Polymer–Colloid Complexes Based on Cationic Imidazolium Amphiphile, Polyacrylic Acid and DNA Decamer
Abstract
1. Introduction
2. Results and Discussion
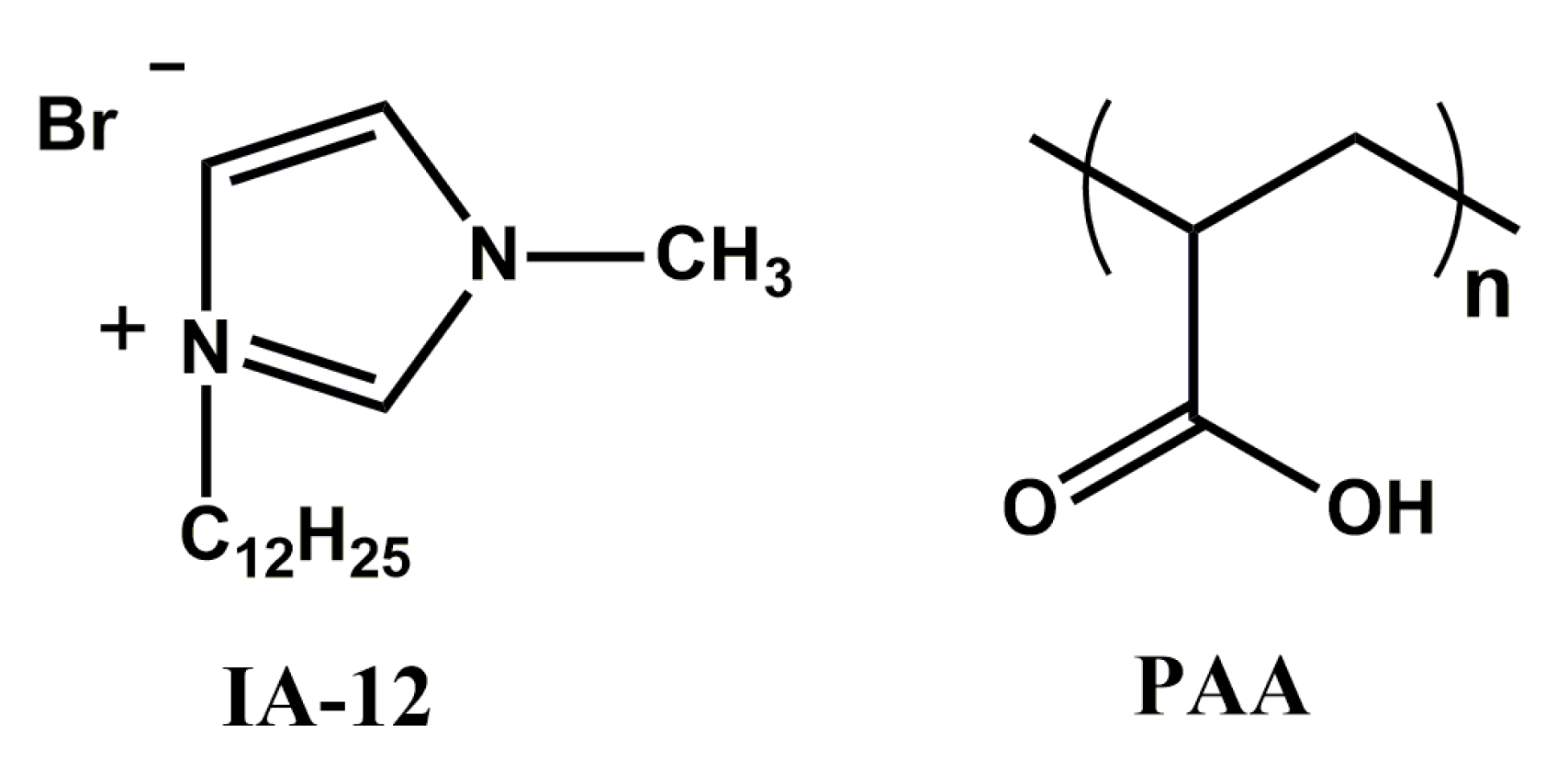
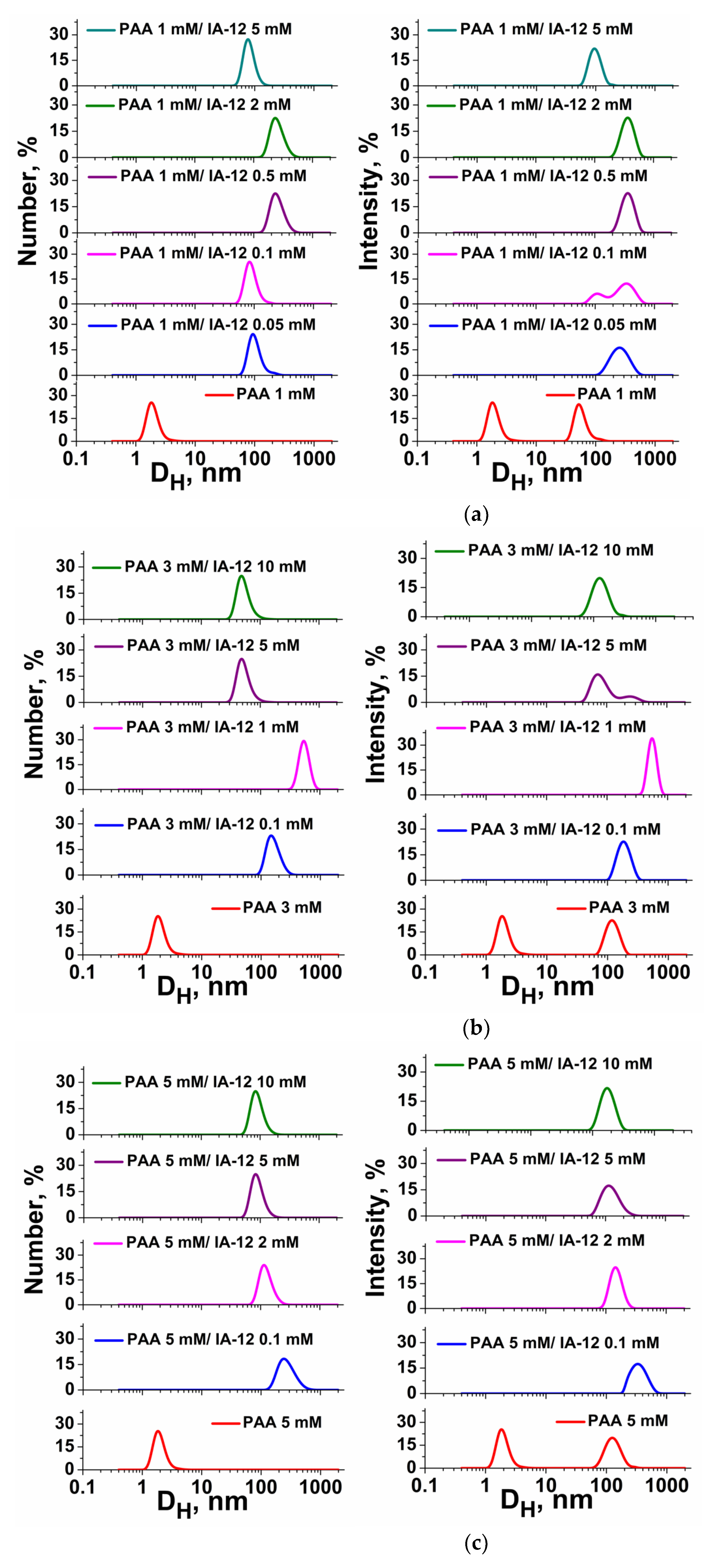
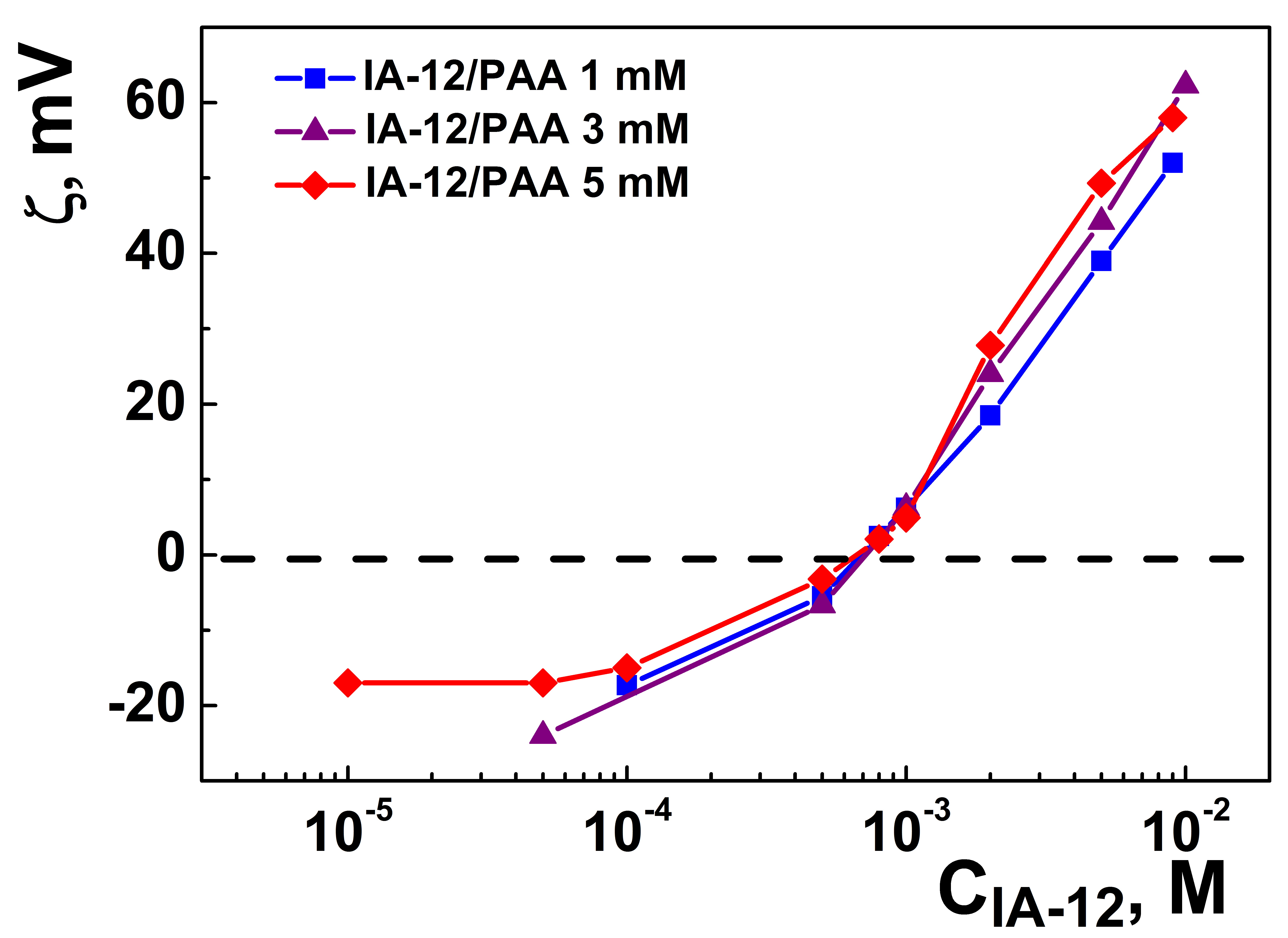
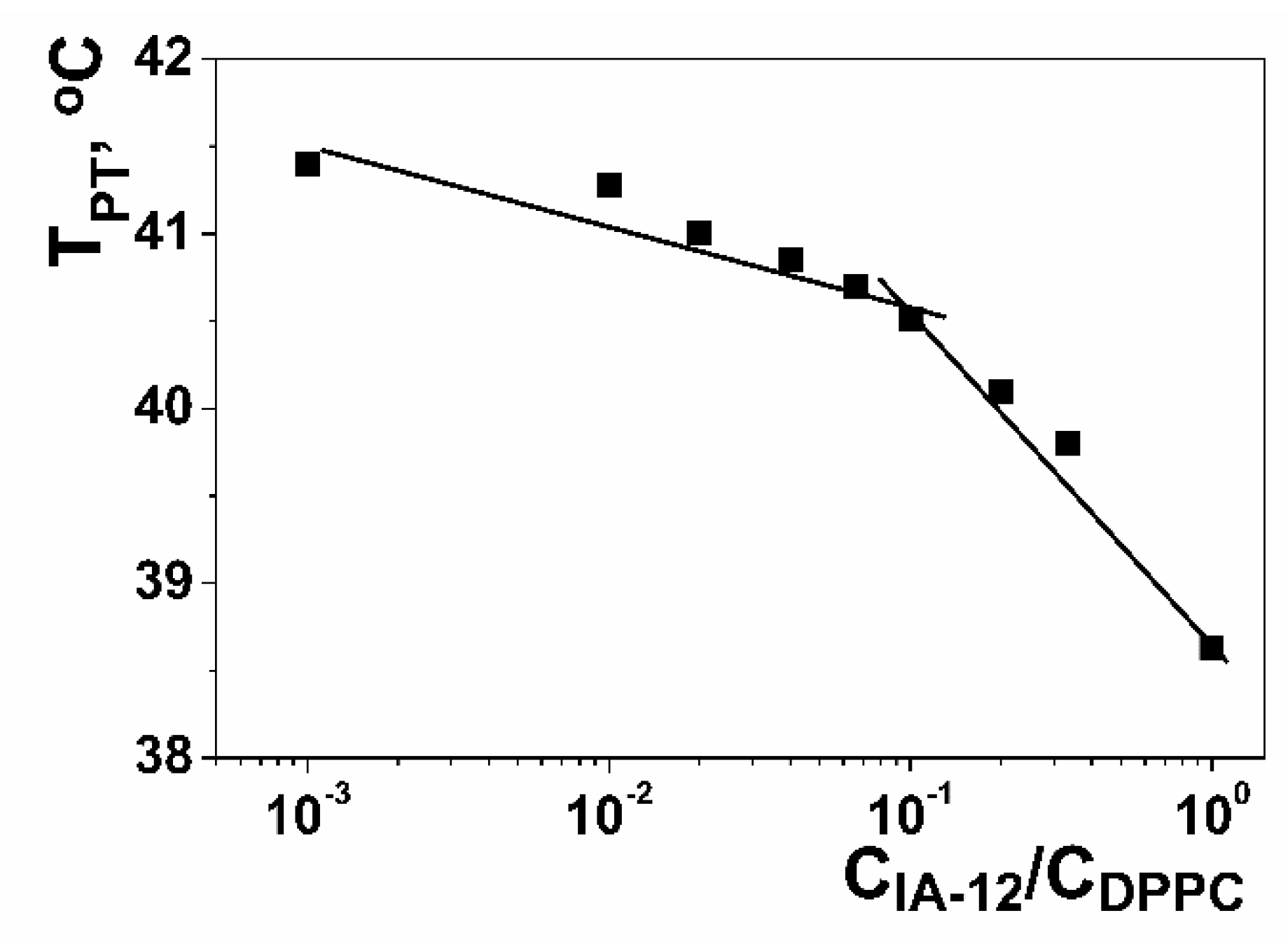
2.1. Mixed Systems Based on IA-12 and Polyacrylic Acid
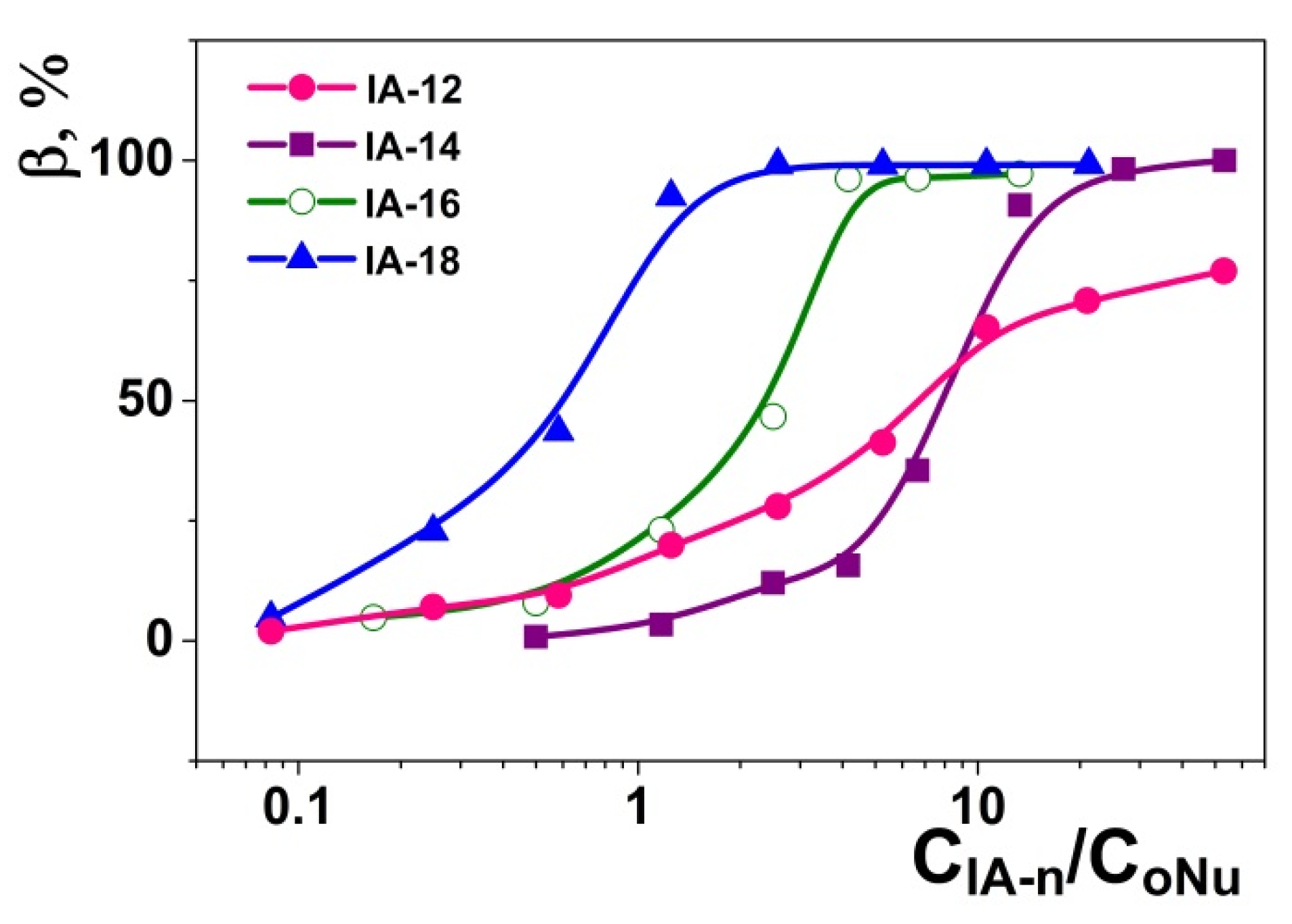
2.2. Amphiphile/DNA Decamer Interactions
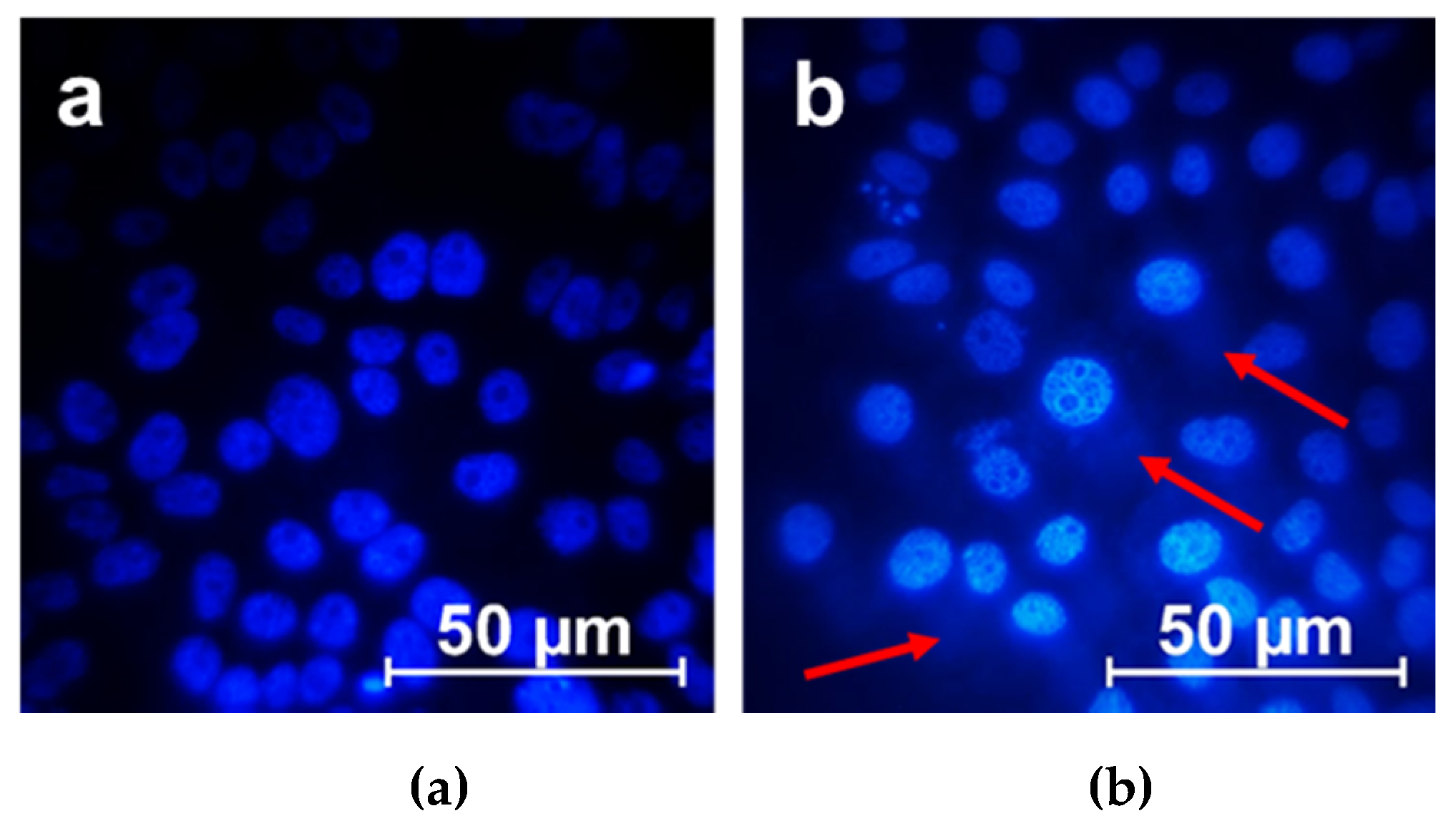
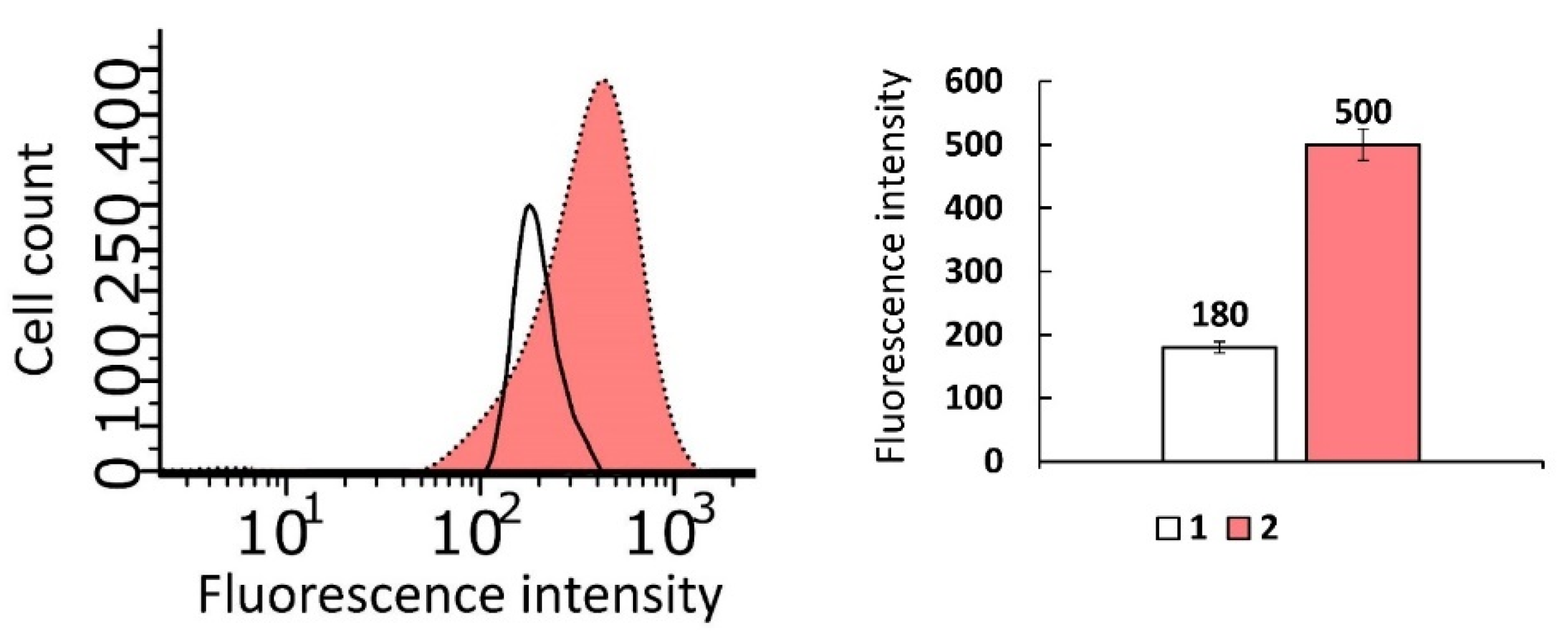
2.3. Membranotropic Properties and Cell Penetration
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Samples
3.3. Methods
3.3.1. Tensiometry
3.3.2. Conductometry
3.3.3. Fluorescence Spectroscopy
3.3.4. Dynamic and Electrophoretic Light Scattering
3.3.5. Electrophoretic Analysis
3.3.6. Turbidimetry
3.3.7. Fluorescence Microscopy
3.3.8. Flow Cytometry Assay
3.3.9. In Vitro Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Varga, I.; Campbell, R.A. General physical description of the behavior of oppositely charged polyelectrolyte/surfactant mixtures at the air/water interface. Langmuir 2017, 33, 5915–5924. [Google Scholar] [CrossRef] [PubMed]
- Lele, B.J.; Tilton, R.D. Colloidal depletion and structural force synergism or antagonism in solutions of mutually repelling polyelectrolytes and ionic surfactants. Langmuir 2019, 35, 15937–15947. [Google Scholar] [CrossRef] [PubMed]
- Gradzielski, M.; Hoffmann, I. Polyelectrolyte-surfactant complexes (PESCs) composed of oppositely charged components. Curr. Opin. Colloid Interface Sci. 2018, 35, 124–141. [Google Scholar] [CrossRef]
- Guzmán, E.; Fernández-Peña, L.; Ortega, F.; Rubio, R.G. Equilibrium and kinetically trapped aggregates in polyelectrolyte–oppositely charged surfactant mixtures. Curr. Opin. Colloid Interface Sci. 2020, 48, 91–108. [Google Scholar] [CrossRef]
- Guzmán, E.; Llamas, S.; Maestro, A.; Fernández-Peña, L.; Akanno, A.; Miller, R.; Ortega, F.; Rubi, R.G. Polymer–surfactant systems in bulk and at fluid interfaces. Adv. Colloid Interface Sci. 2016, 233, 38–64. [Google Scholar] [CrossRef]
