Graphene Oxide-Based Stimuli-Responsive Platforms for Biomedical Applications
Abstract
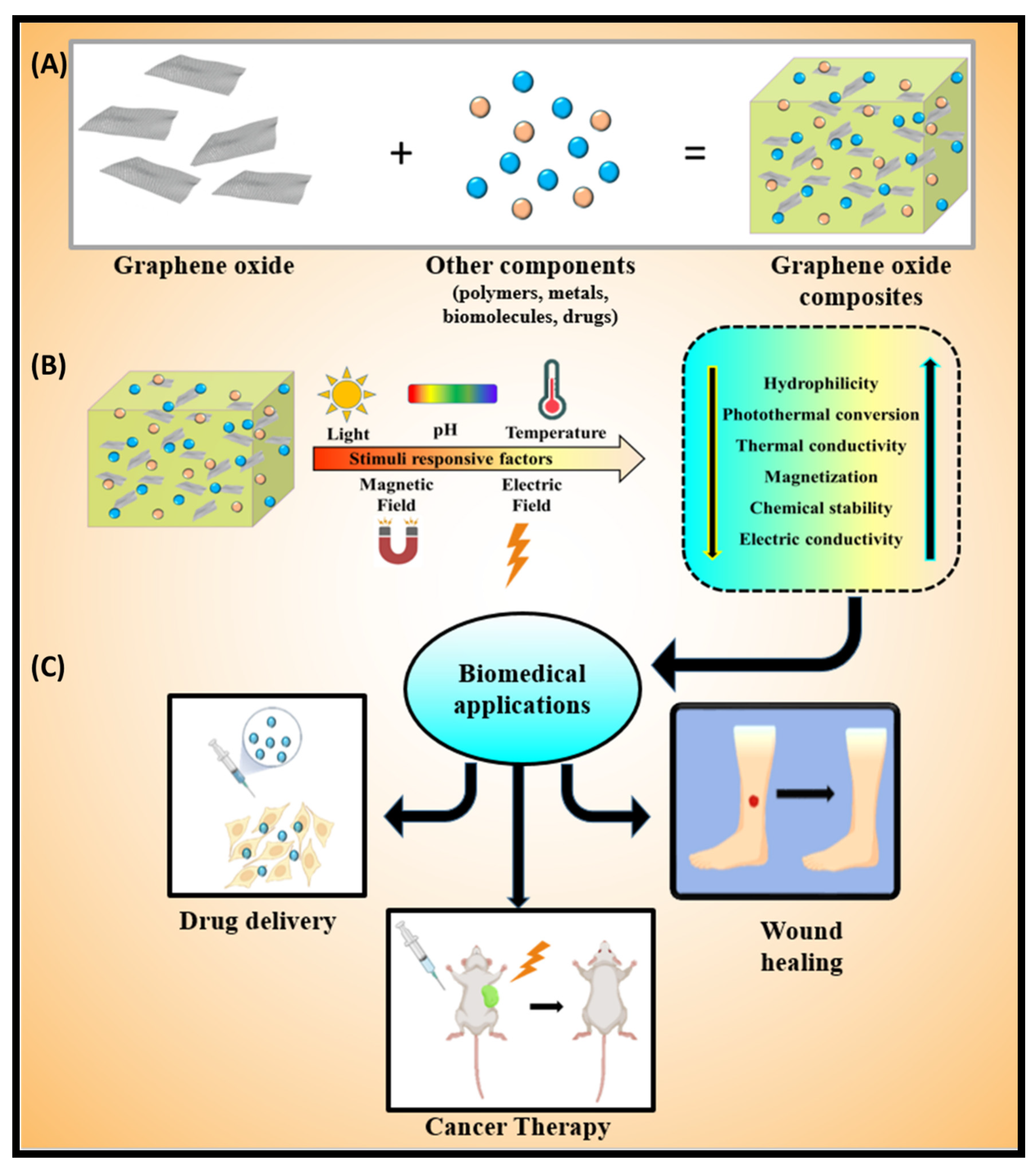
1. Introduction
2. Stimuli-Responsive Factors
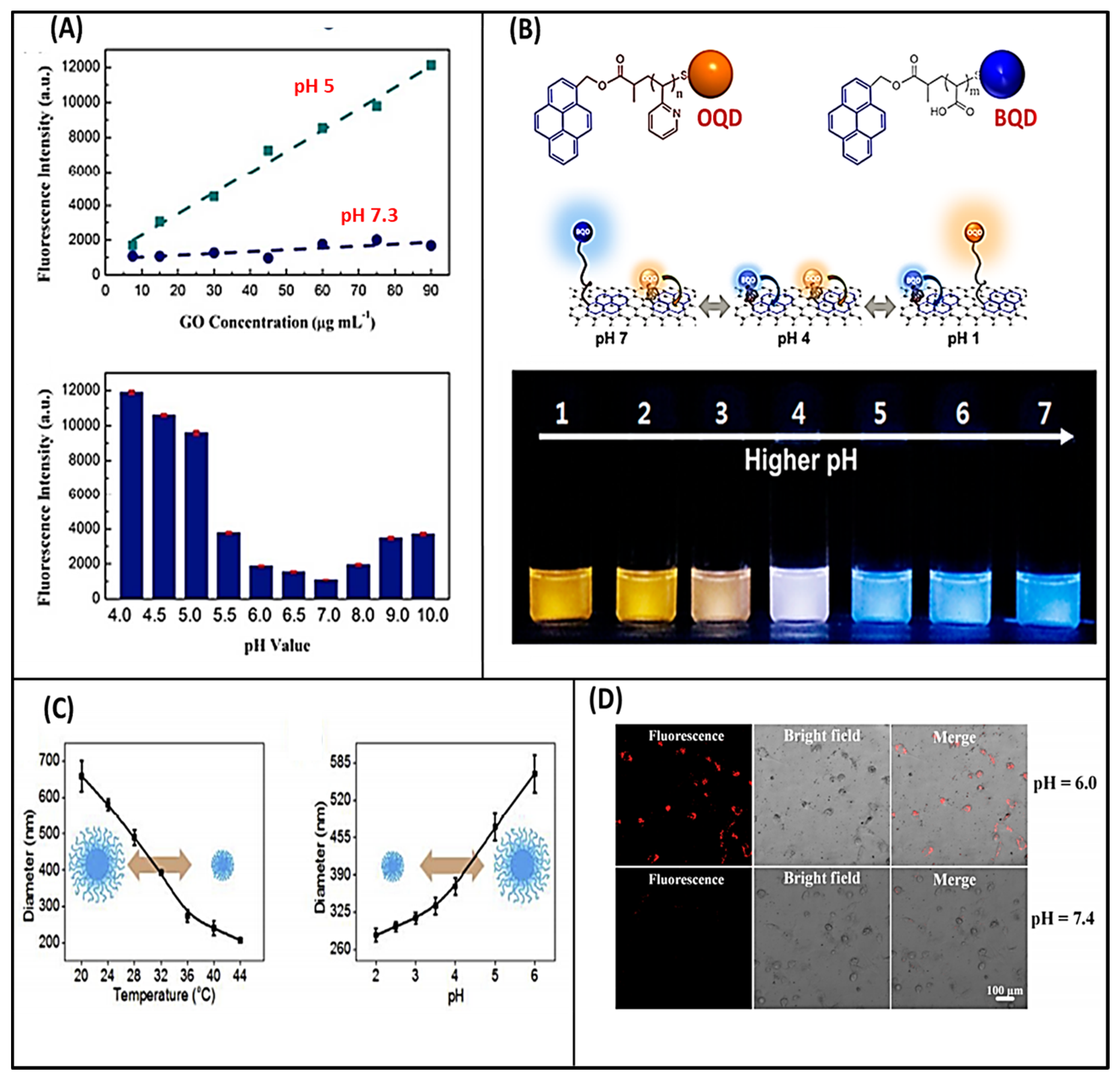
2.1. Effect of pH
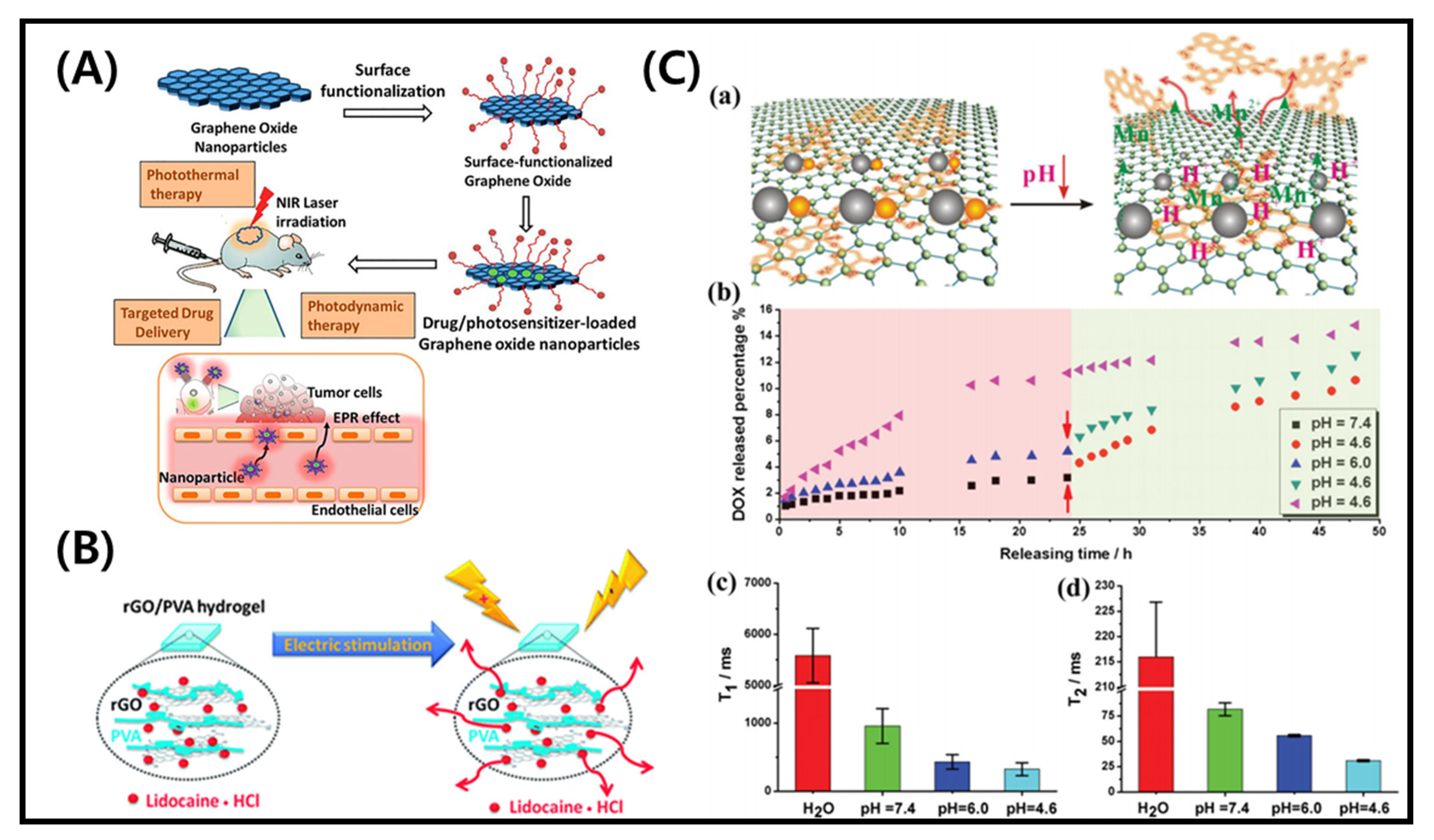
2.2. Effect of Light
2.3. Effect of Temperature
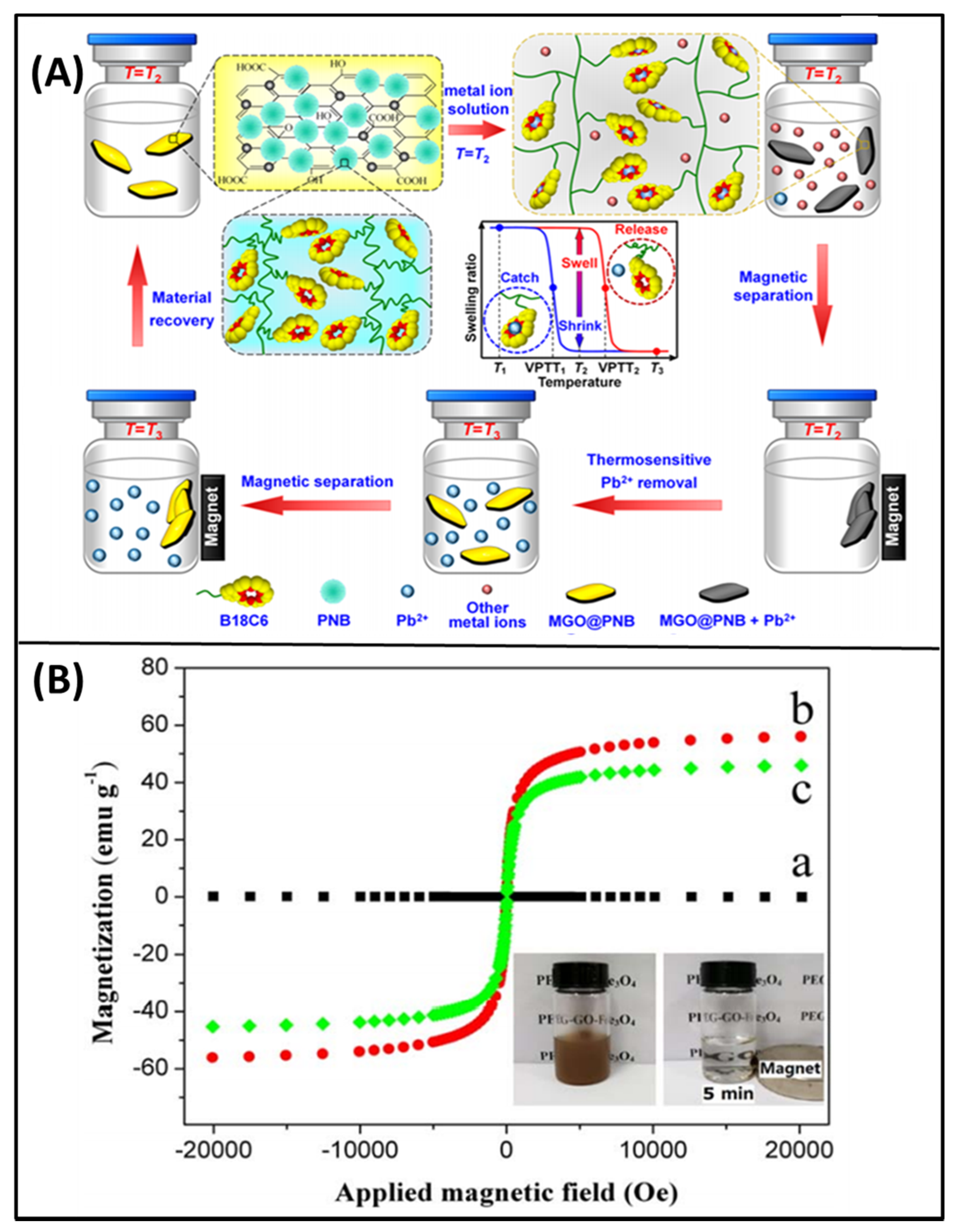
2.4. Effect of Magnetic field
2.5. Effect of Electric Field
3. Biomedical Applications
3.1. Wound Healing
3.2. Application in Cancer Therapy and Drug Delivery
4. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Liu, Y.; So, S.; Yi, H.; Park, S.; Lee, J.K.; Lim, S.C.; Yun, M. Effects of Surface Modifications to Single and Multilayer Graphene Temperature Coefficient of Resistance. ACS Appl. Mater. Interfaces 2020, 12, 48890–48898. [Google Scholar] [CrossRef]
- Liu, W.; Speranza, G. Tuning the Oxygen Content of Reduced Graphene Oxide and Effects on Its Properties. ACS Omega 2021, 6, 6195–6205. [Google Scholar] [CrossRef]
- Genorio, B.; Harrison, K.L.; Connell, J.G.; Dražić, G.; Zavadil, K.R.; Markovic, N.M.; Strmcnik, D. Tuning the Selectivity and Activity of Electrochemical Interfaces with Defective Graphene Oxide and Reduced Graphene Oxide. ACS Appl. Mater. Interfaces 2019, 11, 34517–34525. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, S.; Pooresmaeil, M.; Namazi, H. Green one-pot synthesis of carboxymethylcellulose/Zn-based metal-organic framework/graphene oxide bio-nanocomposite as a nanocarrier for drug delivery system. Carbohydr. Polym. 2019, 208, 294–301. [Google Scholar] [CrossRef]
- Yao, K.; Tan, P.; Luo, Y.; Feng, L.; Xu, L.; Liu, Z.; Li, Y.; Peng, R. Graphene Oxide Selectively Enhances Thermostability of Trypsin. ACS Appl. Mater. interfaces 2015, 7, 12270–12277. [Google Scholar] [CrossRef] [PubMed]
- Morales-Narvaez, E.; Merkoci, A. Graphene Oxide as an Optical Biosensing Platform: A Progress Report. Adv. Mater. 2019, 31, e1805043. [Google Scholar] [CrossRef] [PubMed]
- Yogesh, G.K.; Shuaib, E.P.; Roopmani, P.; Gumpu, M.B.; Krishnan, U.M.; Sastikumar, D. Synthesis, characterization and bioimaging application of laser-ablated graphene-oxide nanoparticles (nGOs). Diam. Relat. Mater. 2020, 104, 107733. [Google Scholar] [CrossRef]
- Martín, C.; Ruiz, A.; Keshavan, S.; Reina, G.; Murera, D.; Nishina, Y.; Fadeel, B.; Bianco, A. A Biodegradable Multifunctional Graphene Oxide Platform for Targeted Cancer Therapy. Adv. Funct. Mater. 2019, 29, 1901761. [Google Scholar] [CrossRef]
- Khalili, R.; Zarrintaj, P.; Jafari, S.H.; Vahabi, H.; Saeb, M.R. Electroactive poly (p-phenylene sulfide)/r-graphene oxide/chitosan as a novel potential candidate for tissue engineering. Int. J. Biol. Macromol. 2020, 154, 18–24. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Mansour, S.F.; Al-Wafi, R.; Menazea, A.A. Composition and design of nanofibrous scaffolds of Mg/Se- hydroxyapatite/graphene oxide @ ε-polycaprolactone for wound healing applications. J. Mater. Res. Technol. 2020, 9, 7472–7485. [Google Scholar] [CrossRef]
- Di Crescenzo, A.; Zara, S.; Di Nisio, C.; Ettorre, V.; Ventrella, A.; Zavan, B.; Di Profio, P.; Cataldi, A.; Fontana, A. Graphene Oxide Foils as an Osteoinductive Stem Cell Substrate. ACS Appl. Bio Mater. 2019, 2, 1643–1651. [Google Scholar] [CrossRef]
- Thebo, K.H.; Qian, X.; Zhang, Q.; Chen, L.; Cheng, H.M.; Ren, W. Highly stable graphene-oxide-based membranes with superior permeability. Nat. Commun. 2018, 9, 1486. [Google Scholar] [CrossRef]
- Pei, S.; Wei, Q.; Huang, K.; Cheng, H.M.; Ren, W. Green synthesis of graphene oxide by seconds timescale water electrolytic oxidation. Nat. Commun. 2018, 9, 145. [Google Scholar] [CrossRef]
