Abstract
Recent findings suggest that tumor microenvironment (TME) plays an important regulatory role in the occurrence, proliferation, and metastasis of tumors. Different from normal tissue, the condition around tumor significantly altered, including immune infiltration, compact extracellular matrix, new vasculatures, abundant enzyme, acidic pH value, and hypoxia. Increasingly, researchers focused on targeting TME to prevent tumor development and metastasis. With the development of nanotechnology and the deep research on the tumor environment, stimulation-responsive intelligent nanostructures designed based on TME have attracted much attention in the anti-tumor drug delivery system. TME-targeted nano therapeutics can regulate the distribution of drugs in the body, specifically increase the concentration of drugs in the tumor site, so as to enhance the efficacy and reduce adverse reactions, can utilize particular conditions of TME to improve the effect of tumor therapy. This paper summarizes the major components and characteristics of TME, discusses the principles and strategies of relevant nano-architectures targeting TME for the treatment and diagnosis systematically.
1. Introduction
Owing to the complex and continuously evolving tumor microenvironment (TME), cancer becomes one of the most difficult diseases to cure all over the world. The bidrectional interactions between tumor and the TME bring about the progression, therapeutic resistance, and metastasis of cancer [1]. TME composes various supporting cells such as immune cells, fibroblasts, endothelial cells, and extra components like exosomes, cytokines, enzymes, growth factors, and extracellular matrix (ECM), etc. [2,3]. In addition, the tumor microenvironment displays unique pH values, hypoxic condition, high ATP concentration, and abundant tumor microvasculature [4,5,6]. The communications between tumor cells and the microenvironment result in drug resistance by changing the phenotypes of tumor cells as well [7]. Therefore, treatment targeting the microenvironment has attracted increasing attention.
The rapid development of nanotechnology has provided a good platform for early diagnosis and more effective therapy of tumors [8]. Nanoparticles (NPs) can effectively improve the pharmacokinetic and pharmacodynamics properties of drugs and improve the therapeutic effect due to its special size, shape, and material [9]. Coated with folic acid, hyaluronic acid, and other molecules, nanoparticles can be used as good carriers concentrating drugs at the tumor site much better. Due to the high biocompatibility, good targeting property and low toxicity of organic nanomaterials, related materials have been developed in large quantities. Some organic nanomaterials like liposomes (pegylated liposomal doxorubicin, paclitaxel liposome, vincristine sulfate liposome, etc.) have been used in clinical chemotherapy very well [9]. Inorganic materials are also widely used in the preparation of nanomaterials. Mesoporous silica nanoparticles (MSNs) have great advantages in the fields of adsorption, separation, catalysis, and drug delivery [10]. Magnetic Nanoparticles (MNPs), by means of an external magnetic field, can increase the aggregation of MNPs at the tumor site and reduce the distribution in normal tissues. Furthermore, MNPs have the functions of hyperthermia and imaging, and its super paramagnetism makes it an obvious advantage as MRI (magnetic resonance imaging) contrast agent [11]. Other metal nanoparticles, such as gold nanoparticles (GNPs), can inhibit tumor angiogenesis by themselves and have photothermal effects as well [12]. However, these nanomaterials have shown great success in treating tumors and reducing adverse reactions. The presence of TME still bring limitations for nanomedicines to treat tumors. Aiming for the acidic pH, hypoxia and abundant ATP quantity conditions of TME, NPs response to different stimuli were developed, which could remove the obstacle of low accumulation with enhanced permeation and retention (EPR) effect in the tumor [13]. Nano-architectures established by virus-like particles, polymer, inorganics, micelle, self-assembled proteins, liposomes, polypeptides with suitable volume ratio, and tunable morphologies can achieve the purpose of broad spectrum, low toxicity, and low drug resistance. These NPs can not only reach the tumor site precisely, but also load much more lipophilic drug molecules by its special hollow structure, cut off the interaction between tumor cells and the microenvironment, and inhibit the proliferation of tumor cells more efficiently [14,15,16].
The role of the TME during nano-targeted tumor treatment strategies has been reviewed somewhere, however, most research just focused on part of the compositions or stimuli categories [17,18,19]. This review aims to elaborate the components and physiological conditions of TME, summarize the nano-architectures response to physiological barriers or unique constituents, and discuss the prospect of nano therapeutics in TME (Figure 1).
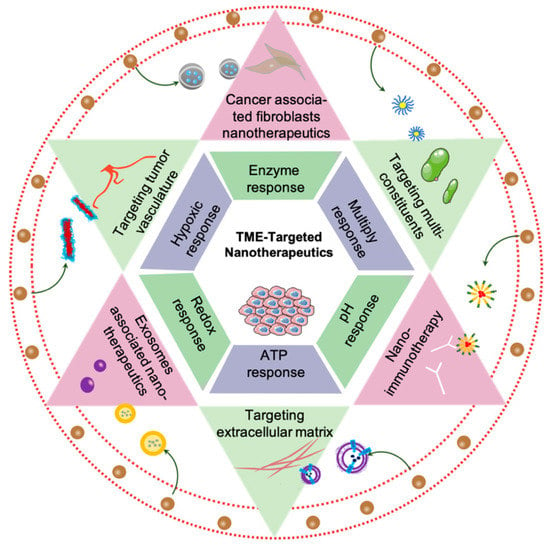
Figure 1.
Schematic of nano therapeutics target tumor microenvironment.
2. Special Characteristics of Tumor Microenvironment
2.1. Major Constituents of TME
Immune cells, ECM, cancer associated fibroblasts (CAFs), tumor vessels, exosomes and chemokines are vital constituents of TME, all of which participate in tumor progression and invasion particularly (Figure 2). Here, we will concentrate on the principles of their respective activities.

Figure 2.
The main components of the tumor microenvironment, including tumor cell, immune cells (such as B cells, T cells, DC cells, NK cells, M1/M2 macrophages), tumor vasculature, ECM, fibroblast, exosomes and cytokines.
2.1.1. Immune Cells and Chemokines
The functions of immune cells and chemokines are controversial in TME. Current research showed that there were a variety of immune cells in the inflammatory microenvironment, including adaptive immune cells as T lymphocytes (T cells) and B lymphocytes (B cells), innate immune defense cells as macrophages, natural killer (NK) cells, dendritic cells (DCs), and myeloid-derived suppressor cells (MDSCs) [20,21,22]. In the inflammatory microenvironment, the special phenomenon-“immune escape” prevents cancer cells from being recognized by killer cells such as CD8+ T cells and NK cells, making it easier for cancer cells to survive, infiltrate, and metastasize [5,23,24]. Among them, tumor associated macrophages (TAMs), regulatory T cells (Tregs) and MDSCs play vital roles in the tumor immunosuppression environment. Recruitment and differentiation of these immunosuppressive cells depend on the presence of numerous cytokines in the microenvironment [25,26,27].
Macrophages can be divided into M1 and M2 type according to different phenotypes and functions. M1 macrophages with tumoricidal effects can inhibit tumor growth and secrete pro-inflammatory cytokines like tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-12 [26,28]. M2 macrophages activated by IL-4, IL-10, and IL-13 hold the function of secreting cytokines such as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and transforming growth factor-β (TGF-β). All of them participate in promoting repair, suppressing immune response, and angiogenesis [29,30]. M2 macrophages in the tumor microenvironment account for a much higher proportion than M1 macrophages [26]. Programmed cell death 1 (PD-1) expressed on M2 macrophages combining with the programmed cell death ligand 1 (PD-L1) expressed on tumor cells exerted immunosuppressive effect [31]. Meanwhile, tumor cells highly express CD47, which is the ligand of signaling regulatory protein α (SIRPα), an immune checkpoint found on macrophages [32,33]. Binding of CD47-SIRPα axis will suppress phagocytosis effectively. Therefore, effectively improving phagocytosis of macrophages or transforming M2 macrophages into M1 type is the main therapeutic direction in current research.
Tregs, which are abundant in the tumor stroma is a specific subgroup of CD4+ T cells expressing the transcription factor Foxp3, CD25 and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) [34,35,36]. The chemokine ligand (CCL) 22 produced by macrophages and tumor cells can bind to chemokine receptor (CCR) 4 expressed on Tregs, consequently recruiting Treg into TME and leading to tumor growth and poor patients’ outcomes with its immunosuppressive function [37]. Tregs create an immunosuppressive environment through the activities of cell surface molecules (Foxp3, CTLA-4, CD25, CD39, CD73, TIGIT), secretion of cytokines (IL-2, IL-10, TGF-β, CCR4) and immune molecules (granzyme, cyclic AMP, and indole-amine-2,3-dioxygenase (IDO)) [36,38,39,40,41,42]. Therefore, blocking the functional molecules expressed on Tregs such as CTLA-4, PD-1, CCR4, TGF-β, Foxp3, or completely eliminating the presence of Tregs can improve the immune escape effect of tumor inflammatory microenvironment.
MDSCs are a group of heterogeneous cells that lack lymphoid markers with multi-directional differentiation potential and immunosuppressive function. This group includes immature DCs, macrophages, granulocytes, and other myeloid cells in the early stage of differentiation [43]. Immune suppression by MDSC involves several complex mechanisms. Due to its suppression of T cells and NK cells in TME, the accumulation of MDSCs is one of the main reasons for tumor immune unresponsiveness [44]. MDSCs can reduce local tryptophan levels due to the activity of IDO to reduce the proliferation of T cells [45]. Peroxynitrite (PNT) produced by MDSCs can alter chemokines and block the entrance of CD8+ T cells. MDSC also induce Tregs and affect function of NK cells by producing immunosuppressive cytokines like IL-10 and TGF-β [46]. Besides the influence of differentiation of TAMs, MDSCs also promote angiogenesis by secreting factors compensating for VEGF [27,47]. Immunotherapy targeting MDSCs provides a new therapeutic strategy for anti-tumor therapy.
2.1.2. Extracellular Matrix
ECM contains proteins, glycoproteins, proteoglycans, polysaccharides, and other components. All of them provide structural support for tissue organization and promote information transmission between cells [48]. In normal tissues, connective proteins and adhesive proteins in ECM keep connection between cells and maintain tissue homeostasis [49]. However, in solid tumors, remodeled ECM affects the migration and invasion of cells, and promotes the occurrence and malignant progression of tumors. Working as information transmitter between ECM and other cells, integrins are highly expressed on tumor cells and vascular endothelial cells, and usually affect the function of some immune cells and fibroblasts. Integrins on the surface of tumor cells regulate cell protrusion and adhesion in the process of tumor migration. Meanwhile, they mediate the function of multiple matrix metalloproteinases (MMPs) like MMP2, MMP9, and MMP14 to remodel ECM [50,51]. ECM remodeling is mainly regulated by MMPs, and proteases such as serine acid/cysteine [52]. In addition, the remodeled dense ECM slows the penetration and diffusion of large molecules to create a high-pressure environment, thereby resulting in therapy limitation [53,54].
2.1.3. Cancer Associated Fibroblasts
CAFs are one of the main components of TME. Unlike resting fibroblasts, CAFs metabolize vigorously and secrete large amounts of proteome, including cytokines, chemokines, and various protease CAF spindles [55,56]. CAFs also provide structural support for tissues and act as a transmitter of information between cells [57]. Due to the expression of serine protease, fibrinogen activator, and MMPs on CAFs, ECM is hydrolyzed and reconstructed. In addition, CAFs can also express a variety of cytokines and proteases, such as stromal cell- derived factor 1 (SDF1), VEGF, MMPs, and monocyte chemotactic protein-1 (MCP-1) to promote tumor growth, metastasis, and angiogenesis [58,59,60]. In addition, CAFs support cancer progression through changes of metabolism. In tumors, p38 signal of CAFs activates by cancer cells, the fibroblast-derived p38-regulated cytokines mobilize glycogen in cancer cells, which utilizes by cancer cells for glycolysis, promoting cancer invasion and metastasis [61]. In breast cancer, glycolytic CAFs provide extra pyruvate and lactate for augmentation of mitochondrial activity of tumor cells, which confers tumor cells with multiple drug resistance [62]. CAFs also regulate cancer cell metabolism independently of genetic mutations of cancer cell. FAK-depletion in CAFs promoting chemokin production, enhancing malignant cell glycolysis by activating protein kinase A via CCR1/CCR2 axis [63]. Therefore, NPs targeting CAFs can prevent tumor cells growing in numerous ways.
2.1.4. Exosomes
Exosomes are extracellular vesicles typically ~30 to ~200 nm in diameter and containing genetic material, proteins, and lipids [64]. They act as powerful signaling molecules connecting cancer cells and the surrounding components. Exosomes secreted by tumor cells carry miRNAs to regulate vascular endothelial cells. This phenomenon destroys the barrier of endothelial cells, and then allows cancer cells to enter the blood vessels, promoting tumor spread and metastasis [65]. Exosomes secreted by leukemia cells can promote the activation of CAFs. In breast cancer research, exosomes secreted by CAFs promote the invasion and metastasis of cancer by activating the Wnt-pathway [66]. Astrocytes in the brain metastatic microenvironment secrete exosomes loading miRNAs, which specifically downregulate tumor suppressor gene PTEN and lead to metastatic colonization [67]. Cancer exosomes inhibit the cytotoxic of CD4+ T cells, CD8+ T cells, and NK cells [68]. Exosomes also inhibit the differentiation of DCs and MDSCs [69,70]. Exosomes derived from cancer cells definitely have short-and long-term effects on cancer progress. Treatments targeting exosomes might be new directions of tumor therapy.
