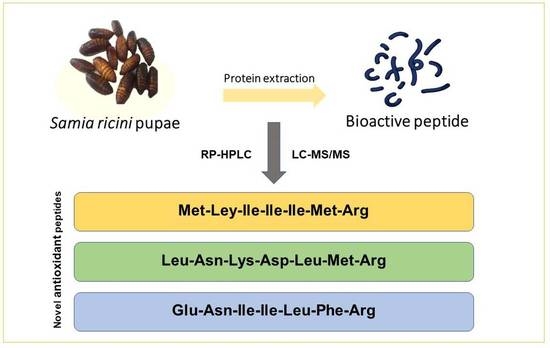
Purification and Identification of Novel Antioxidant Peptides from Enzymatically Hydrolysed Samia ricini Pupae
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Protein Extraction S. ricini
2.3. Preparation of Hydrolysates and Fractionation with Ultrafiltration Membranes
2.4. Purification of the Antioxidant Peptides from S. ricini Pupae
2.4.1. Ultrafiltration
2.4.2. RT-HPLC
2.4.3. LC-MS/MS
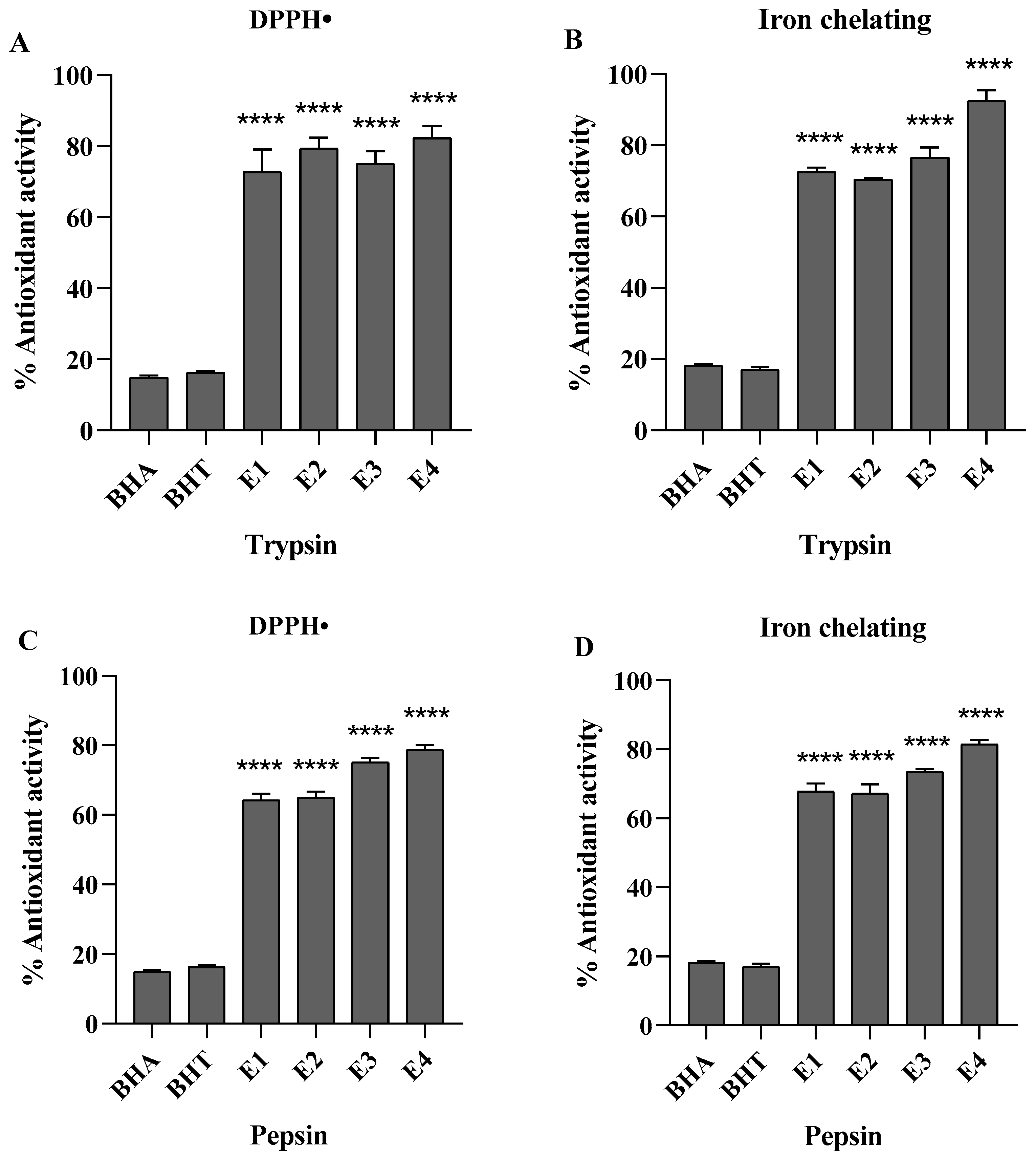
2.5. Determination of Antioxidant Activity
2.5.1. DPPH Radical Scavenging Activity
2.5.2. Iron Chelating Activity
2.6. Statistical Analysis
3. Results
3.1. Preparation of Protein Hydrolysates of S. ricini Pupae
3.2. Purification of Antioxidant Peptides from S. ricini Pupae
3.2.1. Ultrafiltration
3.2.2. Isolation of Peptide by Reversed-Phase High Performance Liquid Chromatography (RT-HPLC)
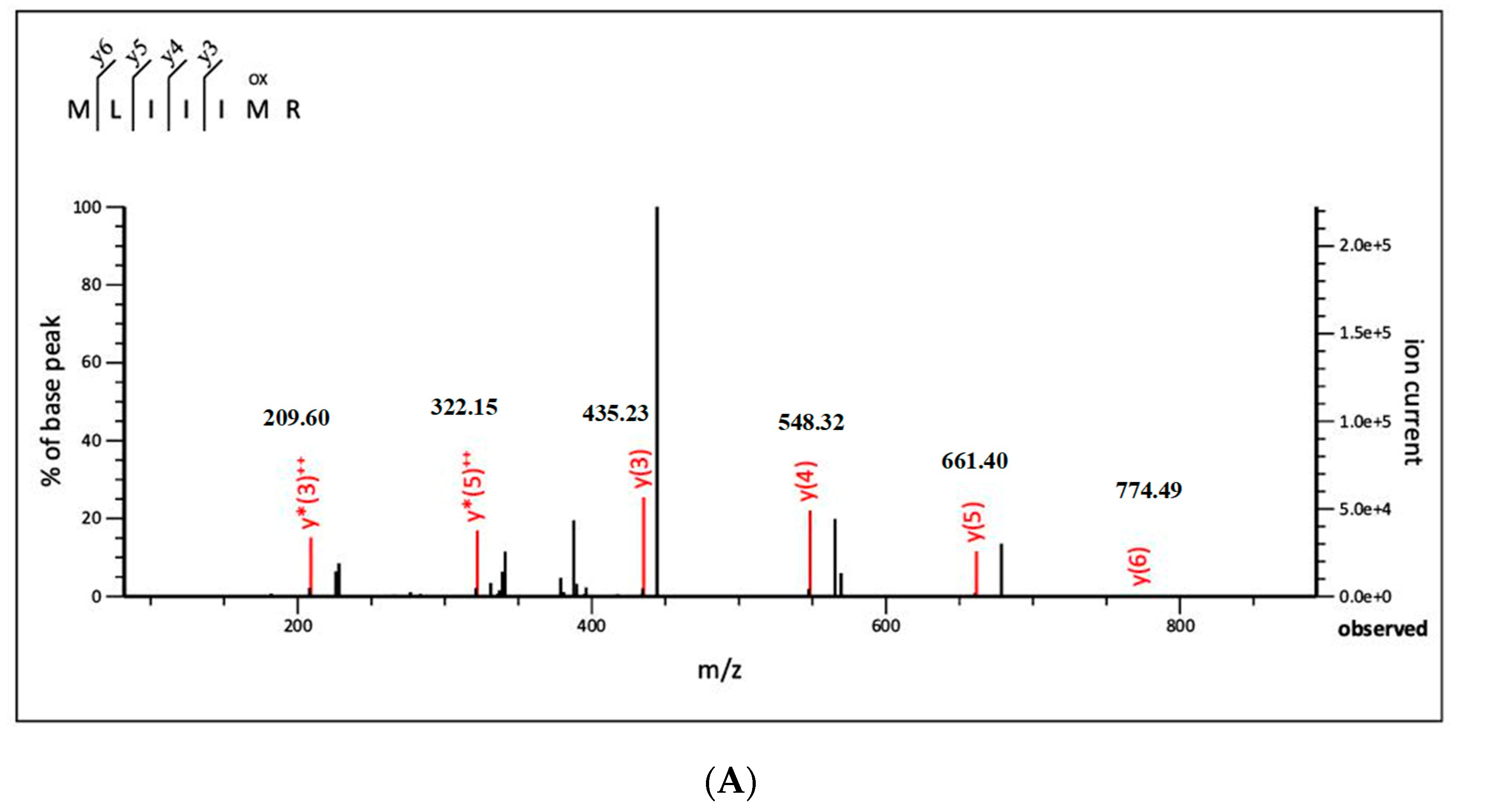
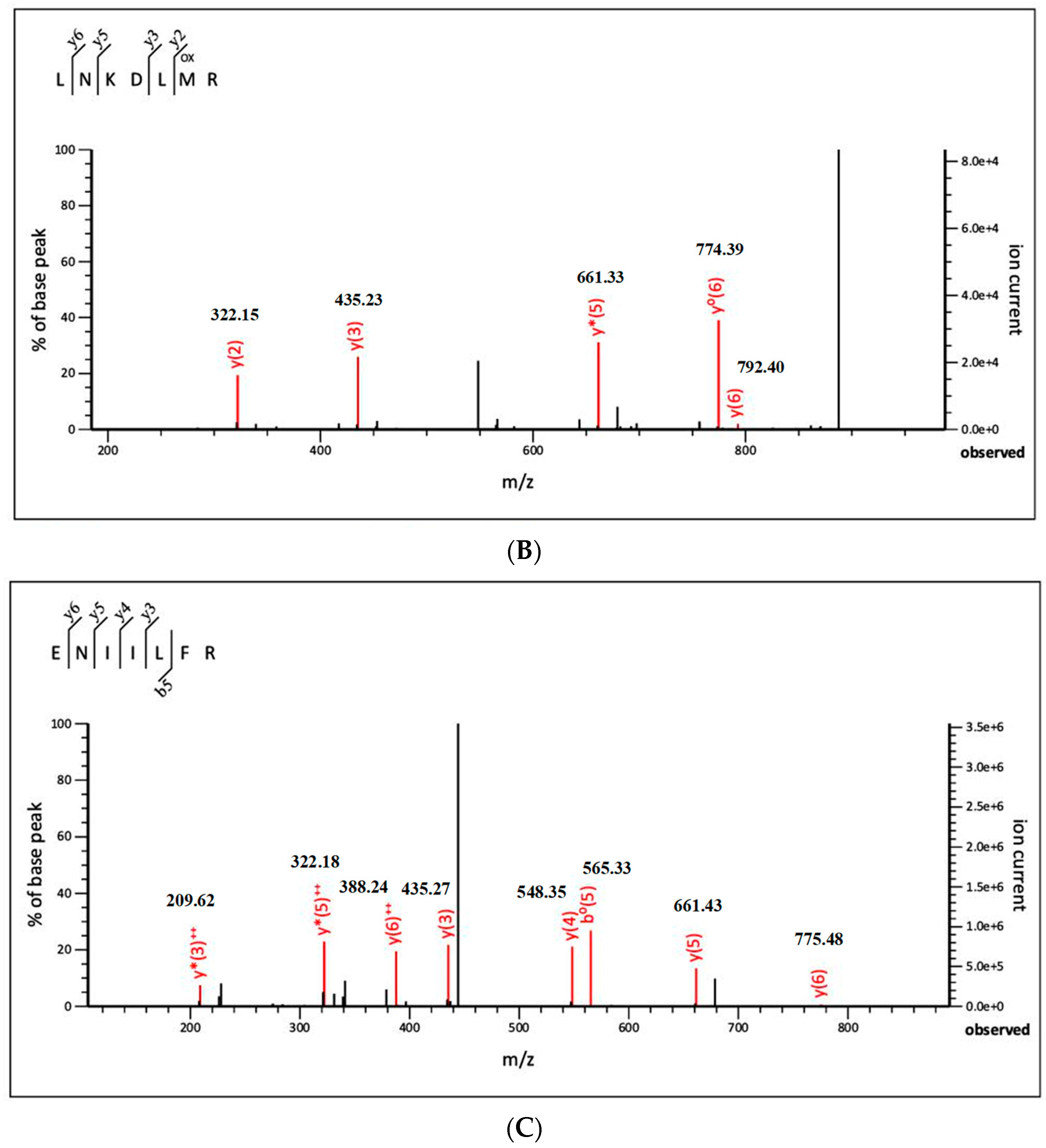
3.2.3. Identification of Antioxidant Peptide by Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chatterjee, S. Oxidative Stress, Inflammation, and Disease, in Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–58. [Google Scholar]
- Rowe, L.A.; Degtyareva, N.; Doetsch, P.W. DNA damage-induced reactive oxygen species (ROS) stress response in Saccharomyces cerevisiae. Free Radic. Biol. Med. 2008, 45, 1167–1177. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. Chandel, ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Decker, E.A.; Elias, R.J.; McClements, D.J. McClements, Oxidation in Foods and Beverages and Antioxidant Applications: Understanding Mechanisms of Oxidation and Antioxidant Activity; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Hellwig, M. The Chemistry of Protein Oxidation in Food. Angew. Chem. Int. Ed. 2019, 58, 16742–16763. [Google Scholar] [CrossRef]
- Conning, D.; Phillips, J. Comparative metabolism of BHA, BHT and other phenolic antioxidants and its toxicological relevance. Food Chem. Toxicol. 1986, 24, 1145–1148. [Google Scholar] [CrossRef]