- Klučáková, M.; Jarábková, S.; Velcer, T.; Kalina, M.; Pekař, M. Transport of a model diffusion probe in polyelectrolyte-surfactant hydrogels. Colloids Surf. A 2019, 573, 73–79. [Google Scholar] [CrossRef]
- Vasilieva, E.A.; Samarkina, D.A.; Gaynanova, G.A.; Lukashenko, S.S.; Gabdrakhmanov, D.R.; Zakharov, V.M.; Vasileva, L.A.; Zakharova, L.Y. Self-assembly of the mixed systems based on cationic surfactants and different types of polyanions: The influence of structural and concentration factors. J. Mol. Liq. 2018, 272, 892–901. [Google Scholar] [CrossRef]
- Tseng, H.-W.; Chen, P.-C.; Tsui, H.-W.; Wang, C.-H.; Hu, T.-Y.; Chen, L.-J. Effect of molecular weight of poly(acrylic acid) on the interaction of oppositely charged ionic surfactant–polyelectrolyte mixtures. J. Taiwan Inst. Chem. Eng. 2018, 92, 50–57. [Google Scholar] [CrossRef]
- Asadov, Z.H.; Nasibova, S.M.; Ahmadova, G.A.; Zubkov, F.I.; Rahimov, R.A. Head-group effect of surfactants of cationic type in interaction with propoxylated sodium salt of polyacrylic acid in aqueous solution. Colloids Surf. A. 2017, 527, 95–100. [Google Scholar] [CrossRef]
- Yan, P.; Jin, C.; Wang, C.; Ye, J.P.; Xiao, J.X. Effect of surfactant head group size on polyelectrolyte–surfactant interactions: Steady-state and time-resolved fluorescence study. J. Colloid Interface Sci. 2005, 282, 188–192. [Google Scholar] [CrossRef]
- Langevin, D. Complexation of oppositely charged polyelectrolytes and surfactants in aqueous solutions. A review. Adv. Colloid Interface Sci. 2009, 147−148, 170–177. [Google Scholar] [CrossRef]
- Wang, C.; Tam, K.C. Interaction between polyelectrolyte and oppositely charged surfactant: Effect of charge density. J. Phys. Chem. B. 2004, 108, 8976–8982. [Google Scholar] [CrossRef]
- Schulze-Zachau, F.; Braunschweig, B. CnTAB/polystyrene sulfonate mixtures at air–water interfaces: Effects of alkyl chain length on surface activity and charging state. Phys. Chem. Chem. Phys. 2019, 21, 7847–7856. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.K.; Kumar, J.; Nalwa, H.S. Handbook of Polyelectrolytes and Their Applications; American Scientific Publishers: Stevenson Ranch, CA, USA, 2002. [Google Scholar]