- Lee, X.J.; Hiew, B.Y.Z.; Lai, K.C.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Rigby, S. Review on graphene and its derivatives: Synthesis methods and potential industrial implementation. J. Taiwan Inst. Chem. Eng. 2019, 98, 163–180. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Lee, Y.; Kim, D.; Rao, K.M.; Kim, J.; Park, S.; Haider, A.; Lee, D.H.; Han, S.S. Effect of crosslinking functionality on microstructure, mechanical properties, and in vitro cytocompatibility of cellulose nanocrystals reinforced poly (vinyl alcohol)/sodium alginate hybrid scaffolds. Int. J. Biol. Macromol. 2017, 95, 962–973. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Riedl, T.; Forster, M.; Klinowski, J. 13C and 1H MAS NMR studies of graphite oxide and its chemically modified derivatives. Solid State Ionics 1997, 101–103, 857–862. [Google Scholar] [CrossRef]
- Lu, Q.; Jang, H.S.; Han, W.J.; Lee, J.H.; Choi, H.J. Stimuli-Responsive Graphene Oxide-Polymer Nanocomposites. Macromol. Res. 2019, 27, 1061–1070. [Google Scholar] [CrossRef]
- Patel, D.K.; Seo, Y.R.; Lim, K.T. Stimuli-Responsive Graphene Nanohybrids for Biomedical Applications. Stem Cells Int. 2019, 17, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Tolvanen, J.; Kilpijärvi, J.; Pitkänen, O.; Hannu, J.; Jantunen, H. Stretchable Sensors with Tunability and Single Stimuli-Responsiveness through Resistivity Switching Under Compressive Stress. ACS Appl. Mater. Interfaces 2020, 12, 14433–14442. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-Y.; Liu, Y.; Jiang, H.-B.; Xue, J.-Z.; Yu, Z.-P.; Li, S.-Y.; Han, Z.-W.; Ren, L.-Q. Janus Soft Actuators with On–Off Switchable Behaviors for Controllable Manipulation Driven by Oil. ACS Appl. Mater. Interfaces 2019, 11, 13742–13751. [Google Scholar] [CrossRef] [PubMed]
- Abu-Thabit, N.Y.; Hamdy, A.S. Stimuli-responsive Polyelectrolyte Multilayers for fabrication of self-healing coatings—A review. Surface and Coatings Technology 2016, 303, 406–424. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chi-leung Hui, P. Review of Stimuli-Responsive Polymers in Drug Delivery and Textile Application. Molecules 2019, 24, 2547. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Hu, L.; Shen, Q.; Jiang, L.; Zhang, Q.; Serpe, M.J. Stimuli-responsive polymer-based systems for diagnostic applications. J. Mater. Chem. B 2020, 8, 7042–7061. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Yoon, C. Advances in Stimuli-Responsive Soft Robots with Integrated Hybrid Materials. Actuators 2020, 9, 115. [Google Scholar] [CrossRef]
- Li, Z.; Yin, Y. Stimuli-Responsive Optical Nanomaterials. Adv. Mater. 2019, 31, 1807061. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Shih, C.J.; Lin, S.; Sharma, R.; Strano, M.S.; Blankschtein, D. Understanding the pH-dependent behavior of graphene oxide aqueous solutions: A comparative experimental and molecular dynamics simulation study. Langmuir ACS J. Surf. Colloids 2012, 28, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Liang, J.; He, S.; Luan, T.; Yu, J.; Zhao, H.; Xu, J.; Tian, L. pH-Responsive Graphene Oxide–DNA Nanosystem for Live Cell Imaging and Detection. Anal. Chem. 2017, 89, 5445–5452. [Google Scholar] [CrossRef]
- Samanta, D.; Ebrahimi, S.B.; Kusmierz, C.D.; Cheng, H.F.; Mirkin, C.A. Protein Spherical Nucleic Acids for Live-Cell Chemical Analysis. J. Am. Chem. Soc. 2020, 142, 13350–13355. [Google Scholar] [CrossRef]
- Paek, K.; Yang, H.; Lee, J.; Park, J.; Kim, B.J. Efficient Colorimetric pH Sensor Based on Responsive Polymer–Quantum Dot Integrated Graphene Oxide. ACS Nano 2014, 8, 2848–2856. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, J.; Hao, L.; Jiang, Y.; van der Bruggen, B.; Sotto, A.; Gao, C.; Shen, J. Thermo- and pH-responsive graphene oxide membranes with tunable nanochannels for water gating and permeability of small molecules. J. Membr. Sci. 2019, 587, 117163. [Google Scholar] [CrossRef]