- Kahl, R.; Kappus, H. Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. z Lebensm Unters Forsch 1993, 196, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Fisherman, E.W. Chemical intolerance to butylated-hydroxyanisole (BHA) and butylated-hydroxytoluene (BHT) and vascular response as an indicator and monitor of drug intolerance. Ann. Allergy 1973, 31, 126. [Google Scholar] [PubMed]
- Gharavi, N.; El-Kadi, A.O.S. Tert-butylhydroquinone is a novel aryl hydrocarbon receptor ligand. Drug Metab. Dispos. 2004, 33, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Deori, M.; Devi, D.; Devi, R. Nutrient composition and antioxidant activities of muga and eri silkworm pupae. Int. J. Sci. Nat. 2014, 5, 636–664. [Google Scholar]
- Longvah, T.; Mangthya, K.; Ramulu, P. Nutrient composition and protein quality evaluation of eri silkworm (Samia ricinii) prepupae and pupae. Food Chem. 2011, 128, 400–403. [Google Scholar] [CrossRef]
- Lokeshwari, R.; Shantibala, T.; Singh, K.M.; Hazarika, B. The nutritional goldmine waste: The spent pupae of mulberry, eri and oak tasar silkworms for combating malnutrition. Int. J. Environ. Ecol. Fam. Urban Stud. 2019, 9. [Google Scholar] [CrossRef]
- Chukiatsiri, S.; Siriwong, S.; Thumanu, K. Pupae protein extracts exert anticancer effects by downregulating the expression of IL-6, IL-1β and TNF-α through biomolecular changes in human breast cancer cells. Biomed. Pharmacother. 2020, 128, 110278. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.-J.; Hu, Y.-Y.; Hu, H.-Y.; Ge, Q.-F.; Zhou, G.-H.; Zhang, W.-G. Purification and identification of antioxidative peptides from dry-cured Xuanwei ham. Food Chem. 2016, 194, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nat. Cell Biol. 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Chaudhary, S.; Chandrashekar, K.S.; Pai, K.S.R.; Setty, M.M.; Devkar, R.A.; Reddy, N.D.; Shoja, M.H. Evaluation of antioxidant and anticancer activity of extract and fractions of Nardostachys jatamansi DC in breast carcinoma. BMC Complement. Altern. Med. 2015, 15, 50. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, J.