Calcium Phosphate Particles Coated with Humic Substances: A Potential Plant Biostimulant from Circular Economy
Abstract
:1. Introduction
2. Results
2.1. Materials Characterization
2.2. Observations on Plant Species
2.2.1. Germination and Seedlings Development
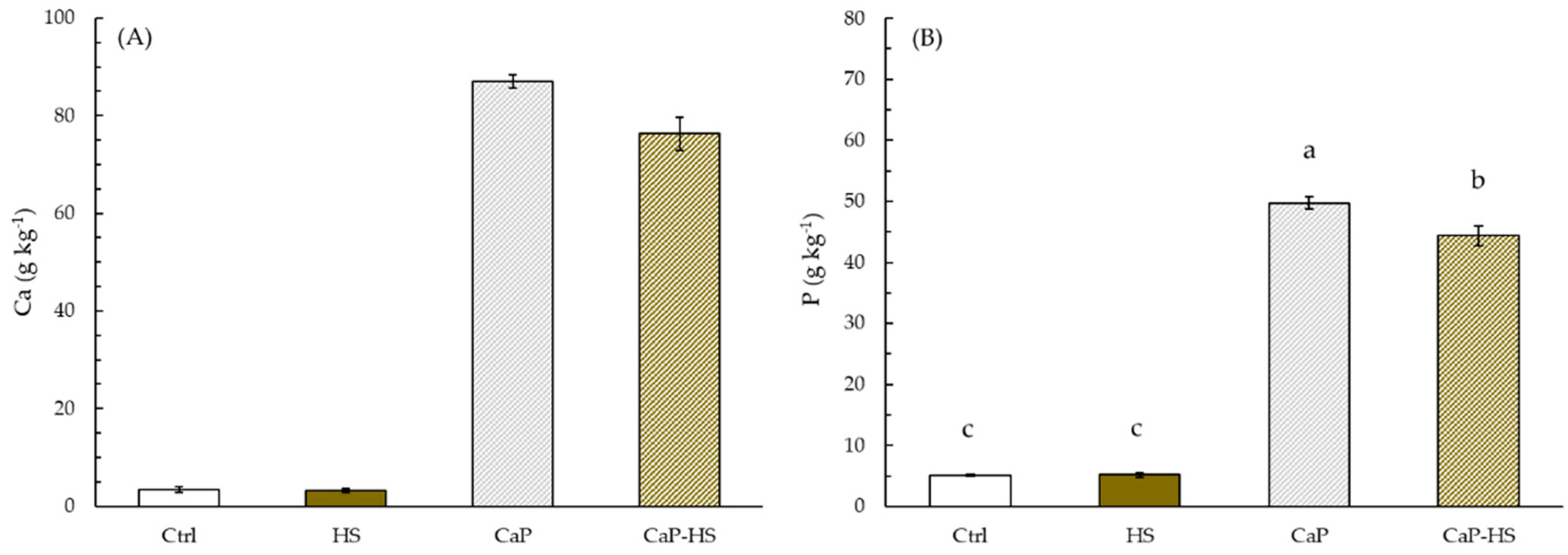
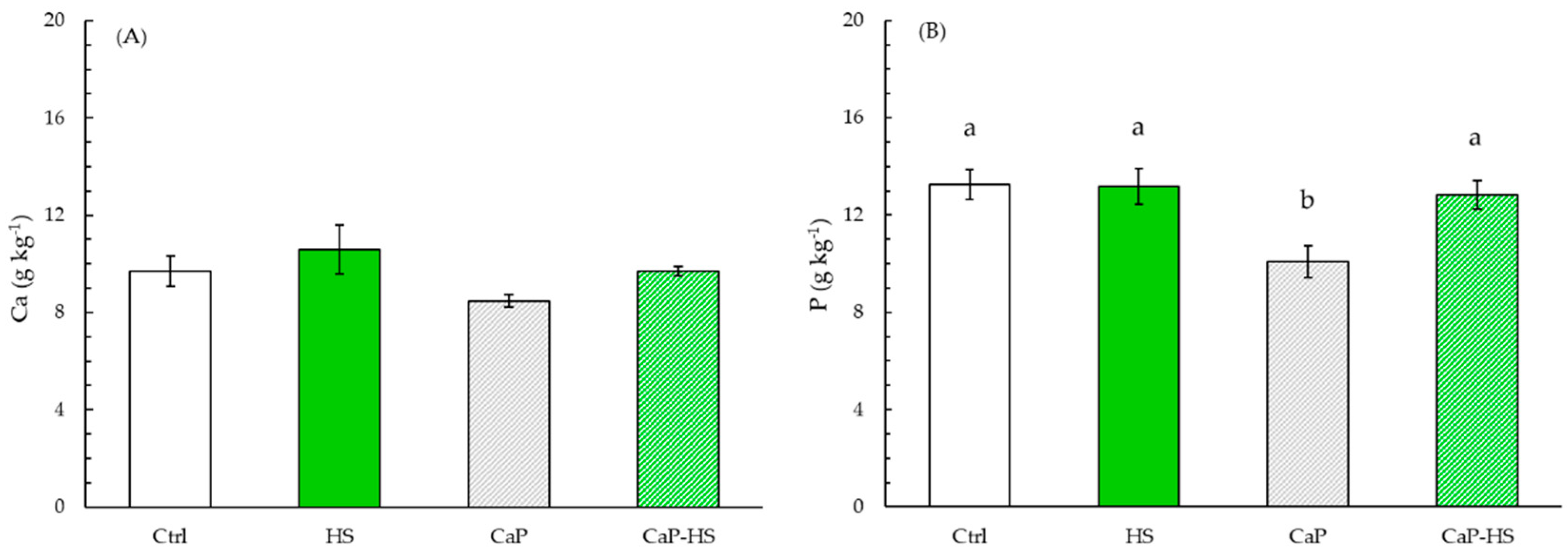
2.2.2. Element Concentration in Seedling Roots and Shoot
3. Discussion
4. Materials and Methods
4.1. CaP-HS Production and Characterization
4.1.1. CaP Extraction from Fish Bones
4.1.2. CaP Coating with HS
4.1.3. Samples Characterization
4.2. Plant Experiment Setup
4.3. Cellular ATP Activity Determination
4.4. Macroelements in Plant Seedlings
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- RHorrigan, L.; Lawrence, R.S.; Walker, P. How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ. Health Perspect. 2002, 110, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Jindo, K.; Canellas, L.P.; Albacete, A.; dos Santos, L.F.; Frinhani Rocha, R.L.; Carvalho Baia, D.; Oliveira Aguiar Canellas, N.; Goron, T.L.; Olivares, F.L. Interaction between humic substances and plant hormones for phosphorous acquisition. Agronomy 2020, 10, 640. [Google Scholar] [CrossRef]
- Olivares, F.L.; Busato, J.G.; de Paula, A.M.; da Silva Lima, L.; Aguiar, N.O.; Canellas, L.P. Plant growth promoting bacteria and humic substances: Crop promotion and mechanisms of action. Chem. Biol. Technol. Agric. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Purwanto, B.H.; Wulandari, P.; Sulistyaningsih, E.; Utami, S.N.H.; Handayani, S.; Lisetskii, F. Improved Corn Yields When Humic Acid Extracted from Composted Manure Is Applied to Acid Soils with Phosphorus Fertilizer. Appl. Environ. Soil Sci. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Gerke, J. Review Article: The effect of humic substances on phosphate and iron acquisition by higher plants: Qualitative and quantitative aspects. J. Plant Nutr. Soil Sci. 2021. [Google Scholar] [CrossRef]
- Çimrin, K.M.; Türkmen, Ö.; Turan, M.; Tuncer, B. Phosphorus and humic acid application alleviate salinity stress of pepper seedling. Afr. J. Biotechnol. 2010, 9, 5845–5851. [Google Scholar]
- García, A.C.; Santos, L.A.; Izquierdo, F.G.; Sperandio, M.V.L.; Castro, R.N.; Berbara, R.L.L. Vermicompost humic acids as an ecological pathway to protect rice plant against oxidative stress. Ecol. Eng. 2012, 47, 203–208. [Google Scholar] [CrossRef]
