Antioxidant Activity in Rheum emodi Wall (Himalayan Rhubarb)
Abstract
1. Introduction
2. Results
2.1. Total Phenolic Contents of R. emodi Fractions
2.2. Antioxidant Activity Analysis
2.3. Correlation of Total Phenolic Content and Antioxidant Activity
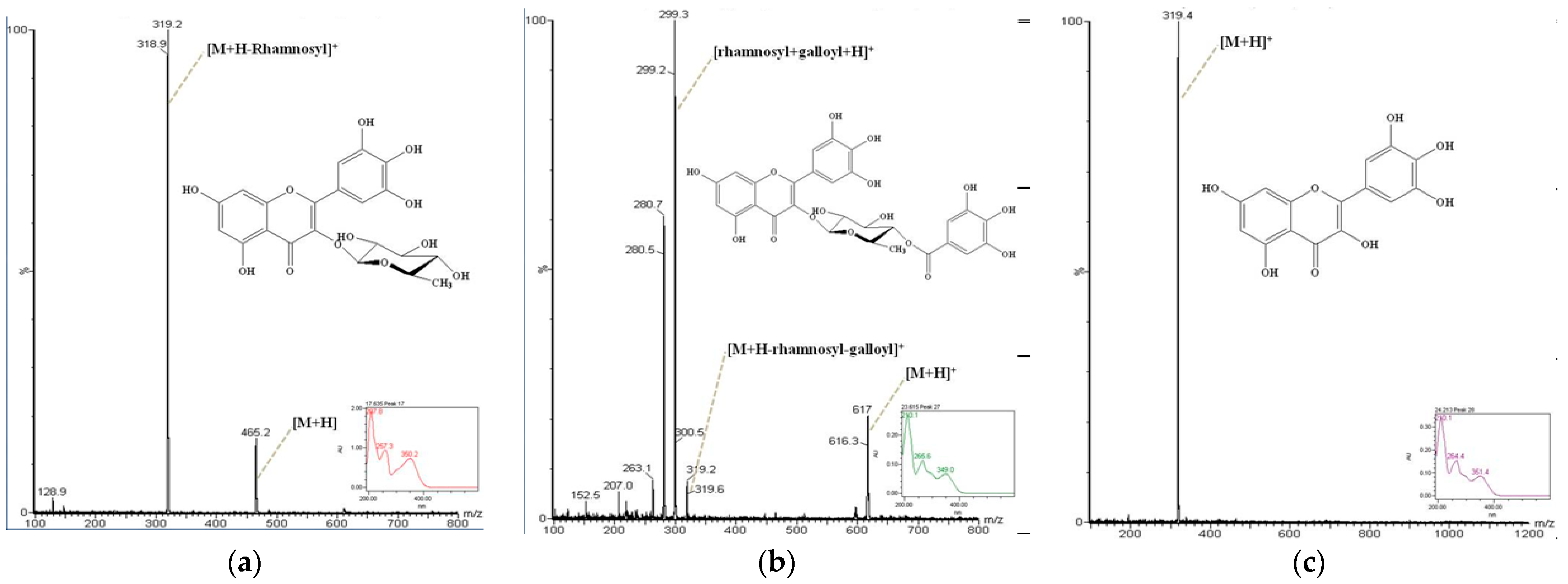
2.4. Identification of the Antioxidant Compounds
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Total Phenolic Compound Analysis
4.3. DPPH Radical Scavenging Activity
4.4. ABTS Radical Scavenging Activity
4.5. Superoxide Dismutase (SOD) Assay
4.6. Isolation and Identification of Antioxidant Compounds
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Nautiyal, B.P.; Prakash, V.; Maithani, U.C.; Chauhan, R.S.; Purohit, H.; Nautiyal, M.C. Germinability, Productivity and economic viabillity of Rheum emodi wall. Ex Meissn cultivated at lower altitude. Curr. Sci. 2003, 84, 143–148. [Google Scholar]
- Matsuda, T.; Someya, T.; Fujimoto, A. Phenolic inhibitors of chemical and enzymatic oxidation in the leaves of Myrica rubra. Biosci. Biotechnol. Biochem. 2010, 74, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Zargar, B.A.; Masoodi, M.H.; Ahmed, B.; Ganie, S.A. Phytoconstituents and therapeutic uses of Rheum emodi wall. Ex Meissn. Food Chem. 2011, 128, 585–589. [Google Scholar] [CrossRef]
- Rajkumar, V.; Guha, G.; Ashok Kumar, R. Antioxidant and Anti-Cancer Potentials of Rheum emodi Rhizome Extracts. Evid. Based Complementary Altern. Med. 2011. [Google Scholar] [CrossRef]
- Erol, N.T.; Sari, F.; Polat, G.; Velioglu, Y.S. Antioxidant and antibacterial activities of various extracts and fractions of fresh tea leaves and green tea. Tarim Bilim. Derg. 2009, 15, 371–378. [Google Scholar]
- Larrauri, M.; Demaria, M.G.; Ryan, L.C.; Asensio, C.M.; Grosso, N.R.; Nepote, V. Chemical and sensory quality preservation in coated almonds with the addition of antioxidants. J. Food Sci. 2016, 81, S208–S215. [Google Scholar] [CrossRef]
- Rolta, R.; Sharma, A.; Kumar, V.; Sourirajan, A.; Baumler, D.J.; Dev, K. Methanolic extracts of the rhizome of R.emodi act as bioenhancer of antibiotics against bacteria and fungi and antioxidant potential. J. Med. Plants Res. 2018, 8, 74–85. [Google Scholar]
- Rolta, R.; Kumar, V.; Sourirajan, A.; Upadhyay, N.K.; Dev, K. Bioassay guided fractionation of rhizome extract of Rheum emodi wall as bio-availability enhancer of antibiotics against bacterial and fungal pathogens. J. Ethnopharmacol. 2020, 257, 112867. [Google Scholar] [CrossRef]
- Singh, R.; Chaturvedi, P. Phytochemical screening and determination of antioxidant activity in callus and different parts of Rheum emodi Wall ex. Messin. J. Pharmacogn. Phytochem. 2018, 7, 2541–2547. [Google Scholar]
- Choi, Y.; Lee, S.M.; Chun, J.; Lee, H.B.; Lee, J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shitake (Lentinus endodes) mushroom. Food Chem. 2006, 99, 381–387. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Oxidative stress and redox regulation: Adaptation, damage, repair, senescence, and death. In Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; pp. 199–283. [Google Scholar]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Marañón, A.M.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship Between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef]
