The Redox Potential of the β-93-Cysteine Thiol Group in Human Hemoglobin Estimated from In Vitro Oxidant Challenge Experiments
Abstract
1. Introduction
2. Results
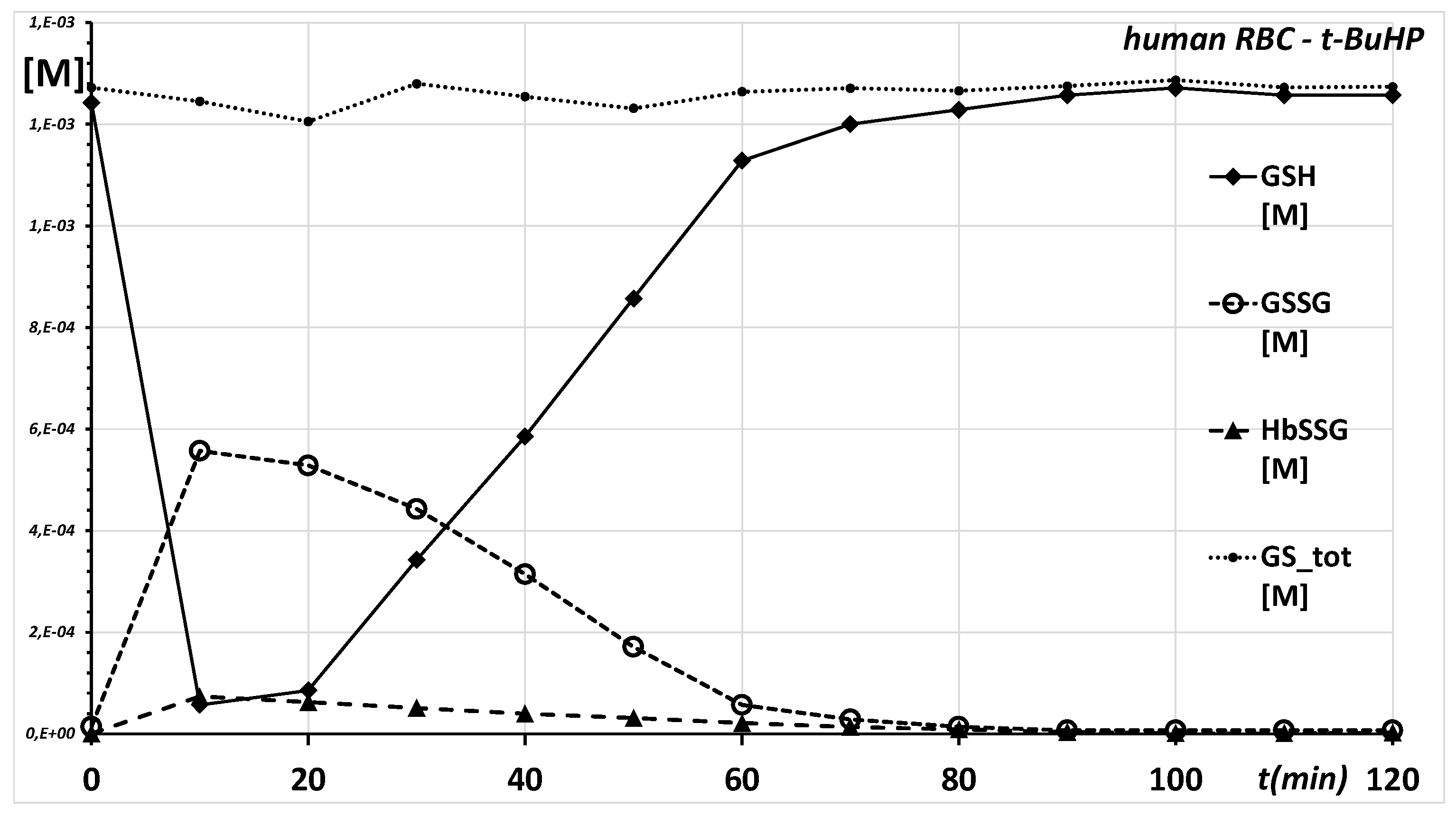
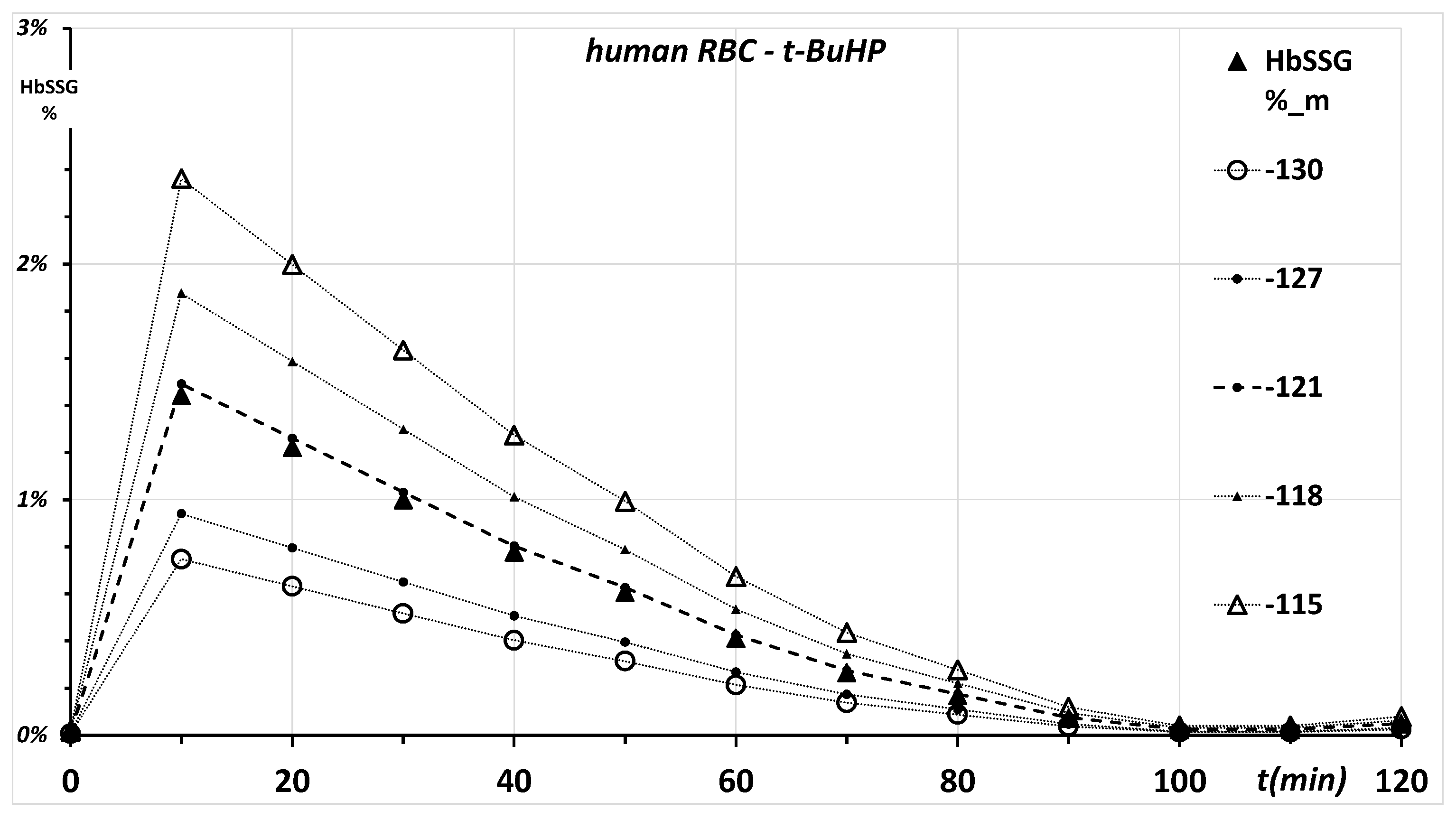
2.1. Ex Vivo, In Vitro Oxidative Challenge of Red Blood Cells by Hydroperoxides
2.2. Redox Equilibria of the Bio-Thiols and Measurement of the Standard Electrochemical Potential (E0)
E0(RefS) + RT/nF ln([OXRefS]/[REDRef]2)
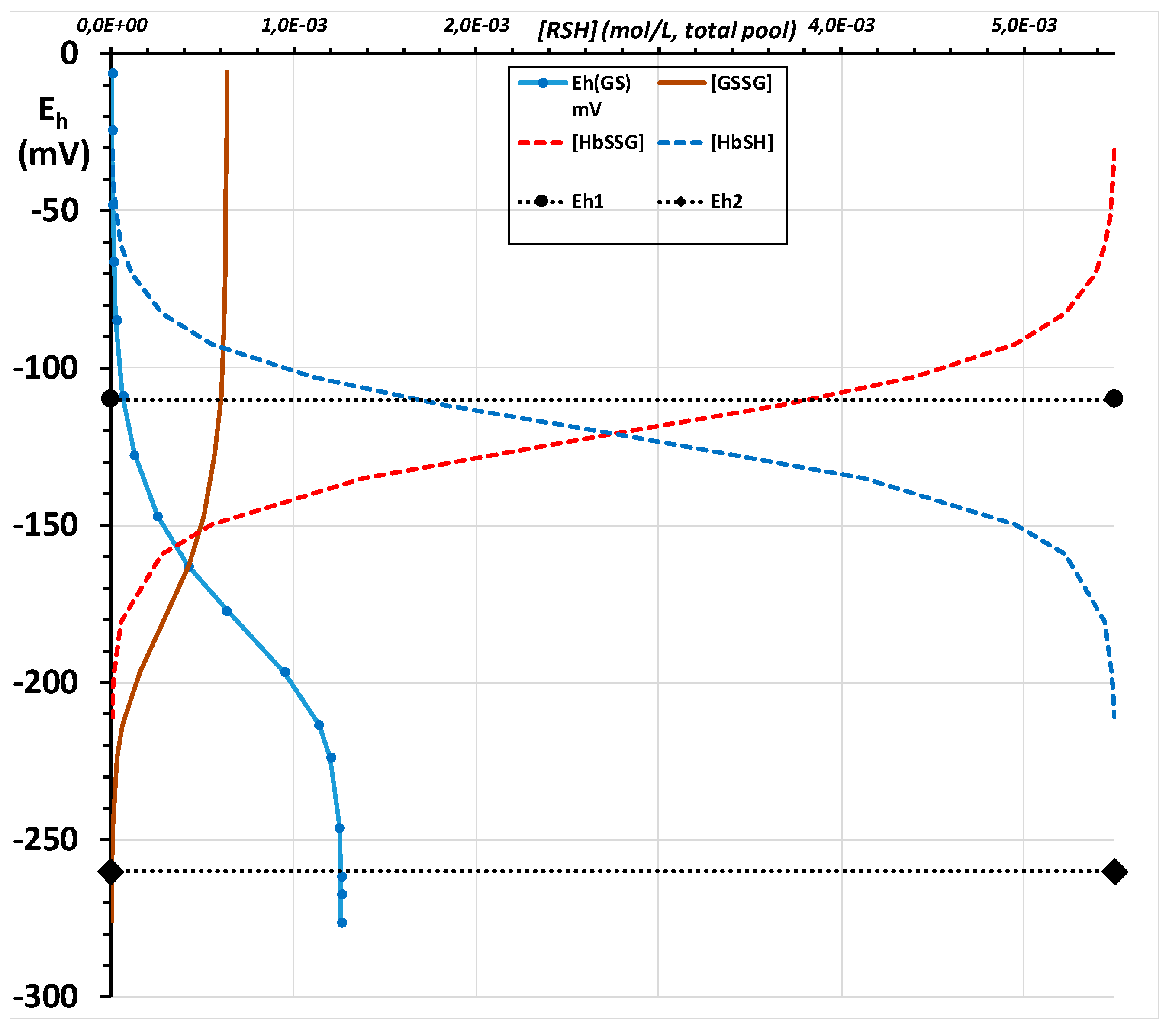
2.3. Calculation of the Redox Potential of the Thiol Group of Hemoglobin’s Beta-93 Cysteine
E0(Hb) + RT/nF ln [HbSSG]/[HbSH]
2.3.1. First-Tier Calculation
2.3.2. Second-Tier Calculation
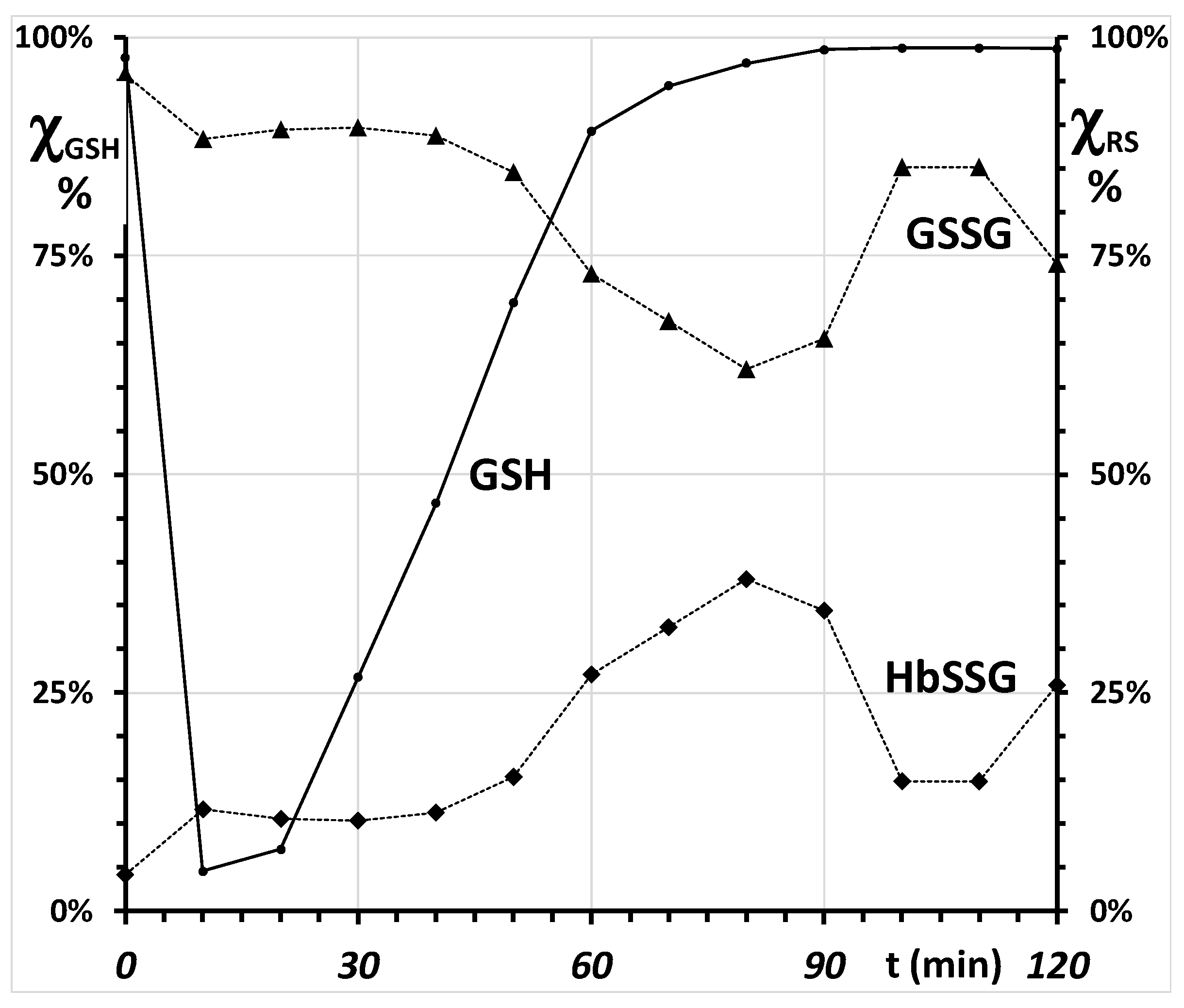
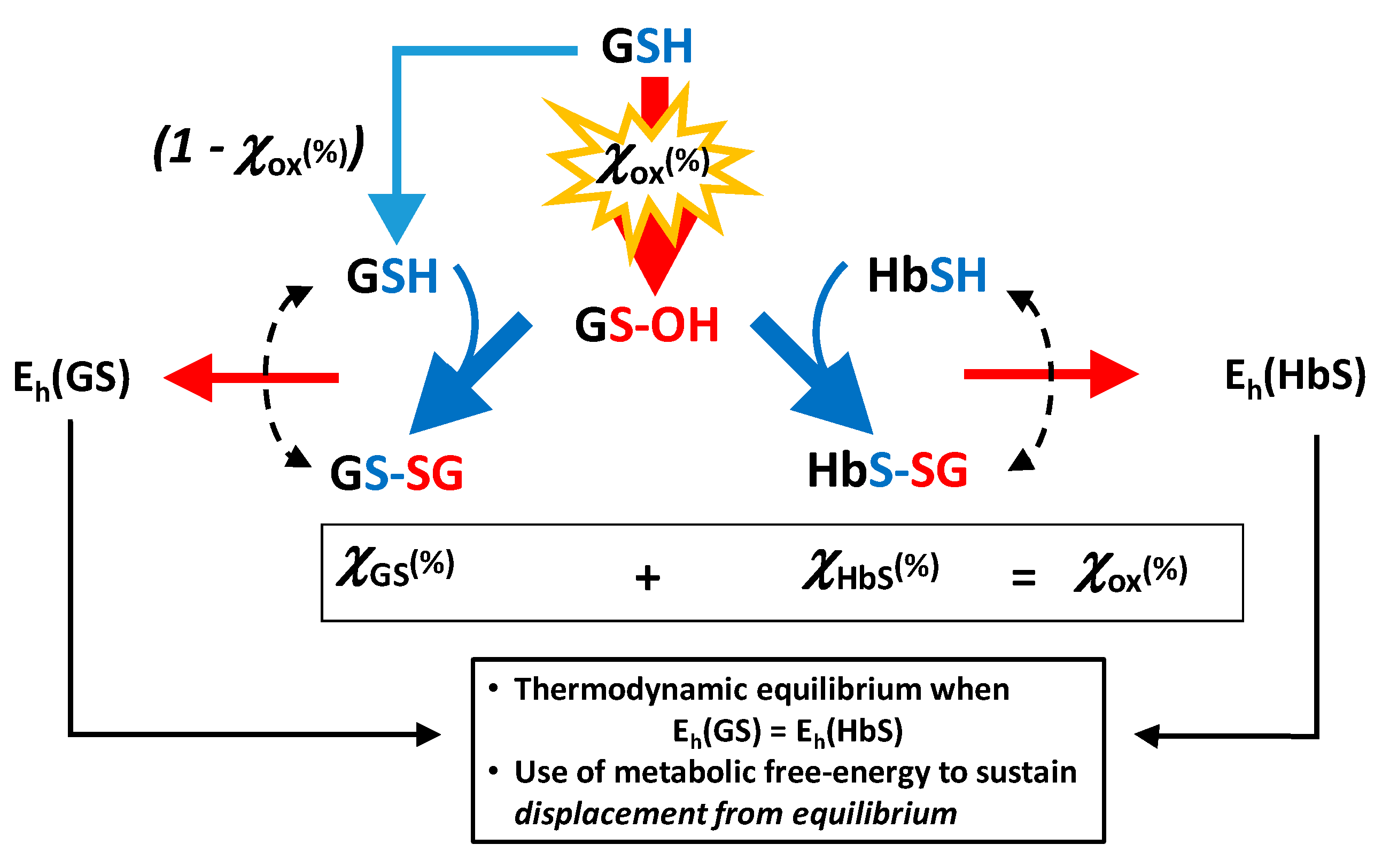
2.4. Coping with Oxidative Stress and Redox Potentials of the Thiols in the Red Blood Cells
2.4.1. Placement of Glutathionyl-Hemoglobin among the Redox Bio-Thiols
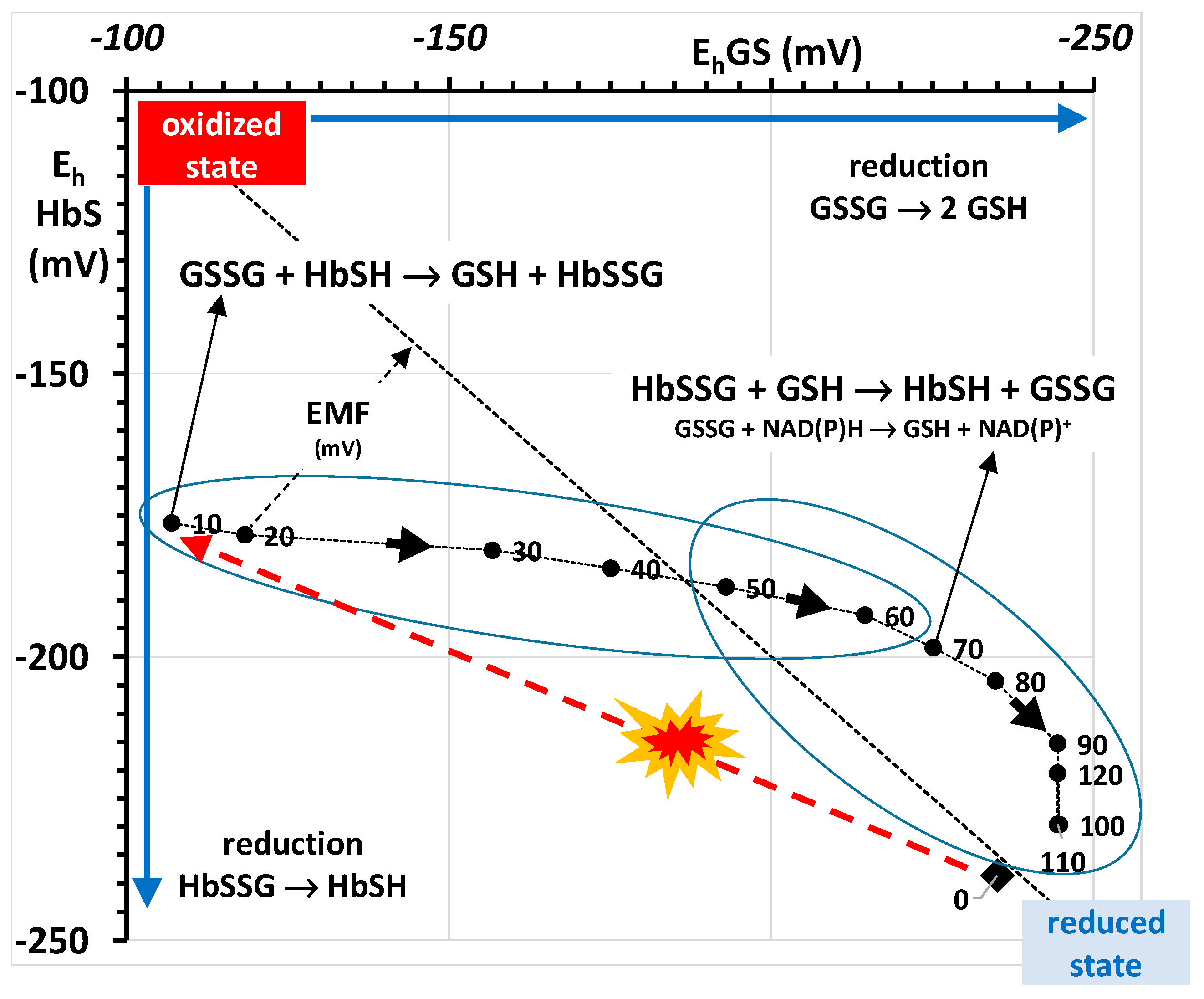
2.4.2. Towards a Mechanistic Understanding of the RBC Response to Oxidative Burst
E0(Hb) + RT/nF ln [HbSSG]/[HbSH]
3. Discussion
4. Materials and Methods
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A
| Time min | GSH [mM] | GSSG [mM] | HbSSG [mM] | GS_tot [mM] | HbSSG % |
|---|---|---|---|---|---|
| 0 | 1.24 | 0.014 | 0.0006 | 1.27 | 0.01% |
| 10 | 0.06 | 0.557 | 0.0738 | 1.25 | 1.44% |
| 20 | 0.09 | 0.529 | 0.0625 | 1.21 | 1.22% |
| 30 | 0.34 | 0.443 | 0.0513 | 1.28 | 1.00% |
| 40 | 0.59 | 0.314 | 0.0400 | 1.25 | 0.78% |
| 50 | 0.86 | 0.171 | 0.0313 | 1.23 | 0.61% |
| 60 | 1.13 | 0.057 | 0.0213 | 1.26 | 0.41% |
| 70 | 1.20 | 0.029 | 0.0138 | 1.27 | 0.27% |
| 80 | 1.23 | 0.014 | 0.0088 | 1.27 | 0.17% |
| 90 | 1.26 | 0.007 | 0.0038 | 1.28 | 0.07% |
| 100 | 1.27 | 0.007 | 0.0013 | 1.29 | 0.02% |
| 110 | 1.26 | 0.007 | 0.0013 | 1.27 | 0.02% |
| 120 | 1.26 | 0.007 | 0.0025 | 1.27 | 0.05% |
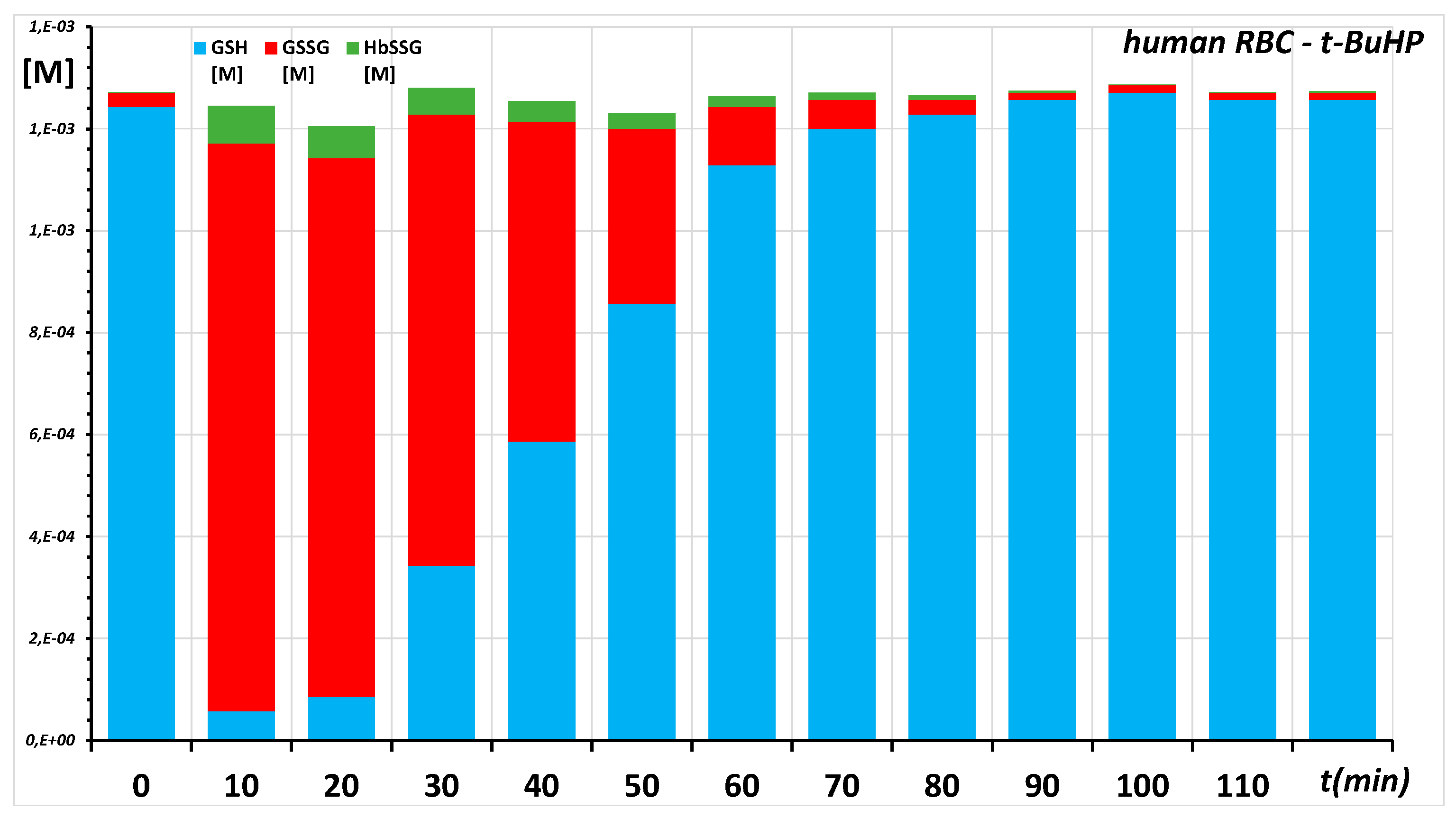
Appendix B
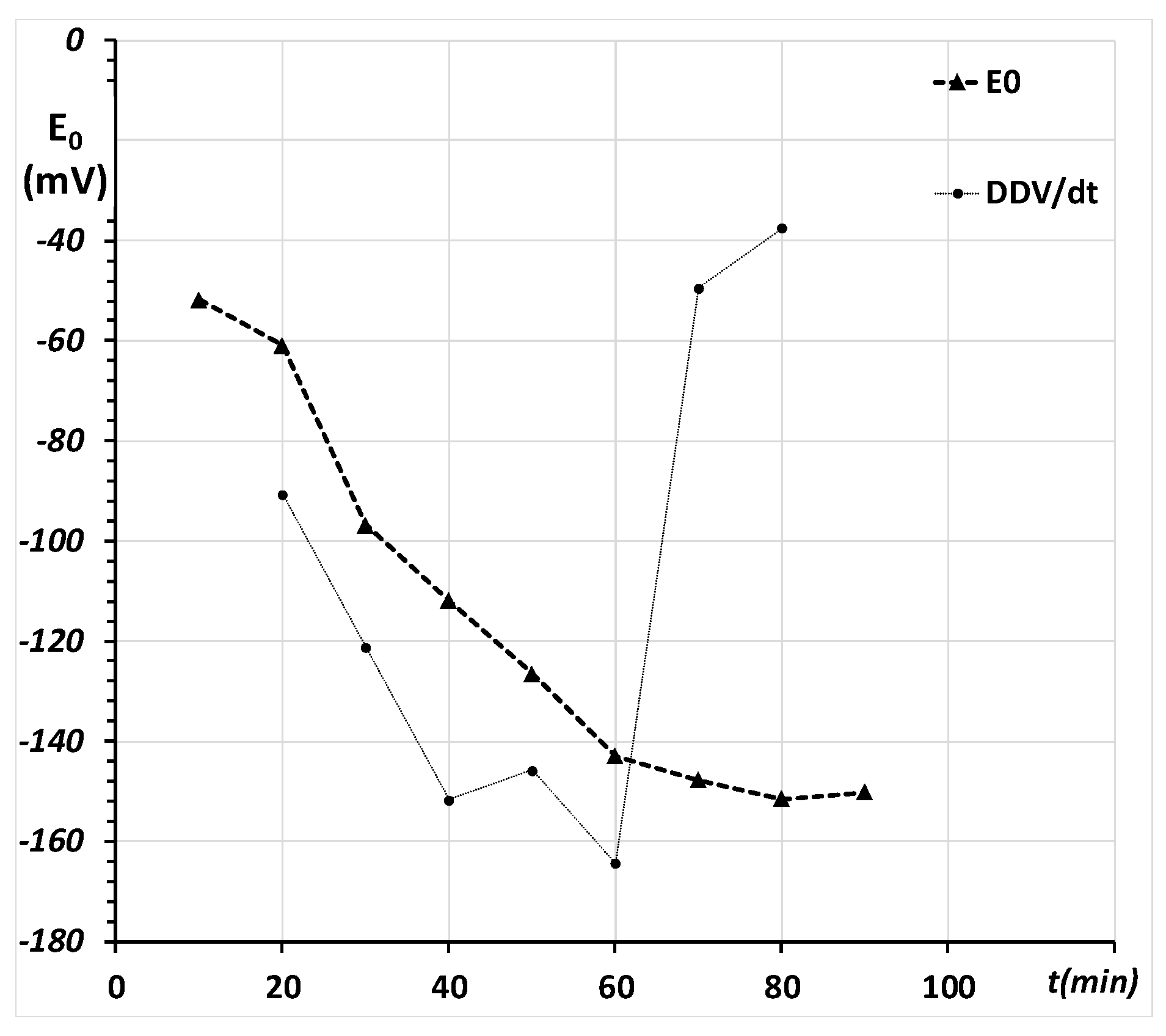

Appendix C
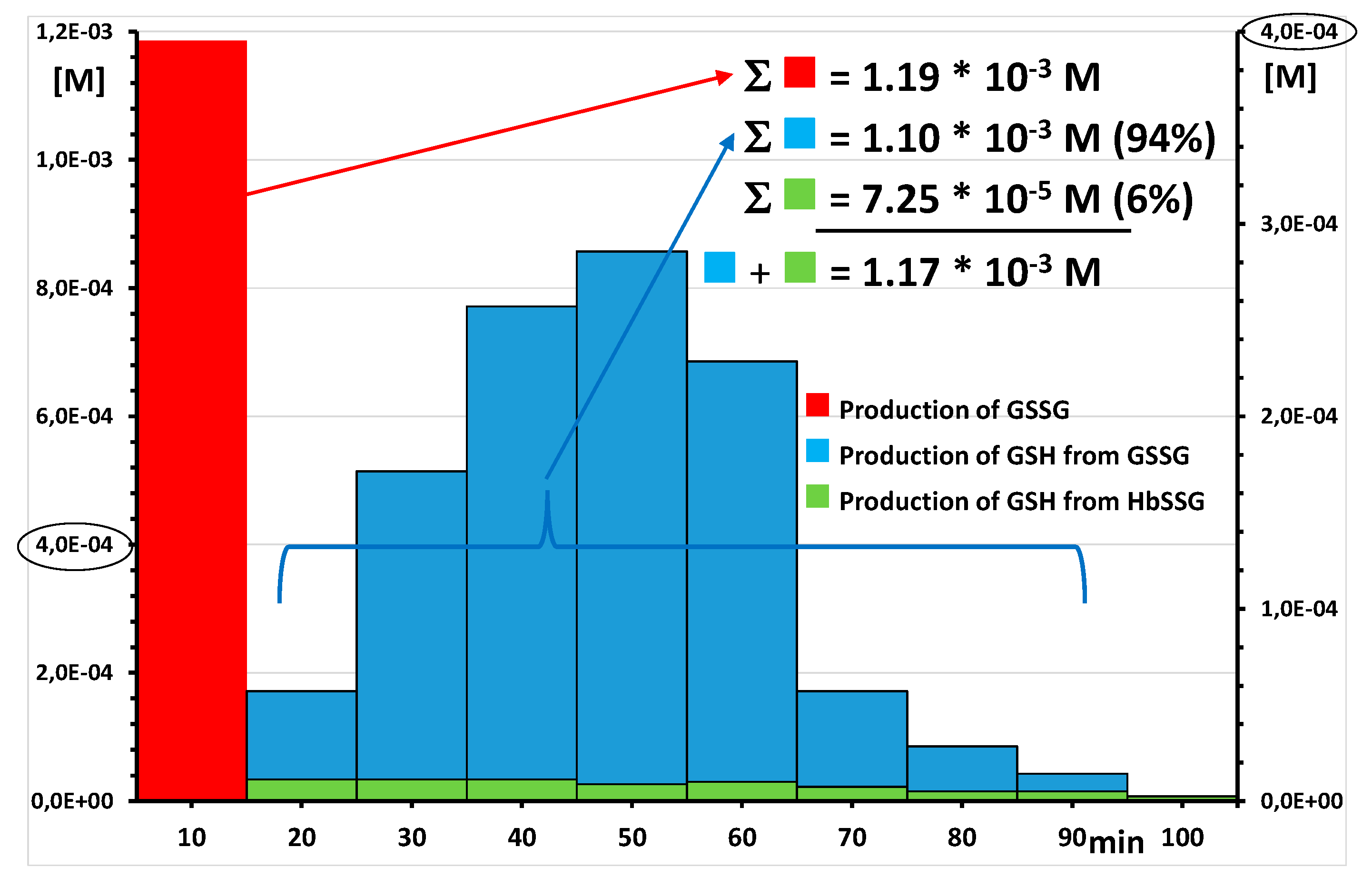
References
- Huisman, T.H.; Dozy, A.M. Studies on the Heterogeneity of Hemoglobin. V. Binding of Hemoglobin with Oxidized Glutathione. J. Lab. Clin. Med. 1962, 60, 302–319. [Google Scholar] [PubMed]
