Design, Synthetic Strategies, and Therapeutic Applications of Heterofunctional Glycodendrimers
Abstract
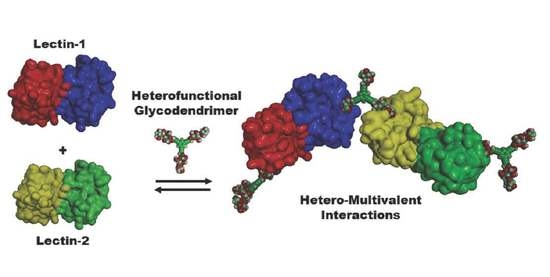
:1. Introduction
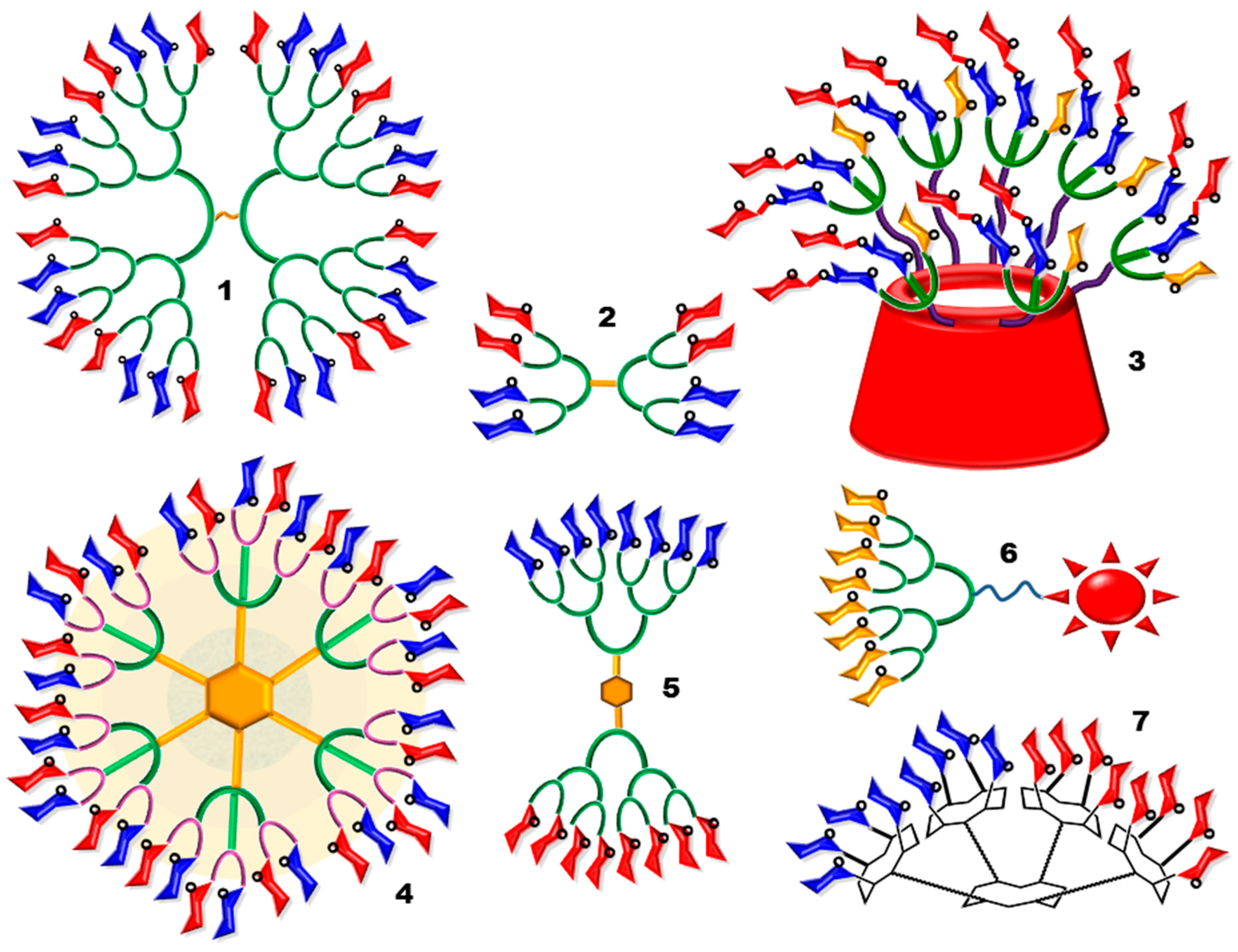
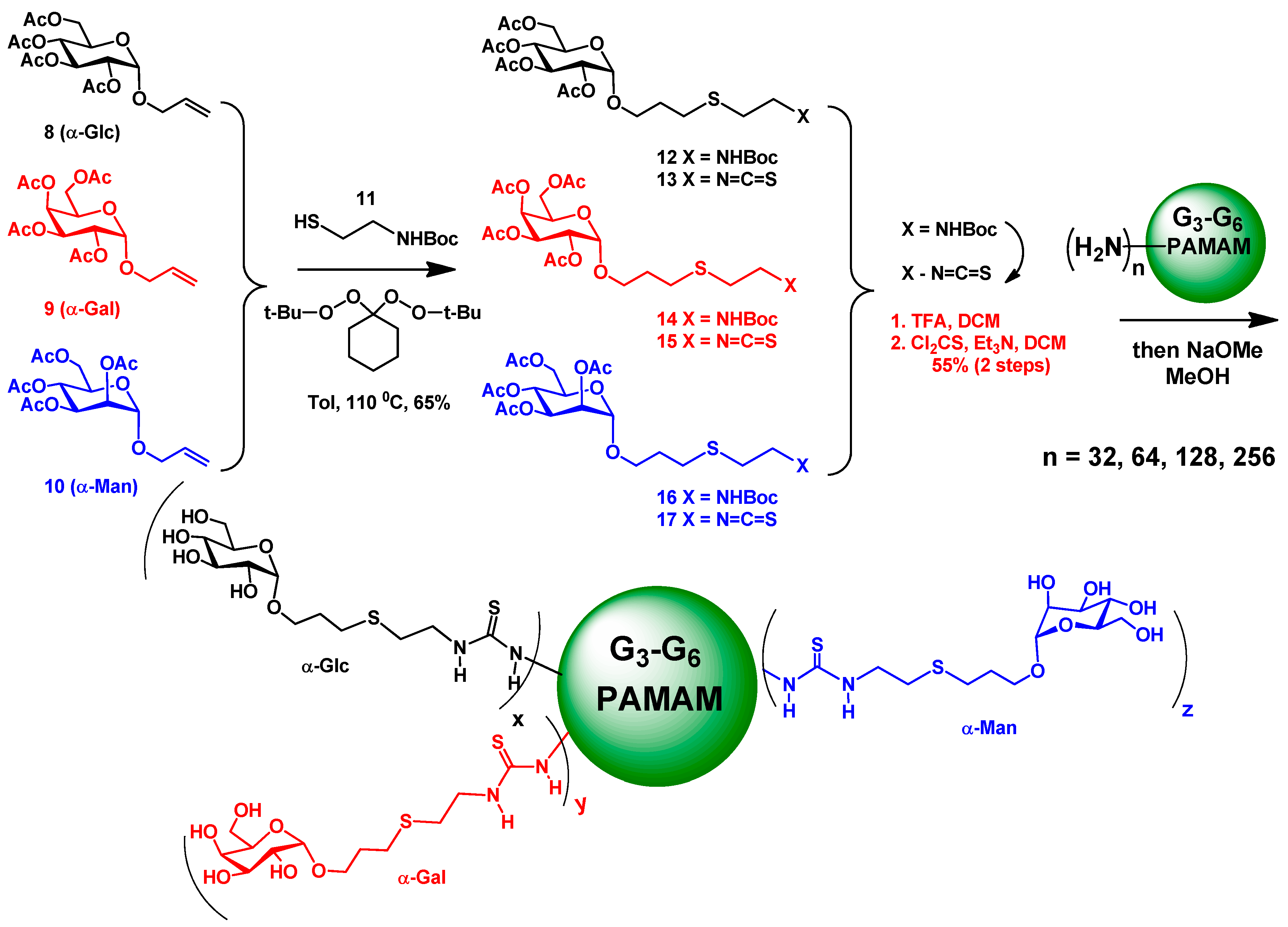
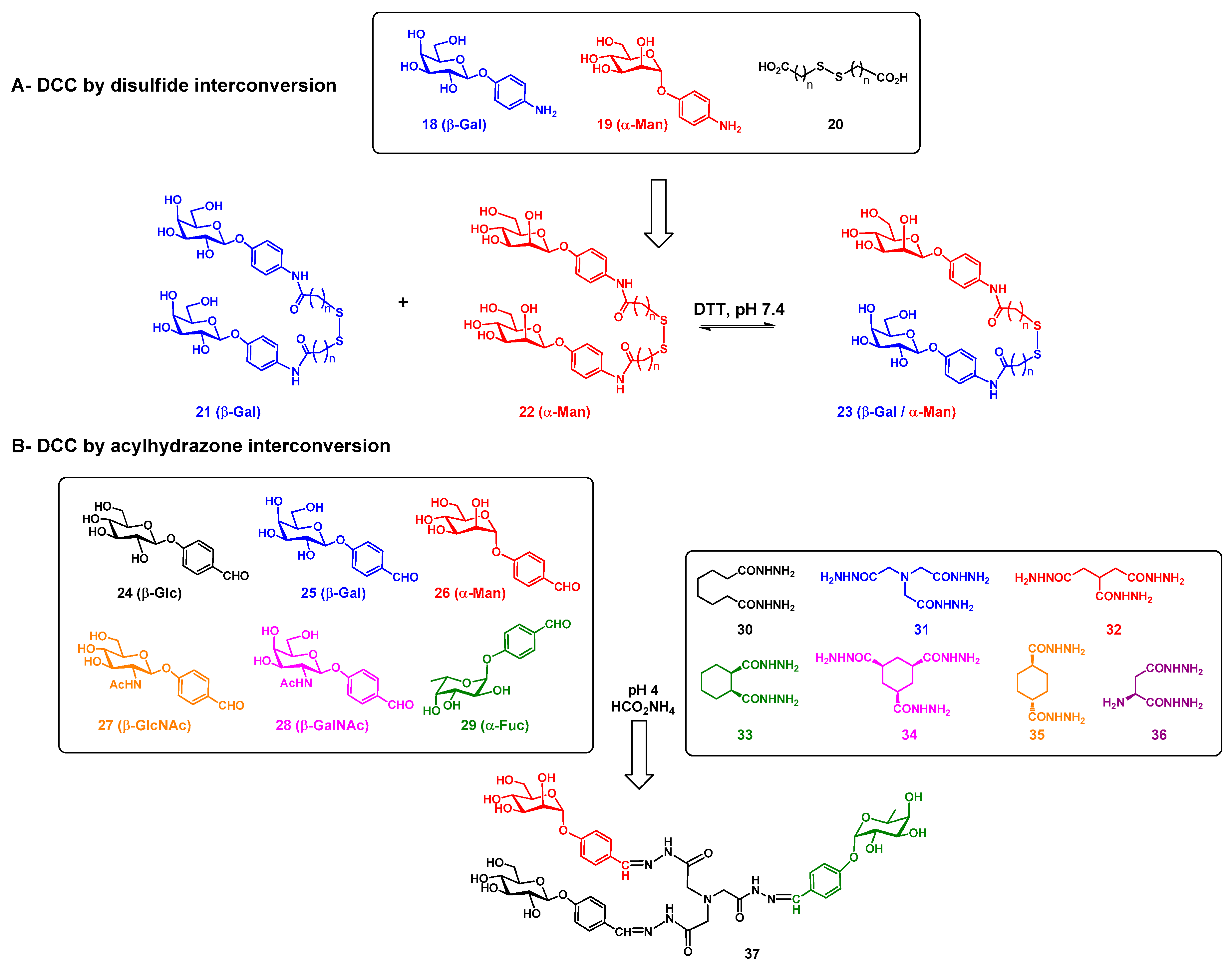
2. Randomized Heterofunctional Glycodendrimers and Dynamic Combinatorial Library
3. Immune Cell Targeting, Immunodiagnostics, and Vaccines
3.1. Heterofunctional Glycodendrimers as Clearing Agents Following Radioimmunotherapy
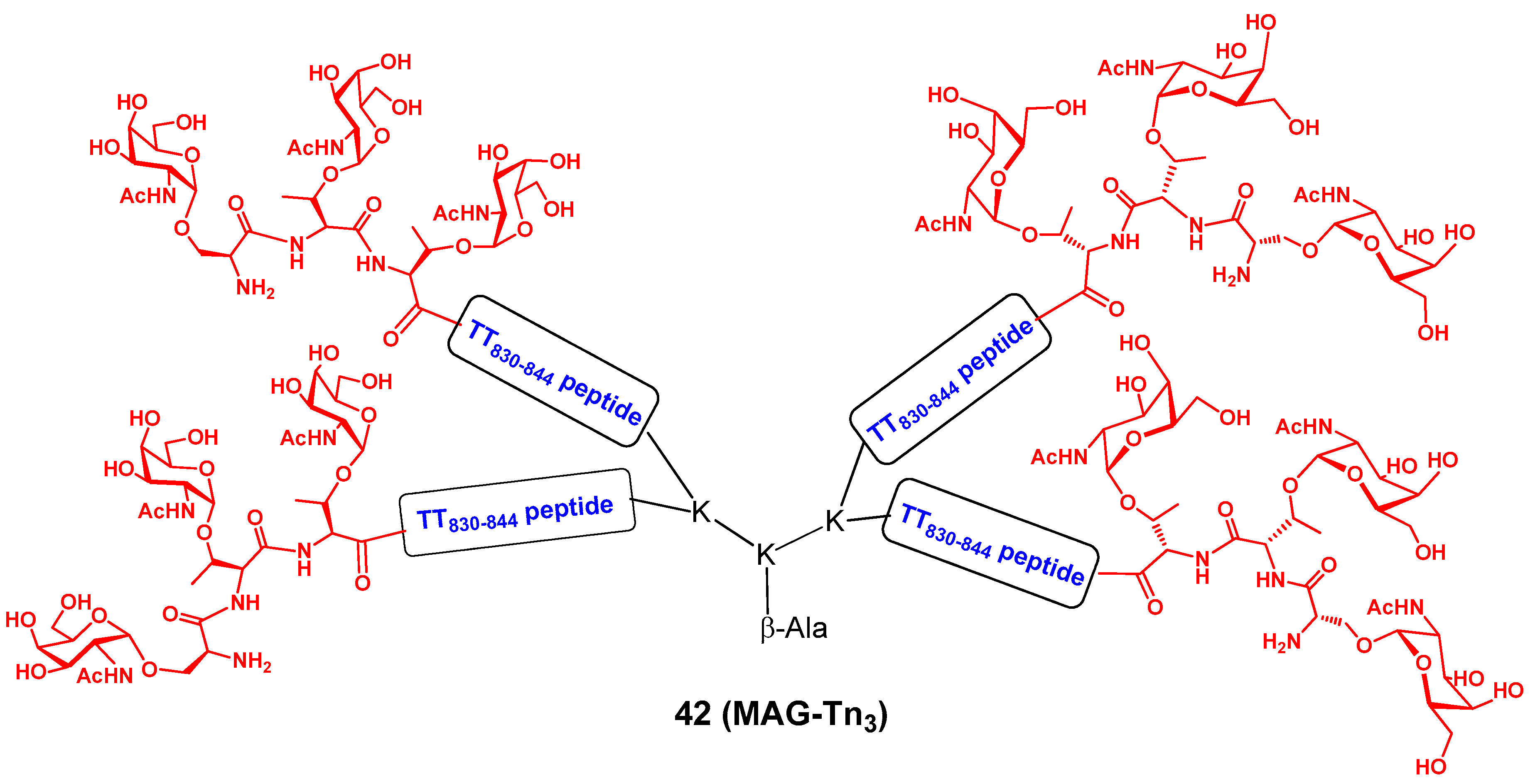
3.2. Heterobifunctional Cancer Vaccines
3.3. Blocking Antibody Formation to Prevent Autoimmune Diseases and Allergy
3.4. Dendritic Glycopeptides as Vaccines against Allergy
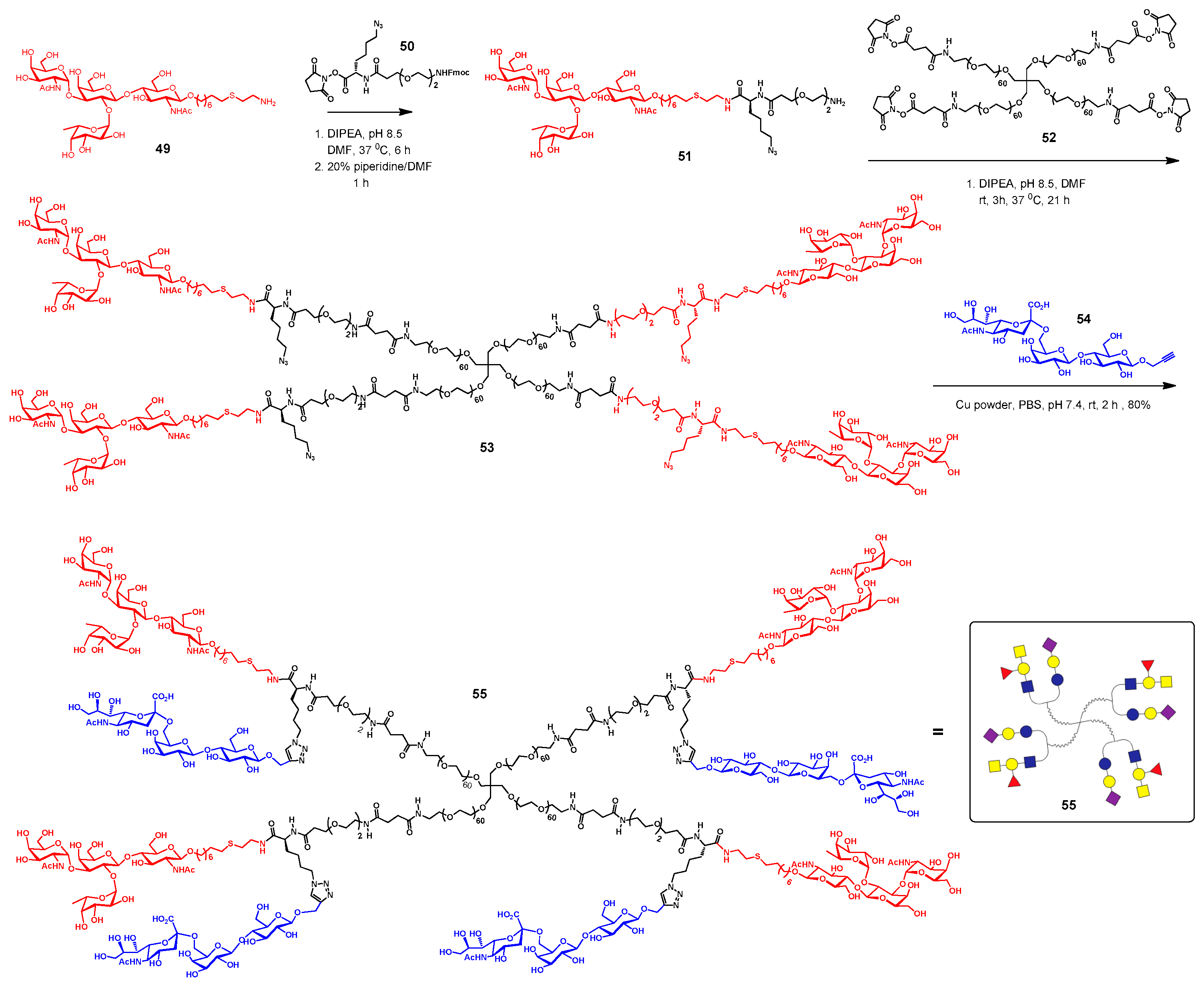
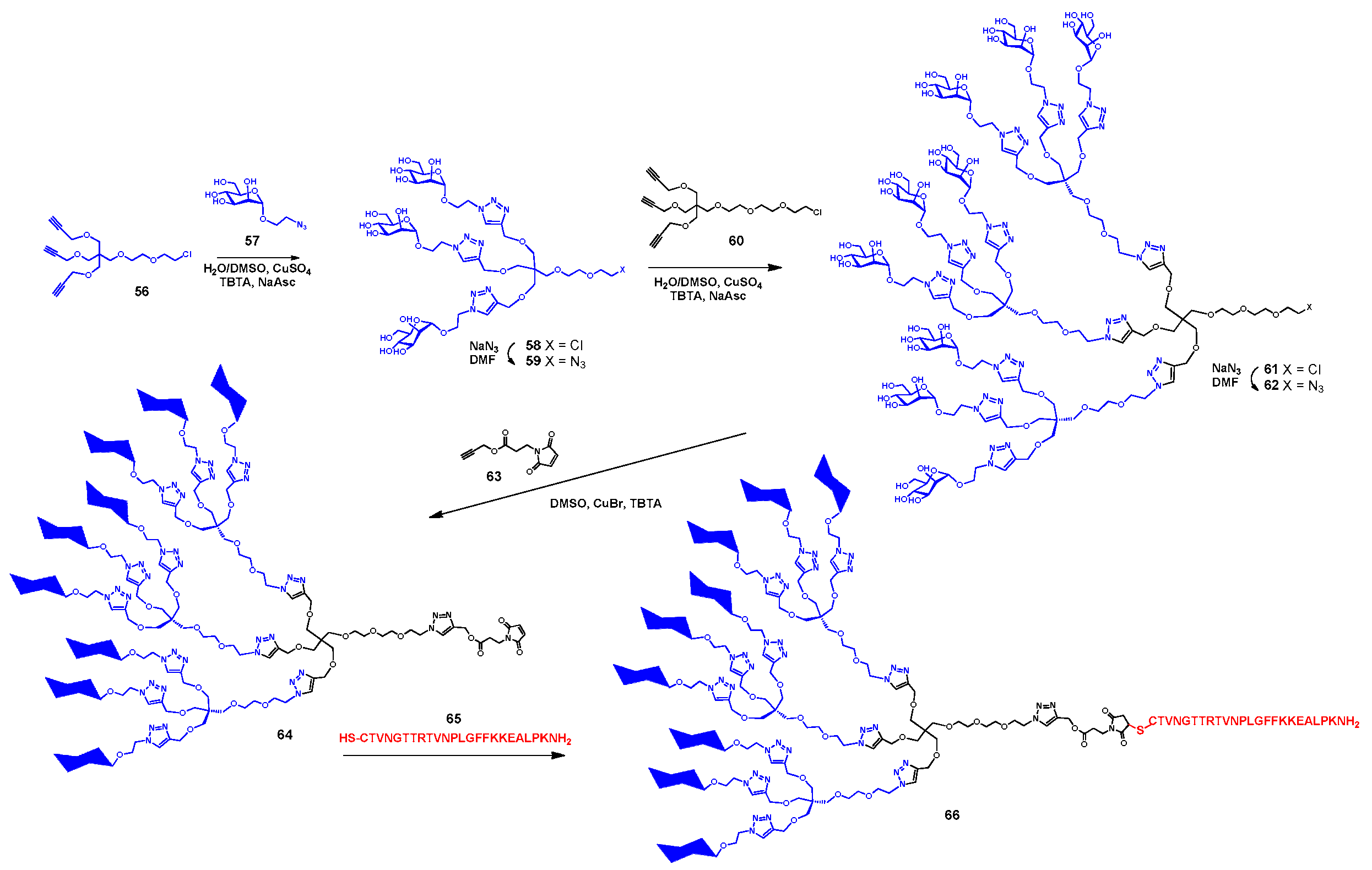
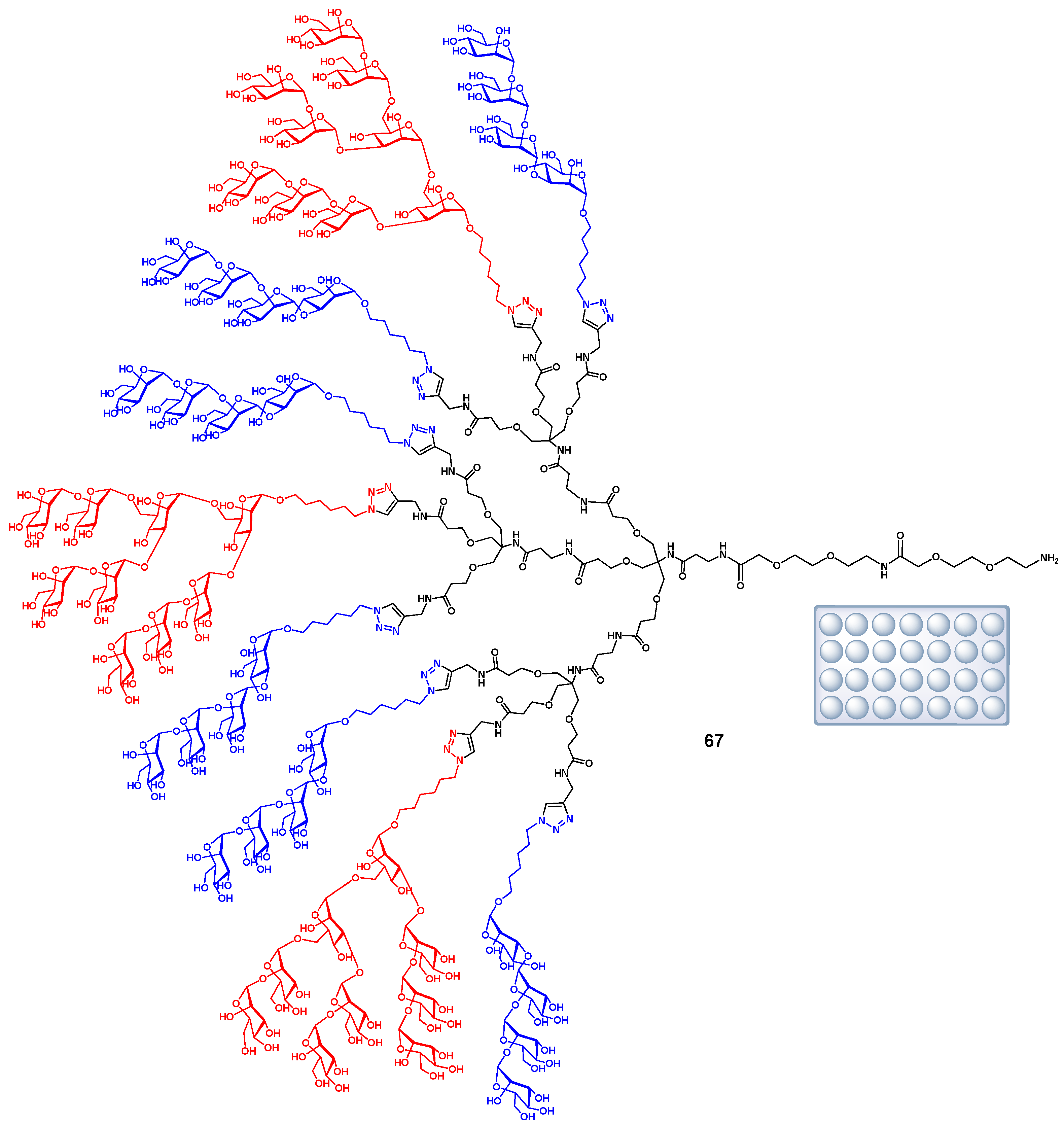
3.5. Immunodiagnostics Using Glycan Microarrays
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Roy, R. A decade of glycodendrimer chemistry. TIGGS 2003, 15, 291–310. [Google Scholar] [CrossRef]
- Chabre, Y.M.; Roy, R. Recent trends in glycodendrimer syntheses and applications. Curr. Top. Med. Chem. 2008, 8, 1237–1285. [Google Scholar] [CrossRef]
- Chabre, Y.M.; Roy, R. Design and Creativity in Multivalent Neoglycoconjugate Synthesis. Adv. Carbohydr. Chem. Biochem. 2010, 63, 165–393. [Google Scholar] [PubMed]
- Chabre, Y.M.; Roy, R. Multivalent glycoconjugate syntheses and applications using aromatic scaffolds. Chem. Soc. Rev. 2013, 42, 4657–4708. [Google Scholar] [CrossRef]
- Roy, R.; Shiao, T.C. Glyconanosynthons as powerful scaffolds and building blocks for the rapid construction of multifaceted, dense and chiral dendrimers. Chem. Soc. Rev. 2015, 44, 3924–3941. [Google Scholar] [CrossRef]