2.1.5. Tumor Vasculature
The regeneration of vasculatures is a very complicated progress in TME. Vascular endothelial cells regulated by the angiogenic factors can affect the migration and proliferation of tumor. The new vessels formed by adhesion of loosely endothelial cells provide chances for tumor growth and distant metastasis [71]. A variety of cells and growth factors are involved in this process, such as vascular cell adhesion molecule-1 (VCAM-1), α(v)β(3) integrin, VEGF, TGF, platelet-derived growth factor (PDGF), and angiogenin. Among them, VCAM-1 and α(v)β(3) integrin not only promote the proliferation and differentiation of endothelial cells but also improve vascular permeability [72,73]. PDGF, angiopoietin, and TGF secreted by tumor cells can also affect the action of peripheral cells, vascular maturation, and integrity. In addition, the highly abnormal and dysfunctional system of tumor blood vessels can also lead to impaired ability of immune effector cells to penetrate solid tumors. Therefore, the normalization of tumor blood vessels can enhance tissue perfusion and improve the infiltration of immune effector cells, thus enhancing therapy effects [74,75].
2.2. Physiological Condition of TME for Imaging and Targeting
2.2.1. Hypoxic Condition and Acid Microenvironment
Normal tissue is powered by mitochondrial oxidative decomposition, while cancer cells are mostly powered by glycolysis, a reprogrammed way known as the “Warburg effect” [76]. The majority of tumors are lack of adequate blood supply, and then hypoxic regions appear, where metabolize glucose into lactic acid through anaerobic glycolysis. When a large amount of lactic acid accumulates in the tumor cell, the proton pump transports H+ to the extracellular environment, resulting in an acidic extracellular environment (pH = 5.6–6.8) [77]. During glycolysis, the hypoxia inducible factor (HIF) can regulate glycolysis enzymes (HK1, HK3, TGF-2, et al.) to affect the energy metabolism and proliferation of tumor cells [78,79]. Therefore, utilizing the acid environment of the TME to design a platform acting on HIF-1 could be a new treatment strategy for tumors [80].
2.2.2. Extracellular ATP Content
It is an established notion that extracellular adenosine-5’-triphos-phate (ATP) is one of the major biochemical constituents of TME [81]. Mitochondria are where ATP is produced. It is different between normal cells and cancer cells with mitochondrial metabolism. Although there is little oxygen in cancer cells, in mitochondria, glycolysis is preferential for providing energy, which is called Warburg effect. The reprogramming metabolism in cancer is regulated by central regulators of glycolysis such as HIF-1, Myc, p53, and the PI3K/Akt/mTOR pathways [82]. In tumor tissues, these active pathways promote glycolysis in hypoxia, promoting mitochondria to produce large amounts of ATP. The sharp different concentration of ATP contrast between extracellular (<0.4 mM) and intracellular (1–10 mM) is a characteristic of TME, and the use of ATP can be a practical way for regulating drug release [83,84]. In addition, tumor cells usually metabolize vigorously, and once there is a lack of energy, cell damage happens. The damage of plasma membrane is a recognizable origin of ATP upregulating. Besides the cell injury, the hypoxic-induced stress of TME is also a strong stimulus for ATP release [85]. The ATP concentration in TME is remarkably more than those in normal tissues (10–100 nM) [84]. Based on such a concentration difference, the ATP stimulating response system can be designed to ensure the drugs reach the tumor site more accurately.
2.2.3. Redox Condition
Many organelles, such as cytosol, mitochondrion, and nucleus, contain very high concentrations of glutathione (GSH). In cancer cells, the concentration of GSH is 100–1000 folds of the normal tissue. Due to the existence of thiol groups, GSH can act as electron donors (reducing agents) for developing smart NPs [86].
3. Microenvironment-Targeted Nano-Delivery System as a Promising Strategy
The drug therapy for cancer has been unable to exert its maximum effect due to insufficient orientation, pharmacokinetic obstacles. In order to overcome the shortcomings of traditional drug delivery methods, a new method, a nano-delivery system, is being researched. It can bring drugs accurately to the tumor site and prolong the half-life of the drug in vivo. According to different conditions between TME and normal tissue, NPs are designed to be new solutions for tumor imaging and treatment (Figure 3). All the works mentioned were summarized in Table 1 to reflect the latest development of nano-target strategies applied to TME.
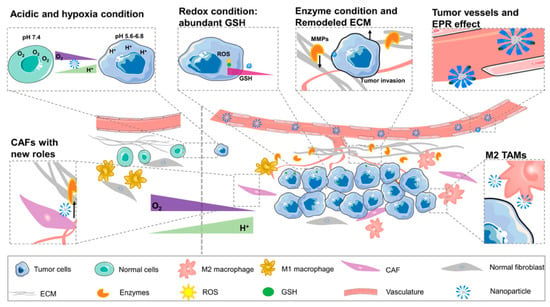
Figure 3.
The difference between tumor microenvironment and normal cellular environment (N: normal cellular environment, T: tumor microenvironment). The picture shows new curved blood vessels in TME and EPR effect. Redox condition created by the high content of GSH in tumor cells. Compared with the normal tissue, TME contains a large number of enzymes, anoxic and acidic environments. The percentage of M2 TAM in tumor tissue is larger than that in normal tissue. Special ECM and CAF cells contact with vasculature provides a good condition for angiogenesis and tumor progression.

Table 1.
Nanoparticle approaches for targeting TME in this review.
3.1. Major Composition Mediated Nanoparticles in TME
3.1.1. Nanoparticles Targeting Immune Cells
The immunosuppressive microenvironment is one of the main reasons for the poor antitumor effect in vivo [87]. For M2 macrophages, the most popular methods are reducing M2 macrophages, blocking the immune suppressive function of M2 by blocking PD-1/PD-L1 and the CD47-SIRPα axis [88]. Qian and others designed a fibrin gel capsulate calcium carbonate NPs used in the surgical wound which would polarize M2-like macrophages to a M1-like phenotype. The pre-loaded anti-CD47 antibody in this vector further block the “don’t eat me” signal in cancer cells [89]. Shi et al. utilized precision nanopart icle- based reactive oxygen species photogeneration to reprogram M2 macrophages to M1 macrophages, then recruited cytotoxic lymphocyte (CTL) and direct memory T-cells to make the tumoricidal response more effective [90]. Both gold nanoparticles (AuNPs) and silver nanoparticles (AgNPs) can modulate ROS and reactive nitrogen species (RNS) to activate inflammatory signaling pathways, which can re-polarize macrophages to M1-like phenotypes [91]. We built a multifunctional nanoplatform (FA-CuS/DTX@PEI-PpIX-CpG nanocomposites) for synergistic PDT, PTT, loading DTX to enhance immunotherapy of anti-PD-L1, and polarizing myeloid-derived suppressor cells (MDSCs) toward M1 phenotype successfully in breast cancer (Figure 4) [92].
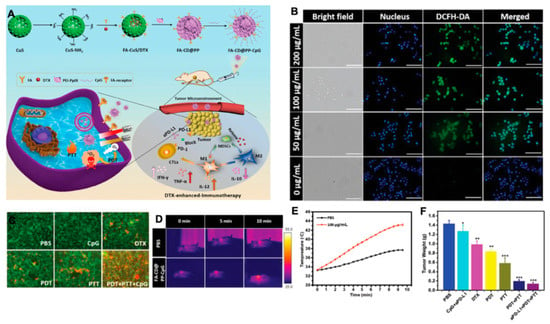
Figure 4.
(A) Rational design and synthesis, its application in cancer treatment (left), and illustration of FA-CD@PP-CpG for docetaxel-enhanced immunotherapy (right); (B) intracellular ROS detection in 4T1 cells incubated with various concentrations of FA-CD@PP-CpG under 650 nm irradiation; (C) corresponding fluorescence images of 4T1 cells constrained with calcein AM (live cells, green) and propidium iodide (dead cells, red) after being treated with different conditions. (D) The in vivo thermal images of the mice after intravenous injection of PBS and FA-CD@PP-CpG under 808 nm irradiation; (E) temperature change curve of tumor sites as a function of irradiation time; (F) the weight of tumor tissue in different groups obtained on day 14, adapted with permission from [92].
Recently, interaction between the tumor metabolism and immunity has been proved to be a potential therapeutic strategy. A mannosylated lactoferrin nanoparticulate system (Man-LF NPs) is developed. It facilitated dual-targeting biomimetic codelivery of shikonin and JQ1 to target the macrophage marker mannose receptor and LRP-1. JQ1 itself is a PD-L1 checkpoint blockage that can combine with Man-LF NPs and reduce the generation of immune cells such as Tregs [93]. Macrophages in TME have also contributed to tumor diagnosis and localization, Kim et al. made imaging macrophages in tumors possible towards a pharmacokinetically optimized, 64Cu-labeled polyglucose nanoparticle (Macrin) for quantitative positron emission tomography (PET). This technique not only detected the number of macrophages, but also contributed to the effective image of tumor location [94].
In general, the expression of receptor tyrosine kinases (RTKs) in cancer cells can activate the STAT3/5 signaling pathway, which promotes the secretion of TH2 cytokines and then promotes the survival of CD4+ Foxp3+ Tregs [95,96]. Thus, the sunitinib-targeting receptor tyrosine kinase drug transferred by nanomaterial has been used to decrease CD4+ Foxp3+ Tregs and MDSCs [97]. Tlyp1 peptide coupled nanoparticles, combined with anti-CTLA4 immuno-checkpoint inhibitors targeting microenvironments, can also enhance imatinib’s ability of decrease Tregs by inhibiting the phosphorylation of STAT3 and STAT5 signaling pathways [98]. A CpG self-crosslinked nanoparticles-loaded IR820-conjugated hydrogel with dual self-fluorescence to exert the combined photothermal-immunotherapy was designed in a melanoma model. These NPs improve the immune response of adjuvant through adjusting the quantity of CD8+ T cells, DCs, Tregs, and MDSCs in TME [99].
Stimulator of interferon genes (STING) could enhance tumor immunogenicity, and researchers found, when packing STING into NPs, that its activity to 2’3’ cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) enhanced [100]. IDO,TGF-β, IL-10, and IL-35 also have the abilities to modulate the immune microenvironment [101]. IDO is a rate-limiting enzyme of human tryptophan metabolic that can oxidize tryptophan into canine urine. IDO directly inhibits the function of T cells and enhances the immunosuppressant effect of Tregs, thereby mediating the effect of local immune tolerance and promoting the immune escape of tumors [102,103]. IDO is increasingly incorporated into the nano therapeutics system to regulate the outcome of immunotherapy interventions. In melanoma, Cheng and colleagues built a peptide assembling nanoparticle, which concurrently blockade immune checkpoints and tryptophan metabolism towards on-demand release of a short d-peptide antagonist of programmed cell PD-1 and an inhibitor of IDO [104]. Aimed at TGF-β, Xu et al. silenced TGF-β in microenvironments to solve the problem that the combined vaccine of tumor antigen (Trp 2 peptide) and adjuvant (CpG oligonucleotide) has a poor effect on melanoma [105]. Our team constructed CpG capsuled Cu9S5@mSiO2-PpIX@MnO2 NPs to promote infiltration of CTLs in tumor tissue, and further upregulated interferon gamma (IFN-γ) to promote immune response [106].
Suppressing the function of immune-tolerant cells and promoting the anti-tumor effect of cells have been the key points of tumor immunotherapy. Regulation of immune tolerance in TME combined with nanomaterials can effectively avoid the obstacles caused by microenvironments for drug entry. Therefore, regulating the function of immune cells in microenvironments is one of the key tasks of the nanomaterials system.
3.1.2. Nanoparticles Targeting CAF
CAF is the main source of growth factors, chemokines, ECM proteins, and matrix degrading enzymes in TME. It can produce a variety of growth factors and cytokines to promote the survival and invasion of tumor cells [107]. Kovacs and colleagues found that gold-core silver-shell hybrid nanomaterials could reduce the tumor promotion by attenuating behavior of CAFs [108].
According to the regulation of CAFs of immune cells, Hou et al. developed a nanoemulsion (NE) formulation to deliver fraxinellone (Frax). This NP was around 145 nm length, could be taken by CAFs efficiently, and accumulated in the TME. Combining with a tumor-specific peptide vaccine will enhance tumor-specific T-cell infiltration and activate death receptors on the tumor cell surface (Figure 5) [109]. Recently, NPs targeting CAFs for tumor therapy mainly focused on destroying the tumor tissue to promote drug penetration and reprogramming immune TME. Since CAFs participate in cancer glucose metabolism immediately, NPs targeting CAFs about decreasing glycolysis of cancer cells remain to be developed.
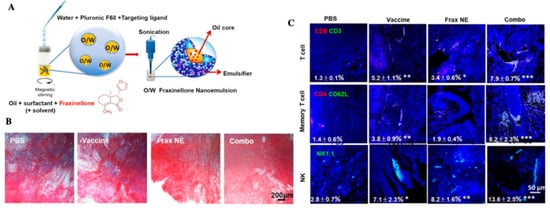
Figure 5.