- Garel, M.-C.; Domenget, C.; Caburi-Martin, J.; Prehu, C.; Galacteros, F.; Beuzard, Y. Covalent Binding of Glutathione to He-Moglobin, I. Inhibition of Hemoglobin-S Olymerization. J. Biol. Chem. 1986, 261, 14704–14709. [Google Scholar] [CrossRef]
- Craescu, C.T.; Poyart, C.; Schaeffer, C.; Garel, M.C.; Kister, J.; Beuzard, Y. Covalent Binding of Glutathione to Hemoglobin. II. Functional Consequences and Structural Changes Reflected in NMR Spectra. J. Biol. Chem. 1986, 261, 14710–14716. [Google Scholar] [CrossRef]
- Wodak, S.J.; De Coen, J.L.; Edelstein, S.J.; Demarne, H.; Beuzard, Y. Modification of Human Hemoglobin by Glutathione. III. Perturbations of Hemoglobin Conformation Analyzed by Computer Modeling. J. Biol. Chem. 1986, 261, 14717–14724. [Google Scholar] [CrossRef]
- Murakami, K.; Mawatari, S. Oxidation of Hemoglobin to Methemoglobin in Intact Erythrocyte by a Hydroperoxide Induces Formation of Glutathionyl Hemoglobin and Binding of α-Hemoglobin to Membrane. Arch. Biochem. Biophys. 2003, 417, 244–250. [Google Scholar] [CrossRef]
- Mawatari, S.; Murakami, K. Different Types of Glutathionylation of Hemoglobin can Exist in Intact Erythrocytes. Arch. Biochem. Biophys. 2004, 421, 108–114. [Google Scholar] [CrossRef]
- Metere, A.; Iorio, E.; Scorza, G.; Camerini, S.; Casella, M.; Crescenzi, M.; Minetti, M.; Pietraforte, D. Carbon Monoxide Signaling in Human Red Blood Cells: Evidence for Pentose Phosphate Pathway Activation and Protein Deglutathionylation. Antioxid. Redox Signal. 2014, 20, 403–416. [Google Scholar] [CrossRef]
- Böhmer, A.; Pich, A.; Schmidt, M.; Haghikia, A.; Tsikas, D. Evidence by Chromatography and Mass Spectrometry that Inorganic Nitrite Induces S -Glutathionylation of Hemoglobin in Human Red Blood Cells. J. Chromatogr. B 2016, 1019, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Banerjee, S.; Mitra, A.; Muralidharan, M.; Roy, B.; Banerjee, R.; Mandal, A.K.; Chatterjee, I.B. Interaction of p -Benzoquinone with Hemoglobin in Smoker’s Blood Causes Alteration of Structure and Loss of Oxygen Binding Capacity. Toxicol. Rep. 2016, 3, 295–305. [Google Scholar] [CrossRef][Green Version]
- Mitra, A.; Mandal, A.K. Conjugation of Para-Benzoquinone of Cigarette Smoke with Human Hemoglobin Leads to Unstable Tetramer and Reduced Cooperative Oxygen Binding. J. Am. Soc. Mass Spectrom. 2018, 29, 2048–2058. [Google Scholar] [CrossRef]