-S.; Baek, H.-H.; Lee, H.G. Purification and characterization of antioxidant peptides from soy protein hydrolysate. J. Food Biochem. 2010, 34, 120–132. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Jyothirmayi, T.; Diwan, P.V.; Kumar, B.D. Antioxidant activity and functional properties of enzymatic protein hydrolysates from common carp (Cyprinus carpio) roe (egg). J. Food Sci. Technol. 2015, 52, 5817–5825. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Alam, N.; Bristi, N.J. Rafiquzzaman Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Shavandi, A.; Hu, Z.; Teh, S.; Zhao, J.; Carne, A.; Bekhit, A.; Bekhit, A.E.-D.A. Antioxidant and functional properties of protein hydrolysates obtained from squid pen chitosan extraction effluent. Food Chem. 2017, 227, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Adjimani, J.P.; Asare, P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol. Rep. 2015, 2, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Heli, H.; Mirtorabi, S.; Karimian, K. Advances in iron chelation: An update. Expert Opin. Ther. Patents 2011, 21, 819–856. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Caiazzo, F.; Drioli, E. Separation and purification of phenolic compounds from pomegranate juice by ultrafiltration and nanofiltration membranes. J. Food Eng. 2017, 195, 1–13. [Google Scholar] [CrossRef]
- Gholam Hossein Gudarzi, B.; Sadri, R.; Asghari, A.; Karami, S. Purification of Trypsin from Bovine Pancreas in Laboratory; Razi Vaccine and Serum Research Institute: Karaj, Iran, 2008. [Google Scholar]
- Chi, C.-F.; Cao, Z.-H.; Wang, B.; Hu, F.-Y.; Li, Z.-R.; Zhang, B. Antioxidant and Functional Properties of Collagen Hydrolysates from Spanish Mackerel Skin as Influenced by Average Molecular Weight. Molecules 2014, 19, 11211–11230. [Google Scholar] [CrossRef]
- Chi, C.-F.; Wang, B.; Deng, Y.-Y.; Wang, Y.-M.; Deng, S.-G.; Ma, J.-Y. Isolation and characterization of three antioxidant pentapeptides from protein hydrolysate of monkfish (Lophius litulon) muscle. Food Res. Int. 2014, 55, 222–228. [Google Scholar] [CrossRef]