- Lin, B.; Chen, H.; Liang, D.; Lin, W.; Qi, X.; Liu, H.; Deng, X. Acidic pH and High-H2O2 Dual Tumor Microenvironment-Responsive Nanocatalytic Graphene Oxide for Cancer Selective Therapy and Recognition. ACS Appl. Mater. Interfaces 2019, 11, 11157–11166. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Chithra Sekhar, V.; Athira, V.S. Graphene oxide based functionalized chitosan polyelectrolyte nanocomposite for targeted and pH responsive drug delivery. Int. J. Biol. Macromol. 2020, 150, 468–479. [Google Scholar] [CrossRef]
- Hsieh, C.J.; Chen, Y.C.; Hsieh, P.Y.; Liu, S.R.; Wu, S.P.; Hsieh, Y.Z.; Hsu, H.Y. Graphene Oxide Based Nanocarrier Combined with a pH-Sensitive Tracer: A Vehicle for Concurrent pH Sensing and pH-Responsive Oligonucleotide Delivery. ACS Appl. Mater. Interfaces 2015, 7, 11467–11475. [Google Scholar] [CrossRef]
- Chen, R.J.; Zhang, Y. Controlled Precipitation of Solubilized Carbon Nanotubes by Delamination of DNA. J. Phys. Chem. B 2006, 110, 54–57. [Google Scholar] [CrossRef]
- Acik, M.; Lee, G.; Mattevi, C.; Chhowalla, M.; Cho, K.; Chabal, Y.J. Unusual infrared-absorption mechanism in thermally reduced graphene oxide. Nat. Mater. 2010, 9, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Xu, Y.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.; Li, F.; Guo, T.; Chen, Y. Infrared-Triggered Actuators from Graphene-Based Nanocomposites. J. Phys. Chem. C 2009, 113, 9921–9927. [Google Scholar] [CrossRef]
- Loomis, J.; Fan, X.; Khosravi, F.; Xu, P.; Fletcher, M.; Cohn, R.W.; Panchapakesan, B. Graphene/elastomer composite-based photo-thermal nanopositioners. Sci. Rep. 2013, 3, 1900. [Google Scholar] [CrossRef]
- Shuai, H.-H.; Yang, C.-Y.; Harn, H.I.C.; York, R.L.; Liao, T.-C.; Chen, W.-S.; Yeh, J.A.; Cheng, C.-M. Using surfaces to modulate the morphology and structure of attached cells—a case of cancer cells on chitosan membranes. Chem. Sci. 2013, 4, 3058–3067. [Google Scholar] [CrossRef]
- Tong, Y.; Shao, L.; Li, X.; Lu, J.; Sun, H.; Xiang, S.; Zhang, Z.; Wu, Y.; Wu, X. Adhesive and Stimulus-Responsive Polydopamine-Coated Graphene Oxide System for Pesticide-Loss Control. J. Agric. Food Chem. 2018, 66, 2616–2622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, X.; Tan, L.; Cui, Z.; Li, Z.; Liang, Y.; Zhu, S.; Yeung, K.W.K.; Zheng, Y.; Wu, S. An UV to NIR-driven platform based on red phosphorus/graphene oxide film for rapid microbial inactivation. Chem. Eng. J. 2020, 383, 123088. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, J.; Liu, Y.; Xu, Y.; Zhang, A. NIR-UV Responsive Actuator with Graphene Oxide/Microchannel-Induced Liquid Crystal Bilayer Structure for Biomimetic Devices. ACS Appl. Mater. Interfaces 2020, 12, 6727–6735. [Google Scholar] [CrossRef]
- Xie, J.; Huang, L.; Wang, R.; Ye, S.; Song, X. Novel visible light-responsive graphene oxide/Bi2WO6/starch composite membrane for efficient degradation of ethylene. Carbohydr. Polym. 2020, 246, 116640. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Andres, C.M.; Xu, J.; Ramamoorthy, A.; Tsotsis, T.; Kotov, N.A. Pseudonegative Thermal Expansion and the State of Water in Graphene Oxide Layered Assemblies. ACS Nano 2012, 6, 8357–8365. [Google Scholar] [CrossRef]
- Lima-Sousa, R.; de Melo-Diogo, D.; Alves, C.G.; Cabral, C.S.D.; Miguel, S.P.; Mendonca, A.G.; Correia, I.J. Injectable in situ forming thermo-responsive graphene based hydrogels for cancer chemo-photothermal therapy and NIR light-enhanced antibacterial applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111294. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, G.; Huang, X.; Li, Y.; Cao, F.; Chen, H.; Liu, W. Thermo-Responsive Graphene Oxide/Poly(Ethyl Ethylene Phosphate) Nanocomposite via Ring Opening Polymerization. Nanomaterials 2019, 9, 207. [Google Scholar] [CrossRef]
- Ganguli, S.; Roy, A.K.; Anderson, D.P. Improved thermal conductivity for chemically functionalized exfoliated graphite/epoxy composites. Carbon 2008, 46, 806–817. [Google Scholar] [CrossRef]