- Muralidharan, M.; Mitra, A.; Maity, D.; Pal, D.; Mandal, A.K. Structural Analysis of Glutathionyl Hemoglobin Using Native Mass Spectrometry. J. Struct. Biol. 2019, 208, 107386. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Weisbach, V.; Sticht, H.; Seebahn, A.; Bussmann, J.; Zimmermann, R.; Becker, C.-M. Oxidative Stress-Induced Post-Translational Modifications of Human Hemoglobin in Erythrocytes. Arch. Biochem. Biophys. 2013, 529, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Minetti, M.; Pietraforte, D.; Carbone, V.; Salzano, A.M.; Scorza, G.; Marino, G. Scavenging of Peroxynitrite by Oxyhemoglobin and Identification of Modified Globin Residues. Biochemistry 2000, 39, 6689–6697. [Google Scholar] [CrossRef]
- Giulivi, C.; Davies, K.J. Dityrosine and Tyrosine Oxidation Products are Endogenous Markers for the Selective Proteolysis of Oxidatively Modified Red Blood Cell Hemoglobin by (the 19 S) Proteasome. J. Biol. Chem. 1993, 268, 8752–8759. [Google Scholar] [CrossRef]
- Daneshvar, B.; Frandsen, H.; Dragsted, L.O.; Knudsen, L.E.; Autrup, H. Analysis of Native Human Plasma Proteins and Haemoglobin for the Presence of Bityrosine by High-Performance Liquid Chromatography. Pharmacol. Toxicol. 1997, 81, 205–208. [Google Scholar] [CrossRef]
- Stepuro, A.I.; Adamchuk, R.I.; Oparin, A.Y.; Stepuro, I.I. Thiamine Inhibits Formation of Dityrosine, a Specific Marker of Oxidative Injury, in Reactions Catalyzed by Oxoferryl Forms of Hemoglobin. Biochemistry (Moscow) 2008, 73, 1031–1041. [Google Scholar] [CrossRef]
- Giustarini, D.; Dalle-Donne, I.; Milzani, A.; Braconi, D.; Santucci, A.; Rossi, R. Membrane Skeletal ProteinS-Glutathionylation in Human Red Blood Cells as Index of Oxidative Stress. Chem. Res. Toxicol. 2019, 32, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Naito, C.; Kajita, M.; Niwa, T. Determination of Glutathionyl Hemoglobin in Hemodialysis Patients Using Electrospray Ionization Liquid Chromatography-Mass Spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1999, 731, 121–124. [Google Scholar] [CrossRef]
- Takayama, F.; Tsutsui, S.; Horie, M.; Shimokata, K.; Niwa, T. Glutathionyl Hemoglobin in Uremic Patients Undergoing Hemodialysis and Continuous Ambulatory Peritoneal Dialysis. Kidney Int. 2001, 59, 155–158. [Google Scholar] [CrossRef]