- Cross, C.E.; Halliwell, B.; Borish, E.T.; Pryor, W.A.; Ames, B.N.; Saul, R.L.; McCord, J.M.; Harman, D. Oxygen radicals and human disease. Ann. Intern. Med. 1987, 107, 526–545. [Google Scholar] [CrossRef]
- Sozmen, E.Y.; Tanyalcin, T.; Onat, T.; Kutay, F.; Erlacin, S. Ethanol induced oxidative stress and membrane injury in rat erythrocytes. Eur. J. Clin. Chem. Clin. Biochem. 1994, 32, 741–744. [Google Scholar] [CrossRef]
- Frei, B. Natural Antioxidants in Human Health and Disease, 1st ed.; Academic Press: San Diego, CA, USA, 1994; pp. 25–55. [Google Scholar]
- Mavelli, I.; Ciriolo, M.R.; Rotilio, G.; De Sole, P.; Castorino, M.; Stabile, A. Superoxide dismutase, glutathione peroxidase and catalase in oxidative hemolysis. A study of Fanconi’s anemia erythrocytes. Biochem. Biophys. Res. Commun. 1982, 106, 286–290. [Google Scholar] [CrossRef]
- Sen, C.K. Oxidants and antioxidants on exercise. J. Appl. Phys. 1995, 79, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Antioxidants versus Food Antioxidant Additives and Food Preservatives. Antioxidants 2019, 8, 542. [Google Scholar] [CrossRef]
- Pressman, P.; Clemens, R.; Hayes, W.; Reddy, C. Food additive safety: A review of toxicologic and regulatory issues. Toxicol. Res. Appl. 2017, 1, 1–22. [Google Scholar] [CrossRef]
- Blando, F.; Russo, R.; Negro, C.; De Bellis, L.; Frassinetti, S. Antimicrobial and Antibiofilm Activity against Staphylococcus aureus of Opuntia ficus-indica (L.) Mill. Cladode Polyphenolic Extracts. Antioxidants 2019, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Q.U.; Alhassan, A.M.; Khatib, A.; Shah, S.A.A.; Hasan, M.M.; Sarian, M.N. Antiradical and Xanthine Oxidase Inhibitory Activity Evaluations of Averrhoa bilimbi L. Leaves and Tentative Identification of Bioactive Constituents through LC-QTOF-MS/MS and Molecular Docking Approach. Antioxidants 2018, 7, 137. [Google Scholar] [CrossRef]
- Dienaite, L.; Pukalskiene, M.; Pukalskas, A.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Isolation of Strong Antioxidants from Paeonia Officinalis Roots and Leaves and Evaluation of Their Bioactivities. Antioxidants 2019, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Razali, N.; Razab, R.; Junit, S.M.; Aziz, A.A. Radical scavenging and reducing properties of extracts of cashew shoot (Anacardium occidental). Food Chem. 2008, 111, 38–44. [Google Scholar] [CrossRef]
- Chang, S.T.; Wu, J.H.; Wang, S.Y.; Kang, P.L.; Yang, N.S.; Shyur, L.F. Antioxidant activity of extracts from Acacia bark and heartwood. J. Agric. Food Chem. 2001, 49, 3420–3424. [Google Scholar] [CrossRef]
- Akowuah, G.A.; Ismail, Z.; Norhayati, I.; Sadikum, A. The effect of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical scavenging. Food Chem. 2005, 93, 311–317. [Google Scholar] [CrossRef]
- Chai, Y.; Wang, F.; Li, Y.; Liu, K.; Xu, H. Antioxidant activities of stilbenoids from Rhem emodi wall. Evid. Based Complementary Altern. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Han, J.P. Antioxidant activities and nitrite scavenging abilities of extracts from Ulmus devidianan. J. Korean Soc. Food Sci. Nutr. 2000, 29, 893–899. [Google Scholar]