- Yu, A.; Ramesh, P.; Sun, X.; Bekyarova, E.; Itkis, M.E.; Haddon, R.C. Enhanced Thermal Conductivity in a Hybrid Graphite Nanoplatelet—Carbon Nanotube Filler for Epoxy Composites. Adv. Mater. 2008, 20, 4740–4744. [Google Scholar] [CrossRef]
- Liang, J.; Chen, B.; Hu, J.; Huang, Q.; Zhang, D.; Wan, J.; Hu, Z.; Wang, B. pH and Thermal Dual-Responsive Graphene Oxide Nanocomplexes for Targeted Drug Delivery and Photothermal-Chemo/Photodynamic Synergetic Therapy. ACS Appl. Biol. Mater. 2019, 2, 5859–5871. [Google Scholar] [CrossRef]
- Loo, A.H.; Sofer, Z.; Bouša, D.; Ulbrich, P.; Bonanni, A.; Pumera, M. Carboxylic Carbon Quantum Dots as a Fluorescent Sensing Platform for DNA Detection. ACS Appl. Mater. Interfaces 2016, 8, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Lingamdinne, L.P.; Koduru, J.R.; Karri, R.R. A comprehensive review of applications of magnetic graphene oxide based nanocomposites for sustainable water purification. J. Environ. Manag. 2019, 231, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Ma, Y.; Huang, Y.; Wang, Y.; Chen, Y. Superparamagnetic graphene oxide–Fe3O4 nanoparticles hybrid for controlled targeted drug carriers. J. Mater. Chem. 2009, 19, 2710. [Google Scholar] [CrossRef]
- Tang, T.; Liu, F.; Liu, Y.; Li, X.; Xu, Q.; Feng, Q.; Tang, N.; Du, Y. Identifying the magnetic properties of graphene oxide. Appl. Phys. Lett. 2014, 104, 123104. [Google Scholar] [CrossRef]
- Liu, M.; Lu, Y.; Yu, Q.; Yu, S.-H. In situ assembly of magnetic nanocrystals/graphene oxide nanosheets on tumor cells enables efficient cancer therapy. Nano Res. 2020, 13, 1133–1140. [Google Scholar] [CrossRef]
- Pan, L.; Zhai, G.; Yang, X.; Yu, H.; Cheng, C. Thermosensitive Microgels-Decorated Magnetic Graphene Oxides for Specific Recognition and Adsorption of Pb(II) from Aqueous Solution. ACS Omega 2019, 4, 3933–3945. [Google Scholar] [CrossRef]
- Qi, J.; Chen, Y.; Xue, T.; Lin, Y.; Huang, S.; Cao, S.; Wang, X.; Su, Y.; Lin, Z. Graphene oxide-based magnetic nanocomposites for the delivery of melittin to cervical cancer HeLa cells. Nanotechnology 2020, 31, 065102. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Müller, M.B.; Gilmore, K.J.; Wallace, G.G.; Li, D. Mechanically Strong, Electrically Conductive, and Biocompatible Graphene Paper. Adv. Mater. 2008, 20, 3557–3561. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Zheng, W.-G.; Yan, Q.; Yang, Y.; Wang, J.-W.; Lu, Z.-H.; Ji, G.-Y.; Yu, Z.-Z. Electrically conductive polyethylene terephthalate/graphene nanocomposites prepared by melt compounding. Polymer 2010, 51, 1191–1196. [Google Scholar] [CrossRef]
- Marsden, A.J.; Papageorgiou, D.G.; Vallés, C.; Liscio, A.; Palermo, V.; Bissett, M.A.; Young, R.J.; Kinloch, I.A. Electrical percolation in graphene–polymer composites. 2D Mater. 2018, 5, 032003. [Google Scholar] [CrossRef]
- Weaver, C.L.; LaRosa, J.M.; Luo, X.; Cui, X.T. Electrically Controlled Drug Delivery from Graphene Oxide Nanocomposite Films. ACS Nano 2014, 8, 1834–1843. [Google Scholar] [CrossRef] [PubMed]
- di Luca, M.; Vittorio, O.; Cirillo, G.; Curcio, M.; Czuban, M.; Voli, F.; Farfalla, A.; Hampel, S.; Nicoletta, F.P.; Iemma, F. Electro-responsive graphene oxide hydrogels for skin bandages: The outcome of gelatin and trypsin immobilization. Int. J. Pharm. 2018, 546, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Kedambaimoole, V.; Kumar, N.; Shirhatti, V.; Nuthalapati, S.; Nayak, M.M.; Konandur, R. Electric Spark Induced Instantaneous and Selective Reduction of Graphene Oxide on Textile for Wearable Electronics. ACS Appl. Mater. Interfaces 2020, 12, 15527–15537. [Google Scholar] [CrossRef]
- Yun, Y.; Wu, H.; Gao, J.; Dai, W.; Deng, L.; Lv, O.; Kong, Y. Facile synthesis of Ca(2+)-crosslinked sodium alginate/graphene oxide hybrids as electro- and pH-responsive drug carrier. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110380. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for wound healing: Scope and advancement. Nanomed. Nanotechnol. Biol. Med. 2015, 10, 2593–2612. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, M.; Zhang, Z.; Ren, G.; Liu, Y.; Wu, S.; Shen, J. Facile synthesis of ZnO QDs@GO-CS hydrogel for synergetic antibacterial applications and enhanced wound healing. Chem. Eng. J. 2019, 378, 122043. [Google Scholar] [CrossRef]