(A) Scheme depicting the preparation process of Frax NE; (B) Masson’s trichrome stain for collagen; (C) confocal and flow cytometric analysis of immune cells infiltration in the TME, adapted with permission from [109].
3.1.3. Nanoparticles Targeting ECM
People try to find ways to destruct the structure of ECM. As the most well-known matrix enzyme, MMPs, particularly MMP2 and MMP9, were frequently applied in NPs [110,111]. Many systems that respond to enzymes such as MMPs are also used in drug delivery and imaging. Ji et al. utilized pirfenidone (PFD) loaded MRPL (MRPL-PFD), a MMP2 responsive peptide-hybrid liposome, to downregulate the components of ECM, thus increasing the penetration of drugs in pancreatic cancer tissue (Figure 6) [112]. Other enzymes acting on the microenvironment can also degrade the structure of ECM. Hyaluronidase (HAase) can break down hyaluronan and then enhance the efficacy of nanoparticle-based PDT. Utilizing HAase and DOX together will also increase cancer mortality [113,114,115]. Blocking collagen and integrin signaling for anti-fibrotic therapeutic strategy can also be considered in future treatment for ECM to improve drug delivery [116].
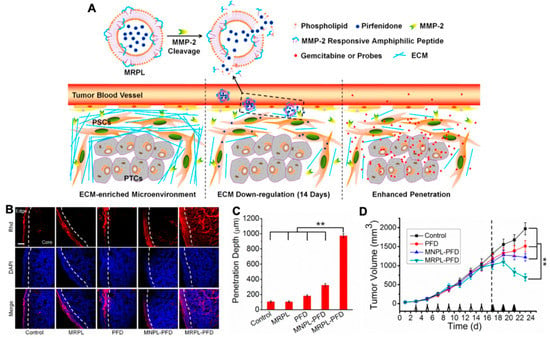
Figure 6.
(A) Mechanism of MMP-2 Responsive Peptide Hybrid Liposome (MRPL) for downregulation of ECM in pancreatic tumors; (B) Rhd penetration and distribution in pancreatic tumor (PSCs/Mia-paca-2 co-implanted) tissues after 2 weeks’ treatment of the different PFD formulations; (C) quantification of the depth of Rhd penetration in tumors treated by the PFD formulations; (D) the growth curves of PSCs and Mia-paca-2 co-implanted pancreatic tumors in mice treated by the different PFD formulations, adapted with permission from [112].
3.1.4. Nanoparticles Targeting Exosomes
As a natural intercellular shuttle of miRNA, exosomes affect a series of physiological and pathological processes in receptor cells or tissues, and are ideal nano carriers for nucleic acid targeted delivery in vivo [117]. The antigen presenting function of dendritic cells was utilized to develop a single membrane vesicle-based vaccine, which would participate in repressing both melanoma (B16) and Lewis lung carcinoma (LLC) tumor growth [118]. Utilizing exosomes to load NPs showed perfect biocompatibility. Xiong and co-workers built NPs together laurate-functionalized Pt (IV) prodrug (Pt(lau)) and human serum albumin (HSA) with lecithin, capsuled by the exosomes, had a good platinum chemotherapy efficiency (Figure 7) [119].

Figure 7.
(A) Schematic illustration of the Pt(lau)HSA NP-loaded exosome platform (NPs/Rex) for efficient chemotherapy of breast cancer; (B) biodistribution of DiR, DiR-Pt(lau)HSA NPs, and DiR-NPs/Rex in 4T1 tumor-bearing BALB/c mice; (C) the volume of orthotopic tumors; (D) typical lung tissues with visualized metastatic nodules (black arrows) and H&E for metastatic nodules of lungs in each group, adapted with permission from [119].
Exosomes shed by cancer cells have also been designed on cancer diagnosis. Liu et al. made exosomes immobilize on magnetic microbeads to produce fluorescent signal. They qualified the exosomes in plasma samples from breast cancer patients for early diagnosis of cancer in vitro [120]. Lewis et al. built an in vitro probe screening of bio-membrane chips, which was composed of the captured exosomes and other extracellular vesicles in the plasma, and tested the express of glypican-1 and CD63 to diagnose pancreatic ductal adenocarcinom in vitro [121].
3.1.5. Nanoparticles Targeting Tumor Vasculature
Using EPR more effectively and enhancing the permeability of vascular have been widely studied in the latest nano therapeutics [122,123]. There are some reports that used NO to improve the EPR effect in pancreatic cancer and other diseases with low vascular permeability [122,124,125]. Other delivery systems like actively targeting VEGF and α(v)β(3) integrin were also used widely [126]. Integrins play important roles in cell adhesion and cell signaling, and α(v)β(3) integrin is one type of them that can modulate angiogenic endothelial cells. Graf et al. described a NP using cyclic pentapeptide c(RGDfK) to active target α(v)β(3) integrin on cancer cells and tumor neovasculature [127].
To improve the diagnosis of tumor, Youbin and co-workers proposed the poly(acrylicacid) (PAA)-modified NaLnF4:40Gd/20Yb/2Er nanorods ((Ln = Y, Yb, Lu, PAA-Ln-NRs) to enhance the shifting of NIR-IIb (a general in vivo fluorescence imaging technology), which successfully imaged the vessels of small tumors (about 4 mm), metastatic tissue (about 3 mm), and even brain vasculatures (Figure 8) [128]. Cecchini et al. reported a nanoMIPs against VEGF coupled with quantum dots (QDs) for tumor imaging in melanoma [129]. In cholangiocarcinoma, α(v)β(3) integrin also combined with aggregation induced emission (AIE) for image-guided PDT, and presented a good antitumor response [130].

Figure 8.
(A) Schematic illustration of the enhanced NIR-IIb emission of PAA-Ln-NRs via Ce3+ doping for non-invasive tumor metastasis/vascular visualization and brain vessel imaging; (B) schematic illustration of in vivo small tumor diagnosis by using PAA-Lu-NRs, and the NIR-IIb bioimaging of tumor-bearing mouse after intravenously injecting PAA-Lu-NRs at different time periods; (C) schematic illustration of in vivo noninvasive brain vessel imaging by using the in vivo imaging system, fast brain vascular imaging of a mouse with hair removed and cross-sectional fluorescence intensity profiles along the green lines of the mouse, adapted with permission from [128].
3.1.6. Nanoparticles Targeting Multiconstituents
In addition to targeting single constituents, some NPs can regulate multiple barriers and have also been simultaneously designed for tumor therapies. Targeting tumor cells and immune cells simultaneously can effectively reduce the immune escape phenomenon of TME. Shi et al. designed a versatile calcium ion nanogenerator. The degradation and release of Ca2 + by nanoparticles can promote the maturation of DCs by promoting autophagy of DCs, and it can promote tumor cells to produce damage-associated molecular patterns (DAMPs), further maturing DCs and the enhanced infiltration of CTLs at the same time [131]. In addition, researchers have combined exosomes with immunotherapy. Xie’s team synthesized responsive exosome nano-bioconjugates. They modified exosomes derived from M1 macrophages with antibodies of CD47 and SIRPα. The broken benzoic-imine bonds are cleaved to release antibodies of SIRPα and CD47 in the acidic TME abolished the “don’t eat me” signal between tumor cell and macrophages [132]. The native M1 exosomes reprogram the M2 macrophages to M1 effectively at the same time [132].
3.2. Physiological Condition Mediated Nanoparticles in TME
3.2.1. Hypoxic Stimulus
The hypoxic condition in tumor microenvironment is considered to play an important role in malignancy and progression of cancer. Hypoxic areas of tumors also bring obstacles to radiation therapy due to the oxygen free radicals [133]. Utilizing this characteristic, the low oxygen response nano-delivery systems were produced.
The approaches developed thus far can be classified into three categories: countering hypoxia, exploiting hypoxia, and disregarding hypoxia [134]. (i) Directly or indirectly elevating O2 concentration to counter hypoxia is a promising way to improve the efficiency of tumor therapy, especially for photodynamic therapy (PDT) and radiation therapy (RT). PDT generates reactive oxygen species (ROS) by using light-excited photosensitizer (PS), resulting in cell apoptosis and microvascular damage [125]. Red blood cells (RBCs) carrying hemoglobin molecules are primary oxygen sources in our body. Because the efficacy of PDT is deeply oxygen-dependent, a technique named RBC-facilitated PDT was developed to improve hypoxia conditions in tumors. Wei et al. showed a nanocapsule that encapsulated photosensitizers and tethered the conjugates onto RBC surface. By using biotin-neutravidin-mediated coupling, they conjugated ZnF16Pc (photosensitizer)-loaded ferritins onto each RBC [135]. This new structure, which could overcome low oxygen conditions, showed efficient 1O2 production to overcome low oxygen conditions and enhanced PDT capacity [135]. For RT, an artificial nanoscale RBC will remarkably enhance the treatment efficacy as well. For example, an artificial blood substitute perfluorocarbon (PFC) was encapsulated with biocompatible poly(d,l-lactide-co-glycolide) (PLGA) and then further coated with a red-blood-cell membrane (RBCM), showing efficient loading of oxygen and significantly enhanced treatment efficiency during RT [136]. With increasing production of H2O2 in cancer cells, NPs converting endogenous H2O2 to toxic ROS and decomposing endogenous H2O2 to O2 were rapidly developed. Noble metal nanoparticles like Mn, Au, Pt, and Ir are well known for their catalytic performances in various fields [137]. MnO2 is a common material to enhance PDT treatment and imaging. Mn2+ could react with H2O2 in the tumor, then downregulate the expression of HIF-1 to increase oxygen content and optimize MRI imaging [138,139]. A nanoplatform based on mesoporous polydopamine (MPDA) modified with Pt also produces O2 by decomposing overexpressed H2O2 in the tumor. Meanwhile, the existence of Pt can act as a nano-factory to provide support for PDT (Figure 9) [140]. (ii) Taking advantage of the deficiency of oxygen molecules is a new approach for drug release and PDT. Yin et al. developed a novel amphiphilic block copolymer radiosensitizers. After optimizing the ratios of carboxyl and metronidazole (MN) groups, PEG-b-P(LG-g-MN) micelles could be used to encapsulate doxorubicin (DOX@HMs) efficiently [141]. Hypoxia-responsive structural transformation of MN into hydrophilic aminoimidazole triggers fast DOX release from DOX@HMs, which acted as high-efficiency radiosensitizers and hypoxia-responsive DOX nanocarriers [141]. Some drugs that are selectively toxic to hypoxic cells like Tirapazamine (TPZ) were designed to combine with oxygen-dependent PDT to enhance bioreductive therapy. Shao’s group developed a core–shell upconversion nanoparticle@porphyrinic MOFs (UCSs) for combinational therapy against hypoxic tumors [142]. TPZ was encapsulated in nanopores of the MOF shell of the heterostructures (TPZ/UCSs), which enables the near-infrared light-triggered production of cytotoxic reactive oxygen species [142]. Furthermore, with the combination of PD-L1, this nanoplatform recruited specific tumor infiltration of cytotoxic T cells and inhibited the metastasis of the tumor as well. Other methods like eliminating the oxygen in the tumor, inhibiting the growth of tumor vessels, and stopping the nutrient delivery to starve the tumor cells still have many challenges [143,144]. (iii) Using new anticancer modalities to disregard hypoxia conditions becomes another innovative antitumor strategy. PDT with diminished O2 dependence will effectively overcome its strong oxygen dependence and limitation of treating deep tumors. It has been reported that fractional light delivery may be a superior way to enhance PDT effects due to the reduction of short-term oxygen consumption during PDT [145]. Since the generation of oxygen-irrelevant free radicals is oxygen-independent, and the exploration of UCNP is an inner light source to activate most organic photosensitizers (PSs) to create cytotoxic 1O2, researchers discovered that the Ru complex displayed excellent type I PDT activity [142,146]. Due to its special Fenton reaction, Fe nanoparticles can produce reactive •OH species with endogenous H2O2 (Fe2+ + H2O2 → Fe3+ + •OH + OH−) and produce cytotoxic effects without external energy through chemotherapeutic therapy (CDT). Yu et al. fabricated a core–shell structured iron-based NPs (Fe5C2@Fe3O4) to release ferrous ions in acidic environments to disproportionate H2O2 into •OH radicals, and its high magnetization is favorable for both magnetic targeting and T2-weighted MRI [147]. In addition, gold nanospheres, graphene oxide, polydopamine (PDA), and other materials have been widely used as PTT reagents and nano carriers to deliver PDT reagents, so as to overcome the therapeutic limitations of PDT [148,149,150].
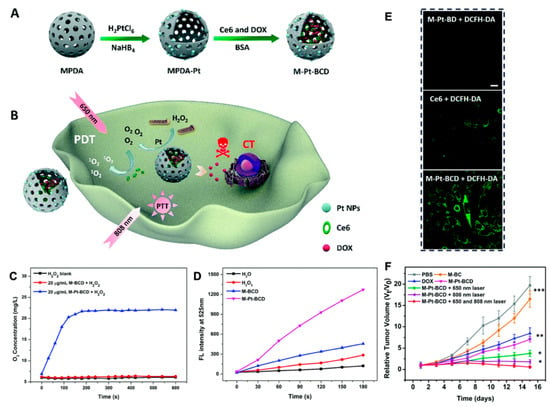
Figure 9.