- Yao, Y.; Lin, G.; Xie, Y.; Ma, P.; Li, G.; Meng, Q.; Wu, T. Performulation studies of myricetin: A natural antioxidant flavonoid. Pharmazie 2014, 69, 19–26. [Google Scholar]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florencio, M.H.; Jenninqs, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, T. Myricetin facilitates potassium currents and inhibits neuronal activity of PVN neurons. Neurochem. Res. 2012, 37, 1450–1456. [Google Scholar] [CrossRef]
- Wu, J.H.; Huang, C.Y.; Tung, Y.T.; Chang, S.T. Online RP-HPLC-DPPH screening method for detection of radical-scavenging phytochemicals from flowers of Acacia confusa. J. Agric. Food Chem. 2008, 56, 328–332. [Google Scholar] [CrossRef]
- Domitrović, R.; Rashed, K.; Cvijanović, O.; Vladimir-Knežević, S.; Škoda, M.; Višnić, A. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem. Biol. Interact. 2015, 230, 21–29. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.J.; Paganga, G. Structure antioxidant activity relationship of flavonoid and phenolic acids. Free Radic. Biol. Med. 1996, 20, 1533–1540. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Yoo, K.M.; Lee, C.H.; Lee, H.J.; Moon, B.Y.; Lee, C.Y. Relative antioxidant and cytoprotective activities of common herbs. Food Chem. 2008, 106, 929–936. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
| Fraction | Total Phenolic Content (mg GAE/g 1) |
|---|---|
| n-hexane | 27.76 ± 0.49 a |
| n-butanol | 133.49 ± 0.58 c |
| Ethyl acetate | 209.21 ± 5.81 d |
| Dichloromethane | 66.27 ± 0.34 b |
| Water | 139.00 ± 2.12 c |
| Antioxidant Activities | Fraction | IC50 (μg/mL) |
|---|---|---|
| DPPH radical scavenging | n-hexane | 2448.79 ± 174.14 c |
| n-butanol | 38.67 ± 18.80 a | |
| Ethyl acetate | 21.52 ± 2.78 a | |
| Dichloromethane | 348.42 ± 26.98 b | |
| Water | 43.04 ± 0.39 a | |
| Ascorbic acid * | 70.33 ± 0.30 a | |
| ABTS+ radical scavenging | n-hexane | 1718.05 ± 308.38 b |
| n-butanol | 128.96 ± 1.95 a | |
| Ethyl acetate | 90.25 ± 0.62 a | |
| Dichloromethane | 260.67 ± 6.51 a | |
| Water | 141.97 ± 2.20 a | |
| Ascorbic acid * | 111.06 ± 2.05 a | |
| SOD activity | n-hexane | ND |
| n-butanol | 24.36 ± 0.84 b | |
| Ethyl acetate | 2.31 ± 1.1 a | |
| Dichloromethane | 43.87 ± 9.03 c | |
| Water | 32.25 ± 4.11 b | |
| Ascorbic acid * | 64.78 ± 4.65 d |
| Total Phenolic Content | Antioxidant Activity | |||
|---|---|---|---|---|
| DPPH | ABTS+ | SOD | ||
| Total Phenolic Content | 1 | |||
| DPPH | −0.765 ** | 1 | ||
| ABTS+ | −0.740 ** | −0.988 ** | 1 | |
| SOD | −0.928 ** | −0.700 * | −0.853 ** | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.K.; Lee, Y.K. Antioxidant Activity in Rheum emodi Wall (Himalayan Rhubarb). Molecules 2021, 26, 2555. https://doi.org/10.3390/molecules26092555
Park SK, Lee YK. Antioxidant Activity in Rheum emodi Wall (Himalayan Rhubarb). Molecules. 2021; 26(9):2555. https://doi.org/10.3390/molecules26092555
Chicago/Turabian StylePark, Sang Koo, and Yoon Kyung Lee. 2021. "Antioxidant Activity in Rheum emodi Wall (Himalayan Rhubarb)" Molecules 26, no. 9: 2555. https://doi.org/10.3390/molecules26092555
APA StylePark, S. K., & Lee, Y. K. (2021). Antioxidant Activity in Rheum emodi Wall (Himalayan Rhubarb). Molecules, 26(9), 2555. https://doi.org/10.3390/molecules26092555