- Aydin, A.; Kant, C.; Turan, M. Humic acid application alleviate salinity stress of bean (Phaseolus vulgaris L.) plants decreasing membrane leakage. Afr. J. Agric. Res. 2012, 7, 1073–1086. [Google Scholar] [CrossRef]
- Alewell, C.; Ringeval, B.; Ballabio, C.; Robinson, D.A.; Panagos, P.; Borrelli, P. Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Lee, J.G.; Esposti, L.D.; Iafisco, M.; Kim, P.J.; Shin, S.G.; Jeon, J.-R.; Adamiano, A. Synergistic release of crop nutrients and stimulants from hydroxyapatite nanoparticles functionalized with humic substances: Toward a multifunctional nanofertilizer. ACS Omega 2020, 5, 6598–6610. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, R.; Evans, L.A.; Milham, P.J.; Wilson, M.A. Effects of humic material on the precipitation of calcium phosphate. Geoderma 2004, 118, 245–260. [Google Scholar] [CrossRef]
- Carella, F.; Seck, M.; Degli Esposti, L.; Diadiou, H.; Maienza, A.; Baronti, S.; Vignaroli, P.; Vaccari, F.P.; Iafisco, M.; Adamiano, A. Thermal conversion of fish bones into fertilizers and biostimulants for plant growth–A low tech valorization process for the development of circular economy in least developed countries. J. Environ. Chem. Eng. 2021, 9, 104815. [Google Scholar] [CrossRef]
- Piccirillo, C.; Adamiano, A.; Tobaldi, D.M.; Montalti, M.; Manzi, J.; Castro, P.M.L.; Panseri, S.; Montesi, M.; Sprio, S.; Tampieri, A. Luminescent calcium phosphate bioceramics doped with europium derived from fish industry byproducts. J. Am. Ceram. Soc. 2017, 100, 3402–3414. [Google Scholar] [CrossRef]
- Ben-Nissan, B. Advances in Calcium Phosphate Biomaterials; Springer: Berlin/Heidelberg, Germany; Hong-Kong, China, 2014. [Google Scholar]
- Fowler, B.; Moreno, E.; Brown, W. Infra-red spectra of hydroxyapatite, octacalcium phosphate and pyrolysed octacalcium phosphate. Arch. Oral. Biol. 1966, 11, 477–492. [Google Scholar] [CrossRef]
- Adamiano, A.; Sangiorgi, N.; Sprio, S.; Ruffini, A.; Sandri, M.; Sanson, A.; Gras, P.; Grossin, D.; Francès, C.; Chatzipanagis, K. Biomineralization of a titanium-modified hydroxyapatite semiconductor on conductive wool fibers. J. Mat. Chem. B 2017, 5, 7608–7621. [Google Scholar] [CrossRef]
- Tatzber, M.; Stemmer, M.; Spiegel, H.; Katzlberger, C.; Haberhauer, G.; Mentler, A.; Gerzabek, M.H. FTIR-spectroscopic characterization of humic acids and humin fractions obtained by advanced NaOH, Na4P2O7, and Na2CO3 extraction procedures. J. Plant. Nutr. Soil Sci. 2007, 170, 522–529. [Google Scholar] [CrossRef]