- Ray, D.; Das, B. Micellization of ionic liquid surfactants induced by sodium polystyrenesulfonate in aqueous solutions. J. Solut. Chem. 2019, 48, 1576–1590. [Google Scholar] [CrossRef]
- Koolivand-Salooki, M.; Javadi, A.; Bahramian, A.; Abdollahi, M. Dynamic interfacial properties and foamability of polyelectrolyte-surfactant mixtures. Colloids Surf. A 2019, 562, 345–353. [Google Scholar] [CrossRef]
- Gabdrakhmanov, D.R.; Valeeva, F.G.; Samarkina, D.A.; Lukashenko, S.S.; Mirgorodskaya, A.B.; Zakharova, L.Y. The first representative of cationic amphiphiles bearing three unsaturated moieties: Self-assembly and interaction with polypeptide. Colloids Surf. A. 2018, 558, 463–469. [Google Scholar] [CrossRef]
- Jain, N.; Trabelsi, S.; Guillot, S.; McLoughlin, D.; Langevin, D.; Letellier, P.; Turmine, M. Citical aggregation concentration in mixed solutions of anionic polyelectrolytes and cationic surfactants. Langmuir 2004, 20, 8496–8503. [Google Scholar] [CrossRef]
- Taylor, D.J.F.; Thomas, R.K.; Li, P.X. Adsorption of oppositely charged polyelectrolyte/surfactant mixtures. neutron reflection from alkyl trimethylammonium bromides and sodium poly(styrenesulfonate) at the air/water interface: The effect of surfactant chain length. Langmuir 2003, 19, 3712–3719. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Vasilieva, E.A.; Lukashenko, S.S.; Ahtamyanova, L.R.; Siraev, I.S.; Zakharova, L.Y. Supramolecular catalytic systems based on a cationic amphiphile and sodium polystyrene sulfonate for decomposition of organophosphorus pollutants. Russ. J. Org. Chem. 2019, 55, 11–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Delivery Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Faizullin, D.A.; Zuev, Y.F.; Nizameev, I.R.; Kadirov, M.K.; Kuznetsov, D.M.; Zakharova, L.Y. Interaction of bovine serum albumin with cationic imidazoliumcontaining amphiphiles bearing urethane fragment: Effect of hydrophobic tail length. J. Mol. Liq. 2020, 307, 113001. [Google Scholar] [CrossRef]