- Mandal, A.K.; Woodi, M.; Sood, V.; Krishnaswamy, P.R.; Rao, A.; Ballal, S.; Balaram, P. Quantitation and Characterization of Glutathionyl Haemoglobin as an Oxidative Stress Marker in Chronic Renal Failure by Mass Spectrometry. Clin. Biochem. 2007, 40, 986–994. [Google Scholar] [CrossRef]
- Pastore, A.; Tozzi, G.; Gaeta, L.M.; Giannotti, A.; Bertini, E.; Federici, G.; Digilio, M.C.; Piemonte, F. Glutathione Metabolism and Antioxidant Enzymes in Children with Down Syndrome. J. Pediatr. 2003, 142, 583–585. [Google Scholar] [CrossRef] [PubMed]
- Piemonte, F.; Pastore, A.; Tozzi, G.; Tagliacozzi, D.; Santorelli, F.M.; Carrozzo, R.; Casali, C.; Damiano, M.; Federici, G.; Bertini, E. Glutathione in Blood of Patients with Friedreich’s Ataxia. Eur. J. Clin. Investig. 2001, 31, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Mathew, B.; Srinivasan, K.; Johnson, P.; Thomas, T. Elevated Levels of Glutathionyl Haemoglobin as an Oxidative Stress Marker in Patients with Major Depressive Disorder. Indian J. Med. Res. 2019, 149, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Niketic, V.; Beslo, D.; Raicevic, S.; Sredic, S.; Stojkovic, M. Glutathione Adduct of Haemoglobin (Hb ASSG) in Hemolysates of Patients on Long-Term Antiepileptic Therapy. Int. J. Biochem. 1992, 24, 503–507. [Google Scholar] [CrossRef]
- Naito, C.; Niwa, T. Analysis of Glutathionyl Hemoglobin Levels in Diabetic Patients by Electrospray Ionization Liquid Chroma-Tography-Mass Spectrometry: Effect of Vitamin E Administration. J. Chromatogr. B Biomed. Sci. Appl. 2000, 746, 91–94. [Google Scholar] [CrossRef]
- Niwa, T.; Naito, C.; Mawjood, A.H.; Imai, K. Increased Glutathionyl Hemoglobin in Diabetes Mellitus and Hyperlipidemia Demonstrated by Liquid Chromatography/Electrospray Ionization-Mass Spectrometry. Clin. Chem. 2000, 46, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Al-Abed, Y.; Vanpatten, S.; Li, H.; Lawson, J.A.; Fitzgerald, G.A.; Manogue, K.R.; Bucala, R. Characterization of a Novel Hemoglobin-Glutathione Adduct That Is Elevated in Diabetic Patients. Mol. Med. 2001, 7, 619–623. [Google Scholar] [CrossRef]