- Iafisco, M.; Marchetti, M.; Gómez Morales, J.; Hernández-Hernández, M.A.; Garcia Ruiz, J.M.; Roveri, N. Silica gel template for calcium phosphates crystallization. Cryst. Growth Des. 2009, 9, 4912–4921. [Google Scholar] [CrossRef]
- Terzioglu, P.; Ogut, H.; Kalemtas, A. Natural calcium phosphates from fish bones and their potential biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Suzuki, S. Microstructural development of natural hydroxyapatite originated from fish-bone waste through heat treatment. J. Am. Ceram. Soc. 2002, 85, 1315–1317. [Google Scholar] [CrossRef]
- Hamada, M.; Nagai, T.; Kai, N.; Tanoue, Y.; Mae, H.; Hashimoto, M.; Miyoshi, K.; Kumagai, H.; Saeki, K. Inorganic constituents of bone of fish. Fish Sci. 1995, 61, 517–520. [Google Scholar] [CrossRef] [Green Version]
- Marchiol, L.; Iafisco, M.; Fellet, G.; Adamiano, A. Nanotechnology support the next agricultural revolution: Perspectives to enhancement of nutrient use efficiency. Adv. Agron. 2020, 161, 27–116. [Google Scholar]
- García, A.C.; De Souza, L.G.A.; Pereira, M.G.; Castro, R.N.; García-Mina, J.M.; Zonta, E.; Lisboa, F.J.G.; Berbara, R.L.L. Structure-property-function relationship in humic substances to explain the biological activity in plants. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Vekariya, R.L.; Sonigara, K.K.; Fadadu, K.B.; Vaghasiya, J.V.; Soni, S.S. Humic acid as a sensitizer in highly stable dye solar cells: Energy from an abundant natural polymer soil component. ACS Omega 2016, 1, 14–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, H.J.; Cha, J.-Y.; Choi, J.H.; Jang, K.-S.; Lim, J.; Kim, W.-Y.; Seo, D.-C.; Jeon, J.-R. One-pot transformation of technical lignins into humic-like plant stimulants through fenton-based advanced oxidation: Accelerating natural fungus-driven humification. ACS Omega 2018, 3, 7441–7453. [Google Scholar] [CrossRef] [Green Version]
- Savy, D.; Mazzei, P.; Drosos, M.; Cozzolino, V.; Lama, L.; Piccolo, A. Molecular characterization of extracts from biorefinery wastes and evaluation of their plant biostimulation. ACS Sustain. Chem. Eng. 2017, 5, 9023–9031. [Google Scholar] [CrossRef]
- David, P.; Nelson, P.; Sanders, D. A humic acid improves growth of tomato seedling in solution culture. J. Plant Nutr. 1994, 17, 173–184. [Google Scholar] [CrossRef]