- Samarkina, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Nizameev, I.R.; Kadirov, M.K.; Zakharova, L.Y. Homologous series of amphiphiles bearing imidazolium head group: Complexation with bovine serum albumin. J. Mol. Liq. 2019, 275, 232–240. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, A.; Miyata, K.; Kataoka, K. Recent progress in development of siRNA delivery vehicles for cancer therapy. Adv. Drug Deliv. Rev. 2016, 104, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Wittrup, A.; Lieberman, J. Knocking down disease: A progress report on siRNA therapeutics. Nat. Rev. Genet. 2015, 16, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Wettig, S.D.; Verrall, R.E.; Foldvari, M. Gemini surfactants: A new family of building blocks for non-viral gene delivery systems. Curr. Gene Ther. 2008, 8, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Feng, L.; Dong, R.; Hao, J.; Dong, S. Transfection efficiency of DNA enhanced by association with salt-free catanionic vesicles. Biomacromolecules 2013, 14, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, W.; Wilkowska, M.; Peplinska, B.; Skrzypczak, A.; Kozak, M. Structural characterization of transfection nanosystems based on tricationic surfactants and short double stranded oligonucleotides. Biochem. Biophys. Res. Commun. 2019, 518, 706–711. [Google Scholar] [CrossRef]
- Szala, S. Terapia Genowa; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2003. [Google Scholar]
- Leitner, S.; Grijalvo, S.; Solans, C.; Eritja, R.; García-Celma, M.J.; Calderó, G. Ethylcellulose nanoparticles as a new “in vitro” transfection tool for antisense oligonucleotide delivery. Carbohydr. Polym. 2020, 229, 115451. [Google Scholar] [CrossRef]
- López-López, M.; López-Cornejo, P.; Martín, V.I.; Ostos, F.J.; Checa-Rodríguez, C.; Prados-Carvajal, R.; Lebrón, J.A.; Huertas, P.; Moyá, M.L. Importance of hydrophobic interactions in the single-chained cationic surfactant-DNA complexation. J. Colloid Interface Sci. 2018, 521, 197–205. [Google Scholar] [CrossRef]
- Limeresa, M.J.; Suñé-Poua, M.; Prieto-Sánchez, S.; Moreno-Castro, C.; Nusblat, A.D.; Hernández-Munain, C.; Castro, G.R.; Suñé, C.; Suñé-Negre, J.M.; Cuetsas, M.L. Development and characterization of an improved formulation of cholesteryl oleate-loaded cationic solid-lipid nanoparticles as an efficient non-viral gene delivery system. Colloids Surf. B. 2019, 184, 110533. [Google Scholar] [CrossRef]