- Sampathkumar, R.; Balasubramanyam, M.; Sudarslal, S.; Rema, M.; Mohan, V.; Balaram, P. Increased Glutathionylated he-Moglobin (HbSSG) in Type 2 Diabetes Subjects with Microangiopathy. Clin. Biochem. 2005, 38, 892–899. [Google Scholar] [CrossRef]
- Rubino, F.M.; Della Noce, C.; Vigna, L.; De Giuseppe, R.; Novembrino, C.; De Liso, F.; Maiavacca, R.; Patrini, L.; Riboldi, L.; Bamonti, F. Measurement of Glutathionylated Haemoglobin by MAL-DI-ToF Mass Spectrometry as a Biomarker of Oxidative Stress in Heavy Smokers and in Occupational Obese Subjects. Int. J. Anal. Mass Spectrom. Chromatogr. 2013, 1, 22–30. [Google Scholar] [CrossRef][Green Version]
- Shet, A.S.; Pinto, S.M.; Mitra, G.; Mandal, A.K. Glutathionyl Hemoglobin Is Elevated in Iron Deficiency Anemia. Acta Haematol. 2012, 127, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Primavera, A.; Fustinoni, S.; Biroccio, A.; Ballerini, S.; Urbani, A.; Bernardini, S.; Federici, G.; Capucci, E.; Manno, M.; Bello, M.L. Glutathione Transferases and Glutathionylated Hemoglobin in Workers Exposed to Low Doses of 1,3-Butadiene. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3004–3012. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ottolenghi, S.; Rubino, F.M.; Sabbatini, G.; Coppola, S.; Veronese, A.; Chiumello, D.; Paroni, R. Oxidative Stress Markers to Investigate the Effects of Hyperoxia in Anesthesia. Int. J. Mol. Sci. 2019, 20, 5492. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, G.; Rubino, F.M.; Pitton, M.; Massetto, N.; Bissi, M.; Bianciardi, P.; Samaja, M.; Carelli, S. Glutathionyl-Hemoglobin Levels in Carotid Endarterectomy: A Pilot Study on 12 Cases Clinically Uneventful. J. Cardiovasc. Surg. (Torino) 2013, 53, 1–7. [Google Scholar]
- Dalle–Donne, I.; Milzani, A.; Gagliano, N.; Colombo, R.; Giustarini, D.; Rossi, R. Molecular Mechanisms and Potential Clinical Significance of S-Glutathionylation. Antioxid. Redox Signal. 2008, 10, 445–474. [Google Scholar] [CrossRef] [PubMed]
- Choury, D.; Wajcman, H.; Boissel, J.; Kaplan, J. Evidence for Endogenous Proteolytic Solubilization of Human Red-Cell Membrane NADH-Cytochromeb5reductase. FEBS Lett. 1981, 126, 172–174. [Google Scholar] [CrossRef]