- Grimble, G.; Rees, R.; Keohane, P.; Cartwright, T.; Desreumaux, M.; Silk, D. Effect of peptide chain length on absorption of egg protein hydrolysates in the normal human jejunum. Gastroenterology 1987, 92, 136–142. [Google Scholar] [CrossRef]
- Ko, J.-Y.; Lee, J.-H.; Samarakoon, K.; Kim, J.-S.; Jeon, Y.-J. Purification and determination of two novel antioxidant peptides from flounder fish (Paralichthys olivaceus) using digestive proteases. Food Chem. Toxicol. 2013, 52, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gong, Y.-D.; Li, Z.-R.; Yu, D.; Chi, C.-F.; Ma, J.-Y. Isolation and characterisation of five novel antioxidant peptides from ethanol-soluble proteins hydrolysate of spotless smoothhound (Mustelus griseus) muscle. J. Funct. Foods 2014, 6, 176–185. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Hemalatha, R.; Jyothirmayi, T.; Diwan, P.V.; Bhaskarachary, K.; Vajreswari, A.; Kumar, R.R.; Kumar, B.D. Chemical composition and immunomodulatory effects of enzymatic protein hydrolysates from common carp (Cyprinus carpio) egg. Nutrition 2015, 31, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Tanzadehpanah, H.; Asoodeh, A.; Chamani, J. An antioxidant peptide derived from Ostrich (Struthio camelus) egg white protein hydrolysates. Food Res. Int. 2012, 49, 105–111. [Google Scholar] [CrossRef]
- He, R.; Alashi, A.; Malomo, S.A.; Girgih, A.T.; Chao, D.; Ju, X.; Aluko, R.E. Antihypertensive and free radical scavenging properties of enzymatic rapeseed protein hydrolysates. Food Chem. 2013, 141, 153–159. [Google Scholar] [CrossRef]
- Pan, X.-Y.; Wang, Y.-M.; Li, L.; Chi, C.-F.; Wang, B. Four Antioxidant Peptides from Protein Hydrolysate of Red Stingray (Dasyatis akajei) Cartilages: Isolation, Identification, and In Vitro Activity Evaluation. Mar. Drugs 2019, 17, 263. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, X.; Li, Y.; Wang, H.; Wang, Z.; Qi, B.; Sui, X.; Jiang, L. Purification and Characterization of Antioxidant Peptides from Alcalase-Hydrolyzed Soybean (Glycine max L.) Hydrolysate and Their Cytoprotective Effects in Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2019, 67, 5772–5781. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; He, H.; Xie, B.J. Novel Antioxidant Peptides from Fermented MushroomGanoderma lucidum. J. Agric. Food Chem. 2004, 52, 6646–6652. [Google Scholar] [CrossRef]