- Röckendorf, N.; Lindhorst, T.K. Glycodendrimers. Top. Curr. Chem. 2001, 217, 201–238. [Google Scholar]
- Müller, C.; Despras, G.; Lindhorst, T.K. Organizing multivalency in carbohydrate recognition. Chem. Soc. Rev. 2016, 45, 3275–3302. [Google Scholar] [CrossRef] [Green Version]
- Touaibia, M.; Roy, R. Glycodendrimers as anti-adhesion drugs against type 1 fimbriated E. coli uropathogenic infections. Mini Rev. Med. Chem. 2007, 7, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Touaibia, M. Application of multivalent mannosylated dendrimers in glycobiology. In Comprehensive Glycoscience; Kamerling, H., Ed.; Elsevier: Oxford, UK, 2007; pp. 821–870. [Google Scholar]
- Johannssen, T.; Lepenies, B. Glycan-Based Cell Targeting to Modulate Immune Responses. Trends Biotechnol. 2017, 35, 334–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirois, S.; Touaibia, M.; Chou, K.-C.; Roy, R. Glycosylation of HIV-1 gp120 V3 Loop: Towards the Rational Design of a Synthetic Carbohydrate Vaccine. Curr. Med. Chem. 2007, 14, 3232–3242. [Google Scholar] [CrossRef] [PubMed]
- Mousavifar, L.; Roy, R. Recent development in the design of small ‘drug-like’ and nanoscale glycomimetics against Escherichia coli infections. Drug Discov. Today 2021, in press. [Google Scholar] [CrossRef]
- Imberty, A.; Chabre, Y.M.; Roy, R. Glycomimetics and glycodendrimers as high affinity microbial anti-adhesins. Chem. Eur. J. 2008, 14, 7490–7499. [Google Scholar] [CrossRef] [PubMed]
- Pieters, R.J. Intervention with bacterial adhesion by multivalent carbohydrates. Med. Res. Rev. 2007, 27, 796–816. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lee, R.T. Carbohydrate-Protein Interactions: Basis of Glycobiology. Acc. Chem. Res. 1995, 28, 321–327. [Google Scholar] [CrossRef]