(A) Schematic illustration of the synthesis of MPDA-Pt-BSA/ Ce6/DOX (M-Pt-BCD); (B) schematic illustration of the application of M-Pt-BCD for enhanced- PDT and synergistic therapy; (C) O2 generation of H2O2 blank, M-BCD and M-Pt-BCD; (D) 1O2 production efficiency of H2O, H2O2, M-BCD and M-Pt-BCD; (E) confocal microscopic images of cellular 1O2 levels detected by DCFH-DA staining upon 650 nm irradiation; (F) tumor growth curves, reproduced by permission of The Royal Society of Chemistry [140].
3.2.2. pH Response
pH responsive nano-vectors are one of the typical carriers for TME. Chemical bond response to pH is one of the most widely used strategies in pH responsive nano delivery systems. The most common pH sensitive bonds include hydrazone bond, imine bond, oxime bond, amide bond, benzoic-imine bond, orthoesters, polyacetals, and ketals [151,152]. These chemical bonds break in acidic environments to degrade the carrier, and then increase the uptake of tumor cells or accelerate drug release. When it comes to the design of pH-sensitive materials, besides pH sensitive chemical bonds, other main strategies are conformational change, protonation, and charge reversal with pH change [151]. For example, Chen’s team developed a DNA-based stimulus-responsive drug delivery system precisely responding to pH variations in the range of 5.0–7.0. On the face of the gold nanoparticles, one DNA strand was an acti-MUC1 aptamer targeting tumor membrane, the other DNA strand was switchable DNA, which has a linear conformation under neutral or alkaline conditions and self-folds into a triplex under acidic conditions [153].
In cells, nano-switches can react with endosomes and lysosomes and switch to triplex in lysosomes, so as to achieve the goal of accurately drug release (Figure 10) [153]. Nanocarriers involved in protonation/deprotonation are mainly nano liposomes, peptides, and polymers. The phospholipid components in liposomes are usually destabilized under acidic conditions, so as to deliver the contents of liposomes to cells. In mouse cancer models, Guangna et al. combined platelet membrane with the functionalized synthetic liposome; because of its camouflage based on the platelet membrane, this platform enhanced tumor affinity and released DOX in acidic microenvironment more selectively and efficiently [154]. The anionic/cationic polymers with different groups deform various nanocarriers through the change of their hydrophilicity, which lead to drug release [155]. Inorganic salts such as MnO2, CaP, and CaCO3 are widely used for pH response NPs because of their acid solubility [156,157,158]. Ma et al. designed a pH-sensitive dye linked peptide substrate of MMP-9 with Fe3O4 nanoparticles, establishing a Forster resonance energy transfer (FRET) system to detect the invasion and metastasis of tumor by detecting the overexpression of MMP9 [159]. A pH responsive magnetic nanoparticle can combine magnetic hyperthermia with drug delivery dependent on magnetic stimulation, achieving the purpose of targeting TME and tumor treatment at the same time [160].

Figure 10.
(A) Schematic illustration of the DNA-based stimulus-responsive drug delivery system; (B) pH-responsive regulation of the nanoswitch and drug release; (C) schematic illustration of DNA strands and the multidrug loaded on the surface of AuNPs; (D) intracellular pH-responsive multidrug delivery and release, adapted with permission from [153].
3.2.3. ATP Response
Since the concentration of ATP in tumor cells is much higher than extracellular environments and ATP is involved in many biochemical reactions in cells, NPs response to ATP were widely developed. ATP sensitive NPs can release drugs without the help of external forces. Zhenqi et al. developed nano ZIF-90 self-assembled from zinc ions and imidazole-2-carboxaldehyde (2-ICA) to deliver DOX. Because the coordination between ATP and Zn2+ is much stronger than that between imidazole and Zn2+, nano ZIF-90 can be decomposed and respond to ATP [161]. Graphene oxide (GO) has been shown to bind single stranded DNA. When the template DNA contains ATP binding domain and reached ATP specific recognition, it could be circularized upon proximity ligation after hybridizing to linker DNA on the surface of GO [162]. Then, rolling circle amplification was initiated from the 3′-end of the template DNA, and the elongated sequence was hybridized with thousands of signal DNA (conjugated with Cy3), so as to amplify the template DNA, generate fluorescent signals, and achieve the purpose of tumor monitoring [162]. Yuan et al. exploited a ATP binding natural protein, GroEL (a bacterial chaperonin) loading DOX, once in the presence of a critical concentration of ATP in tumor site, it releases drugs [163]. In addition to using ATP as a switch for drug release, another way in which ATP participates is to regulate its expression in cells with nanosystems. Xiao’s team exploited a multifunctional theranostic platform combing CDT with limotherapy. While enhancing the CDT effect to induce apoptosis of cancer cells, nano Se and Mn2+ ions inhibited the production of ATP, which made cancer cells starve and further killed tumor cells, monitoring the treatment of tumors by MRI simultaneously [164].
3.2.4. Reduction Response
In addition to GSH, tumor cells also contain thioprotein, Fe2+, cysteine, and other reductive substances, and the difference of GSH concentration between tumor cells and TME makes a reduction-responsive drug carrier come true [165,166]. GSH/glutathione disulfide (GSSG) is one of the major redox couples in cells, and adding disulfide bonds to drug carriers is one of the most commonly used methods to build GSH responsive drug carriers. There are many forms of GSH-responsive nano-vehicles (like micelles, nanogels, nanoparticles), so as to improve the drug release successfully. For example, in order to solve the problems of drug resistance caused by cancer stem cells (CSC), Rubone (RUB, a miR-34 activator for targeting CSCs) and DTX were utilized to treat taxane resistant prostate patients. A self-assembled DTX/p-RUB micelles showed good stability in vitro and could be accurately delivered to tumor cells though the EPR effect. After the tumor cells endocytosed the micelles, the micelles expanded and disintegrated due to the protonation of diisopropylaminoethanol (DIPAE) and GSH induced disulfide bond cleavage of acid endocytosis vesicles, which led to the rapid release of DTX and RUB. The release of RUB upregulated miR-34a and regulated the expression of chemoresistance related proteins, thus making tumor cells sensitive to DTX, significantly inhibiting the progress of drug resistance [167]. Ling and colleagues constructed a self-assembled NP platform composed of amphiphilic lipid polyethylene glycol (PEG), and it can effectively deliver Pt (IV) precursor drugs through the elimination of GSH [168].
Some metal oxides like MnO2 also have the potential of GSH response. MnO2 reacts with GSH in cells to form glutathione disulfide and Mn 2+, which leads to the consumption of GSH and enhancement of CDT. In addition to the MRI features of Mn2+, MRI monitored chemo- chemical combination therapy is realized [167].
3.2.5. Enzyme Response
Many enzymes like MMPs regulate the function of cellular components and take part in tumor progression. From this prospect, the presence of these abnormal enzymes gives the chance for researchers to build a sensitive system for drug release. The presence of NO can activate endogenous MMP1 and MMP2, and researchers have developed an MSN loaded with a doxorubicin (DOX) and NO donor to enhance the antitumor effect [169]. In addition, PLGLAG peptide and gelatin are both main target proteins of MMP9 and MMP2, which can be widely used to MMP responsive NPs [170,171,172].
According to the Warburg effect, the proliferation of tumor cells mainly depends on aerobic glycolysis, so tumor cells are more sensitive to the change of glucose concentration. Glucose oxidase (GOx), an endogenous oxidoreductase, reacts with glucose and O2 in cells then produce gluconic acid and H2O2, which can inhibit the proliferation of cancer cells through starvation therapy. In addition, H2O2 can be transformed into · OH free radical to kill cancer cells and enhance the oxidative stress response of cancer cells [173]. Through GOx, starvation therapy can together with chemotherapy, CDT, PDT, or immunotherapy to explore new strategies for cancer treatment. For example, Mengyu et al. constructed a multifunctional cascade bioreactor based on hollow mesoporous Cu2MoS4 (CMS) loaded with GOx for synergetic cancer therapy by CDT/starvation therapy/phototherapy/immunotherapy [174]. First of all, CMS containing multivalent elements (Cu1+/2+, Mo4+/6+) showed Fenton like activity, which could produce · OH and reduce GSH, thus reducing the antioxidant capacity of tumor. Secondly, in hypoxic TME conditions, hydrogen peroxide like CMS can react with H2O2 to generate O2, activate the effect of GOx, start starvation therapy, and regenerate H2O2. Finally, the regenerated H2O2 can participate in Fenton like reactions to realize GOx-catalysis-enhanced CDT. At the same time, because of the excellent photothermal conversion efficiency under 1064 nm laser irradiation, CMS killed tumor cells significantly in PDT. More importantly, the PEGylated CMS@GOx-based synergistic therapy combined with anti-CTLA4 can stimulate a robust immune response [174].
3.2.6. Multiply Response
A mesoporous silica-coated gold cube-in-cubes core/shell nanocomposites loading DOX was combined with ArgGlyAsp (RGD) peptide to achieve a platform that can deliver drugs and produce O2 in situ. This nano platform simultaneously enhanced photodynamic efficacy, achieving heat- and pH-sensitive drug release and location imaging (Figure 11) [175]. Lan et al. decorated an emerging class of highly tunable two-dimensional material: cationic Hf12-Ru nanoscale metal-organic frameworks (Hf12-Ru nMOF), then functionalized with pH-sensitive fluorescein isothiocyanate and targeting mitochondria, utilized the pH and quantities of O2 in the mitochondria to image living cells [176]. Yi and colleagues developed a redox/ATP switchable theranostic NPs. They conjugated a fluorescent probe (FAM) and a quencher (BHQ-1) to ATP, complexed with a GSH-sensitive cationic polymer. This smart NPs loading fluorescent probes can monitor drug release in vivo [177].
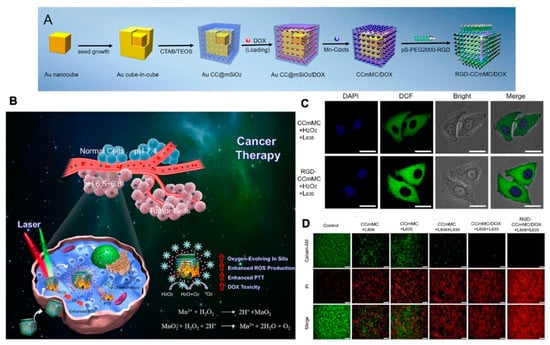
Figure 11.
(A) Schematic illustration of the synthesis process for the versatile RGD-CCmMC/DOX nanovehicles; (B) schematic illustration of the therapeutic mechanism of the RGD-CCmMC/DOX nanoplatforms to enhance the overall anticancer efficiency of triple-combination photodynamic/photothermal/chemo-therapy in a solid tumor; (C) CLSM images of 4T1 cells treated with different formulations under laser irradiation. The production of intracellular ROS and O2 generation were measured by the green fluorescence intensity of DCF; (D) fluorescence microscopy images of 4T1 cells with various treatments using Calcein AM/PI staining, adapted with permission from [175].
4. Conclusions and Outlook
Traditional cancer diagnosis, chemotherapy, radiotherapy, surgery, and other treatments have kept the high mortality rate of cancer patients, and this led us to develop new strategies with more accurate diagnoses and more effective treatments. Using NPs to treat cancers is an emerging approach. In addition to targeting tumors themselves, utilizing TME and physicochemical properties to treat and orientate tumors have been a great inspiration and challenge for the development of nanoparticles. More evidence is needed for the clinical application of NPs, and here we summarize current results and several challenges of NPs.
The special conditions of the tumor microenvironment give us superior delivery conditions. The immunosuppressed environment of tumor causes “immune escape” and serves as a good direction for the treatment of tumors. The extracellular matrix, enzymes, and inflammatory factors also provide promising therapeutic targets. The physiological features such as hypoxia, acidic microenvironment, and abundant angiogenesis also give NPs good access conditions to reach tumor site and release drugs.
How to use the particular microenvironment of tumor to design delivered nanoparticles is a big hurdle. A question that remains to be solved is how to deliver drugs to tumor tissues more efficiently and specifically. PEG and zwitterionic materials can effectively reduce the blood clearance rate. In order to improve the biocompatibility, it is also a breakthrough for people to use the biological membranes to cover the material. According to the EPR effect and the abundance of blood vessels in tumors, drugs will be delivered to tumor tissue precisely, hence improving therapeutic efficiency for tumors. However, there is a huge difference between the internal environment of human beings and that of animal models, and how to reduce the side effects of materials and systems is what we need to work on. Clinical trials on nanoparticles are yet to be developed, and we should make more efforts to develop safe and efficient therapy strategies.
Author Contributions
Conceptualization, methodology, software, writing-original draft preparation, editing, Y.S. and Y.L.; project administration, and review, S.S. and C.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Commission of Shanghai Municipality (Grant No. 14DZ2261100, 15DZ1940106), the Joint Project of Health and Family Planning Committee of Pudong New Area (Grant No. PW2017D-10) and the National Natural Science Foundation of China (Grant Nos. 81860547, 81573008, 21671150, 21877084, 81171646, 31170776, and 21472139).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
There are no conflict to declare.
