Antibacterial and Antifungal Studies of Biosynthesized Silver Nanoparticles against Plant Parasitic Nematode Meloidogyne incognita, Plant Pathogens Ralstonia solanacearum and Fusarium oxysporum
Abstract
1. Introduction
2. Results and Discussion
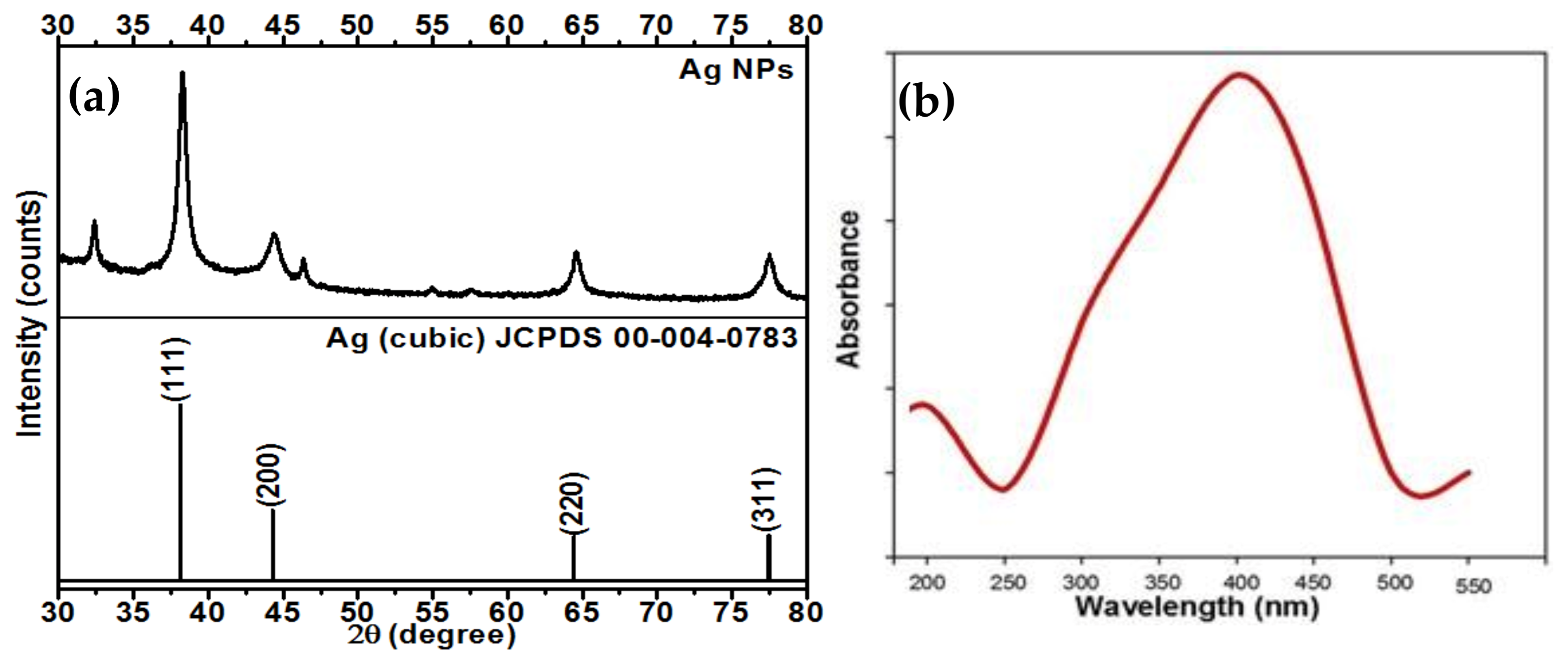
2.1. XRD Analysis of the Synthesized AgNPs
2.2. UV-Vis Spectroscopic Analysis of the Synthesized AgNPs
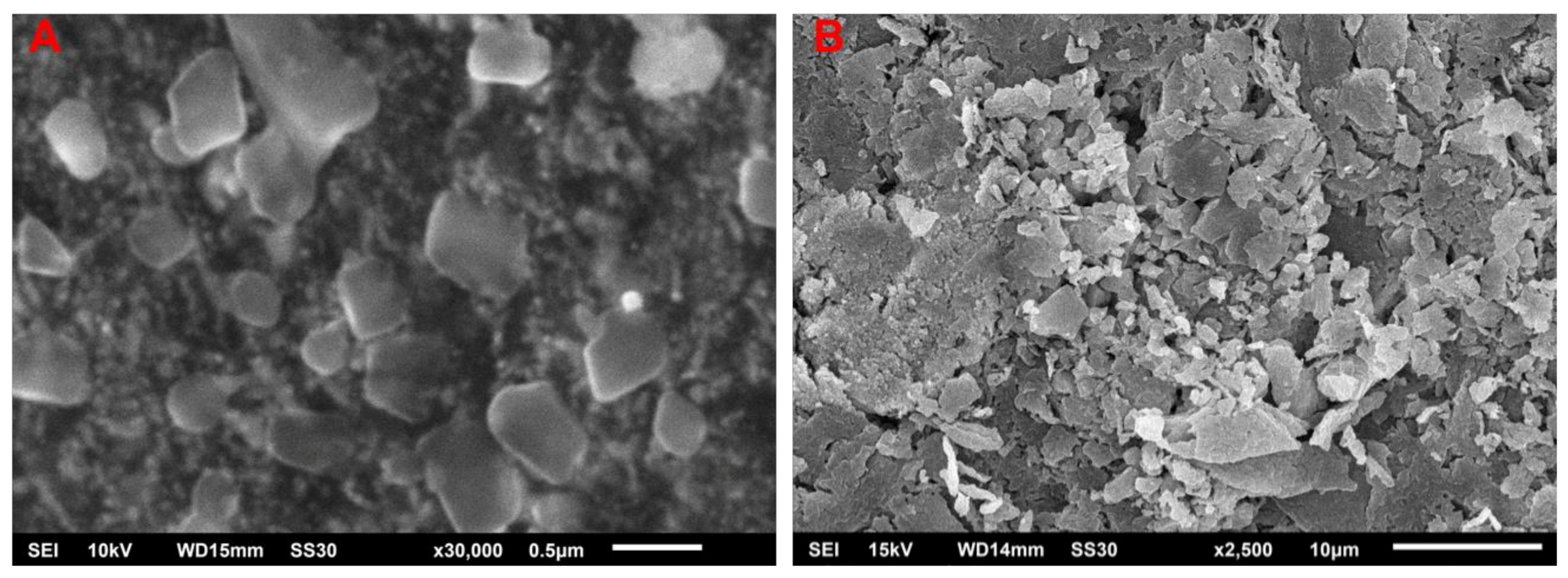