- Lundquist, J.L.; Toone, E.J. The cluster glycoside effect. Chem. Rev. 2002, 102, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Mammen, M.; Choi, S.-K.; Whitesides, G.M. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew. Chem. Int. Ed. 1998, 37, 2754–2794. [Google Scholar] [CrossRef]
- Lee, R.T.; Lee, Y.C. Affinity enhancement by multivalent lectin—Carbohydrate interaction. Glycoconj. J. 2000, 17, 543–551. [Google Scholar] [CrossRef]
- Roy, R. Syntheses and some applications of chemically defined multivalent glycoconjugates. Curr. Opin. Struct. Biol. 1996, 6, 692–702. [Google Scholar] [CrossRef]
- Kiessling, L.L.; Gestwicki, J.E.; Strong, L.E. Synthetic multivalent ligands as probes of signal transduction. Angew. Chem. Int. Ed. Engl. 2006, 45, 2348–2368. [Google Scholar] [CrossRef]
- Wolfenden, M.L.; Cloninger, M.J. Mannose/Glucose-Functionalized Dendrimers to Investigate the Predictable Tunability of Multivalent Interactions. J. Am. Chem. Soc. 2005, 127, 12168–12169. [Google Scholar] [CrossRef]
- Wolfenden, M.L.; Cloninger, M.J. Carbohydrate-Functionalized Dendrimers to Investigate the Predictable Tunability of Multivalent Interactions. Bioconjugate Chem. 2006, 17, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Deguise, I.; Lagnoux, D.; Roy, R. Synthesis of glycodendrimers containing both fucoside and galactoside residues and their binding properties to Pa-IL and PA-IIL lectins from Pseudomonas Aeruginosa. New J. Chem. 2007, 31, 1321–1331. [Google Scholar] [CrossRef]
- Jiménez Blanco, J.L.; Ortiz Mellet, C.; Garciá Fernández, J.M. Multivalency in heterogeneous glycoenvironments: Hetero-glycoclusters, -glycopolymers and -glycoassemblies. Chem. Soc. Rev. 2013, 42, 4518–4531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Cuesta, M.; Ortiz Mellet, C.; García Fernández, J.M. Carbohydrate supramolecular chemistry: Beyond the multivalent effect. Chem. Commun. 