- Mashal, M.; Attia, N.; Soto-Sánchez, C.; Martínez-Navarrete, G.; Fernández, E.; Puras, G.; Pedraz, J.L. Non-viral vectors based on cationic niosomes as efficient gene delivery vehicles to central nervous system cells into the brain. Int. J. Pharm. 2018, 552, 48–55. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Lin, W.; Chen, S. Gene transfection in complex media using PCBMAEE-PCBMA copolymer with both hydrolytic and zwitterionic blocks. Biomaterials 2014, 35, 7909–7918. [Google Scholar] [CrossRef]
- Soni, S.; Sarkar, S.; Mirzadeh, N.; Selvakannan, P.; Bhargava, S. Self-assembled functional nanostructure of plasmid DNA with ionic liquid [Bmim][PF6]: Enhanced efficiency in bacterial gene transformation. Langmuir 2015, 31, 4722–4732. [Google Scholar] [CrossRef]
- Huang, Q.; Ou, W.; Chen, H.; Feng, Z.; Wang, J.-Y.; Zhang, J.; Zhu, W.; Yu, X.-Q. Novel cationic lipids possessing protonated cyclen and imidazolium salt for gene delivery. Eur. J. Pharm. Biopharm. 2011, 78, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Llizo, A.; Li, P.; Wang, C.; Guo, Y.; Ao, M.; Bai, L. High transfection efficiency of homogeneous DNA nanoparticles induced by imidazolium gemini surfactant as nonviral vector. J. Phys. Chem. C 2013, 117, 26573–26581. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, G.; Ao, M.; Yang, Y.; Wang, C. DNA compaction to multi-molecular DNA condensation induced by cationic imidazolium gemini surfactants. Colloids Surf. A. 2012, 414, 33–40. [Google Scholar] [CrossRef]
- Manning, G.S. Limiting Laws and Counterion Condensation in Polyelectrolyte Solutions I. Colligative Properties. J. Chem. Phys. 1969, 51, 924–938. [Google Scholar] [CrossRef]
- Yoshida, K.; Dubin, P.L. Complex formation between polyacrylic acid and cationic:nonionic mixed micelles: Effect of pH on electrostatic interaction and hydrogen bonding. Colloids Surf. A. 1999, 147, 161–167. [Google Scholar] [CrossRef]