2.3. SEM of the Biosynthesized AgNPs
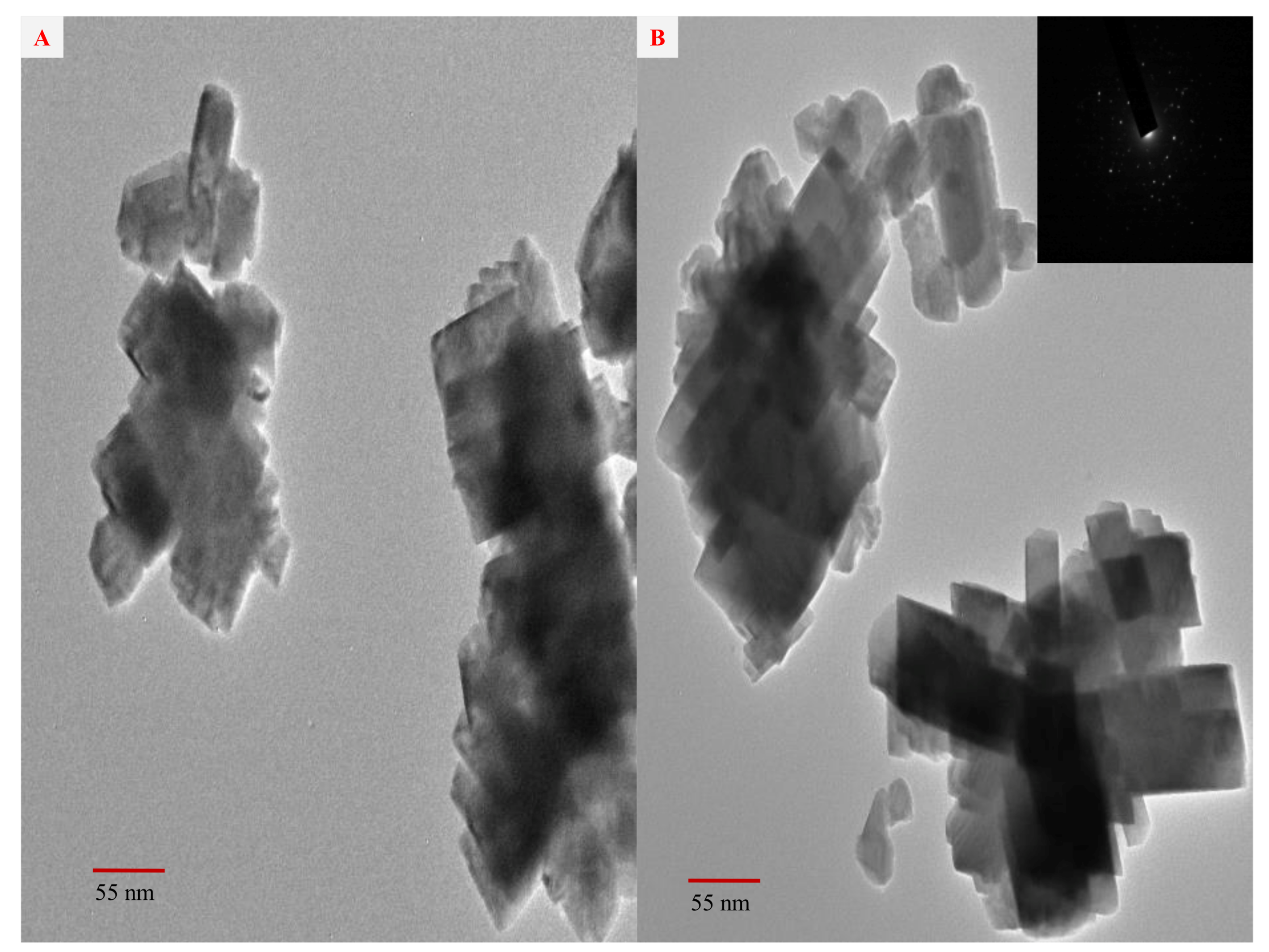
2.4. TEM and SAED Studies of the Biosynthesized AgNPs
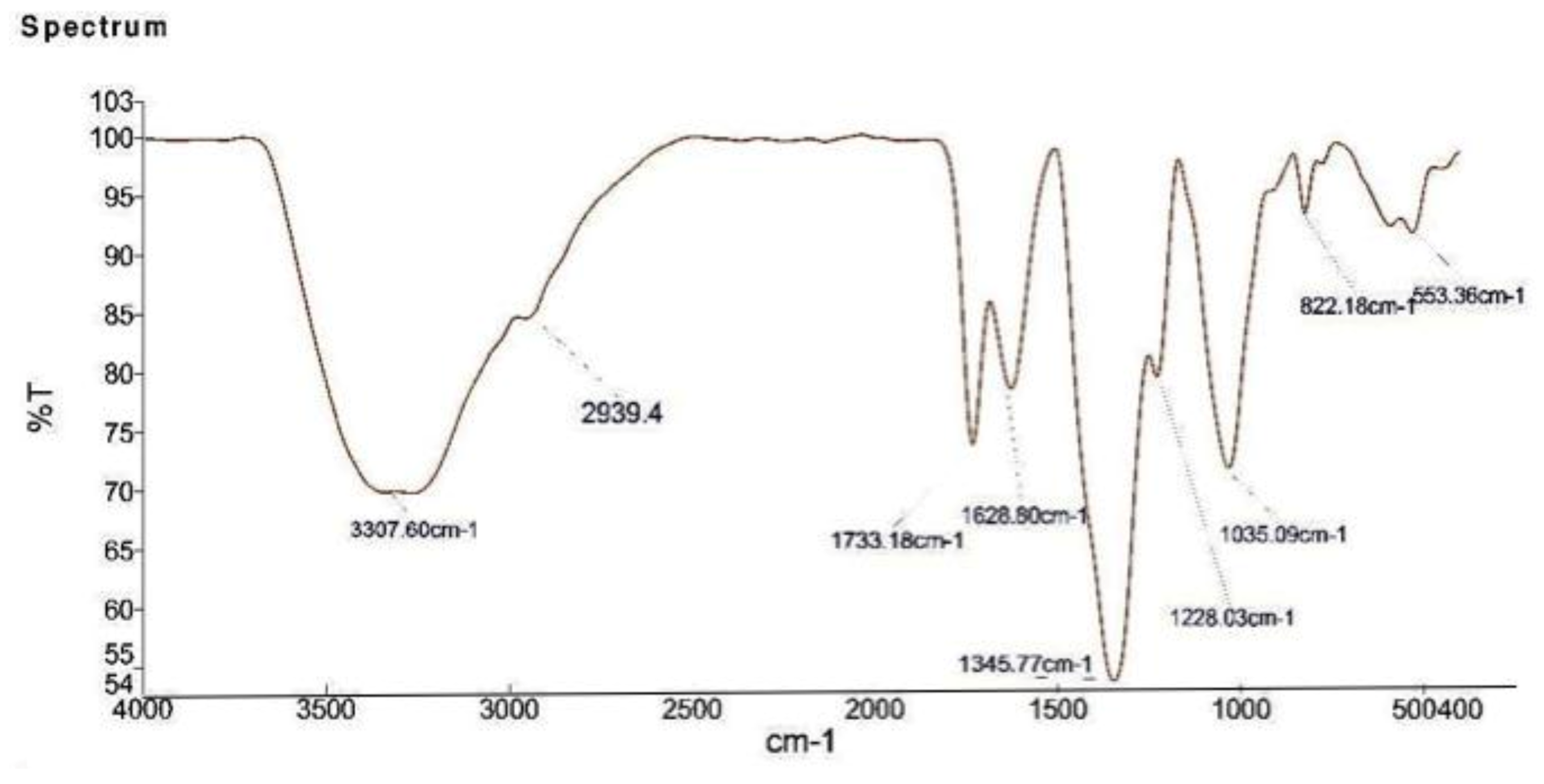
2.5. FTIR Analysis of the Biosynthesized AgNPs
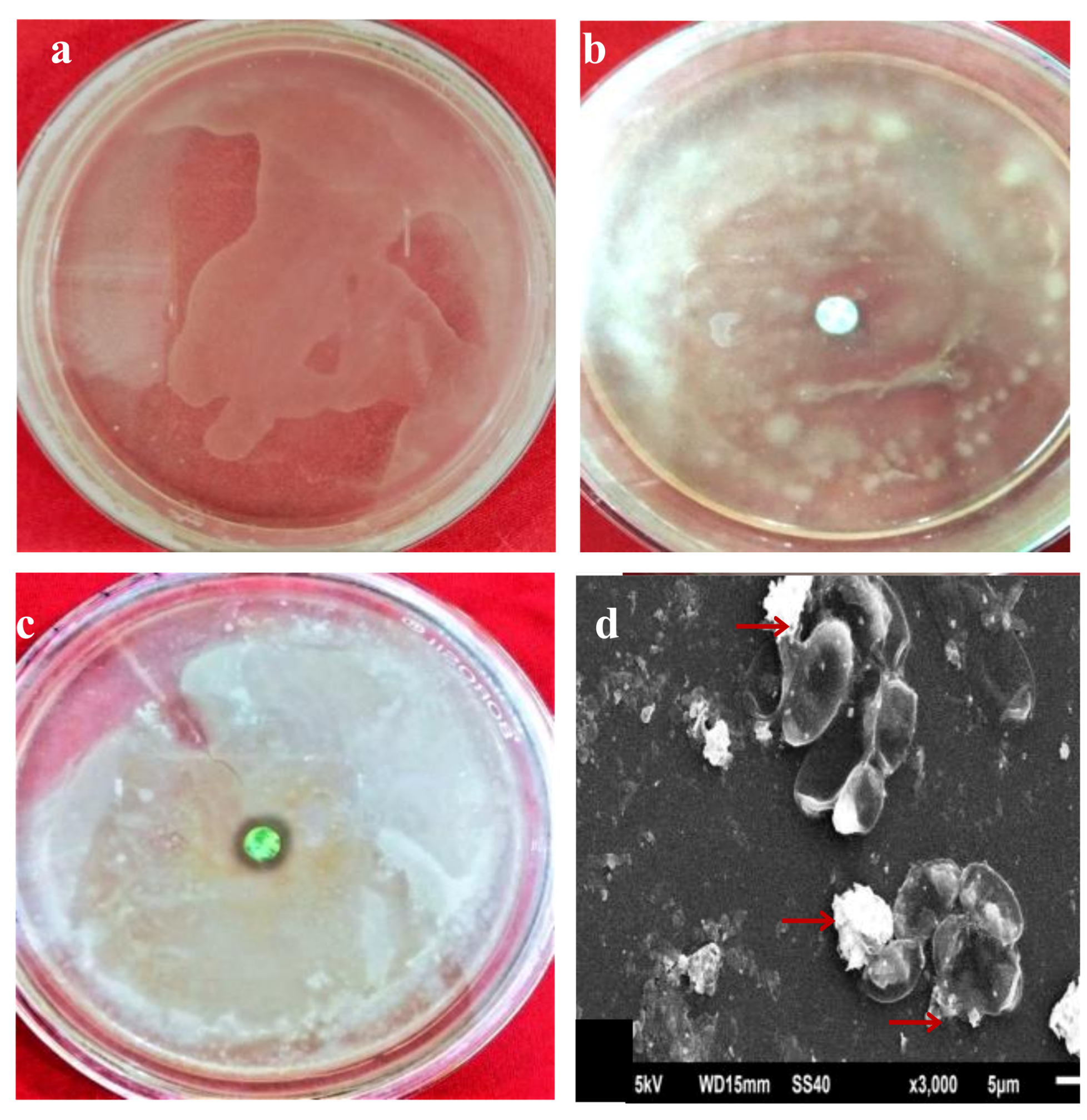
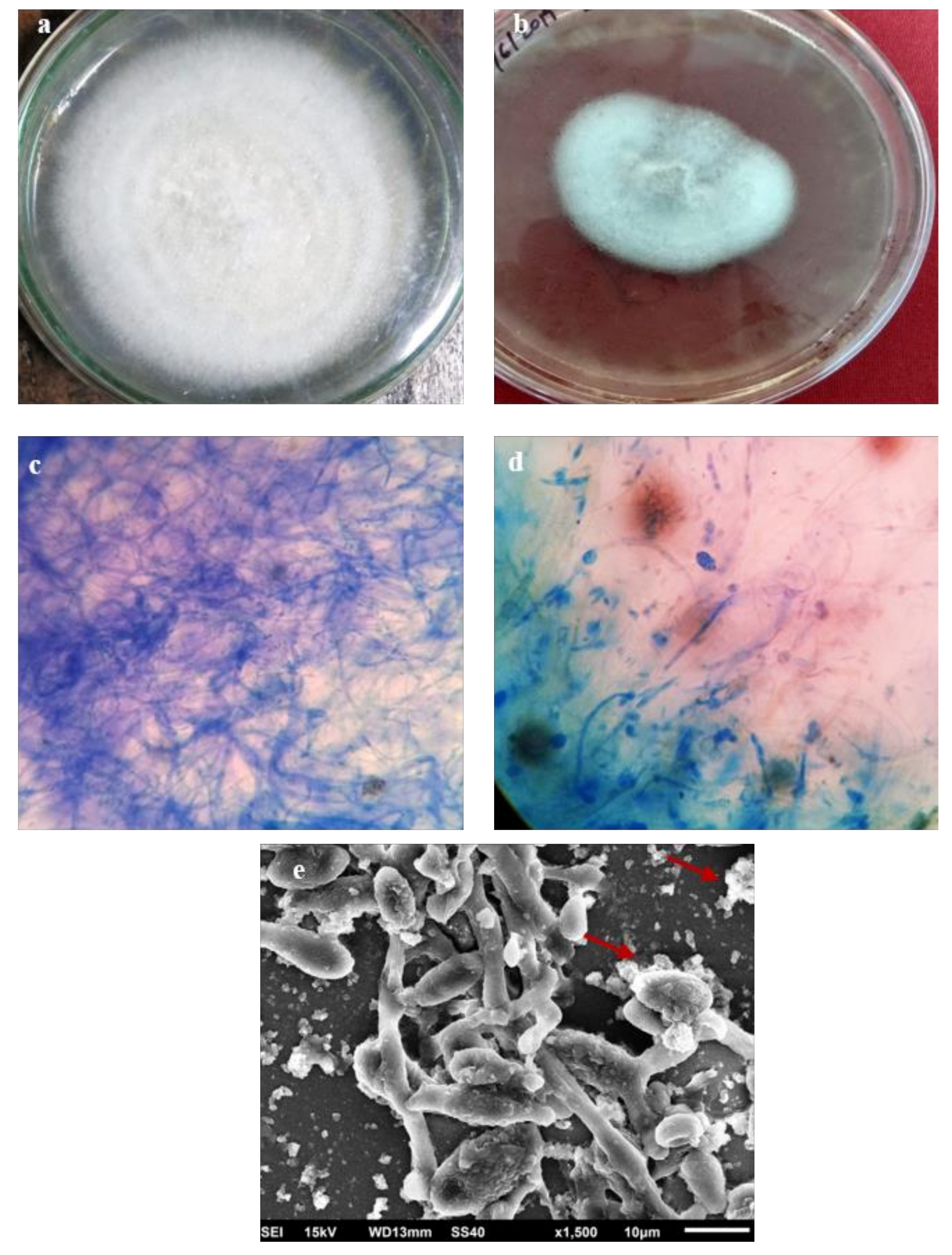
2.6. In Vitro Antifungal and Antibacterial Activity of AgNPs
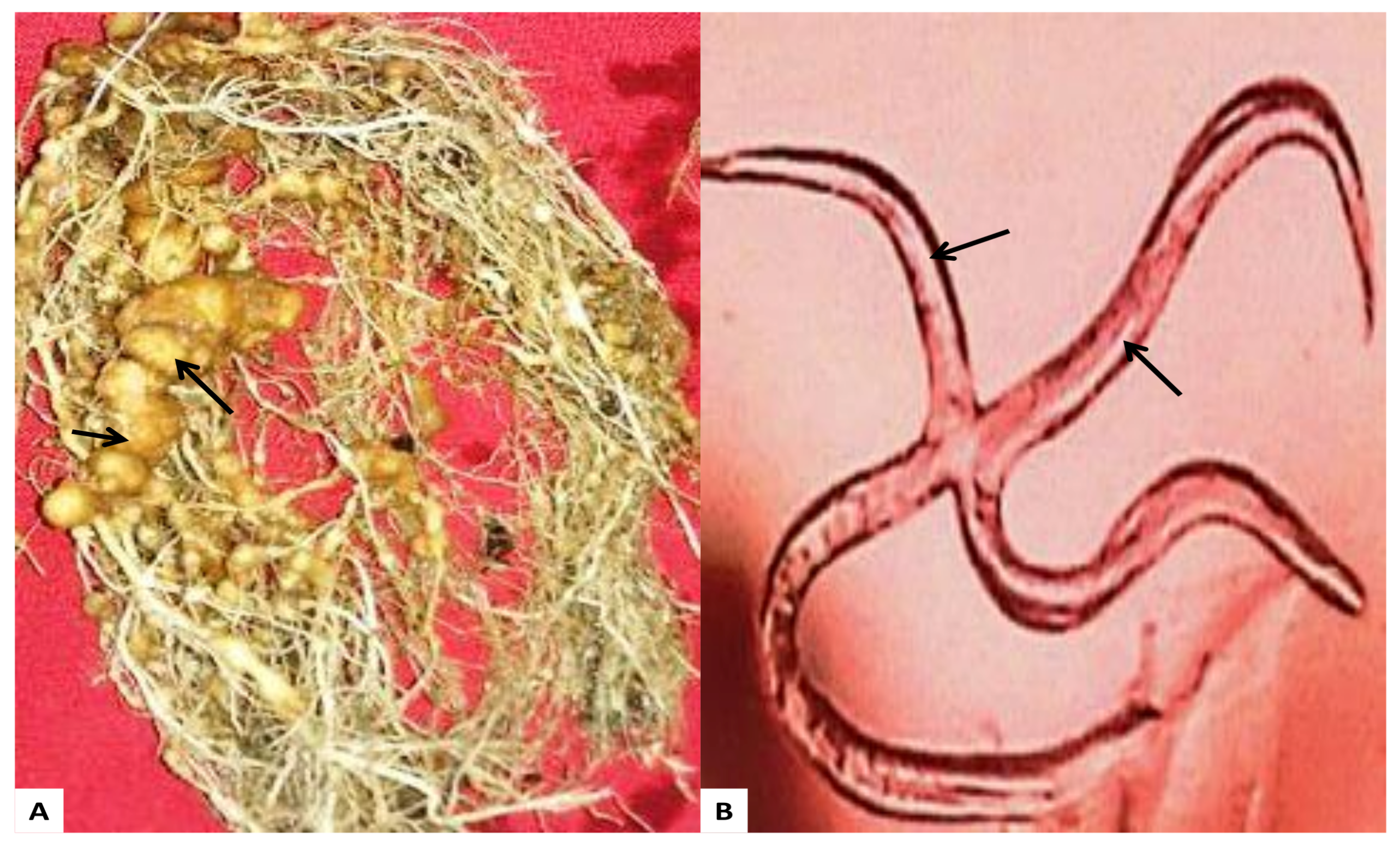