Abbreviations
| Abbreviation | Full terms |
| TME | Tumor microenvironment |
| ECM | Extracellular matrix |
| NPs | Nanoparticles |
| EPR | Enhanced permeation and retention |
| MSNs | Mesoporous silica nanoparticles |
| MNPs | Magnetic nanoparticles |
| GNPs | Gold nanoparticles |
| CAFs | Cancer associated fibroblasts |
| T cell | T lymphocyte |
| B cell | B lymphocyte |
| ROS | Reactive oxygen species |
| NK | Natural killer |
| DCs | Dendritic cells |
| SIRPα | Signaling regulatory protein α |
| Tregs | Regulatory T cells |
| TAMs | Tumor associated macrophages |
| TNF-α | Tumor necrosis factor-α-T |
| IL | Interleukin |
| CTLA-4 | Cytotoxic T-lymphocyte-associated antigen-4 |
| CCL | Chemokine ligand |
| CCR | Chemokine recepter |
| MDSCs | Myeloid-derived suppressor cells |
| VEGF | Vascular endothelial growth factor |
| EGF | Epidermal growth factor |
| TGF-β | Transforming growth factor-β |
| MMP | Matrix metalloproteinase |
| SDF1 | Stromal cell- derived factor 1 |
| MCP-1 | Monocyte chemotactic protein-1 |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| PDGF | Platelet-derived growth factor |
| DAMPs | produce damage-associated molecular patterns |
| HIF | Hypoxia inducible factor |
| ATP | Adenosine-5’-triphos-phate |
| GSH | Glutathione |
| MPDA | Mesoporous polydopamine |
| FRET | Forster resonance energy transfer |
| ssDNA | Single-stranded DNA |
| GSSG | GSH/glutathione disulfide |
| CSC | Cancer stem cells |
| PEG | polyethylene glycol |
| DOX | Doxorubicin |
| CTL | Cytotoxic lymphocyte |
| AuNPs | Gold nanoparticles |
| AgNPs | Silver nanoparticles |
| RNS | Reactive nitrogen species |
| PTT | Photothermal therapy |
| PDT | Photodynamic therapy |
| DTX | Docetaxel |
| ROS | Reactive oxygen species |
| Man-LF NPs | Mannosylated lactoferrin nanoparticulate system |
| cGAMP | Cyclic guanosine monophosphate-adenosine monophosphate |
| STING | Stimulator of interferon genes |
| PD-1 | Programmed cell death 1 |
| PD-L1 | Programmed cell death ligand 1 |
| IDO | Indole-amine-2,3-dioxygenase |
| NE | Nanoemulsion |
| PFD | Pirfenidone |
| LLC | Lewis lung carcinoma |
| HSA | Human serum albumin |
| QDs | Quantum dots |
| ACF | Acriflavine |
References
- Cheng, H.S.; Lee, J.X.T.; Wahli, W.; Tan, N.S. Exploiting vulnerabilities of cancer by targeting nuclear receptors of stromal cells in tumor microenvironment. Mol. Cancer 2019, 18, 51. [Google Scholar] [CrossRef]
- Lord, E.M.; Penney, D.P.; Sutherland, R.M.; Cooper, R.A., Jr. Morphological and functional characteristics of cells infiltrating and destroying tumor multicellular spheroids in vivo. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1979, 31, 103–116. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, T.; Perche, F.; Taigind, A.; Torchilin, V.P. Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety. Proc. Natl. Acad. Sci. USA 2013, 110, 17047–17052. [Google Scholar] [CrossRef]
- Fane, M.; Weeraratna, A.T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 2020, 20, 89–106. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. The Microenvironmental Landscape of Brain Tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Dou, B.; Tan, H.; Feng, Y.; Wang, N.; Wang, D. Tumor microenvironment-driven non-cell-autonomous resistance to antineoplastic treatment. Mol. Cancer 2019, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Musetti, S.; Huang, L. Nanoparticle-Mediated Remodeling of the Tumor Microenvironment to Enhance Immunotherapy. ACS Nano 2018, 12, 11740–11755. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.A.; Patil, Y.; La-Beck, N.M. New insights and evolving role of pegylated liposomal doxorubicin in cancer therapy. Drug. Resist. Update 2016, 29. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Pastucha, M.; Farka, Z.; Lacina, K.; Mikušová, Z.; Skládal, P. Magnetic nanoparticles for smart electrochemical immunoassays: A review on recent developments. Mikrochim. Acta 2019, 186, 312. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Keren, L.; Bosse, M.; Marquez, D.; Angoshtari, R.; Jain, S.; Varma, S.; Yang, S.-R.; Kurian, A.; Van Valen, D.; West, R.; et al. A Structured Tumor-Immune Microenvironment in Triple Negative Breast Cancer Revealed by Multiplexed Ion Beam Imaging. Cell 2018, 174, 1373–1387.e1319. [Google Scholar] [CrossRef] [PubMed]
- Meurette, O.; Mehlen, P. Notch Signaling in the Tumor Microenvironment. Cancer Cell 2018, 34, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Cong, V.T.; Gaus, K.; Tilley, R.D.; Gooding, J.J. Rod-shaped mesoporous silica nanoparticles for nanomedicine: Recent progress and perspectives. Expert Opin. Drug Deliv. 2018, 15, 881–892. [Google Scholar] [CrossRef] [PubMed]
- El-Sawy, H.S.; Al-Abd, A.M.; Ahmed, T.A.; El-Say, K.M.; Torchilin, V.P. Stimuli-Responsive Nano-Architecture Drug-Delivery Systems to Solid Tumor Micromilieu: Past, Present, and Future Perspectives. ACS Nano 2018, 12, 10636–10664. [Google Scholar] [CrossRef]
- Shi, Y.; Lammers, T. Combining Nanomedicine and Immunotherapy. Acc. Chem. Res. 2019, 52, 1543–1554. [Google Scholar] [CrossRef]
- Peng, J.; Yang, Q.; Shi, K.; Xiao, Y.; Wei, X.; Qian, Z. Intratumoral fate of functional nanoparticles in response to microenvironment factor: Implications on cancer diagnosis and therapy. Adv. Drug Deliv. Rev. 2019, 143, 37–67. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, A.; Memon, D.; Pourpe, S.; Veeraraghavan, H.; Li, Y.; Vargas, H.A.; Gill, M.B.; Park, K.J.; Zivanovic, O.; Konner, J.; et al. Heterogeneous Tumor-Immune Microenvironments among Differentially Growing Metastases in an Ovarian Cancer Patient. Cell 2017, 170, 927–938.e920. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Guo, C.; Xiong, F.; Yu, J.; Ge, J.; Wang, H.; Liao, Q.; Zhou, Y.; Gong, Q.; Xiang, B.; et al. Single cell RNA-seq reveals the landscape of tumor and infiltrating immune cells in nasopharyngeal carcinoma. Cancer Lett. 2020, 477, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Thommen, D.S.; Schumacher, T.N. T Cell Dysfunction in Cancer. Cancer Cell 2018, 33, 547–562. [Google Scholar] [CrossRef]
- Zhang, Y.; Kurupati, R.; Liu, L.; Zhou, X.Y.; Zhang, G.; Hudaihed, A.; Filisio, F.; Giles-Davis, W.; Xu, X.; Karakousis, G.C.; et al. Enhancing CD8 T Cell Fatty Acid Catabolism within a Metabolically Challenging Tumor Microenvironment Increases the Efficacy of Melanoma Immunotherapy. Cancer Cell 2017, 32, 377–391.e379. [Google Scholar] [CrossRef]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Manic, G.; Coussens, L.M.; Kroemer, G.; Galluzzi, L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019, 30, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Baert, T.; Vankerckhoven, A.; Riva, M.; Van Hoylandt, A.; Thirion, G.; Holger, G.; Mathivet, T.; Vergote, I.; Coosemans, A. Myeloid Derived Suppressor Cells: Key Drivers of Immunosuppression in Ovarian Cancer. Front. Immunol. 2019, 10, 1273. [Google Scholar] [CrossRef]
- Shabani, M.; Sadeghi, A.; Hosseini, H.; Teimouri, M.; Babaei Khorzoughi, R.; Pasalar, P.; Meshkani, R. Resveratrol alleviates obesity-induced skeletal muscle inflammation via decreasing M1 macrophage polarization and increasing the regulatory T cell population. Sci. Rep. 2020, 10, 3791. [Google Scholar] [CrossRef]
- Mathivet, T.; Bouleti, C.; Van Woensel, M.; Stanchi, F.; Verschuere, T.; Phng, L.-K.; Dejaegher, J.; Balcer, M.; Matsumoto, K.; Georgieva, P.B.; et al. Dynamic stroma reorganization drives blood vessel dysmorphia during glioma growth. EMBO Mol. Med. 2017, 9, 1629–1645. [Google Scholar] [CrossRef]
- Nawaz, A.; Aminuddin, A.; Kado, T.; Takikawa, A.; Yamamoto, S.; Tsuneyama, K.; Igarashi, Y.; Ikutani, M.; Nishida, Y.; Nagai, Y.; et al. CD206 M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nat. Commun. 2017, 8, 286. [Google Scholar] [CrossRef]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef]
- Logtenberg, M.E.W.; Jansen, J.H.M.; Raaben, M.; Toebes, M.; Franke, K.; Brandsma, A.M.; Matlung, H.L.; Fauster, A.; Gomez-Eerland, R.; Bakker, N.A.M.; et al. Glutaminyl cyclase is an enzymatic modifier of the CD47- SIRPα axis and a target for cancer immunotherapy. Nat. Med. 2019, 25, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Veillette, A.; Tang, Z. Signaling Regulatory Protein (SIRP)α-CD47 Blockade Joins the Ranks of Immune Checkpoint Inhibition. J. Clin. Oncol. 2019, 37, 1012–1014. [Google Scholar] [CrossRef] [PubMed]
- Arce Vargas, F.; Furness, A.J.S.; Solomon, I.; Joshi, K.; Mekkaoui, L.; Lesko, M.H.; Miranda Rota, E.; Dahan, R.; Georgiou, A.; Sledzinska, A.; et al. Fc-Optimized Anti-CD25 Depletes Tumor-Infiltrating Regulatory T Cells and Synergizes with PD-1 Blockade to Eradicate Established Tumors. Immunity 2017, 46, 577–586. [Google Scholar] [CrossRef]
- Matoba, T.; Imai, M.; Ohkura, N.; Kawakita, D.; Ijichi, K.; Toyama, T.; Morita, A.; Murakami, S.; Sakaguchi, S.; Yamazaki, S. Regulatory T cells expressing abundant CTLA-4 on the cell surface with a proliferative gene profile are key features of human head and neck cancer. Int. J. Cancer 2019, 144, 2811–2822. [Google Scholar] [CrossRef]
- Lu, L.; Barbi, J.; Pan, F. The regulation of immune tolerance by FOXP3. Nat. Rev. Immunol. 2017, 17, 703–717. [Google Scholar] [CrossRef]
- Pierini, A.; Nishikii, H.; Baker, J.; Kimura, T.; Kwon, H.-S.; Pan, Y.; Chen, Y.; Alvarez, M.; Strober, W.; Velardi, A.; et al. Foxp3 regulatory T cells maintain the bone marrow microenvironment for B cell lymphopoiesis. Nat. Commun. 2017, 8, 15068. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Mikami, N.; Wing, J.B.; Tanaka, A.; Ichiyama, K.; Ohkura, N. Regulatory T Cells and Human Disease. Annu Rev. Immunol. 2020, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liao, X.; Kang, Y. Tregs: Where We Are and What Comes Next? Front. Immunol. 2017, 8, 1578. [Google Scholar] [CrossRef]
- Kryczek, I.; Wang, L.; Wu, K.; Li, W.; Zhao, E.; Cui, T.; Wei, S.; Liu, Y.; Wang, Y.; Vatan, L.; et al. Inflammatory regulatory T cells in the microenvironments of ulcerative colitis and colon carcinoma. Oncoimmunology 2016, 5, e1105430. [Google Scholar] [CrossRef]
- Altorki, N.K.; Markowitz, G.J.; Gao, D.; Port, J.L.; Saxena, A.; Stiles, B.; McGraw, T.; Mittal, V. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat. Rev. Cancer 2019, 19, 9–31. [Google Scholar] [CrossRef] [PubMed]
- Riera-Domingo, C.; Audigé, A.; Granja, S.; Cheng, W.-C.; Ho, P.-C.; Baltazar, F.; Stockmann, C.; Mazzone, M. Immunity, Hypoxia, and Metabolism-the Ménage à Trois of Cancer: Implications for Immunotherapy. Physiol. Rev. 2020, 100, 1–102. [Google Scholar] [CrossRef] [PubMed]