- Vasilieva, E.A.; Lukashenko, S.S.; Vasileva, L.A.; Pavlov, R.V.; Gaynanova, G.A.; Zakharova, L.Y. Aggregation behavior of the surfactant bearing pyrrolidinium head group in the presence of polyacrylic acid. Russ. Chem. Bull. 2019, 68, 341–346. [Google Scholar] [CrossRef]
- Vasilieva, E.A.; Ibragimova, A.R.; Lukashenko, S.S.; Konovalov, A.I.; Zakharova, L.Y. Mixed self-assembly of polyacrylic acid and oppositely charged gemini surfactants differing in the structure of head group. Fluid Phase Equilib. 2014, 376, 172–180. [Google Scholar] [CrossRef]
- Samarkina, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Khamatgalimov, A.R.; Zakharova, L.Y. Aggregation capacity and complexation properties of a system based on an imidazole-containing amphiphile and bovine serum albumin. Russ. J. Gen. Chem. 2017, 87, 2826–2831. [Google Scholar] [CrossRef]
- Hunt, K.K.; Vorburger, S.A.; Swisher, S.G. Gene Therapy for Cancer; Humana Press: Totowa, NJ, USA, 2007. [Google Scholar]
- Samarkina, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Khamatgalimov, A.R.; Kovalenko, V.I.; Zakharova, L.Y. Cationic amphiphiles bearing imidazole fragment: From aggregation properties to potential in biotechnologies. Colloids Surf. A. 2017, 529, 990–997. [Google Scholar] [CrossRef]
- Salakhieva, D.; Shevchenko, V.; Németh, C.; Gyarmati, B.; Szilágyi, A.; Abdullin, T. Structure-biocompatibility and transfection activity relationships of cationic polyaspartamides with (dialkylamino)alkyl and alkyl or hydroxyalkyl side groups. Int. J. Pharm. 2017, 517, 234–246. [Google Scholar] [CrossRef]
- Jumbri, K.; Ahmad, H.; Abdulmalek, E.; Basyaruddin, M.; Rahman, A. Binding energy and biophysical properties of ionic liquid-DNA complex: Understanding the role of hydrophobic interactions. J. Mol. Liq. 2016, 223, 1197–1203. [Google Scholar] [CrossRef]
- Grigoriev, I.V.; Korobeynikov, V.A.; Cheresiz, S.V.; Pokrovskiy, A.G.; Zakharova, L.Y.; Voronin, M.A.; Lukashenko, S.S.; Konovalov, A.I.; Zuev, Y.F. Cationic gemini surfactants as new agents for plasmid DNA delivery into cells. Dokl. Biochem. Biophys. 2012, 445, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Voloshina, A.D.; Sapunova, A.S.; Kashapov, R.R.; Zakharova, L.Y. Self-assembled systems based on novel hydroxyethylated imidazolium-containing amphiphiles: Interaction with DNA decamer, protein and lipid. Chem. Phys. Lipids. 2019, 223, 104791. [Google Scholar] [CrossRef]