2.7. Effect of AgNPs on Nematode M. Incognita
3. Experimental
3.1. Reagents
3.2. Instruments
3.3. Preparation of Aqueous Extract of Strawberry Waste
3.4. Biosynthesis of AgNPs Using Aqueous Extract of Strawberry Waste
3.5. Bacterial Inoculum
3.6. Fungus Inoculum
3.7. Root Knot Nematodes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Aslani, F.; Bagheri, S.; Muhd, J.N.; Juraimi, A.S.; Hashemi, F.S.G.; Baghdadi, A. Effects of engineered nanomaterials on plants growth: An overview. Sci. World J. 2014, 2014, 1–28. [Google Scholar] [CrossRef]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Padnya, P.; Gorbachuk, V.; Stoikov, I. The Role of Calix[n]arenes and Pillar[n]arenes in the Design of Silver Nanoparticles: Self-Assembly and Application. Int. J. Mol. Sci. 2020, 21, 1425. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.; Mungray, A.A.; Mungray, A.K. Fabricated nanoparticles. Rev. Environ. Contam. Toxicol. 2014, 230, 83–110. [Google Scholar]
- BCC Research. 2014. Global Markets for Nanocomposites, Nanoparticles, Nanoclays, and Nanotubes. Available online: https://www.bccresearch.com/market-research/nanotechnology/nanocomposites-market-nan021f.html?vsmaid=203 (accessed on 4 February 2021).