- Wang, X.J.; Wang, Z.Q.; Li, S. The effect of humic acids on the availability of phosphorus fertilizers in alkaline soils. Soil Use Manage. 1995, 11, 99–102. [Google Scholar] [CrossRef]
- Delgado, A.; Madrid, A.; Kassem, S.; Andreu, L.; Del Campillo, M.D.C. Phosphorus fertilizer recovery from calcareous soils amended with humic and fulvic acids. Plant Soil 2002, 245, 277–286. [Google Scholar] [CrossRef]
- Benedetti, A.; Figliolia, A.; Izza, C.; Canali, S.; Rossi, G. Some thoughts on the physiological effects of humic acids: Interactions with mineral fertilisers. Agrochimica 1996, 40, 229–240. [Google Scholar]
- Bermudez, D.; Juarez, M.; Sanchez-Andreu, J.; Jorda, J. Role of eddha and humic acids on the solubility of soil phosphorus. Commun. Soil Sci. Plant Anal. 1993, 24, 673–683. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.; Schmidt, R. Physiological effects of liquid applications of a seaweed extract and a humic acid on creeping bentgrass. J. Am. Soc. Hortic. Sci. 2003, 128, 492–496. [Google Scholar] [CrossRef] [Green Version]
- Nikbakht, A.; Kafi, M.; Babalar, M.; Xia, Y.P.; Luo, A.; Etemadi, N.-A. Effect of humic acid on plant growth, nutrient uptake, and postharvest life of gerbera. J. Plant Nutr. 2008, 31, 2155–2167. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J. Eats roots and leaves. Can edible horticultural crops address dietary calcium, magnesium and potassium deficiencies? Proc. Nutr. Soc. 2010, 69, 601–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamiano, A.; Fabbri, D.; Falini, G.; Belcastro, M.G. A complementary approach using analytical pyrolysis to evaluate collagen degradation and mineral fossilisation in archaeological bones: The case study of Vicenne-Campochiaro necropolis (Italy). J. Anal. Aplp. Pyrolysis 2013, 100, 173–180. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-Ray Diffraction Procedures: For Polycrystalline and Amorphous Materials; WIley: Hoboken, NJ, USA, 1974. [Google Scholar]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinf. 2017, 18, 1–26. [Google Scholar] [CrossRef]
- Mattiello, A.; Filippi, A.; Pošćić, F.; Musetti, R.; Salvatici, M.C.; Giordano, C.; Vischi, M.; Bertolini, A.; Marchiol, L. Evidence of phytotoxicity and genotoxicity in Hordeum vulgare L. exposed to CeO2 and TiO2 nanoparticles. Front. Plant. Sci. 2015, 6, 1043. [Google Scholar] [CrossRef] [Green Version]
- Team RDC. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010; Available online: http://www.R-project.org (accessed on 4 February 2021).