- You, D.; Li, K.; Guo, W.; Zhao, G.; Fu, C. Poly (lactic-co-glycolic acid)/graphene oxide composites combined with electrical stimulation in wound healing: Preparation and characterization. Int. J. Nanomed. 2019, 14, 7039–7052. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Zheng, Y.; Yeung, K.W.K.; Chu, P.K.; Wu, S. Rapid Sterilization and Accelerated Wound Healing Using Zn2+ and Graphene Oxide Modified g-C3N4 under Dual Light Irradiation. Adv. Funct. Mater. 2018, 28, 1800299. [Google Scholar] [CrossRef]
- Zhong, X.; Tong, C.; Liu, T.; Li, L.; Liu, X.; Yang, Y.; Liu, R.; Liu, B. Silver nanoparticles coated by green graphene quantum dots for accelerating the healing of MRSA-infected wounds. Biomater. Sci. 2020, 8, 6670–6682. [Google Scholar] [CrossRef]
- Cibecchini, G.; Veronesi, M.; Catelani, T.; Bandiera, T.; Guarnieri, D.; Pompa, P.P. Antiangiogenic Effect of Graphene Oxide in Primary Human Endothelial Cells. ACS Appl. Mater Interfaces 2020, 12, 22507–22518. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.R.u.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Hasan, A. Graphene Oxide Loaded Hydrogel for Enhanced Wound Healing in Diabetic Patients. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 3943–3946. [Google Scholar]
- Low, Y.Z.; Li, L.; Tan, L.P. Investigating the Behavior of Mucoadhesive Polysaccharide-Functionalized Graphene Oxide in Bladder Environment. ACS Appl. Biol. Mater. 2021, 4, 630–639. [Google Scholar] [CrossRef]
- Tang, P.; Han, L.; Li, P.; Jia, Z.; Wang, K.; Zhang, H.; Tan, H.; Guo, T.; Lu, X. Mussel-Inspired Electroactive and Antioxidative Scaffolds with Incorporation of Polydopamine-Reduced Graphene Oxide for Enhancing Skin Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 7703–7714. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, Y.; Chu, J.; Wang, X.; Yan, W.; Zhang, Q.; Liu, H. Reduced Graphene Oxide Incorporated Acellular Dermal Composite Scaffold Enables Efficient Local Delivery of Mesenchymal Stem Cells for Accelerating Diabetic Wound Healing. ACS Biol. Sci. Eng. 2019, 5, 4054–4066. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Liu, B.; Wang, J.; Zhang, S.; Lin, Q.; Gong, P.; Ma, L.; Yang, S. A Novel Wound Dressing Based on Ag/Graphene Polymer Hydrogel: Effectively Kill Bacteria and Accelerate Wound Healing. Adv. Funct. Mater. 2014, 24, 3933–3943. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Han, D.; Kong, L.; Wang, J.; Zhao, M.; Cheng, W.; Ju, H.; Yang, Z.; Ding, S. Eco-Friendly Preparation of Epoxy-Rich Graphene Oxide for Wound Healing. ACS Biomater. Sci. Eng. 2021, 7, 752–763. [Google Scholar] [CrossRef]
- Zhang, B.; He, J.; Shi, M.; Liang, Y.; Guo, B. Injectable self-healing supramolecular hydrogels with conductivity and photo-thermal antibacterial activity to enhance complete skin regeneration. Chem. Eng. J. 2020, 400, 125994. [Google Scholar] [CrossRef]
- Hussein, K.H.; Abdelhamid, H.N.; Zou, X.; Woo, H.-M. Ultrasonicated graphene oxide enhances bone and skin wound regeneration. Mater. Sci. Eng. C 2019, 94, 484–492. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Karimi, Y.; Naghieh, S.; Alizadeh Sardroud, H.; Gorji, M.; Chen, X. Electrospinning of Scaffolds from the Polycaprolactone/Polyurethane Composite with Graphene Oxide for Skin Tissue Engineering. Appl. Biochem. Biotechnol. 2020, 191, 567–578. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Zhao, Z.; Sun, L.; Zou, M.; Ren, J.A.; Zhao, Y. Responsive graphene oxide hydrogel microcarriers for controllable cell capture and release. Sci. China Mater. 2018, 61, 1314–1324. [Google Scholar] [CrossRef]