- Kaabipour, S.; Hemmati, S. A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J. Nanotechnol. 2021, 12, 102–136. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Sedaghat, S.; Moradi, O. Review on green nano-biosynthesis of silver nanoparticles and their biological activities: With an emphasis on medicinal plants. Inorg. Nano Met. Chem. 2021, 51, 133–142. [Google Scholar] [CrossRef]
- Velgosova, O.; Veselovský, L. Synthesis of Ag nanoparticle using R. officinalis, U. dioica and V. vitis-idaea extracts. Mater. Lett. 2019, 248, 150–152. [Google Scholar]
- Velgosova, O.; Dolinská, S.; Mražíková, S.; Briančin, J. Effect of P. kessleri extracts treatment on AgNPs synthesis. Inorg. Nano-Met. Chem. 2020, 50, 842–852. [Google Scholar] [CrossRef]
- Velgosova, O.; Mudra, E.; Vojtko, M. Preparing, Characterization and Anti-Biofilm Activity of Polymer Fibers Doped by Green Synthesized AgNPs. Polymers 2021, 13, 605. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. Green synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Singh, J.; Kukkar, P.; Sammi, H.; Rawat, M.; Singh, G.; Kukkar, D. Enhanced catalytic reduction of 4-nitrophenol and congo red dye By silver nanoparticles prepared from Azadirachta indica leaf extract under direct sunlight exposure. Particul. Sci. Technol. 2019, 37, 434–443. [Google Scholar] [CrossRef]
- Singh, K.; Kukkar, D.; Singh, R.; Kukkar, P.; Bajaj, N.; Singh, J.; Kim, K.H. In situ green synthesis of Au/Ag nanostructures on a metal-organic framework surface for photocatalytic reduction of p-nitrophenol. J. Ind. Eng. Chem. 2020, 81, 196–205. [Google Scholar] [CrossRef]
- Rani, P.; Kumar, V.; Singh, P.P.; Matharu, A.S.; Zhang, W.; Kim, K.H.; Singh, J.; Rawat, M. Highly stable AgNPs prepared via a novel green approach for catalytic and photocatalytic removal of biological and non-biological pollutants. Environ. Int. 2020, 143, 105924. [Google Scholar] [CrossRef]
- Verma, S.K.; Das, A.K.; Patel, M.K.; Shah, A.; Kumar, V.; Gantait, S. Engineered nanomaterials for plant growth and development: A perspective analysis. Sci. Total Environ. 2018, 630, 1413–1435. [Google Scholar] [CrossRef]
- Mohseniazar, M.; Barin, M.; Zarredar, H.; Alizadeh, S.; Shanehbandi, D. Potential of Microalgae and Lactobacilli in biosynthesis of silver nanoparticles. BioImpacts 2011, 1, 149–152. [Google Scholar]
- Khan, A.U.; Khan, M.; Khan, M.M. Selected nanotechnology and nanostructure for drug delivery, nanomedicine and cure. Bioprocess. Biosyst. Eng. 2020, 43, 1339–1357. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N. On the Basic Concept of Nano Technology. In Proceedings of the International Conference on Production Engineering, Tokyo, Japan, 26–29 August 1974; pp. 18–23. [Google Scholar]
- Ocsoy, I.; Mathews, L.; Paret, M.A.; Ocsoy, S.; Kunwar, T.; Chen, M.Y.; Tan, W. Nanotechnology in Plant Disease Management: DNA-Directed Silver Nanoparticles on Graphene Oxide as an Antibacterial against Xanthomonas perforans. ACS Nano 2013, 7, 8972–8980. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Kalathil, S.; Lee, J.; Cho, M.H. Synthesis of Cysteine Capped Silver Nanoparticles by Electrochemically Active Biofilm and their Antibacterial Activities. Bull. Korean Chem. Soc. 2012, 33, 2592–2596. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Redhwi, H.H.; Achilias, D.S.; Kosmidou, E.; Vakalopoulou, E.; Ioannidou, M.D. Green Synthesis of Silver Nanoparticles and Study of Their Antimicrobial Properties. J. Polym. Environ. 2018, 26, 423–433. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, M.; Khan, M.M. Antifungal and Antibacterial Assay by Silver Nanoparticles Synthesized from Aqueous Leaf Extract of Trigonellafoenum-graecum. BioNanoScience 2019, 9, 597–602. [Google Scholar] [CrossRef]
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D.; García Mendoza, E.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A.; et al. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020, 10, 12805. [Google Scholar] [CrossRef]
- Min, J.S.; Kim, K.S.; Kim, S.W.; Jung, J.H.; Lamsal, K.; Kim, S.B.; Jung, M.; Lee, Y.S. Effects of colloidal silver nanoparticles on sclerotium-forming phytopathogenic fungi. Plant. Pathol. J. 2009, 25, 376–380. [Google Scholar] [CrossRef]
- Valli, J.S.; Vaseeharan, B. Biosynthesis of silver nanoparticles by Cissus quadrangularis extracts. Mater. Lett. 2012, 82, 171–173. [Google Scholar] [CrossRef]
- Padali, H.; Moteriya, P.; Chanda, S. Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arab. J. Chem. 2015, 8, 732–741. [Google Scholar] [CrossRef]
- Khan, M.M.; Saadah, N.H.; Khan, M.E.; Harunsani, M.H.; Tan, A.L.; Cho, M.H. Potentials of Costus woodsonii leaf extract in producing narrow band gap ZnO nanoparticles. Mater. Sci. Semicond. Process. 2019, 91, 194–200. [Google Scholar] [CrossRef]
- Matussin, S.; Harunsani, M.H.; Tan, A.L.; Mansoob Khan, M. Plant-Extract-Mediated SnO2 Nanoparticles: Synthesis and Applications. ACS Sustain. Chem. Eng. 2020, 8, 3040–3054. [Google Scholar] [CrossRef]
