, 7, e1701357. [Google Scholar] [CrossRef]
- Haume, K.; Rosa, S.; Grellet, S.; Śmiałek, M.A.; Butterworth, K.T.; Solov’Yov, A.V.; Prise, K.M.; Golding, J.; Mason, N.J. Gold nanoparticles for cancer radiotherapy: A review. Cancer Nanotechnol. 2016, 7, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Liang, R.; Fang, F. Applications of nanomaterials in radiotherapy for malignant tumors. J. Nanosci. Nanotechnol. 2015, 15, 5487–5500. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Cheng, L.; Chao, Y.; Yang, K.; Liu, Z. Emerging nanotechnology and advanced materials for cancer radiation therapy. Adv. Mater. 2017, 29, 1700996. [Google Scholar] [CrossRef]
- Xie, J.; Gong, L.; Zhu, S.; Yong, Y.; Gu, Z.; Zhao, Y. Emerging strategies of nanomaterial-mediated tumor radiosensitization. Adv. Mater. 2019, 31, e1802244. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, D.; Venugopal, A.; Anant, S. Nanoparticles in radiation therapy: A summary of various approaches to enhance radiosensitization in cancer. Transl. Cancer Res. 2013, 2, 330–342. [Google Scholar]
- Prasad, P.; Gordijo, C.R.; Abbasi, A.Z.; Maeda, A.; Ip, A.; Rauth, A.M.; DaCosta, R.S.; Wu, X.Y. Multifunctional albumin-MnO2 nanoparticles modulate solid tumor microenvironment by attenuating hypoxia, acidosis, vascular endothelial growth factor and enhance radiation response. ACS Nano 2014, 8, 3202–3212. [Google Scholar] [CrossRef]
- Bristow, R.G.; Hill, R.P. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Zhou, L.; Zhang, S.; Yan, L.; Gu, Z.; Zhang, G.; Zhao, Y. Gadolinium polytungstate nanoclusters: A new theranostic with ultrasmall size and versatile properties for dual-modal MR/CT imaging and photothermal therapy/radiotherapy of cancer. NPG Asia Mater. 2016, 8, e273. [Google Scholar] [CrossRef] [Green Version]
- Zang, Y.; Gong, L.; Mei, L.; Gu, Z.; Wang, Q. Bi2WO6 semiconductor nanoplates for tumor radiosensitization through high-Z effects and radiocatalysis. ACS Appl. Mater. Interfaces 2019, 11, 18942–18952. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, H.; Yang, Y.; Zhang, C.; Dong, X.; Du, J.; Yan, L.; Zhang, G.; Gu, Z.; Zhao, Y. Tumor microenvironment-manipulated radiocatalytic sensitizer based on bismuth heteropolytungstate for radiotherapy enhancement. Biomaterials 2019, 189, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Pizzuti, V.J.; Misra, R.; Lee, J.; Torregrosa-Allen, S.E.; Currie, M.P.; Clark, S.R.; Patel, A.P.; Schorr, C.R.; Jones-Hall, Y.; Childress, M.O.; et al. Folic acid-conjugated radioluminescent calcium tungstate nanoparticles as radio-sensitizers for cancer radiotherapy. ACS Biomater. Sci. Eng. 2019, 5, 4776–4789. [Google Scholar] [CrossRef] [PubMed]
- Rakotoarisoa, M.; Angelov, B.; Espinoza, S.; Khakurel, K.; Bizien, T.; Angelova, A. Cubic liquid crystalline nanostructures involving catalase and curcumin: BioSAXS study and catalase peroxidatic function after cubosomal nanoparticle treatment of differentiated SH-SY5Y cells. Molecules 2019, 24, 3058. [Google Scholar] [CrossRef] [Green Version]
- Morozov, K.V.; Kolyvanova, M.A.; Kartseva, M.E.; Shishmakova, E.M.; Dement’Eva, O.V.; Isagulieva, A.K.; Salpagarov, M.H.; Belousov, A.V.; Rudoy, V.M.; Shtil, A.A.; et al. Radiosensitization by gold nanoparticles: Impact of the size, dose rate, and photon energy. Nanomaterials 2020, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qi, W.; Cao, H.; Qi, Y.; Zhang, S.; Xu, S.; Sun, J.; Guo, S. BSA-binding properties and anti-proliferative effects of amino acids functionalized polyoxomolybdates. Biomed. Pharmacother. 2016, 79, 78–86. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhang, Y.; Zhang, X.; Lv, R.; Sheng, R.; Sun, R.; Du, T.; Li, Y.; Qi, Y. A GdW10@PDA-CAT Sensitizer with High-Z Effect and Self-Supplied Oxygen for Hypoxic-Tumor Radiotherapy. Molecules 2022, 27, 128. https://doi.org/10.3390/molecules27010128
Chen L, Zhang Y, Zhang X, Lv R, Sheng R, Sun R, Du T, Li Y, Qi Y. A GdW10@PDA-CAT Sensitizer with High-Z Effect and Self-Supplied Oxygen for Hypoxic-Tumor Radiotherapy. Molecules. 2022; 27(1):128. https://doi.org/10.3390/molecules27010128
Chicago/Turabian StyleChen, Lixia, Yang Zhang, Xinming Zhang, Ruijuan Lv, Rongtian Sheng, Ruimeng Sun, Ting Du, Yuhan Li, and Yanfei Qi. 2022. "A GdW10@PDA-CAT Sensitizer with High-Z Effect and Self-Supplied Oxygen for Hypoxic-Tumor Radiotherapy" Molecules 27, no. 1: 128. https://doi.org/10.3390/molecules27010128
APA StyleChen, L., Zhang, Y., Zhang, X., Lv, R., Sheng, R., Sun, R., Du, T., Li, Y., & Qi, Y. (2022). A GdW10@PDA-CAT Sensitizer with High-Z Effect and Self-Supplied Oxygen for Hypoxic-Tumor Radiotherapy. Molecules, 27(1), 128. https://doi.org/10.3390/molecules27010128