- Weng, Z.; Yu, F.; Leng, Q.; Zhao, S.; Xu, Y.; Zhang, W.; Zhu, Z.; Ye, J.; Wei, Q.; Wang, X. Electrical and visible light dual-responsive ZnO nanocomposite with multiple wound healing capability. Mater. Sci. Eng. C 2021, 124, 112066. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Song, L.; Fan, Y.; Tian, L.; Luan, S.; Niu, S.; Ren, L.; Ming, W.; Zhao, J. Synergistic Photodynamic and Photothermal Antibacterial Nanocomposite Membrane Triggered by Single NIR Light Source. ACS Appl. Mater. Interfaces 2019, 11, 26581–26589. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, C.; Xing, Y.; Fang, W.; Ren, J.; Yu, H.; Wang, G. NIR-Light- and pH-Responsive Graphene Oxide Hybrid Cyclodextrin-Based Supramolecular Hydrogels. Langmuir ACS J. Surf. Colloids 2019, 35, 1021–1031. [Google Scholar] [CrossRef]
- Altinbasak, I.; Jijie, R.; Barras, A.; Golba, B.; Sanyal, R.; Bouckaert, J.; Drider, D.; Bilyy, R.; Dumych, T.; Paryzhak, S.; et al. Reduced Graphene-Oxide-Embedded Polymeric Nanofiber Mats: An “On-Demand” Photothermally Triggered Antibiotic Release Platform. ACS Appl. Mater. Interfaces 2018, 10, 41098–41106. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Du, Y.; Wang, Z.; Wang, H.; Pu, F.; Ren, J.; Qu, X. Hyaluronic Acid-Templated Ag Nanoparticles/Graphene Oxide Composites for Synergistic Therapy of Bacteria Infection. ACS Appl. Mater. Interfaces 2017, 9, 19717–19724. [Google Scholar] [CrossRef]
- Jia, R.; Teng, L.; Gao, L.; Su, T.; Fu, L.; Qiu, Z.; Bi, Y. Advances in Multiple Stimuli-Responsive Drug-Delivery Systems for Cancer Therapy. Int. J. Nanomed. 2021, 16, 1525–1551. [Google Scholar] [CrossRef]
- Devnarain, N.; Osman, N.; Fasiku, V.O.; Makhathini, S.; Salih, M.; Ibrahim, U.H.; Govender, T. Intrinsic stimuli-responsive nanocarriers for smart drug delivery of antibacterial agents—An in-depth review of the last two decades. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1664. [Google Scholar] [CrossRef]
- He, S.; Li, J.; Chen, M.; Deng, L.; Yang, Y.; Zeng, Z.; Xiong, W.; Wu, X. Graphene Oxide-Template Gold Nanosheets as Highly Efficient Near-Infrared Hyperthermia Agents for Cancer Therapy. Int. J. Nanomed. 2020, 15, 8451–8463. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, Z.; Li, H.; Hao, Y.; Liu, C.; Zhu, L.; Liu, J.; Lu, B.; Li, R. Multifunctional Nanographene Oxide for Targeted Gene-Mediated Thermochemotherapy of Drug-resistant Tumour. Sci. Rep. 2017, 7, 43506. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. Fabrication of a smart and biocompatible brush copolymer decorated on magnetic graphene oxide hybrid nanostructure for drug delivery application. Eur. Polym. J. 2021, 142, 110126. [Google Scholar] [CrossRef]
- Borandeh, S.; Hosseinbeigi, H.; Abolmaali, S.S.; Monajati, M.; Tamaddon, A.M. Steric stabilization of β-cyclodextrin functionalized graphene oxide by host-guest chemistry: A versatile supramolecule for dual-stimuli responsive cellular delivery of doxorubicin. J. Drug Deliv. Sci. Technol. 2021, 63, 102536. [Google Scholar] [CrossRef]
- Liu, H.-W.; Hu, S.-H.; Chen, Y.-W.; Chen, S.-Y. Characterization and drug release behavior of highly responsive chip-like electrically modulated reduced graphene oxide–poly(vinyl alcohol) membranes. J. Mater. Chem. 2012, 22, 17311–17320. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, P.; Shu, Z.; Wu, M.; Wang, L.; Zhang, S.; Zheng, Y.; Chen, H.; Wang, J.; Li, Y.; et al. Multifunctional Graphene Oxide-based Triple Stimuli-Responsive Nanotheranostics. Adv. Funct. Mater. 2014, 24, 4386–4396. [Google Scholar] [CrossRef]
- Ashjaran, M.; Babazadeh, M.; Akbarzadeh, A.; Davaran, S.; Salehi, R. Stimuli-responsive polyvinylpyrrolidone-NIPPAm-lysine graphene oxide nano-hybrid as an anticancer drug delivery on MCF7 cell line. Artif. Cells Nanomed. Biotechnol. 2019, 47, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Vinothini, K.; Rajendran, N.K.; Rajan, M.; Ramu, A.; Marraiki, N.; Elgorban, A.M. A magnetic nanoparticle functionalized reduced graphene oxide-based drug carrier system for a chemo-photodynamic cancer therapy. New J. Chem. 2020, 44, 5265–5277. [Google Scholar] [CrossRef]