- Hayward, A.C. Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu. Rev. Phytopathol. 1991, 29, 65–87. [Google Scholar] [CrossRef]
- Laurence, M.H.; Summerell, B.A.; Burgess, L.W.; Liew, E.C.Y. Genealogical concordance phylogenetic species recognition in the Fusarium oxysporum species complex. Fungal Biol. 2014, 118, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Malik, N.; Khan, M.; Cho, M.H.; Khan, M.M. Fungi-assisted silver nanoparticle synthesis and their applications. Bioprocess. Biosyst. Eng. 2018, 41, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ogunyemi, S.O.; Abdallah, Y.; Zhang, M.; Fouad, H.; Hong, X.; Ibrahim, E.; Masum, M.I.; Hossain, A.; Mo, J.; Li, B. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif. Cells Nanomed. Biotechnol. 2019, 47, 341–352. [Google Scholar] [CrossRef]
- Stephano-Hornedo, J.L.; Torres-Gutiérrez, O.; Toledano-Magaña, Y.; Gradilla-Martínez, I.; Pestryakov, A. ArgovitTM silver nanoparticles to fight huanglongbing disease in Mexican limes (Citrus aurantifolia Swingle). RSC Adv. 2020, 10, 6146–6155. [Google Scholar] [CrossRef]
- Ghazy, N.A.; Abd El-Hafez, O.A.; El-Bakery, A.M.; El-Geddawy, D.I. Impact of silver nanoparticles and two biological treatments to control soft rot disease in sugar beet (Beta vulgaris L.). Egypt. J. Biol. Pest. Control. 2021, 31, 1–2. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A.U.; Alam, M.J.; Park, S.; Alam, M. Biosynthesis of silver nanoparticles and its application against phytopathogenic bacterium and fungus. Int. J. Environ. Anal. Chem. 2019, 100, 1390–1401. [Google Scholar] [CrossRef]
- Ashraf, H.; Anjum, T.; Riaz, S.; Naseem, S. Microwave-assisted green synthesis and characterization of silver nanoparticles using Melia azedarach for the management of Fusarium wilt in tomato. Front. Microbiol. 2020, 11, 238. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, B.R.; Singh, A. Biofabricated silver nanoparticles act as a strong fungicide against Bipolaris sorokiniana causing Spot Blotch disease in wheat. PLoS ONE 2014, 9, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Lee, S.M.; Iydroose, M.; Lee, K.J.; Oh, B.T. Pine cone-mediated green synthesis of silver nanoparticles and their antibacterial activity against agricultural pathogens. Appl. Microbiol. Biotech. 2013, 97, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Ramachandran, R.; Mohan, K.; Kalaichelvan, P. Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim. Acta A 2012, 93, 95–99. [Google Scholar] [CrossRef]
- Nassar, A.M.K. Effectiveness of Silver Nano-particles of Extracts of Urtica urens (Urticaceae) Against Root-knot Nematode Meloidogyne incognita. Asian J. Nematol. 2016, 5, 14–19. [Google Scholar] [CrossRef]
- Gupta, D.; Chauhan, P. Fungicidal activity of silver nanoparticles against Alternaria brassicicola. AIP Conf. Proc. 2015, 1724, 020031. [Google Scholar]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holk, K.; Kouri, J.B.; Tapía Ramírez, J.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Baronia, R.; Kumar, P.; Singh, S.P.; Walia, R.K. Silver nanoparticles as a potential nematicide against Meloidogyne graminicola. J. Nematol. 2020, 52, 1–9. [Google Scholar] [CrossRef]
- Ardakani, A.S. Toxicity of silver, titanium and silicon nanoparticles on the root-knot nematode, Meloidogyne incognita, and growth parameters of tomato. Nematology 2013, 15, 671–677. [Google Scholar] [CrossRef]
- Kumar, D.A.; Palanichamy, V.; Roopan, S.M. Green synthesis of silver nanoparticles using Alternanthera dentate leaf extract at room temperature and their antimicrobial activity. Spectrochim. Acta A 2014, 127, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Riker, A.J.; Riker, R.S. Introduction to Research on Plant Diseases; John’s Swift Co.: New York, NY, USA; 1936. [Google Scholar]
- Saharan, V. Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int. J. Biol. Macromol. 2013, 62, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Sharvelle, E.G. The Nature and Uses of Modern Fungicides. Nat. Uses Modern Fungicides; Burgess Pub. Co.: Minneapolis, MN, USA, 1961; p. 308. [Google Scholar]
- Vincent, J. Distortion of fungal hyphae in the presence of certain inhibitors. Nature 1947, 159, 850. [Google Scholar] [CrossRef]
| Phytopathogens | Bioresource | Concentration of AgNPs | Stabilizer Type | Size of NPs | Zeta Potential, mV | FTIR Study | XRD Study | SPR Peak (nm) | AgNPs Effect on Phytopathogens | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Ralstonia solanacearum, Fusarium oxysporum and Meloidogyne incognita | strawberry waste (solid waste after fruit juice extraction) | 100 μg/mL | Secondary metabolites in extract | 55–70 nm | n.d | Peaks located at 3307 (NH), 1733 carbonyl group (C=O), and 1628 (stretching -C=C-) aromatic ring stretching vibrations. | Peaks at 38.4°, 46.3°, 64.7°, and 78.2°, which correspond to the (111), (200), (220), and (311) planes of metallic silver. | ~415 | Inhibit growth of tested phytopa-thogens | Our work |