2020, 56, 5207–5222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagul, R.S.; Hosseini, M.M.; Shiao, T.C.; Roy, R. “Onion peel” glycodendrimer syntheses using mixed triazine and cyclotriphosphazene scaffolds. Can. J. Chem. 2017, 95, 975–983. [Google Scholar] [CrossRef]
- Sharma, R.; Naresh, K.; Chabre, Y.M.; Rej, R.; Saadeh, N.K.; Roy, R. “Onion peel” dendrimers: A straightforward synthetic approach towards highly diversified architectures. Polym. Chem. 2014, 5, 4321–4331. [Google Scholar] [CrossRef]
- Sharma, R.; Zhang, I.; Abbassi, L.; Rej, R.; Maysinger, D.; Roy, R. A fast track strategy toward highly functionalized dendrimers with different structural layers: An “onion peel approach”. Polym. Chem. 2015, 6, 1436–1444. [Google Scholar] [CrossRef]
- Sharma, R.; Kottari, N.; Chabre, Y.M.; Abbassi, L.; Shiao, T.C.; Roy, R. A highly versatile convergent/divergent “onion peel” synthetic strategy toward potent multivalent glycodendrimers. Chem. Commun. 2014, 50, 13300–13303. [Google Scholar] [CrossRef]
- Jiang, S.; Niu, S.; Zhao, Z.-H.; Li, Z.-J.; Li, Q. Synthesis of a series of novel heteroglycoclusters and homoglycoclusters and the study of their anti-adhesion activities. Carbohydr. Res. 2015, 414, 39–45. [Google Scholar] [CrossRef]
- Cheal, S.M.; Patel, M.; Yang, G.; Veach, D.; Xu, H.; Guo, H.F.; Zanzonico, P.B.; Axworthy, D.B.; Cheung, N.K.V.; Ouerfelli, O.; et al. An N-Acetylgalactosamino Dendron-Clearing Agent for High-Therapeutic-Index DOTA-Hapten Pretargeted Radioimmunotherapy. Bioconjugate Chem. 2020, 31, 501–506. [Google Scholar] [CrossRef]