- Sharma, H.; Mondal, S. Functionalized Graphene Oxide for Chemotherapeutic Drug Delivery and Cancer Treatment: A Promising Material in Nanomedicine. Int. J. Mol. Sci. 2020, 21, 6280. [Google Scholar] [CrossRef]
- Magaz, A.; Ashton, M.D.; Hathout, R.M.; Li, X.; Hardy, J.G.; Blaker, J.J. Electroresponsive Silk-Based Biohybrid Composites for Electrochemically Controlled Growth Factor Delivery. Pharmaceutics 2020, 12, 742. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Maity, P.P.; Mondal, S.; Ghosh, S.; Dhara, S.; Das, N.C. Green Reduced Graphene Oxide Toughened Semi-IPN Monolith Hydrogel as Dual Responsive Drug Release System: Rheological, Physicomechanical, and Electrical Evaluations. J. Phys. Chem. B 2018, 122, 7201–7218. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, N.; Peng, H.; Li, J.; Yan, R.; Shi, X.; Ma, P.; Lv, M.; Wang, L.; Tang, Z.; et al. Multi-triggered and enzyme-mimicking graphene oxide/polyvinyl alcohol/G-quartet supramolecular hydrogels. Nanoscale 2020, 12, 5186–5195. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cao, C.; Gao, J.; Li, K.; Kang, J.; Wu, D.; Kong, Y. Dual stimuli-responsive nanoplatform based on core-shell structured graphene oxide/mesoporous silica@alginate. Int. J. Biol. Macromol. 2021, 175, 209–216. [Google Scholar] [CrossRef]
- Jiang, W.; Mo, F.; Lin, Y.; Wang, X.; Xu, L.; Fu, F. Tumor targeting dual stimuli responsive controllable release nanoplatform based on DNA-conjugated reduced graphene oxide for chemo-photothermal synergetic cancer therapy. J. Mater. Chem. B 2018, 6, 4360–4367. [Google Scholar] [CrossRef]
- Poudel, K.; Banstola, A.; Tran, T.H.; Thapa, R.K.; Gautam, M.; Ou, W.; Pham, L.M.; Maharjan, S.; Jeong, J.-H.; Ku, S.K.; et al. Hyaluronic acid wreathed, trio-stimuli receptive and on-demand triggerable nanoconstruct for anchored combinatorial cancer therapy. Carbohydr. Polym. 2020, 249, 116815. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, Y.; Du, J.; Wang, X.; Duan, A.; Gao, R.; Liu, J.; Li, B. Graphene oxide loaded with tumor-targeted peptide and anti-cancer drugs for cancer target therapy. Sci. Rep. 2021, 11, 1725. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, M.; Luo, T.; Qu, J.; Chen, W.R. Photo-activated chemo-immunotherapy for metastatic cancer using a synergistic graphene nanosystem. Biomaterials 2021, 265, 120421. [Google Scholar] [CrossRef]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.; Liu, Z. Photothermally Enhanced Photodynamic Therapy Delivered by Nano-Graphene Oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef] [PubMed]
- Abbasian, M.; Roudi, M.M.; Mahmoodzadeh, F.; Eskandani, M.; Jaymand, M. Chitosan-grafted-poly(methacrylic acid)/graphene oxide nanocomposite as a pH-responsive de novo cancer chemotherapy nanosystem. Int. J. Biol. Macromol. 2018, 118, 1871–1879. [Google Scholar] [CrossRef]
- He, D.; He, X.; Wang, K.; Zou, Z.; Yang, X.; Li, X. Remote-Controlled Drug Release from Graphene Oxide-Capped Mesoporous Silica to Cancer Cells by Photoinduced pH-Jump Activation. Langmuir ACS J. Surf. Colloids 2014, 30, 7182–7189. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Bao, H.; Sahoo, N.G.; Wu, T.; Li, L. Water-Soluble Poly(N-isopropylacrylamide)–Graphene Sheets Synthesized via Click Chemistry for Drug Delivery. Adv. Funct. Mater. 2011, 21, 2754–2763. [Google Scholar] [CrossRef]
- Guo, M.; Huang, J.; Deng, Y.; Shen, H.; Ma, Y.; Zhang, M.; Zhu, A.; Li, Y.; Hui, H.; Wang, Y.; et al. pH-Responsive Cyanine-Grafted Graphene Oxide for Fluorescence Resonance Energy Transfer-Enhanced Photothermal Therapy. Adv. Funct. Mater. 2015, 25, 59–67. [Google Scholar] [CrossRef]