- Valanparambil, R.M.; Tam, M.; Gros, P.-P.; Auger, J.-P.; Segura, M.; Gros, P.; Jardim, A.; Geary, T.G.; Ozato, K.; Stevenson, M.M. IRF-8 regulates expansion of myeloid-derived suppressor cells and Foxp3+ regulatory T cells and modulates Th2 immune responses to gastrointestinal nematode infection. PLoS Pathog. 2017, 13, e1006647. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Du, W.; Yan, F.; Wang, Y.; Li, H.; Cao, S.; Yu, W.; Shen, C.; Liu, J.; Ren, X. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J. Immunol. 2013, 190, 3783–3797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef]
- Su, X.; Fan, Y.; Yang, L.; Huang, J.; Qiao, F.; Fang, Y.; Wang, J. Dexmedetomidine expands monocytic myeloid-derived suppressor cells and promotes tumour metastasis after lung cancer surgery. J. Transl. Med. 2018, 16, 347. [Google Scholar] [CrossRef]
- Niklason, L.E. Understanding the Extracellular Matrix to Enhance Stem Cell-Based Tissue Regeneration. Cell Stem Cell 2018, 22, 302–305. [Google Scholar] [CrossRef]
- Kalluri, R. Basement membranes: Structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer 2003, 3, 422–433. [Google Scholar] [CrossRef]
- Chen, H.; Qu, J.; Huang, X.; Kurundkar, A.; Zhu, L.; Yang, N.; Venado, A.; Ding, Q.; Liu, G.; Antony, V.B.; et al. Mechanosensing by the α6-integrin confers an invasive fibroblast phenotype and mediates lung fibrosis. Nat. Commun. 2016, 7, 12564. [Google Scholar] [CrossRef]
- Yosef, G.; Arkadash, V.; Papo, N. Targeting the MMP-14/MMP-2/integrin αβ axis with multispecific N-TIMP2-based antagonists for cancer therapy. J. Biol. Chem. 2018, 293, 13310–13326. [Google Scholar] [CrossRef] [PubMed]
- Castro-Castro, A.; Marchesin, V.; Monteiro, P.; Lodillinsky, C.; Rossé, C.; Chavrier, P. Cellular and Molecular Mechanisms of MT1-MMP-Dependent Cancer Cell Invasion. Annu Rev. Cell Dev. Biol. 2016, 32, 555–576. [Google Scholar] [CrossRef] [PubMed]
- Huels, D.J.; Medema, J.P. Think About the Environment: Cellular Reprogramming by the Extracellular Matrix. Cell Stem Cell 2018, 22, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Hawk, M.A.; Gorsuch, C.L.; Fagan, P.; Lee, C.; Kim, S.E.; Hamann, J.C.; Mason, J.A.; Weigel, K.J.; Tsegaye, M.A.; Shen, L.; et al. RIPK1-mediated induction of mitophagy compromises the viability of extracellular-matrix-detached cells. Nat. Cell Biol. 2018, 20, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Cadamuro, M.; Brivio, S.; Mertens, J.; Vismara, M.; Moncsek, A.; Milani, C.; Fingas, C.; Cristina Malerba, M.; Nardo, G.; Dall'Olmo, L.; et al. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J. Hepatol. 2019, 70, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Roswall, P.; Bocci, M.; Bartoschek, M.; Li, H.; Kristiansen, G.; Jansson, S.; Lehn, S.; Sjölund, J.; Reid, S.; Larsson, C.; et al. Microenvironmental control of breast cancer subtype elicited through paracrine platelet-derived growth factor-CC signaling. Nat. Med. 2018, 24, 463–473. [Google Scholar] [CrossRef]
- Kumar, V.; Donthireddy, L.; Marvel, D.; Condamine, T.; Wang, F.; Lavilla-Alonso, S.; Hashimoto, A.; Vonteddu, P.; Behera, R.; Goins, M.A.; et al. Cancer-Associated Fibroblasts Neutralize the Anti-tumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumors. Cancer Cell 2017, 32, 654–668.e655. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Xiao, M.; Wang, C.; Qin, Z. FSP1+ fibroblasts promote skin carcinogenesis by maintaining MCP-1-mediated macrophage infiltration and chronic inflammation. Am. J. Pathol. 2011, 178, 382–390. [Google Scholar] [CrossRef]
- Moore-Smith, L.D.; Isayeva, T.; Lee, J.H.; Frost, A.; Ponnazhagan, S. Silencing of TGF-β1 in tumor cells impacts MMP-9 in tumor microenvironment. Sci. Rep. 2017, 7, 8678. [Google Scholar] [CrossRef]
- Yang, J.; Lu, Y.; Lin, Y.-Y.; Zheng, Z.-Y.; Fang, J.-H.; He, S.; Zhuang, S.-M. Vascular mimicry formation is promoted by paracrine TGF-β and SDF1 of cancer-associated fibroblasts and inhibited by miR-101 in hepatocellular carcinoma. Cancer Lett. 2016, 383, 18–27. [Google Scholar] [CrossRef]
- Curtis, M.; Kenny, H.A.; Ashcroft, B.; Mukherjee, A.; Johnson, A.; Zhang, Y.; Helou, Y.; Batlle, R.; Liu, X.; Gutierrez, N.; et al. Fibroblasts Mobilize Tumor Cell Glycogen to Promote Proliferation and Metastasis. Cell Metab. 2019, 29. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Yang, G.; Hou, Y.; Tang, X.; Wu, C.; Wu, X.A.; Guo, L.; Zhu, Q.; Luo, H.; Du, Y.E.; et al. Cytoplasmic GPER translocation in cancer-associated fibroblasts mediates cAMP/PKA/CREB/glycolytic axis to confer tumor cells with multidrug resistance. Oncogene 2017, 36, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- Demircioglu, F.; Wang, J.; Candido, J.; Costa, A.S.H.; Casado, P.; de Luxan Delgado, B.; Reynolds, L.E.; Gomez-Escudero, J.; Newport, E.; Rajeeve, V.; et al. Cancer associated fibroblast FAK regulates malignant cell metabolism. Nat. Commun. 2020, 11, 1290. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R.; et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef]
- Luga, V.; Zhang, L.; Viloria-Petit, A.M.; Ogunjimi, A.A.; Inanlou, M.R.; Chiu, E.; Buchanan, M.; Hosein, A.N.; Basik, M.; Wrana, J.L. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012, 151, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S.; Yao, J.; Lowery, F.J.; Zhang, Q.; Huang, W.-C.; Li, P.; Li, M.; Wang, X.; Zhang, C.; et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 2015, 527, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Guo, X.; Qiu, W.; Wang, J.; Liu, Q.; Qian, M.; Wang, S.; Zhang, Z.; Gao, X.; Chen, Z.; Guo, Q.; et al. Glioma exosomes mediate the expansion and function of myeloid-derived suppressor cells through microRNA-29a/Hbp1 and microRNA-92a/Prkar1a pathways. Int. J. Cancer 2019, 144, 3111–3126. [Google Scholar] [CrossRef]
- Guo, X.; Qiu, W.; Liu, Q.; Qian, M.; Wang, S.; Zhang, Z.; Gao, X.; Chen, Z.; Xue, H.; Li, G. Immunosuppressive effects of hypoxia-induced glioma exosomes through myeloid-derived suppressor cells via the miR-10a/Rora and miR-21/Pten Pathways. Oncogene 2018, 37, 4239–4259. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Nichols, J.W.; Toh, K.; Nomoto, T.; Cabral, H.; Miura, Y.; Christie, R.J.; Yamada, N.; Ogura, T.; Kano, M.R.; et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat. Nanotechnol. 2016, 11, 533–538. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, S.; Hong, J.; Soutto, M.; Peng, D.; Belkhiri, A.; Xu, Z.; El-Rifai, W. Gastric tumour-derived ANGPT2 regulation by DARPP-32 promotes angiogenesis. Gut 2016, 65, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Duffy, B.A.; Badar, A.; Lythgoe, M.F.; Årstad, E. Bimodal Imaging of Inflammation with SPECT/CT and MRI Using Iodine-125 Labeled VCAM-1 Targeting Microparticle Conjugates. Bioconjug. Chem. 2015, 26, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kim, B.Y.S.; Chan, C.K.; Hahn, S.M.; Weissman, I.L.; Jiang, W. Improving immune-vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Maman, S.; Witz, I.P. A history of exploring cancer in context. Nat. Rev. Cancer 2018, 18, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liang, Y.; Kang, L.; Liu, Y.; Gao, S.; Chen, S.; Li, Y.; You, W.; Dong, Q.; Hong, T.; et al. Transcriptional Regulation of the Warburg Effect in Cancer by SIX1. Cancer Cell 2018, 33, 368–385. [Google Scholar] [CrossRef]
- Lin, B.; Chen, H.; Liang, D.; Lin, W.; Qi, X.; Liu, H.; Deng, X. Acidic pH and High-HO Dual Tumor Microenvironment-Responsive Nanocatalytic Graphene Oxide for Cancer Selective Therapy and Recognition. ACS Appl. Mater. Interfaces 2019, 11, 11157–11166. [Google Scholar] [CrossRef] [PubMed]
- Keith, B.; Johnson, R.S.; Simon, M.C. HIF1α and HIF2α: Sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 2011, 12, 9–22. [Google Scholar] [CrossRef]
- Massari, F.; Ciccarese, C.; Santoni, M.; Iacovelli, R.; Mazzucchelli, R.; Piva, F.; Scarpelli, M.; Berardi, R.; Tortora, G.; Lopez-Beltran, A.; et al. Metabolic phenotype of bladder cancer. Cancer Treat. Rev. 2016, 45, 46–57. [Google Scholar] [CrossRef]
- Chen, F.; Chen, J.; Yang, L.; Liu, J.; Zhang, X.; Zhang, Y.; Tu, Q.; Yin, D.; Lin, D.; Wong, P.-P.; et al. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat. Cell Biol. 2019, 21, 498–510. [Google Scholar] [CrossRef]
- Kepp, O.; Loos, F.; Liu, P.; Kroemer, G. Extracellular nucleosides and nucleotides as immunomodulators. Immunol. Rev. 2017, 280, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, A.F.; Mahmoud, W.; Al-Harizy, R.M. Targeting glucose metabolism to suppress cancer progression: Prospective of anti-glycolytic cancer therapy. Pharmacol. Res. 2019, 150, 104511. [Google Scholar] [CrossRef]
- Mo, R.; Jiang, T.; DiSanto, R.; Tai, W.; Gu, Z. ATP-triggered anticancer drug delivery. Nat. Commun. 2014, 5, 3364. [Google Scholar] [CrossRef] [PubMed]
- Sameiyan, E.; Bagheri, E.; Dehghani, S.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. Aptamer-based ATP-responsive delivery systems for cancer diagnosis and treatment. Acta Biomater. 2021, 123, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, S.M.; Kjaergaard, J.; Lukashev, D.; Schreiber, T.H.; Belikoff, B.; Abbott, R.; Sethumadhavan, S.; Philbrook, P.; Ko, K.; Cannici, R.; et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci. Transl. Med. 2015, 7, 277ra230. [Google Scholar] [CrossRef]
- Ogiwara, H.; Takahashi, K.; Sasaki, M.; Kuroda, T.; Yoshida, H.; Watanabe, R.; Maruyama, A.; Makinoshima, H.; Chiwaki, F.; Sasaki, H.; et al. Targeting the Vulnerability of Glutathione Metabolism in ARID1A-Deficient Cancers. Cancer Cell 2019, 35, 9–22. [Google Scholar] [CrossRef]
- Yang, J.; Wang, C.; Shi, S.; Dong, C. Nanotechnologies for enhancing cancer immunotherapy. Nano Res. 2020; 1–22. [Google Scholar] [CrossRef]
- Peranzoni, E.; Lemoine, J.; Vimeux, L.; Feuillet, V.; Barrin, S.; Kantari-Mimoun, C.; Bercovici, N.; Guérin, M.; Biton, J.; Ouakrim, H.; et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc. Natl. Acad. Sci. USA 2018, 115, E4041–E4050. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Wang, J.; Wen, D.; Zhang, Y.; Lu, Y.; et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef]
- Shi, C.; Liu, T.; Guo, Z.; Zhuang, R.; Zhang, X.; Chen, X. Reprogramming Tumor-Associated Macrophages by Nanoparticle-Based Reactive Oxygen Species Photogeneration. Nano Lett. 2018, 18, 7330–7342. [Google Scholar] [CrossRef]