- Umayaparvathi, S.; Meenakshi, S.; Vimalraj, V.; Arumugam, M.; Sivagami, G.; Balasubramanian, T. Antioxidant activity and anticancer effect of bioactive peptide from enzymatic hydrolysate of oyster (Saccostrea cucullata). Biomed. Prev. Nutr. 2014, 4, 343–353. [Google Scholar] [CrossRef]
- Li, X.-R.; Chi, C.-F.; Li, L.; Wang, B. Purification and Identification of Antioxidant Peptides from Protein Hydrolysate of Scalloped Hammerhead (Sphyrna lewini) Cartilage. Mar. Drugs 2017, 15, 61. [Google Scholar] [CrossRef]
- Kim, M.; Khan, M.M.; Yoo, J.C. Antimicrobial and antioxidant peptide from Bacillus strain CBS73 isolated from Korean Food. J. Chosun Nat. Sci. 2017, 10, 154–161. [Google Scholar]
- Li, Z.; Zhao, S.; Xin, X.; Zhang, B.; Thomas, A.; Charles, A.; Lee, K.S.; Jin, B.R.; Gui, Z. Purification and characterization of a novel immunomodulatory hexapeptide from alcalase hydrolysate of ultramicro-pretreated silkworm (Bombyx mori) pupa protein. J. Asia Pac. Èntomol. 2019, 22, 633–637. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Xin, X.; Zhang, B.; Thomas, A.; Charles, A.; Lee, K.S.; Jin, B.R.; Gui, Z. Purification, Identification and Functional Analysis of a Novel Immunomodulatory Peptide from Silkworm Pupa Protein. Int. J. Pept. Res. Ther. 2019, 26, 243–249. [Google Scholar] [CrossRef]
- Heo, J.E.; Ryu, J.H.; Jeong, H.K.; Chung, W.T.; Ahn, M.Y. Antioxidant Activity of cholesterol derived from silkworm pupae. Nat. Prod. Sci. 2007, 13, 220. [Google Scholar]
- Anootthato, S.; Therdthai, N.; Ritthiruangdej, P. Characterization of protein hydrolysate from silkworm pupae (Bombyx mori). J. Food Process. Preserv. 2019, 43, 14021. [Google Scholar] [CrossRef]