- Gabdrakhmanov, D.R.; Vasilieva, E.A.; Voronin, M.A.; Kuznetsova, D.A.; Valeeva, F.G.; Mirgorodskaya, A.B.; Lukashenko, S.S.; Zakharov, V.M.; Mukhitov, A.R.; Faizullin, D.A.; et al. Soft nanocontainers based on hydroxyethylated geminis: Role of spacer in self-assembling, solubilization, and complexation with oligonucleotide. J. Phys. Chem. C 2020, 124, 2178–2192. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Ahtamyanova, L.R.; Nizameev, I.R.; Kadirov, M.K.; Zakharova, L.Y. Novel hybrid liposomal formulations based on imidazolium-containing amphiphiles for drug encapsulation. Colloids Surf. B 2019, 178, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, L.; Voronin, M.; Semenov, V.; Gabdrakhmanov, D.; Syakaev, V.; Gogolev, Y.; Giniyatullin, R.; Lukashenko, S.; Reznik, V.; Latypov, S.; et al. Supramolecular systems based on novel mono- and dicationic pyrimidinic amphiphiles and oligonucleotides: A self-organization and complexation study. Chem. Phys. Chem. 2012, 13, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Gabdrakhmanov, D.; Samarkina, D.; Semenov, V.; Syakaev, V.; Giniyatullin, R.; Gogoleva, N.; Zakharova, L. Novel dicationic pyrimidinic surfactant: Self-assembly and DNA complexation. Colloids Surf. A 2015, 480, 113–121. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gaynanova, G.A.; Vasileva, L.A.; Sibgatullina, G.V.; Samigullin, D.V.; Sapunova, A.S.; Voloshina, A.D.; Galkina, I.V.; Petrov, K.A.; Zakharova, L.Y. Mitochondria-targeted cationic liposomes modified with alkyltriphenylphosphonium bromides loaded with hydrophilic drugs: Preparation, cytotoxicity and colocalization assay. J. Mater. Chem. B 2019, 7, 7351–7362. [Google Scholar] [CrossRef] [PubMed]
- Von Smoluchowski, M. Contribution to the theory of electro-osmosis and related phenomena. Bull. Int. Acad. Sci. Cracovie 1903, 3, 184–199. [Google Scholar]
- Aguiar, J.; Carpena, P.; Molina-Bolívar, J.A.; Carnero Ruiz, C. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J. Colloid Interface Sci. 2003, 258, 116–122. [Google Scholar] [CrossRef]