- Pal, R.; Chakraborty, B.; Nath, A.; Singh, L.M.; Ali, M.; Rahman, D.S.; Ghosh, S.K.; Basu, A.; Bhattacharya, S.; Baral, R.; et al. Noble metal nanoparticle-induced oxidative stress modulates tumor associated macrophages (TAMs) from an M2 to M1 phenotype: An in vitro approach. Int. Immunopharmacol. 2016, 38, 332–341. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, L.; Wang, C.; Han, Y.; Lu, Y.; Liu, J.; Hu, X.; Yao, T.; Lin, Y.; Liang, S.; et al. Tumor-Targeted Drug and CpG Delivery System for Phototherapy and Docetaxel-Enhanced Immunotherapy with Polarization toward M1-Type Macrophages on Triple Negative Breast Cancers. Adv. Mater. 2019, 31, e1904997. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, Y.; Fang, Y.; Zhang, M.; Wang, H.; He, Z.; Wang, B.; Xu, Q.; Huang, Y. Reprogramming Tumor Immune Microenvironment (TIME) and Metabolism via Biomimetic Targeting Codelivery of Shikonin/JQ1. Nano Lett. 2019, 19, 2935–2944. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Li, R.; Ng, T.S.C.; Courties, G.; Rodell, C.B.; Prytyskach, M.; Kohler, R.H.; Pittet, M.J.; Nahrendorf, M.; Weissleder, R.; et al. Quantitative Imaging of Tumor-Associated Macrophages and Their Response to Therapy Using Cu-Labeled Macrin. ACS Nano 2018, 12, 12015–12029. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; O'Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Zhao, Y.; Satterlee, A.B.; Wang, Y.; Xu, Y.; Huang, L. Tumor-targeted delivery of sunitinib base enhances vaccine therapy for advanced melanoma by remodeling the tumor microenvironment. J. Control. Release 2017, 245, 81–94. [Google Scholar] [CrossRef]
- Ou, W.; Thapa, R.K.; Jiang, L.; Soe, Z.C.; Gautam, M.; Chang, J.-H.; Jeong, J.-H.; Ku, S.K.; Choi, H.-G.; Yong, C.S.; et al. Regulatory T cell-targeted hybrid nanoparticles combined with immuno-checkpoint blockage for cancer immunotherapy. J. Control. Release 2018, 281, 84–96. [Google Scholar] [CrossRef]
- Dong, X.; Liang, J.; Yang, A.; Qian, Z.; Kong, D.; Lv, F. Fluorescence imaging guided CpG nanoparticles-loaded IR820-hydrogel for synergistic photothermal immunotherapy. Biomaterials 2019, 209, 111–125. [Google Scholar] [CrossRef]
- Shae, D.; Becker, K.W.; Christov, P.; Yun, D.S.; Lytton-Jean, A.K.R.; Sevimli, S.; Ascano, M.; Kelley, M.; Johnson, D.B.; Balko, J.M.; et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 2019, 14, 269–278. [Google Scholar] [CrossRef]
- Prendergast, G.C.; Malachowski, W.P.; DuHadaway, J.B.; Muller, A.J. Discovery of IDO1 Inhibitors: From Bench to Bedside. Cancer Res. 2017, 77, 6795–6811. [Google Scholar] [CrossRef]
- Medzhitov, R.; Shevach, E.M.; Trinchieri, G.; Mellor, A.L.; Munn, D.H.; Gordon, S.; Libby, P.; Hansson, G.K.; Shortman, K.; Dong, C.; et al. Highlights of 10 years of immunology in Nature Reviews Immunology. Nat. Rev. Immunol. 2011, 11, 693–702. [Google Scholar] [CrossRef]
- Smith, C.; Chang, M.Y.; Parker, K.H.; Beury, D.W.; DuHadaway, J.B.; Flick, H.E.; Boulden, J.; Sutanto-Ward, E.; Soler, A.P.; Laury-Kleintop, L.D.; et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012, 2, 722–735. [Google Scholar] [CrossRef]
- Cheng, K.; Ding, Y.; Zhao, Y.; Ye, S.; Zhao, X.; Zhang, Y.; Ji, T.; Wu, H.; Wang, B.; Anderson, G.J.; et al. Sequentially Responsive Therapeutic Peptide Assembling Nanoparticles for Dual-Targeted Cancer Immunotherapy. Nano Lett. 2018, 18, 3250–3258. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y.; Zhang, L.; Huang, L. Nanoparticle-delivered transforming growth factor-β siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano 2014, 8, 3636–3645. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, L.; Hu, X.; Lu, Y.; Liu, W.; Sun, Y.; Yao, T.; Dong, C.; Shi, S. A CuS nanoparticle-based CpG delivery system for synergistic photothermal-, photodynamic- and immunotherapy. Commun. Biol. 2020, 3, 343. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Igaz, N.; Marton, A.; Rónavári, A.; Bélteky, P.; Bodai, L.; Spengler, G.; Tiszlavicz, L.; Rázga, Z.; Hegyi, P.; et al. Core-shell nanoparticles suppress metastasis and modify the tumour-supportive activity of cancer-associated fibroblasts. J. Nanobiotechnol. 2020, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Liu, Q.; Shen, L.; Liu, Y.; Zhang, X.; Chen, F.; Huang, L. Nano-delivery of fraxinellone remodels tumor microenvironment and facilitates therapeutic vaccination in desmoplastic melanoma. Theranostics 2018, 8, 3781–3796. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, L.; Zhang, T.; Shen, R.; Li, C.; Fang, X.; Griffiths, G.; Liu, J. Sensitive Detection of MMP9 Enzymatic Activities in Single Cell-Encapsulated Microdroplets as an Assay of Cancer Cell Invasiveness. ACS Sens. 2017, 2, 626–634. [Google Scholar] [CrossRef]
- Van Rijt, S.H.; Bölükbas, D.A.; Argyo, C.; Datz, S.; Lindner, M.; Eickelberg, O.; Königshoff, M.; Bein, T.; Meiners, S. Protease-mediated release of chemotherapeutics from mesoporous silica nanoparticles to ex vivo human and mouse lung tumors. ACS Nano 2015, 9, 2377–2389. [Google Scholar] [CrossRef]
- Ji, T.; Lang, J.; Wang, J.; Cai, R.; Zhang, Y.; Qi, F.; Zhang, L.; Zhao, X.; Wu, W.; Hao, J.; et al. Designing Liposomes To Suppress Extracellular Matrix Expression To Enhance Drug Penetration and Pancreatic Tumor Therapy. ACS Nano 2017, 11, 8668–8678. [Google Scholar] [CrossRef]
- Gong, H.; Chao, Y.; Xiang, J.; Han, X.; Song, G.; Feng, L.; Liu, J.; Yang, G.; Chen, Q.; Liu, Z. Hyaluronidase To Enhance Nanoparticle-Based Photodynamic Tumor Therapy. Nano Lett. 2016, 16, 2512–2521. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fan, Z.; Deng, J.; Lemons, P.K.; Arhontoulis, D.C.; Bowne, W.B.; Cheng, H. Hyaluronidase Embedded in Nanocarrier PEG Shell for Enhanced Tumor Penetration and Highly Efficient Antitumor Efficacy. Nano Lett. 2016, 16, 3268–3277. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Chen, J.; Hu, Y.; Lin, L.; Sun, P.; Tian, H.; Chen, X. Highly enhanced cancer immunotherapy by combining nanovaccine with hyaluronidase. Biomaterials 2018, 171, 198–206. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Lin, Y.-S.; Brodsky, B. Collagen interactions: Drug design and delivery. Adv. Drug Deliv. Rev. 2016, 97, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Van den Boorn, J.G.; Dassler, J.; Coch, C.; Schlee, M.; Hartmann, G. Exosomes as nucleic acid nanocarriers. Adv. Drug Deliv. Rev. 2013, 65, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhu, M.; Tian, Y.; Ramm, G.A.; Zhao, Y.; Nie, G. A membrane vesicle-based dual vaccine against melanoma and Lewis lung carcinoma. Biomaterials 2012, 33, 6147–6154. [Google Scholar] [CrossRef]
- Xiong, F.; Ling, X.; Chen, X.; Chen, J.; Tan, J.; Cao, W.; Ge, L.; Ma, M.; Wu, J. Pursuing Specific Chemotherapy of Orthotopic Breast Cancer with Lung Metastasis from Docking Nanoparticles Driven by Bioinspired Exosomes. Nano Lett. 2019, 19, 3256–3266. [Google Scholar] [CrossRef]
- Liu, C.; Xu, X.; Li, B.; Situ, B.; Pan, W.; Hu, Y.; An, T.; Yao, S.; Zheng, L. Single-Exosome-Counting Immunoassays for Cancer Diagnostics. Nano Lett. 2018, 18, 4226–4232. [Google Scholar] [CrossRef]
- Lewis, J.M.; Vyas, A.D.; Qiu, Y.; Messer, K.S.; White, R.; Heller, M.J. Integrated Analysis of Exosomal Protein Biomarkers on Alternating Current Electrokinetic Chips Enables Rapid Detection of Pancreatic Cancer in Patient Blood. ACS Nano 2018, 12, 3311–3320. [Google Scholar] [CrossRef]
- Goos, J.A.C.M.; Cho, A.; Carter, L.M.; Dilling, T.R.; Davydova, M.; Mandleywala, K.; Puttick, S.; Gupta, A.; Price, W.S.; Quinn, J.F.; et al. Delivery of polymeric nanostars for molecular imaging and endoradiotherapy through the enhanced permeability and retention (EPR) effect. Theranostics 2020, 10, 567–584. [Google Scholar] [CrossRef]
- Tee, J.K.; Yip, L.X.; Tan, E.S.; Santitewagun, S.; Prasath, A.; Ke, P.C.; Ho, H.K.; Leong, D.T. Nanoparticles' interactions with vasculature in diseases. Chem. Soc. Rev. 2019, 48, 5381–5407. [Google Scholar] [CrossRef]
- Kinoshita, R.; Ishima, Y.; Chuang, V.T.G.; Nakamura, H.; Fang, J.; Watanabe, H.; Shimizu, T.; Okuhira, K.; Ishida, T.; Maeda, H.; et al. Improved anticancer effects of albumin-bound paclitaxel nanoparticle via augmentation of EPR effect and albumin-protein interactions using S-nitrosated human serum albumin dimer. Biomaterials 2017, 140, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Ko, H.; You, D.G.; Kataoka, K.; Park, J.H. Nanomedicines for Reactive Oxygen Species Mediated Approach: An Emerging Paradigm for Cancer Treatment. Acc. Chem. Res. 2019, 52, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Chen, F.; Hong, H.; Valdovinos, H.F.; Hernandez, R.; Shi, S.; Barnhart, T.E.; Cai, W. VEGF121-conjugated mesoporous silica nanoparticle: A tumor targeted drug delivery system. ACS Appl. Mater. Interfaces 2014, 6, 21677–21685. [Google Scholar] [CrossRef] [PubMed]
- Graf, N.; Bielenberg, D.R.; Kolishetti, N.; Muus, C.; Banyard, J.; Farokhzad, O.C.; Lippard, S.J. α(V)β(3) integrin-targeted PLGA-PEG nanoparticles for enhanced anti-tumor efficacy of a Pt(IV) prodrug. ACS Nano 2012, 6, 4530–4539. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, S.; Hao, J. Non-Invasive Optical Guided Tumor Metastasis/Vessel Imaging by Using Lanthanide Nanoprobe with Enhanced Down-Shifting Emission beyond 1500 nm. ACS Nano 2019, 13, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, A.; Raffa, V.; Canfarotta, F.; Signore, G.; Piletsky, S.; MacDonald, M.P.; Cuschieri, A. In Vivo Recognition of Human Vascular Endothelial Growth Factor by Molecularly Imprinted Polymers. Nano Lett. 2017, 17, 2307–2312. [Google Scholar] [CrossRef]
- Li, M.; Gao, Y.; Yuan, Y.; Wu, Y.; Song, Z.; Tang, B.Z.; Liu, B.; Zheng, Q.C. One-Step Formulation of Targeted Aggregation-Induced Emission Dots for Image-Guided Photodynamic Therapy of Cholangiocarcinoma. ACS Nano 2017, 11, 3922–3932. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhang, K.; Wang, B.; Wu, S.; Wang, Y.; Zhang, H.; Zhang, Z.; Liu, J.; Shi, J. Nanoenabled Disruption of Multiple Barriers in Antigen Cross-Presentation of Dendritic Cells Calcium Interference for Enhanced Chemo-Immunotherapy. ACS Nano 2020, 14, 7639–7650. [Google Scholar] [CrossRef]
- Nie, W.; Wu, G.; Zhang, J.; Huang, L.-L.; Ding, J.; Jiang, A.; Zhang, Y.; Liu, Y.; Li, J.; Pu, K.; et al. Responsive Exosome Nano-bioconjugates for Synergistic Cancer Therapy. Angew. Chem. Int. Ed. Engl. 2020, 59, 2018–2022. [Google Scholar] [CrossRef] [PubMed]
- Rey, S.; Schito, L.; Koritzinsky, M.; Wouters, B.G. Molecular targeting of hypoxia in radiotherapy. Adv. Drug. Deliv. Rev. 2017, 109, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, Y.; Zhang, M.; Tang, Z.; He, M.; Bu, W. Modulating Hypoxia via Nanomaterials Chemistry for Efficient Treatment of Solid Tumors. Acc. Chem. Res. 2018, 51, 2502–2511. [Google Scholar] [CrossRef]
- Tang, W.; Zhen, Z.; Wang, M.; Wang, H.; Chuang, Y.-J.; Zhang, W.; Wang, G.D.; Todd, T.; Cowger, T.; Chen, H.; et al. Red Blood Cell-Facilitated Photodynamic Therapy for Cancer Treatment. Adv. Funct. Mater. 2016, 26, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liang, C.; Song, X.; Chen, Q.; Jin, Q.; Wang, C.; Liu, Z. Erythrocyte-Membrane-Enveloped Perfluorocarbon as Nanoscale Artificial Red Blood Cells to Relieve Tumor Hypoxia and Enhance Cancer Radiotherapy. Adv. Mater. 2017, 29, 10. [Google Scholar] [CrossRef]