- Benedé, S.; Ramos-Soriano, J.; Palomares, F.; Losada, J.; Mascaraque, A.; López-Rodríguez, J.C.; Rojo, J.; Mayorga, C.; Villalba, M.; Batanero, E. Peptide Glycodendrimers as Potential Vaccines for Olive Pollen Allergy. Mol. Pharm. 2020, 17, 827–836. [Google Scholar] [CrossRef]
- Roy, R.; Shiao, T.C.; Rittenhouse-Olson, K. Glycodendrimers: Versatile tools for nanotechnology. Braz. J. Pharmac. Sci. 2013, 49, 85–108. [Google Scholar] [CrossRef] [Green Version]
- Pifferi, C.; Thomas, B.; Goyard, D.; Berthet, N.; Renaudet, O. Heterovalent Glycodendrimers as Epitope Carriers for Antitumor Synthetic Vaccines. Chem. Eur. J. 2017, 23, 16283–16296. [Google Scholar] [CrossRef] [Green Version]
- Lis, H.; Sharon, N. Lectins: Carbohydrate-specific proteins that mediate cellular recognition. Chem. Rev. 1998, 98, 637–674. [Google Scholar] [CrossRef]
- Arya, P.; Kutterer, K.M.K.; Quin, H.; Roby, J.; Barnes, M.L.; Kim, J.-M.; Roy, R. Diversity of C-linked Neoglycopeptides for the exploration of subsite-assisted carbohydrate binding interactions. Bioorg. Med. Chem. Lett. 1998, 8, 1127–1132. [Google Scholar] [CrossRef] [Green Version]
- Ramström, O.; Lehn, J.-M. In situ generation and screening of a dynamic combinatorial carbohydrate library against concanavalin A. ChemBioChem 2000, 1, 41–48. [Google Scholar] [CrossRef]
- Ramström, O.; Lohmann, S.; Bunyapaiboonsri, T.; Lehn, J.-M. Dynamic Combinatorial Carbohydrate Libraries: Probing the Binding Site of the Concanavalin a Lectin. Chem. Eur. J. 2004, 10, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Pagé, D.; Roy, R. Synthesis and Biological Properties of Mannosylated Starburst Poly(amidoamine) Dendrimers. Bioconj. Chem. 1997, 8, 714–772. [Google Scholar] [CrossRef] [PubMed]