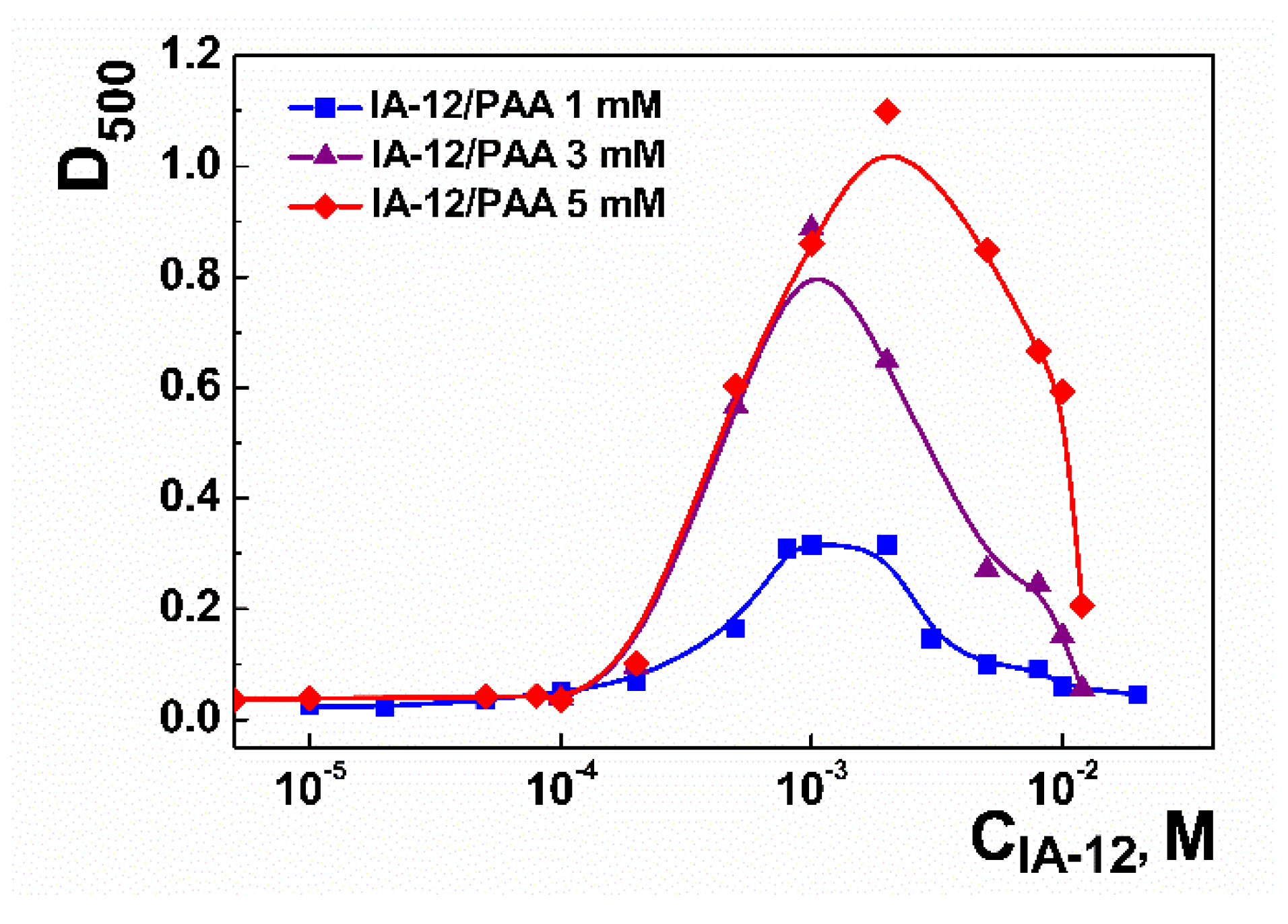
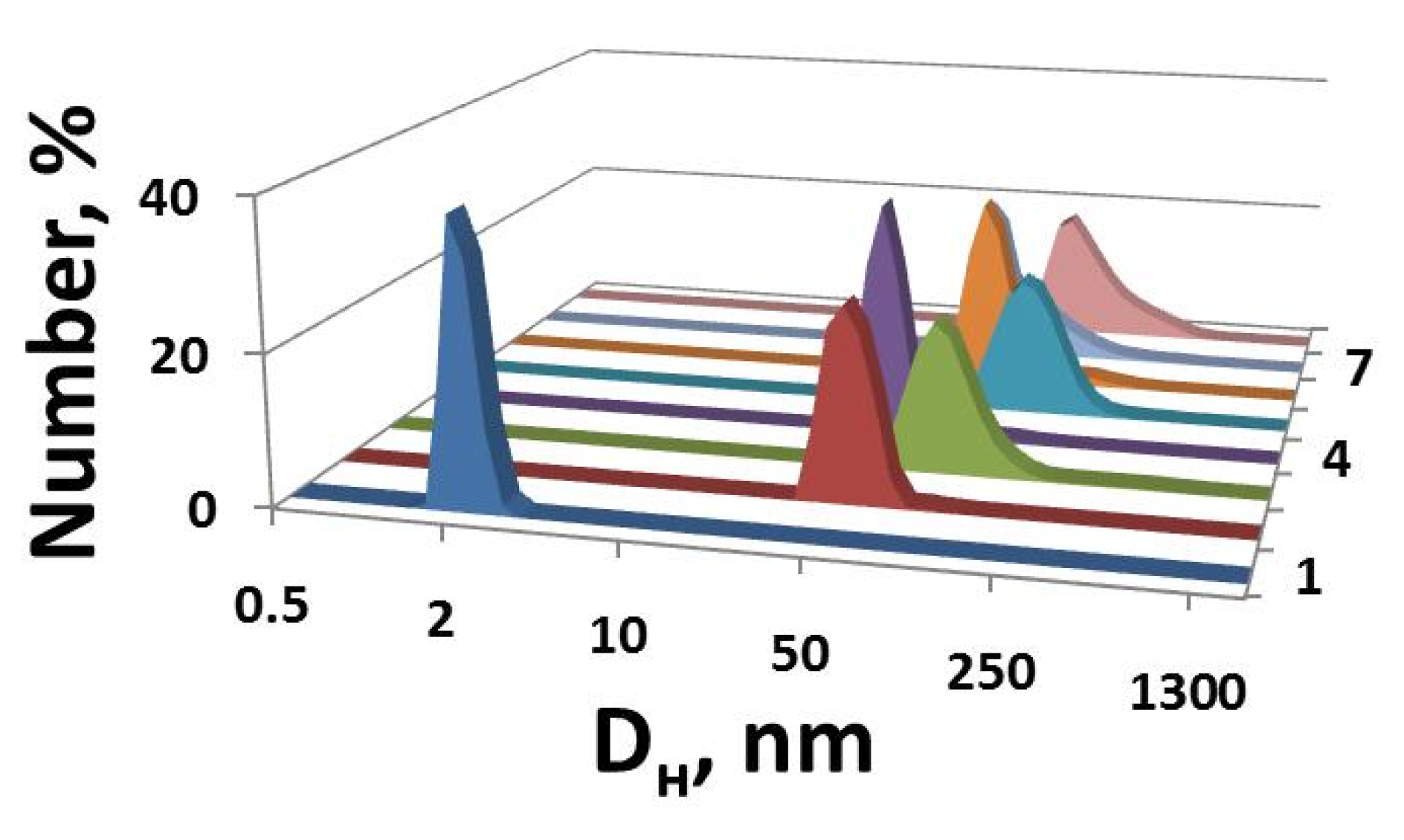
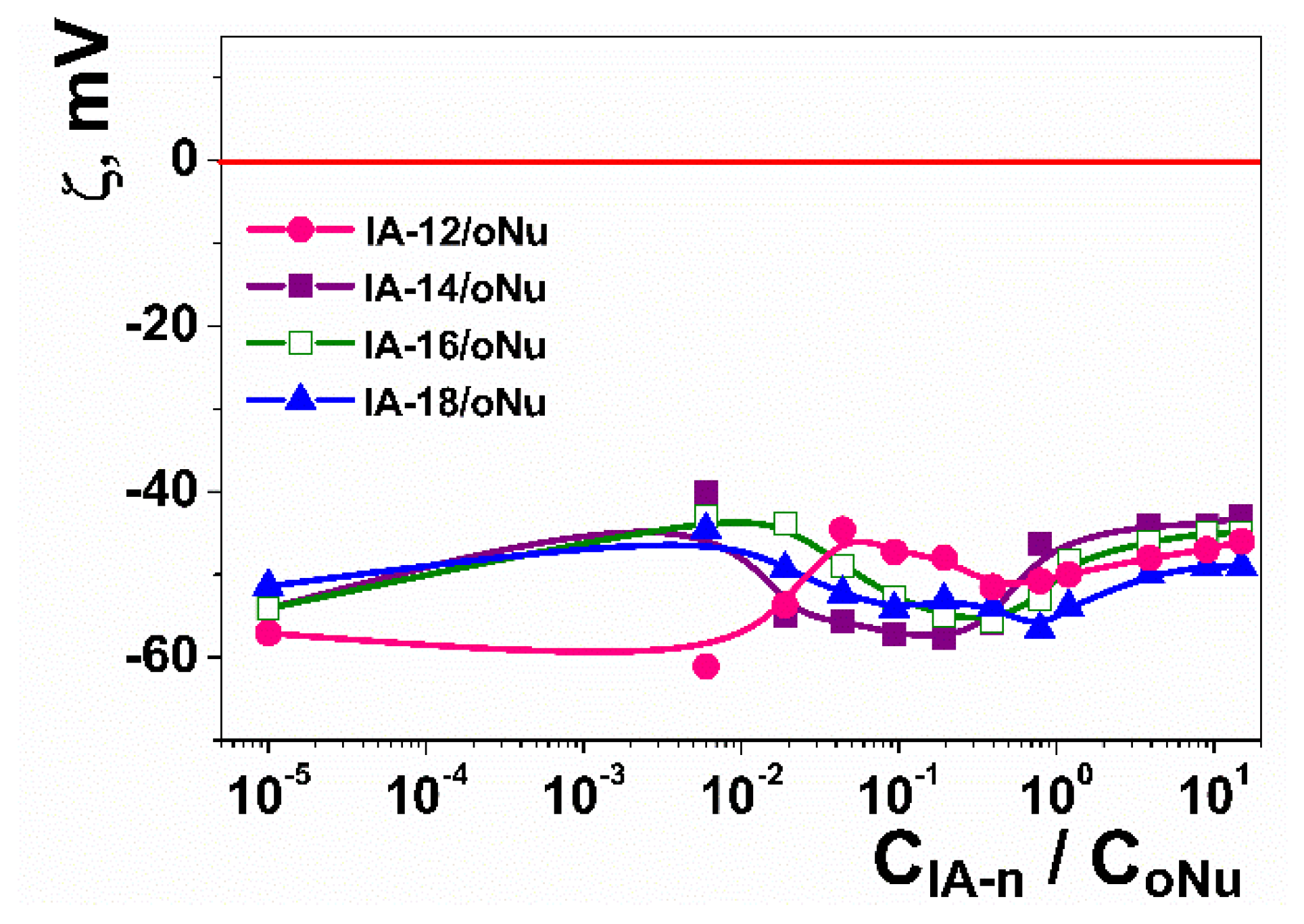
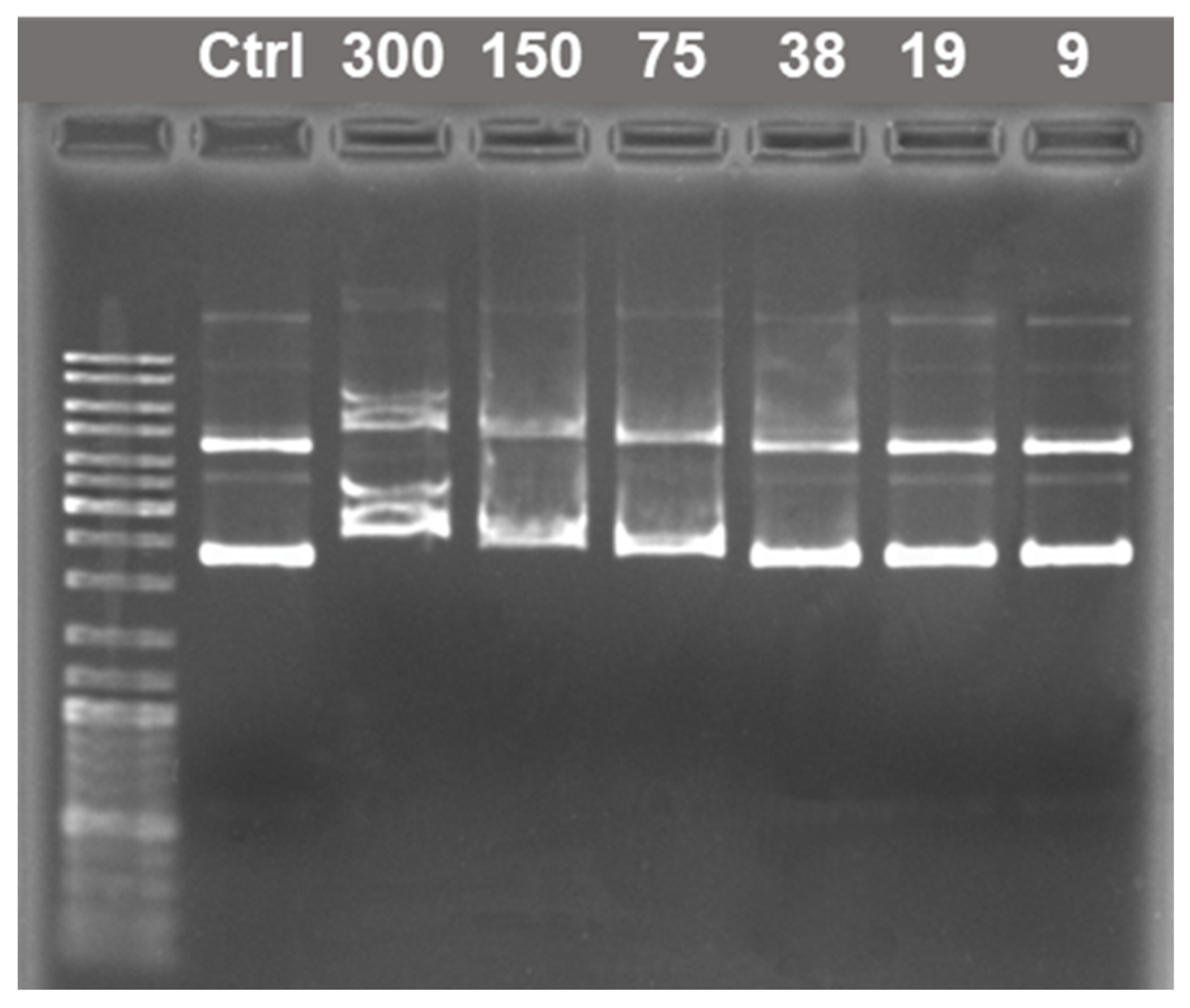
| CPAA, mM | Aggregation Thresholds (mM) | |||
|---|---|---|---|---|
| Tensiometry | Conductometry | Fluorimetry | ||
| CAC1 | CAC2 | |||
| 0 | 9 [43] | - | 10 [43] | 9 [43] |
| 1 | 0.05 | 10.0 | 5 | 0.05 |
| 3 | 0.08 | 9.0 | 4 | 0.05 |
| 5 | 0.10 | 8.0 | 3.5 | 0.07 |
| IC50 µM | |||
|---|---|---|---|
| M-HeLa | Chang Liver | ||
| IA-12 | IA-12/oNu | IA-12 | IA-12/oNu |
| 39 ± 0.1 | 1.1 ± 0.08 | 17.0 ± 0.1 | 21.3 ± 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Kuznetsov, D.M.; Lukashenko, S.S.; Zakharov, V.M.; Sapunova, A.S.; Amerhanova, S.K.; Lyubina, A.P.; Voloshina, A.D.; Salakhieva, D.V.; et al. Polymer–Colloid Complexes Based on Cationic Imidazolium Amphiphile, Polyacrylic Acid and DNA Decamer. Molecules 2021, 26, 2363. https://doi.org/10.3390/molecules26082363
Kuznetsova DA, Gabdrakhmanov DR, Kuznetsov DM, Lukashenko SS, Zakharov VM, Sapunova AS, Amerhanova SK, Lyubina AP, Voloshina AD, Salakhieva DV, et al. Polymer–Colloid Complexes Based on Cationic Imidazolium Amphiphile, Polyacrylic Acid and DNA Decamer. Molecules. 2021; 26(8):2363. https://doi.org/10.3390/molecules26082363
Chicago/Turabian StyleKuznetsova, Darya A., Dinar R. Gabdrakhmanov, Denis M. Kuznetsov, Svetlana S. Lukashenko, Valery M. Zakharov, Anastasiia S. Sapunova, Syumbelya K. Amerhanova, Anna P. Lyubina, Alexandra D. Voloshina, Diana V. Salakhieva, and et al. 2021. "Polymer–Colloid Complexes Based on Cationic Imidazolium Amphiphile, Polyacrylic Acid and DNA Decamer" Molecules 26, no. 8: 2363. https://doi.org/10.3390/molecules26082363
APA StyleKuznetsova, D. A., Gabdrakhmanov, D. R., Kuznetsov, D. M., Lukashenko, S. S., Zakharov, V. M., Sapunova, A. S., Amerhanova, S. K., Lyubina, A. P., Voloshina, A. D., Salakhieva, D. V., & Zakharova, L. Y. (2021). Polymer–Colloid Complexes Based on Cationic Imidazolium Amphiphile, Polyacrylic Acid and DNA Decamer. Molecules, 26(8), 2363. https://doi.org/10.3390/molecules26082363