- Fong, Y.T.; Chen, C.-H.; Chen, J.-P. Intratumoral Delivery of Doxorubicin on Folate-Conjugated Graphene Oxide by In-Situ Forming Thermo-Sensitive Hydrogel for Breast Cancer Therapy. Nanomaterials 2017, 7, 388. [Google Scholar] [CrossRef] [PubMed]


| Graphene Composite | Stimuli Response | Cell Line | Therapeutic Application | Reference |
|---|---|---|---|---|
| GO-CS-QDZnO | Electro/photothermal | NIH-3T3 | Antibacterial activity and wound healing | [69] |
| GO-PLGA | Electro | Balb/c3T3 | Wound repair | [70] |
| GO-SCN-Zn2+ | NIR and Visible light | NIH3T3 | Rapid sterilization and wound healing | [71] |
| GQD-AgNP | pH | NIH3T3 | MRSA- infected wound healing | [72] |
| GO | pH | HeLa | Angiogenesis | [73] |
| GO-GelMA | UV light | HaCaT keratinocyte cells | Proliferation and migration of cells in diabetic patients | [74] |
| GO-CS, GO-CMC | pH | Porcine bladder mucosa | Mucoadhesion to porcine bladder tissues | [75] |
| pGO-CS/SF | electro | C2C12 myoblast cells | ROS scavenging, cell growth | [76] |
| rGO-ADM | pH | MSCs | Rapid re-epithelialization in Diabetic wound healing | [77] |
| G-Ag | pH | L929 | Accelerate healing | [78] |
| erGO | -- | Mice skin, human epithelial cells | Wound healing of mice with skin infection | [79] |
| GO-AD-CD-QCS | photothermal | L929 | Antibacterial activity | [80] |
| Ultrasonicated GO | Sonication | EA.hy 926 and hFOB | Bone and skin healing | [81] |
| GO-PU-PCL | pH | Human skin fibroblast cells | Skin tissue engineering | [82] |
| GO-pNIPAM-GelMA | NIR light | HepG2, Hepa1-6 | Prevent cells from immune system attack | [83] |
| Graphene Composite | Stimuli Response | Therapeutic Applications | Reference |
|---|---|---|---|
| rGO-SF | Electro | Neural tissue engineering | [100] |
| k-carrageenan-rGO | Thermo | Inhibitory effects on human osteosarcoma cells (MG63) | [101] |
| GO-PVA | pH | Peroxide (H2O2) sensing | [102] |
| GO-mSiO2-Alginate | pH and thermal | pH and NIR-responsive burst release of MTX showed higher cytotoxicity on liver hepatoma cells (HepG2) | [103] |
| rGO-PDA | pH and photothermal | Multimodal cancer therapy | [104] |
| γ-Fe2O3@GO | Magnetism-driven assembly | Sharp tumor inhibition in Adenocarcinomic human alveolar basal epithelial cells (A549 cells) | [56] |
| CuS(DOX)-GO-HA | Chemo–photo responsive | Sharp tumor inhibition in CD44 overexpressing tumor cells (SCC-7, MDA-MB-231, BT-474) | [105] |
| DOX@NGO-PEG | pH | OSCC target delivery and improved anti-cancer medicine efficiency | [106] |
| rGO/MTX/SB | NIR light | Triggered host-antitumor immunity in 4T1 mouse mammary tumor model | [107] |
| GO-PEG | pH | Chlorin e6 drug delivery | [108] |
| GO/PMAA-g-CS | pH | Breast cancer chemotherapy | [109] |
| GO-silica | UV light | Doxorubicin drug delivery | [110] |
| GO-PNIPAM | Thermo | Camptothecin drug delivery | [111] |
| GO-cypate | Photothermal | Breast cancer therapy | [112] |
| GO-FA-HACPN | Thermal | Doxorubicin drug delivery for breast cancer treatment | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Lim, K.-T. Graphene Oxide-Based Stimuli-Responsive Platforms for Biomedical Applications. Molecules 2021, 26, 2797. https://doi.org/10.3390/molecules26092797
Patil TV, Patel DK, Dutta SD, Ganguly K, Lim K-T. Graphene Oxide-Based Stimuli-Responsive Platforms for Biomedical Applications. Molecules. 2021; 26(9):2797. https://doi.org/10.3390/molecules26092797
Chicago/Turabian StylePatil, Tejal V., Dinesh K. Patel, Sayan Deb Dutta, Keya Ganguly, and Ki-Taek Lim. 2021. "Graphene Oxide-Based Stimuli-Responsive Platforms for Biomedical Applications" Molecules 26, no. 9: 2797. https://doi.org/10.3390/molecules26092797
APA StylePatil, T. V., Patel, D. K., Dutta, S. D., Ganguly, K., & Lim, K.-T. (2021). Graphene Oxide-Based Stimuli-Responsive Platforms for Biomedical Applications. Molecules, 26(9), 2797. https://doi.org/10.3390/molecules26092797