- Ohmi, Y.; Kambe, M.; Ohkawa, Y.; Hamamura, K.; Tajima, O.; Takeuchi, R.; Furukawa, K.; Furukawa, K. Differential roles of gangliosides in malignant properties of melanomas. PLoS ONE 2018, 13, e0206881. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.M.; Danishefsky, S.J. A Vision for Vaccines Built from Fully Synthetic Tumor-Associated Antigens: From the Laboratory to the Clinic. J. Am. Chem. Soc. 2013, 135, 14462–14472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brockhausen, I.; Melamed, J. Mucins as anti-cancer targets: Perspectives of the glycobiologist. Glycoconj. J. 2021. [Google Scholar] [CrossRef]
- Moffett, S.; Shiao, T.C.; Mousavifar, L.; Mignani, S.; Roy, R. Aberrant glycosylation patterns on cancer cells: Therapeutic opportunities for glycodendrimers/ metallodendrimers oncology. Wires Nanomed. Nanobiotechnol. 2020, 13, e1659. [Google Scholar] [CrossRef]
- Shiao, T.C.; Roy, R. Glycodendrimers as functional antigens and antitumor vaccines. New J. Chem. 2012, 36, 324–339. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconj. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Gildersleeve, J.C.; Blixt, O.; Shin, I. Carbohydrate microarrays. Chem. Soc. Rev. 2013, 42, 4310–4326. [Google Scholar] [CrossRef] [PubMed]
- Narla, S.N.; Nie, H.; Li, Y.; Sun, X.-L. Multi-dimensional glycan microarrays with glyco-macroligands. Glycoconj. J. 2015, 32, 483–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larson, S.M.; Carrasquillo, J.A.; Cheung, N.K.; Press, O.W. Radioimmunotherapy of human tumours. Nat. Rev. Cancer. 2015, 15, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, J.; Kim, H.; Lee, Y.-J.; Kim, J.S.; Lim, S.M. Combination Radioimmunotherapy Strategies for Solid Tumors. Int. J. Mol. Sci. 2019, 20, 5579. [Google Scholar] [CrossRef] [Green Version]