- Dong, Z.; Yang, Z.; Hao, Y.; Feng, L. Fabrication of HO-driven nanoreactors for innovative cancer treatments. Nanoscale 2019, 11, 16164–16186. [Google Scholar] [CrossRef]
- Meng, L.; Cheng, Y.; Tong, X.; Gan, S.; Ding, Y.; Zhang, Y.; Wang, C.; Xu, L.; Zhu, Y.; Wu, J.; et al. Tumor Oxygenation and Hypoxia Inducible Factor-1 Functional Inhibition via a Reactive Oxygen Species Responsive Nanoplatform for Enhancing Radiation Therapy and Abscopal Effects. ACS Nano 2018, 12, 8308–8322. [Google Scholar] [CrossRef]
- Hu, X.; Xu, Z.; Hu, J.; Dong, C.; Lu, Y.; Wu, X.; Wumaier, M.; Yao, T.; Shi, S. A redox-activated theranostic nanoplatform: Toward glutathione-response imaging guided enhanced-photodynamic therapy. Inorg. Chem. Front. 2019, 6, 2865–2872. [Google Scholar] [CrossRef]
- Hu, X.; Lu, Y.; Shi, X.; Yao, T.; Dong, C.; Shi, S. Integrating in situ formation of nanozymes with mesoporous polydopamine for combined chemo, photothermal and hypoxia-overcoming photodynamic therapy. Chem. Commun. 2019, 55, 14785–14788. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Qiang, M.; Ke, W.; Han, Y.; Mukerabigwi, J.F.; Ge, Z. Hypoxia-responsive block copolymer radiosensitizers as anticancer drug nanocarriers for enhanced chemoradiotherapy of bulky solid tumors. Biomaterials 2018, 181, 360–371. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, B.; Di, Z.; Zhang, G.; Sun, L.-D.; Li, L.; Yan, C.-H. Engineering of Upconverted Metal–Organic Frameworks for Near-Infrared Light-Triggered Combinational Photodynamic/Chemo-/Immunotherapy against Hypoxic Tumors. J. Am. Chem. Soc. 2020, 142, 3939–3946. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Cheng, H.; Xie, B.R.; Qiu, W.X.; Zeng, J.Y.; Li, C.X.; Wan, S.S.; Zhang, L.; Liu, W.L.; Zhang, X.Z. Cancer Cell Membrane Camouflaged Cascade Bioreactor for Cancer Targeted Starvation and Photodynamic Therapy. ACS Nano 2017, 11, 7006–7018. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, P.; Pan, W.; Li, N.; Tang, B. A biomimetic nanoreactor for synergistic chemiexcited photodynamic therapy and starvation therapy against tumor metastasis. Nat. Commun. 2018, 9, 5044. [Google Scholar] [CrossRef]
- de Souza, A.L.R.; Marra, K.; Gunn, J.; Samkoe, K.S.; Kanick, S.C.; Davis, S.C.; Chapman, M.S.; Maytin, E.V.; Hasan, T.; Pogue, B.W. Comparing desferrioxamine and light fractionation enhancement of ALA-PpIX photodynamic therapy in skin cancer. Br. J. Cancer 2016, 115, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Wei, H.; Li, Q.; Su, X.; Liu, S.; Zhang, K.Y.; Lv, W.; Zhao, Q.; Li, X.; Huang, W. Achieving efficient photodynamic therapy under both normoxia and hypoxia using cyclometalated Ru(ii) photosensitizer through type I photochemical process. Chem. Sci. 2018, 9, 502–512. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, F.; Gao, W.; Yang, X.; Ju, Y.; Zhao, L.; Guo, W.; Xie, J.; Liang, X.-J.; Tao, X.; et al. Magnetic Reactive Oxygen Species Nanoreactor for Switchable Magnetic Resonance Imaging Guided Cancer Therapy Based on pH-Sensitive FeC@FeO Nanoparticles. ACS Nano 2019, 13, 10002–10014. [Google Scholar] [CrossRef]
- Gu, Z.; Zhu, S.; Yan, L.; Zhao, F.; Zhao, Y. Graphene-Based Smart Platforms for Combined Cancer Therapy. Adv. Mater. 2019, 31, e1800662. [Google Scholar] [CrossRef]
- Poinard, B.; Neo, S.Z.Y.; Yeo, E.L.L.; Heng, H.P.S.; Neoh, K.G.; Kah, J.C.Y. Polydopamine Nanoparticles Enhance Drug Release for Combined Photodynamic and Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 21125–21136. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, J.; Luo, L.; Jiang, M.; Qin, B.; Yin, H.; Zhu, C.; Yuan, X.; Zhang, J.; Luo, Z.; et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat. Commun. 2019, 10, 3349. [Google Scholar] [CrossRef]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef]
- Nie, W.; Wei, W.; Zuo, L.; Lv, C.; Zhang, F.; Lu, G.-H.; Li, F.; Wu, G.; Huang, L.L.; Xi, X.; et al. Magnetic Nanoclusters Armed with Responsive PD-1 Antibody Synergistically Improved Adoptive T-Cell Therapy for Solid Tumors. ACS Nano 2019, 13, 1469–1478. [Google Scholar] [CrossRef]
- Chen, X.; Chen, T.; Ren, L.; Chen, G.; Gao, X.; Li, G.; Zhu, X. Triplex DNA Nanoswitch for pH-Sensitive Release of Multiple Cancer Drugs. ACS Nano 2019, 13, 7333–7344. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhao, X.; Zhang, Y.; Xu, J.; Xu, J.; Li, Y.; Min, H.; Shi, J.; Zhao, Y.; Wei, J.; et al. Engineering Biomimetic Platesomes for pH-Responsive Drug Delivery and Enhanced Antitumor Activity. Adv. Mater. 2019, 31, e1900795. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Wang, J.; Zhu, Z.; Li, R.; Wu, W.; Liu, B.; Jiang, X. Tumor accumulation, penetration, and antitumor response of cisplatin-loaded gelatin/poly(acrylic acid) nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Feng, L.; Hao, Y.; Chen, M.; Gao, M.; Chao, Y.; Zhao, H.; Zhu, W.; Liu, J.; Liang, C.; et al. Synthesis of Hollow Biomineralized CaCO-Polydopamine Nanoparticles for Multimodal Imaging-Guided Cancer Photodynamic Therapy with Reduced Skin Photosensitivity. J. Am. Chem. Soc. 2018, 140, 2165–2178. [Google Scholar] [CrossRef]
- Wei, R.; Gong, X.; Lin, H.; Zhang, K.; Li, A.; Liu, K.; Shan, H.; Chen, X.; Gao, J. Versatile Octapod-Shaped Hollow Porous Manganese(II) Oxide Nanoplatform for Real-Time Visualization of Cargo Delivery. Nano Lett. 2019, 19, 5394–5402. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K.; Bulow, U.; Diehl, W.E.; Durham, N.D.; Senjobe, F.; Chandran, K.; Luban, J.; Munro, J.B. Conformational changes in the Ebola virus membrane fusion machine induced by pH, Ca2+, and receptor binding. PLoS Biol. 2020, 18, e3000626. [Google Scholar] [CrossRef]
- Ma, T.; Hou, Y.; Zeng, J.; Liu, C.; Zhang, P.; Jing, L.; Shangguan, D.; Gao, M. Dual-Ratiometric Target-Triggered Fluorescent Probe for Simultaneous Quantitative Visualization of Tumor Microenvironment Protease Activity and pH in Vivo. J. Am. Chem. Soc. 2018, 140, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Mai, B.T.; Fernandes, S.; Balakrishnan, P.B.; Pellegrino, T. Nanosystems Based on Magnetic Nanoparticles and Thermo- or pH-Responsive Polymers: An Update and Future Perspectives. Acc. Chem. Res. 2018, 51, 999–1013. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, Y.; Sun, L.; Yuan, B.; Tian, Y.; Xiang, L.; Li, Y.; Li, Y.; Li, J.; Wu, A. Dual ATP and pH responsive ZIF-90 nanosystem with favorable biocompatibility and facile post-modification improves therapeutic outcomes of triple negative breast cancer in vivo. Biomaterials 2019, 197, 41–50. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, X.; Liu, J.; Guo, Y. ATP mediated rolling circle amplification and opening DNA-gate for drug delivery to cell. Talanta 2018, 176, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Du, C.; Sun, C.; Zhu, J.; Wu, S.; Zhang, Y.; Ji, T.; Lei, J.; Yang, Y.; Gao, N.; et al. Chaperonin-GroEL as a Smart Hydrophobic Drug Delivery and Tumor Targeting Molecular Machine for Tumor Therapy. Nano Lett. 2018, 18, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, G.; Xu, R.; Chen, H.; Wang, H.; Tian, G.; Wang, B.; Yang, C.; Bai, G.; Zhang, Z.; et al. A pH-responsive platform combining chemodynamic therapy with limotherapy for simultaneous bioimaging and synergistic cancer therapy. Biomaterials 2019, 216, 119254. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Li, R.; Khan, S.; Clanton, R.; Zhang, F.; Lin, Y.-N.; Song, Y.; Wang, H.; Fan, J.; Hernandez, S.; et al. Chemical Design of Both a Glutathione-Sensitive Dimeric Drug Guest and a Glucose-Derived Nanocarrier Host to Achieve Enhanced Osteosarcoma Lung Metastatic Anticancer Selectivity. J. Am. Chem. Soc. 2018, 140, 1438–1446. [Google Scholar] [CrossRef]
- Hu, Y.; Lv, T.; Ma, Y.; Xu, J.; Zhang, Y.; Hou, Y.; Huang, Z.; Ding, Y. Nanoscale Coordination Polymers for Synergistic NO and Chemodynamic Therapy of Liver Cancer. Nano Lett. 2019, 19, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Wen, D.; Wang, X.; Mahato, R.I. Dual responsive micelles capable of modulating miRNA-34a to combat taxane resistance in prostate cancer. Biomaterials 2019, 192, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Tu, J.; Wang, J.; Shajii, A.; Kong, N.; Feng, C.; Zhang, Y.; Yu, M.; Xie, T.; Bharwani, Z.; et al. Glutathione-Responsive Prodrug Nanoparticles for Effective Drug Delivery and Cancer Therapy. ACS Nano 2019, 13, 357–370. [Google Scholar] [CrossRef]
- Dong, X.; Liu, H.-J.; Feng, H.-Y.; Yang, S.-C.; Liu, X.-L.; Lai, X.; Lu, Q.; Lovell, J.F.; Chen, H.-Z.; Fang, C. Enhanced Drug Delivery by Nanoscale Integration of a Nitric Oxide Donor To Induce Tumor Collagen Depletion. Nano Lett. 2019, 19, 997–1008. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Wang, J.; Li, R.; Li, T.; Chang, M.; Yan, F.; Wang, Y. Encapsulation of Curcumin Nanoparticles with MMP9-Responsive and Thermos-Sensitive Hydrogel Improves Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 16315–16326. [Google Scholar] [CrossRef]
- Huang, A.; Honda, Y.; Li, P.; Tanaka, T.; Baba, S. Integration of Epigallocatechin Gallate in Gelatin Sponges Attenuates Matrix Metalloproteinase-Dependent Degradation and Increases Bone Formation. Int. J. Mol. Sci. 2019, 20, 6042. [Google Scholar] [CrossRef]
- Qin, S.-Y.; Feng, J.; Rong, L.; Jia, H.-Z.; Chen, S.; Liu, X.-J.; Luo, G.-F.; Zhuo, R.-X.; Zhang, X.-Z. Theranostic GO-based nanohybrid for tumor induced imaging and potential combinational tumor therapy. Small 2014, 10, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, D.; Chen, Q.; Li, C.; Li, Z.; Lin, J. Recent Advances in Glucose-Oxidase-Based Nanocomposites for Tumor Therapy. Small 2019, 15, e1903895. [Google Scholar] [CrossRef]
- Chang, M.; Wang, M.; Wang, M.; Shu, M.; Ding, B.; Li, C.; Pang, M.; Cui, S.; Hou, Z.; Lin, J. A Multifunctional Cascade Bioreactor Based on Hollow-Structured Cu MoS for Synergetic Cancer Chemo-Dynamic Therapy/Starvation Therapy/Phototherapy/Immunotherapy with Remarkably Enhanced Efficacy. Adv. Mater. 2019, 31, e1905271. [Google Scholar] [CrossRef]
- Zhang, X.; Xi, Z.; Machuki, J.O.a.; Luo, J.; Yang, D.; Li, J.; Cai, W.; Yang, Y.; Zhang, L.; Tian, J.; et al. Gold Cube-in-Cube Based Oxygen Nanogenerator: A Theranostic Nanoplatform for Modulating Tumor Microenvironment for Precise Chemo-Phototherapy and Multimodal Imaging. ACS Nano 2019, 13, 5306–5325. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Ni, K.; You, E.; Wang, M.; Culbert, A.; Jiang, X.; Lin, W. Multifunctional Nanoscale Metal-Organic Layers for Ratiometric pH and Oxygen Sensing. J. Am. Chem. Soc. 2019, 141, 18964–18969. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, Y.; Yan, J.; Chen, J.; Cao, J.; Pu, Y.; Li, L.; He, B. Redox/ATP switchable theranostic nanoparticles for real-time fluorescence monitoring of doxorubicin delivery. J. Mater. Chem. B 2018, 6, 2089–2103. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).