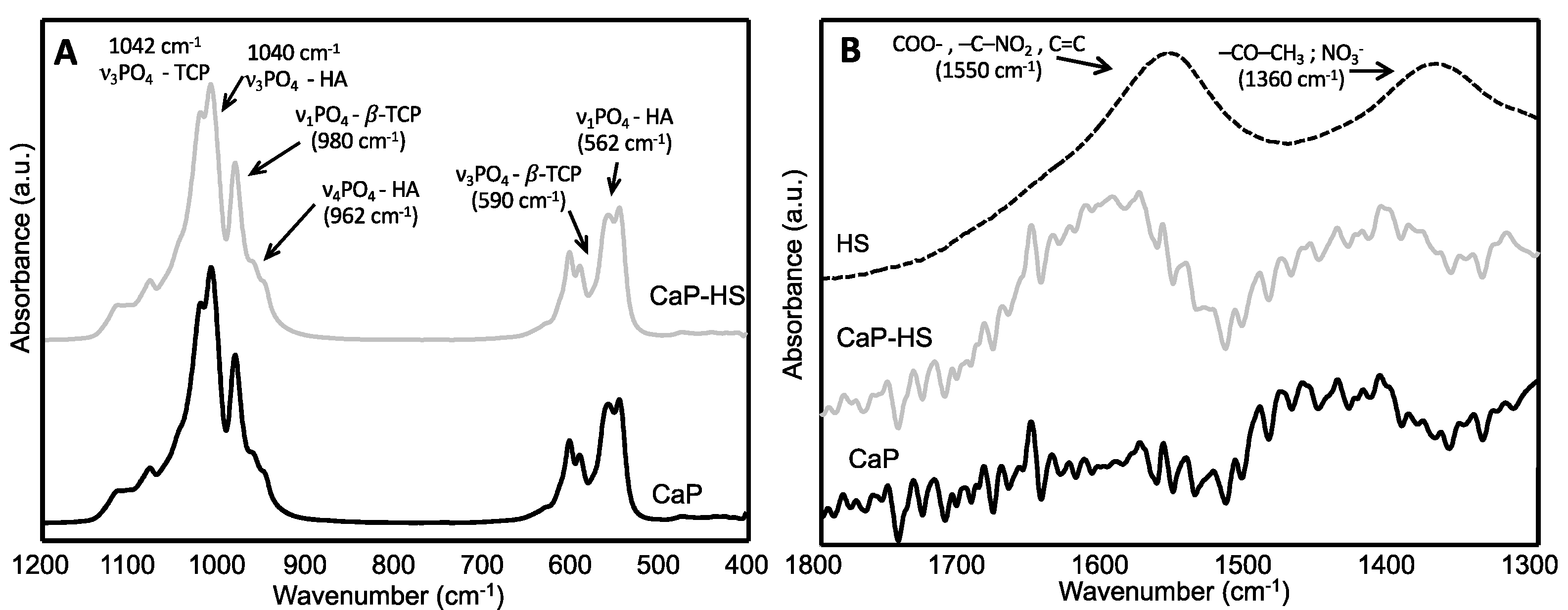
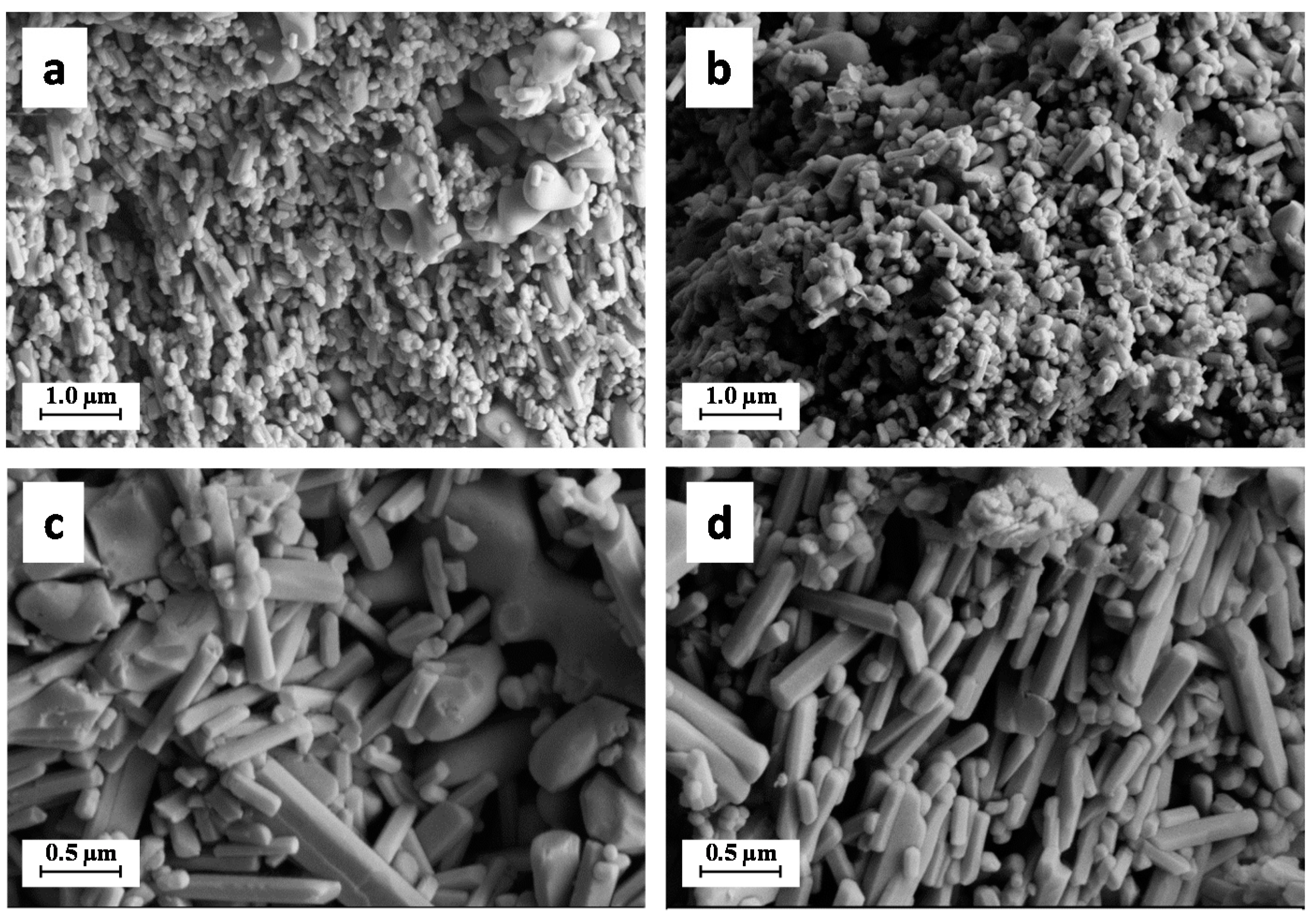


| Sample | CaP | CaP–HS |
|---|---|---|
| HA (wt.%) a | 42.8 ± 1.7 | 42.6 ± 1.3 |
| β-TCP (wt.%)a | 57.1 ± 1.7 | 57.4 ± 1.2 |
| CI (a.u.) a | 61.4 ± 0.5 | 61.2 ± 0.5 |
| Ca (wt.%) b | 34.0 ± 0.3 | 33.5 ± 0.6 |
| P (wt.%)b | 18.7 ± 0.2 | 18.3 ± 0.4 |
| K (wt.%) b | 1.64 ± 0.02 | 1.11 ± 0.01 |
| Mg (wt.%) b | 0.69 ± 0.01 | 0.67 ± 0.01 |
| Na (wt.%) b | 1.80 ± 0.34 | 1.26 ± 0.03 |
| Ca/P molar ratio b | 1.41 ± 0.01 | 1.41 ± 0.01 |
| Humic acid (wt.%) c | - | 0.4 |
| ζ-potential d | −23.0 ± 1.0 | −29.5 ± 0.2 |
| Treatments | Root Length | Roots DW | Shoot ATP | Shoot DW |
|---|---|---|---|---|
| (mm plant−1) | (mg plant−1) | (nmol g−1 DW) | (mg plant−1) | |
| Ctrl | 65.0 ± 8.83 | 5.08 ± 1.75 B * | 0.094 ± 0.026 | 21.0 ± 0.75 b |
| HS | 49.3 ± 10.4 | 3.88 ± 1.72 B | 0.115 ± 0.031 | 24.7 ± 2.33 ab |
| CaP | 56.7 ± 7.39 | 7.63 ± 2.46 A | 0.093 ± 0.023 | 25.0 ± 3.83 a |