- Ganneau, C.; Simenel, C.; Emptas, E.; Courtiol, T.; Coïc, Y.-M.; Artaud, C.; Dériaud, E.; Bonhomme, F.; Delepierre, M.; Leclerc, C.; et al. Large-scale synthesis and structural analysis of a synthetic glycopeptide dendrimer as an anti-cancer vaccine candidate. Org. Biomol. Chem. 2017, 15, 114–123. [Google Scholar] [CrossRef]
- Rosenbaum, P.; Artaud, C.; Bay, S.; Ganneau, C.; Campone, M.; Delaloge, S.; Gourmelon, C.; Loirat, D.; Medioni, J.; Pein, F.; et al. The fully synthetic glycopeptide MAG-Tn3 therapeutic vaccine induces tumor-specific cytotoxic antibodies in breast cancer patients. Cancer Immunol. Immunother. 2020, 69, 703–716. [Google Scholar] [CrossRef]
- Heimburg-Molinaro, J.; Almogren, A.; Morey, S.; Glinskii, O.V.; Roy, R.; Wilding, G.E.; Cheng, R.P.; Glinsky, V.V.; Rittenhouse-Olson, K. Development, characterization, and immunotherapeutic use of peptide mimics of the Thomsen-Friedenreich carbohydrate antigen. Neoplasia 2009, 11, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Daskhan, G.C.; Ton Tran, H.-T.; Meloncelli, P.J.; Lowary, T.L.; West, L.J.; Cairo, C.W. Construction of Multivalent Homo- and Heterofunctional ABO Blood Group Glycoconjugates Using a Trifunctional Linker Strategy. Bioconjugate Chem. 2018, 29, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.L.; Gildersleeve, J.C. Abnormal antibodies to self-carbohydrates in SARS-CoV-2 infected patients. BioRxiv 2020, in press. [Google Scholar] [CrossRef]
- Solís, D.; Bovin, N.V.; Davis, A.P.; Jiménez-Barbero, J.; Romero, A.; Roy, R.; Smetana, K., Jr.; Gabius, H.-J. A guide into glycosciences: How chemistry and biochemistry cooperate to crack the sugar code. Biochim. Biophys. Acta 2015, 1850, 186–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, R.; Murphy, P.V.; Gabius, H.