- Choury, D.; Reghis, A.; Pichard, A.L.; Kaplan, J.C. Endogenous Proteolysis of Membrane-Bound Red Cell Cytochrome-b5 re-Ductase in Adults and Newborns: Its Possible Relevance to the Generation of the Soluble “Methemoglobin Reductase”. Blood 1983, 61, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Giustarini, D.; Milzani, A.; Dalle-Donne, I. Membrane Skeletal Protein S-Glutathionylation and Hemolysis in Human Red Blood Cells. Blood Cells, Mol. Dis. 2006, 37, 180–187. [Google Scholar] [CrossRef]
- Ch, R.; Rey, G.; Ray, S.; Jha, P.K.; Driscoll, P.C.; Dos Santos, M.S.; Malik, D.M.; Lach, R.; Weljie, A.M.; Macrae, J.I.; et al. Rhythmic Glucose Metabolism Regulates the Redox Circadian Clockwork in Human Red Blood Cells. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Khazim, K.; Giustarini, D.; Rossi, R.; Verkaik, D.; Cornell, J.E.; Cunningham, S.E.; Mohammad, M.; Trochta, K.; Lorenzo, C.; Folli, F.; et al. Glutathione Redox Potential is Low and Glutathionylated and Cysteinylated Hemoglobin Levels are Elevated in Maintenance Hemodialysis Patients. Transl. Res. 2013, 162, 16–25. [Google Scholar] [CrossRef]
- Jones, D.P. Redox Potential of [GSH]/[GSSG] Couple: Assay and Biological Significance. Methods Enzym. 2002, 348, 93–112. [Google Scholar]
- Giustarini, D.; Tsikas, D.; Colombo, G.; Milzani, A.; Dalle-Donne, I.; Fanti, P.; Rossi, R. Pitfalls in the Analysis of the Physiological Antioxidant Glutathione (GSH) and its Disulfide (GSSG) in Biological Samples: An Elephant in the Room. J. Chromatogr. B 2016, 1019, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox Environment of the Cell as Viewed Through the Redox State of the Glutathione Disul-Fide/Glutathione Couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Jones, D.P.; Go, Y.-M.; Anderson, C.L.; Ziegler, T.R.; Kinkade, J.J.M.; Kirlin, W.G. Cysteine/Cystine Couple is a Newly Recognized Node in the Circuitry for Biologic Redox Signaling and Control. FASEB J. 2004, 18, 1246–1248. [Google Scholar] [CrossRef]
- Martinovich, G.G.; Cherenkevich, S.N.; Sauer, H. Intracellular Redox State: Towards Quantitative Description. Eur. Biophys. J. 2005, 34, 937–942. [Google Scholar] [CrossRef]
- Kemp, M.; Go, Y.-M.; Jones, D.P. Nonequilibrium Thermodynamics of Thiol/Disulfide Redox Systems: A Perspective on Redox Systems Biology. Free. Radic. Biol. Med. 2008, 44, 921–937. [Google Scholar] [CrossRef]