| CaP–HS | 56.4 ± 2.33 | 6.28 ± 2.56 A | 0.123 ± 0.031 | 21.3 ± 2.27 ab |
| Treatments | Root Length | Roots DW | Shoot ATP | Shoot DW |
|---|---|---|---|---|
| (mm plant−1) | (mg plant−1) | (nmol g−1 DW) | (mg plant−1) | |
| Ctrl | 45.2 ± 4.54 b * | 6.23 ± 1.46 | 0.090 ± 0.011 | 15.6 ± 1.78 b * |
| HS | 48.2 ± 4.79 a | 6.00 ± 0.84 | 0.087 ± 0.005 | 18.7 ± 0.05 a |
| CaP | 41.8 ± 4.50 b | 6.27 ± 0.77 | 0.078 ± 0.011 | 16.1 ± 1.74 b |
| CaP–HS | 54.3 ± 5.05 a | 7.73 ± 0.33 | 0.083 ± 0.006 | 17.7 ± 0.33 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamiano, A.; Fellet, G.; Vuerich, M.; Scarpin, D.; Carella, F.; Piccirillo, C.; Jeon, J.-R.; Pizzutti, A.; Marchiol, L.; Iafisco, M. Calcium Phosphate Particles Coated with Humic Substances: A Potential Plant Biostimulant from Circular Economy. Molecules 2021, 26, 2810. https://doi.org/10.3390/molecules26092810
Adamiano A, Fellet G, Vuerich M, Scarpin D, Carella F, Piccirillo C, Jeon J-R, Pizzutti A, Marchiol L, Iafisco M. Calcium Phosphate Particles Coated with Humic Substances: A Potential Plant Biostimulant from Circular Economy. Molecules. 2021; 26(9):2810. https://doi.org/10.3390/molecules26092810
Chicago/Turabian StyleAdamiano, Alessio, Guido Fellet, Marco Vuerich, Dora Scarpin, Francesca Carella, Clara Piccirillo, Jong-Rok Jeon, Alessia Pizzutti, Luca Marchiol, and Michele Iafisco. 2021. "Calcium Phosphate Particles Coated with Humic Substances: A Potential Plant Biostimulant from Circular Economy" Molecules 26, no. 9: 2810. https://doi.org/10.3390/molecules26092810
APA StyleAdamiano, A., Fellet, G., Vuerich, M., Scarpin, D., Carella, F., Piccirillo, C., Jeon, J.-R., Pizzutti, A., Marchiol, L., & Iafisco, M. (2021). Calcium Phosphate Particles Coated with Humic Substances: A Potential Plant Biostimulant from Circular Economy. Molecules, 26(9), 2810. https://doi.org/10.3390/molecules26092810