- Chatsuwan, N.; Puechkamut, Y.; Pinsirodom, P. Characterization, Functionality and Antioxidant Activity of Water-Soluble Proteins Extracted from Bombyx mori Linn. Curr. Appl. Sci. Technol. 2018, 18, 83–96. [Google Scholar]
- Jung, K.-H.; Choi, Y.-C.; Chun, J.-Y.; Min, S.-G.; Hong, G.-P. Effects of Concentration and Reaction Time of Trypsin, Pepsin, and Chymotrypsin on the Hydrolysis Efficiency of Porcine Placenta. Food Sci. Anim. Resour. 2014, 34, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H. Antioxidant and anti-freezing peptides from salmon collagen hydrolysate prepared by bacterial extracellular protease. Food Chem. 2018, 248, 346–352. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, J.; Tang, X.; Xiong, Y.L. Reducing, Radical Scavenging, and Chelation Properties of in Vitro Digests of Alcalase-Treated Zein Hydrolysate. J. Agric. Food Chem. 2008, 56, 2714–2721. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Wang, L.; Guo, X.; Wang, X.; Yao, H. Antioxidant activities of the rice endosperm protein hydrolysate: Identification of the active peptide. Eur. Food Res. Technol. 2009, 229, 709–719. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.-J.; Zhao, M.-Y.; Lv, L. Purification and identification of antioxidant peptides from egg white protein hydrolysate. Amino Acids 2011, 43, 457–466. [Google Scholar] [CrossRef]
- Wu, H.-C.; Chen, H.-M.; Shiau, C.-Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 2003, 36, 949–957. [Google Scholar] [CrossRef]
- Shaw, C. Peptide Purification by Reverse-Phase HPLC. Basic Protein Pept. Protoc. 1994, 32, 275–288. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Zhu, Z.; Li, X.; Sun, S.; Wang, W.; Sadiq, F.A. Identification and characterization of two novel antioxidant peptides from silkworm pupae protein hydrolysates. Eur. Food Res. Technol. 2021, 247, 343–352. [Google Scholar] [CrossRef]