- Flohé, L. The Fairytale of the GSSG/GSH Redox Potential. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 3139–3142. [Google Scholar] [CrossRef]
- Colombo, G.; Dalle-Donne, I.; Giustarini, D.; Gagliano, N.; Portinaro, N.; Colombo, R.; Rossi, R.; Milzani, A.D.G. Cellular Redox Potential and Hemoglobin S-Glutathionylation in Human and Rat Erythrocytes: A Comparative Study. Blood Cells Mol. Dis. 2010, 44, 133–139. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Awasthi, Y.C.; Beutler, E. Useful Agents for the Study of Glutathione Metabolism in Erythroyctes. Org. Hydroperoxides Biochem. J. 1974, 139, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Dremza, I.; Lapshina, E.; Kujawa, J.; Zavodnik, I. Oxygen-Related Processes in Red Blood Cells Exposed Totert-Butyl Hydroperoxide. Redox. Rep. 2006, 11, 185–192. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Carini, M.; Orioli, M.; Vistoli, G.; Regazzoni, L.; Colombo, G.; Rossi, R.; Milzani, A.; Aldini, G. Protein Carbonylation: 2,4-Dinitrophenylhydrazine Reacts with Both Aldehydes/Ketones and Sulfenic Acids. Free. Radic. Biol. Med. 2009, 46, 1411–1419. [Google Scholar] [CrossRef]
- Regazzoni, L.; Panusa, A.; Yeum, K.-J.; Carini, M.; Aldini, G. Hemoglobin Glutathionylation Can Occur Through Cysteine Sulfenic Acid Intermediate: Electrospray Ionization LTQ-Orbitrap Hybrid Mass Spectrometry Studies. J. Chromatogr. B 2009, 877, 3456–3461. [Google Scholar] [CrossRef]
- Noszál, B.; Visky, D.; Kraszni, M. Population, Acid-Base, and Redox Properties of N-Acetylcysteine Conformers. J. Med. Chem. 2000, 43, 2176–2182. [Google Scholar] [CrossRef] [PubMed]
- Millis, K.K.; Weaver, K.H.; Rabenstein, D.L. Oxidation/Reduction Potential of Glutathione. J. Org. Chem. 1993, 58, 4144–4146. [Google Scholar] [CrossRef]
- Rubino, F.M.; Pitton, M.; Caneva, E.; Pappini, M.; Colombi, A. Thiol-Disulfide Redox Equilibria of Glutathione Metaboloma Compounds Investigated by Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 3935–3948. [Google Scholar] [CrossRef] [PubMed]