| Alternaria alternate, Pseudomonas syringae | Leaf of the Trigonella foenum-graecum | 100 μg/mL | Secondary metabolites in extract | 20–25 nm | n.d | Peaks 3247, 2919, 1587, 1376, and 1019 cm−1, which represents free OH, stretching -C=C- aromatic ring, and C-OH stretching vibrations, respectively | Peaks at 27.9°, 32.4°, 38.2°, and 46.3°, which correspond to the (111), (200), (220), and (311) planes, respectively. | ~410 | Inhibit growth of tested phytopa-thogens | [23] |
| Pectobacterium carotovorum | Fusarium oxysporum | n.d | Secondary metabolites in extract | 16–27 nm | n.d | n.d | n.d | ~430 | Inhibit growth of tested phytopa-thogens | [35] |
| Phomopsis vexans, Ralstonia solanacearum | Corn seeds | 100 μg/mL | Secondary metabolites in extract | 25 nm | n.d | Peaks at 3284 (OH), 1645, 1400, 1336–1145, which represents free OH in molecules and stretching -C=C- aromatic ring and C-OH stretching vibrations. | 2θ values of 27.91°, 32.19°, and 46.64° sets of lattice planes. | 423, 437, 464 | Inhibit growth of tested phytopa-thogens | [36] |
| Fusarium oxysporum | Leaf extract of Melia azedarach | n.d | Secondary metabolites in extract | 12–46 nm | −22.3 | Peaks at 3258.25 and 1634.31 cm−1 represents vibrations of hydroxyl (–OH) group and alkene (C=C) with aromatic ring, respectively | Peaks at 38.12°, 44.23°, 64.51°, and 77.69° that can be assigned to the plane of (111), (200), (220), and (311), respectively | 434 | Inhibit growth of tested phytopa-thogens | [37] |
| Bipolaris sorokiniana | Serratia sp. | n.d | Secondary metabolites in extract | 10–20 nm | n.d | Peaks at 3436.52 and 2942.73 cm−1 were assigned to the stretching vibrations of primary and secondary amines, respectively. | Bragg reflections were obtained at 2θ = 38.4°, 44.5°, 64.6°, and 76.9°, which correspond to the crystal lattice planes (111), (200), (220), and (311) of face centered cubic (fcc) structures of silver (JCPDS files No. 03-0921), respectively | 410 | Inhibit growth of tested phytopa-thogens | [38] |
| Bacillus megaterium, Pseudomonas syringae, Burkholderia glumae, Xanthomonas oryzae, and Bacillus thuringiensis | Pine cone | 108 μg/mL | Secondary metabolites in extract | 5–50 nm | 30 | Peaks at 3449 (vibrations of the O–H groups), 2922 (asymmetric and symmetric C–H stretching), 1718 (carbonyl stretching), 1615, and 1509 (asymmetrical stretching of the carboxylate group), 1369 (symmetrical stretch of the carboxylate group), 1263 (acetyl group), 1160, and 1057 (C–O stretching vibration of ether and alcohol groups), and 3449–3406 cm−1 (binding of silver ions with hydroxyl groups). | Peaks at 38.6°, 44.2°, 46.2°, 65.2°, 68.1°, 78.2°, and 85.2°. The peak at 85.2° was unidentified and indexed to the 111, 200, 220, and 311 planes of the cubic face-centered silver | 414 | Inhibit growth of tested phytopa-thogens | [39] |
| Botrytis cinerea, Alternaria alternata, Curvularia lunata, Rhizoctonia solani, Macrophomina phaseolina, Sclerotinia sclerotiorum | Acalypha indicaleaf | 95 μg/mL | Secondary metabolites in extract | 10–50 nm | n.d | n.d | Peaks at 38.1°, 44.1°, and 64.1°, which indexed the planes 111, 200, and 220 of the cubic face-centered silver | n.d | Inhibit growth of tested phytopa-thogens | [40] |
| Meloidogyne incognita | Urtica uren leaf | n.d | n.d | Ethyl acetate extract: 60–112 nm) Ethanol extract: 80–111 nm | n.d | n.d | n.d | n.d | Inhibit growth of tested phytopa-thogens | [41] |
| Treatments | No. of M. incognita Hatched after 24 h | No. of M. incognita Hatched after 48 h | No. of M. incognita Dead after 48 h |
|---|---|---|---|
| Distilled water | 38 | 81 | 04 |
| AgNPs + Distilled water | 24 | 47 | 09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.; Khan, A.U.; Bogdanchikova, N.; Garibo, D. Antibacterial and Antifungal Studies of Biosynthesized Silver Nanoparticles against Plant Parasitic Nematode Meloidogyne incognita, Plant Pathogens Ralstonia solanacearum and Fusarium oxysporum. Molecules 2021, 26, 2462. https://doi.org/10.3390/molecules26092462
Khan M, Khan AU, Bogdanchikova N, Garibo D. Antibacterial and Antifungal Studies of Biosynthesized Silver Nanoparticles against Plant Parasitic Nematode Meloidogyne incognita, Plant Pathogens Ralstonia solanacearum and Fusarium oxysporum. Molecules. 2021; 26(9):2462. https://doi.org/10.3390/molecules26092462
Chicago/Turabian StyleKhan, Masudulla, Azhar U. Khan, Nina Bogdanchikova, and Diana Garibo. 2021. "Antibacterial and Antifungal Studies of Biosynthesized Silver Nanoparticles against Plant Parasitic Nematode Meloidogyne incognita, Plant Pathogens Ralstonia solanacearum and Fusarium oxysporum" Molecules 26, no. 9: 2462. https://doi.org/10.3390/molecules26092462
APA StyleKhan, M., Khan, A. U., Bogdanchikova, N., & Garibo, D. (2021). Antibacterial and Antifungal Studies of Biosynthesized Silver Nanoparticles against Plant Parasitic Nematode Meloidogyne incognita, Plant Pathogens Ralstonia solanacearum and Fusarium oxysporum. Molecules, 26(9), 2462. https://doi.org/10.3390/molecules26092462