- Esteve, C.; Marina, M.; García, M. Novel strategy for the revalorization of olive (Olea europaea) residues based on the extraction of bioactive peptides. Food Chem. 2015, 167, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Anwar, F.; Abu Bakar, F.; Philip, R.; Saari, N. Identification and characterization of papain-generated antioxidant peptides from palm kernel cake proteins. Food Res. Int. 2014, 62, 726–734. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Oh, D.H.; Lee, B.H. Bioactive Peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Martini, S.; Solieri, L. Bioprospecting for Bioactive Peptide Production by Lactic Acid Bacteria Isolated from Fermented Dairy Food. Fermentation 2019, 5, 96. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; Kumar, V. A Review on Bioactive Peptides: Physiological Functions, Bioavailability and Safety. Int. J. Pept. Res. Ther. 2020, 26, 139–150. [Google Scholar] [CrossRef]

| Samples | DPPH Radical Scavenging Activity (IC50 μg/mL) |
|---|---|
| Crude protein | 1808.1 ± 98.0 b |
| Protein hydrolysate with trypsin | 1406.6 ± 86.9 a |
| Protein hydrolysate with pepsin | 1495.6 ± 101.7 a |
| BHA | 1304.5 ± 75.4 a |
| Samples | Iron Chelating Activity (IC50 μg/mL) |
|---|---|
| Crude protein | 300.7 ± 38.2 a |
| Protein hydrolysate with trypsin | 286.5 ± 48.7 a |
| Protein hydrolysate with pepsin | 189.3 ± 57.1 b |
| BHA | 297.8 ± 29.6 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongsrangsap, N.; Chukiatsiri, S. Purification and Identification of Novel Antioxidant Peptides from Enzymatically Hydrolysed Samia ricini Pupae. Molecules 2021, 26, 2588. https://doi.org/10.3390/molecules26092588
Wongsrangsap N, Chukiatsiri S. Purification and Identification of Novel Antioxidant Peptides from Enzymatically Hydrolysed Samia ricini Pupae. Molecules. 2021; 26(9):2588. https://doi.org/10.3390/molecules26092588
Chicago/Turabian StyleWongsrangsap, Nattakarn, and Suttida Chukiatsiri. 2021. "Purification and Identification of Novel Antioxidant Peptides from Enzymatically Hydrolysed Samia ricini Pupae" Molecules 26, no. 9: 2588. https://doi.org/10.3390/molecules26092588
APA StyleWongsrangsap, N., & Chukiatsiri, S. (2021). Purification and Identification of Novel Antioxidant Peptides from Enzymatically Hydrolysed Samia ricini Pupae. Molecules, 26(9), 2588. https://doi.org/10.3390/molecules26092588