- Van ‘t Erve, T.J.; Wagner, B.A.; Ryckman, K.K.; Raife, T.J.; Buettner, G.R. The Concentration of Glutathione in Human Eryth-Rocytes is a Heritable Trait. Free Radic. Biol. Med. 2013, 65, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Beutler, E. Glutathione Metabolism of the Erythrocyte. The Enzymic Cleavage of Glutathione–Haemoglobin Preparations by Glutathione Reductase. Biochem. J. 1970, 119, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Tursi, F.; Samaia, M.; Salmona, M.; Belvedere, G. Styrene Oxidation to Styrene Oxide in Human Erythrocytes is Catalyzed by Oxyhemoglobin. Cell. Mol. Life Sci. 1983, 39, 593–594. [Google Scholar] [CrossRef][Green Version]
- Belvedere, G.; Tursi, F. Styrene Oxidation to Styrene Oxide in Human Blood Erythrocytes and Lymphocytes. Res. Commun. Chem. Pathol. Pharmacol. 1981, 33, 273–282. [Google Scholar]
- Belvedere, G.; Samaja, M. Oxidation of Olefins Catalyzed by Hemoglobin. Methods Enzymol. 1994, 231, 598–621. [Google Scholar] [CrossRef]

| Thiol | E0′ 1 | E0′ 2 | E0(7.4) 3 | Ref. |
|---|---|---|---|---|
| hemoglobin (human) | - | - | −121 | This work |
| glutathione | −205 | −205 ± 3 | −264 | [54] |
| homocysteine | −196 | −218 ± 3 | - | [54] |
| cysteine | −247 | −246 ± 3 | - | [54] |
| γGluCySH | - | −265 ± 3 | - | [54] |
| penicillamine | −267 | −266 ± 3 | - | [54] |
| N-acetylcysteine | - | −268 ± 3 | - | [54] |
| HSCyGly | - | −272 ± 3 | - | [54] |
| cysteine methyl ester | - | −282 ± 3 | - | [54] |
| N-acetylpenicillamine | - | −295 ± 4 | - | [54] |
| cysteamine | −203 | −372 ± 7 | - | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubino, F.M. The Redox Potential of the β-93-Cysteine Thiol Group in Human Hemoglobin Estimated from In Vitro Oxidant Challenge Experiments. Molecules 2021, 26, 2528. https://doi.org/10.3390/molecules26092528
Rubino FM. The Redox Potential of the β-93-Cysteine Thiol Group in Human Hemoglobin Estimated from In Vitro Oxidant Challenge Experiments. Molecules. 2021; 26(9):2528. https://doi.org/10.3390/molecules26092528
Chicago/Turabian StyleRubino, Federico Maria. 2021. "The Redox Potential of the β-93-Cysteine Thiol Group in Human Hemoglobin Estimated from In Vitro Oxidant Challenge Experiments" Molecules 26, no. 9: 2528. https://doi.org/10.3390/molecules26092528
APA StyleRubino, F. M. (2021). The Redox Potential of the β-93-Cysteine Thiol Group in Human Hemoglobin Estimated from In Vitro Oxidant Challenge Experiments. Molecules, 26(9), 2528. https://doi.org/10.3390/molecules26092528