-J. Multivalent Carbohydrate-Lectin Interactions: How Synthetic Chemistry Enables Insights into Nanometric Recognition. Molecules 2016, 21, 629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krauss, I.J.; Joyce, J.G.; Finnefrock, A.C.; Song, H.C.; Dudkin, V.Y.; Geng, X.; Warren, J.D.; Chastain, M.; Shiver, J.W.; Danishefsky, S.J. Fully Synthetic Carbohydrate HIV Antigens Designed on the Logic of the 2G12 Antibody. J. Am. Chem. Soc. 2007, 129, 11042–11044. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.G.; Krauss, I.J.; Song, H.C.; Opalka, D.W.; Grimm, K.M.; Nahas, D.D.; Esser, M.T.; Hrin, R.; Feng, M.; Dudkin, V.Y.; et al. An oligosaccharide-based HIV-1 2G12 mimotope vaccine induces carbohydrate-specific antibodies that fail to neutralize HIV-1 virions. Proc. Natl. Acad. Sci. USA 2008, 105, 15684–15689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.-H.; Wang, S.-K.; Lin, C.-W.; Wang, L.-C.; Wong, C.-H.; Wu, C.-Y. Effects of Neighboring Glycans on Antibody–Carbohydrate Interaction. Angew. Chem. Int. Ed. 2011, 50, 1608–1613. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavifar, L.; Roy, R. Design, Synthetic Strategies, and Therapeutic Applications of Heterofunctional Glycodendrimers. Molecules 2021, 26, 2428. https://doi.org/10.3390/molecules26092428
Mousavifar L, Roy R. Design, Synthetic Strategies, and Therapeutic Applications of Heterofunctional Glycodendrimers. Molecules. 2021; 26(9):2428. https://doi.org/10.3390/molecules26092428
Chicago/Turabian StyleMousavifar, Leila, and René Roy. 2021. "Design, Synthetic Strategies, and Therapeutic Applications of Heterofunctional Glycodendrimers" Molecules 26, no. 9: 2428. https://doi.org/10.3390/molecules26092428
APA StyleMousavifar, L., & Roy, R. (2021). Design, Synthetic Strategies, and Therapeutic Applications of Heterofunctional Glycodendrimers. Molecules, 26(9), 2428. https://doi.org/10.3390/molecules26092428