Study on Extraction Process of Root of Henry Wood Betony Polysaccharides and Their Antitumor Activity against S180
Abstract
1. Introduction
2. Results and Discussion
2.1. Single-Factor Experiment Analysis
2.2. Optimization of Procedure by Uniform Design
2.3. Characterization of the RHWPs
2.3.1. Analysis of Physicochemical Properties
2.3.2. FTIR Spectroscopy
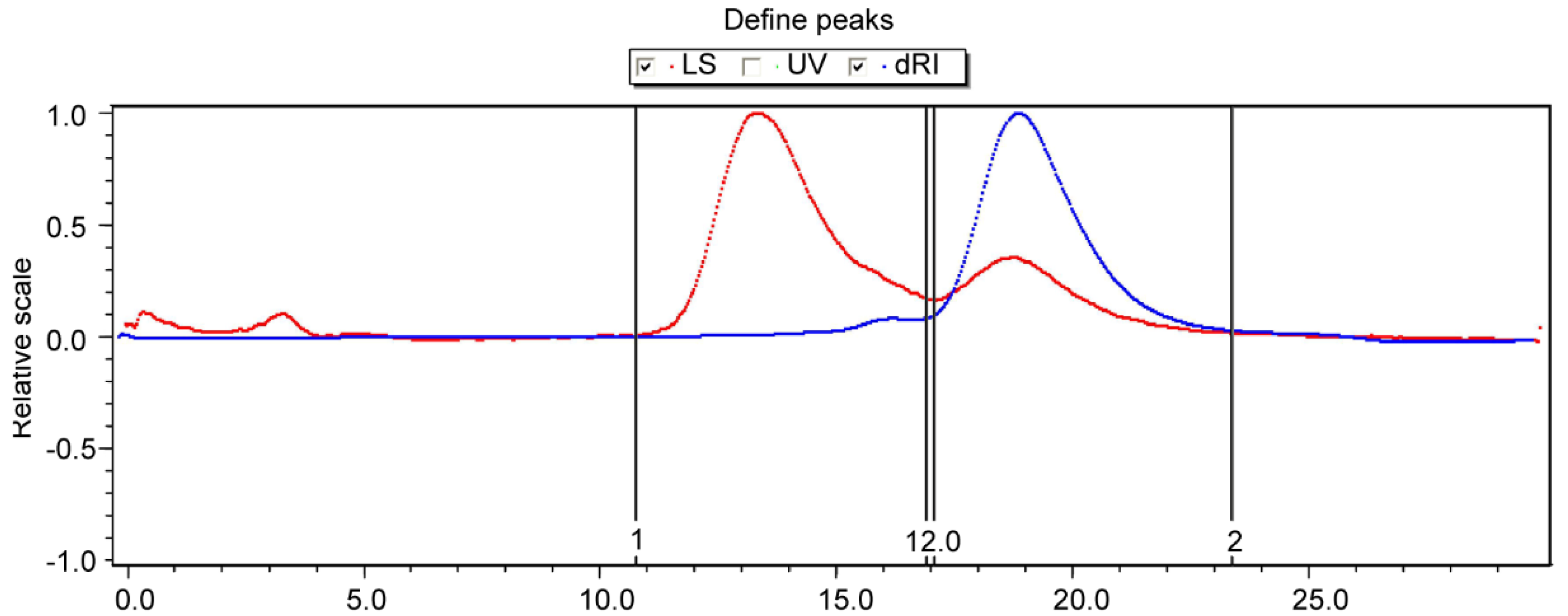
2.3.3. GPC Analysis
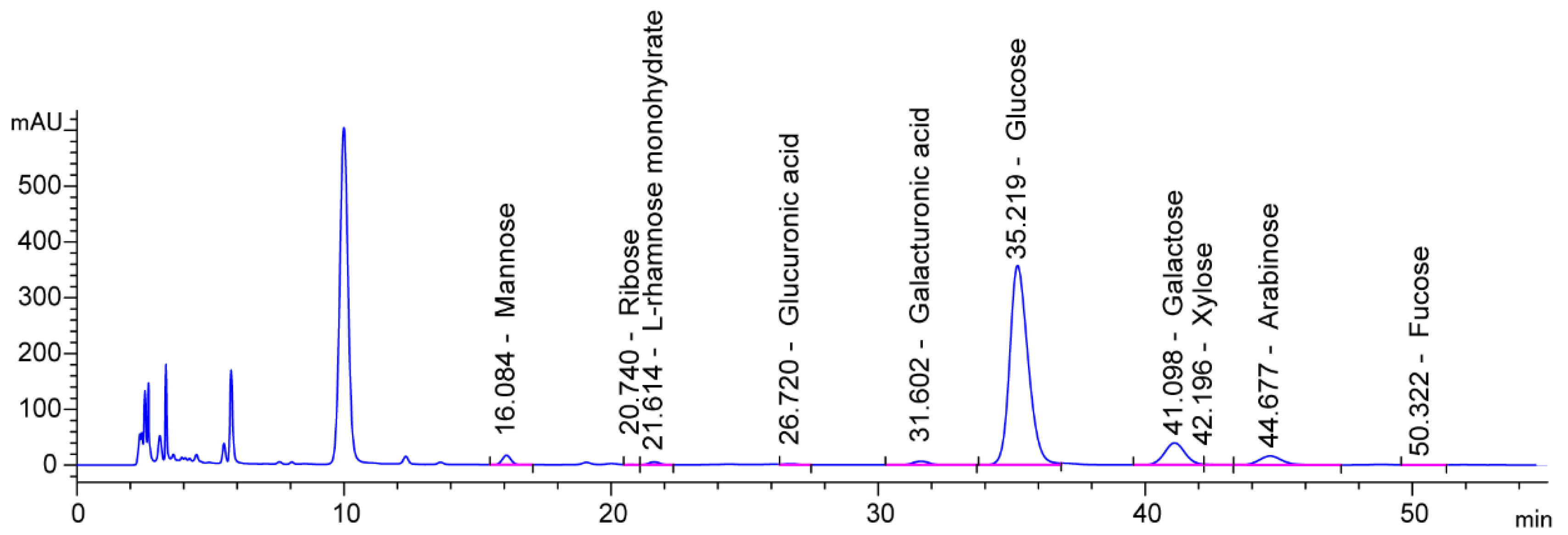
2.3.4. Analysis of Monosaccharide Compositions
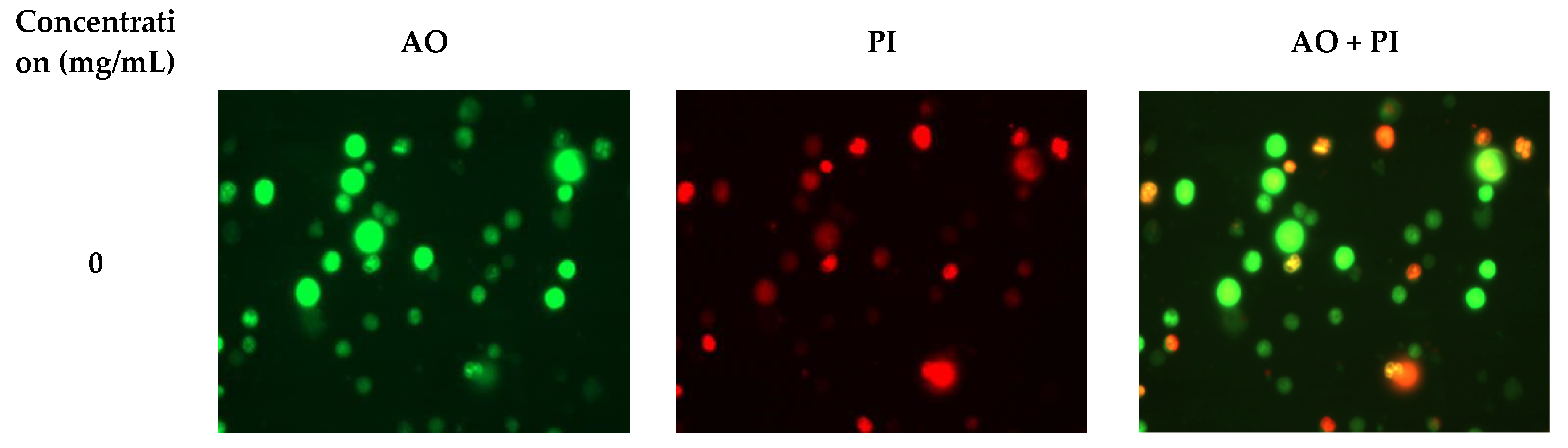
2.4. RHWPs Inhibited the Proliferation of S180 Cells and Induced Apoptosis In Vitro
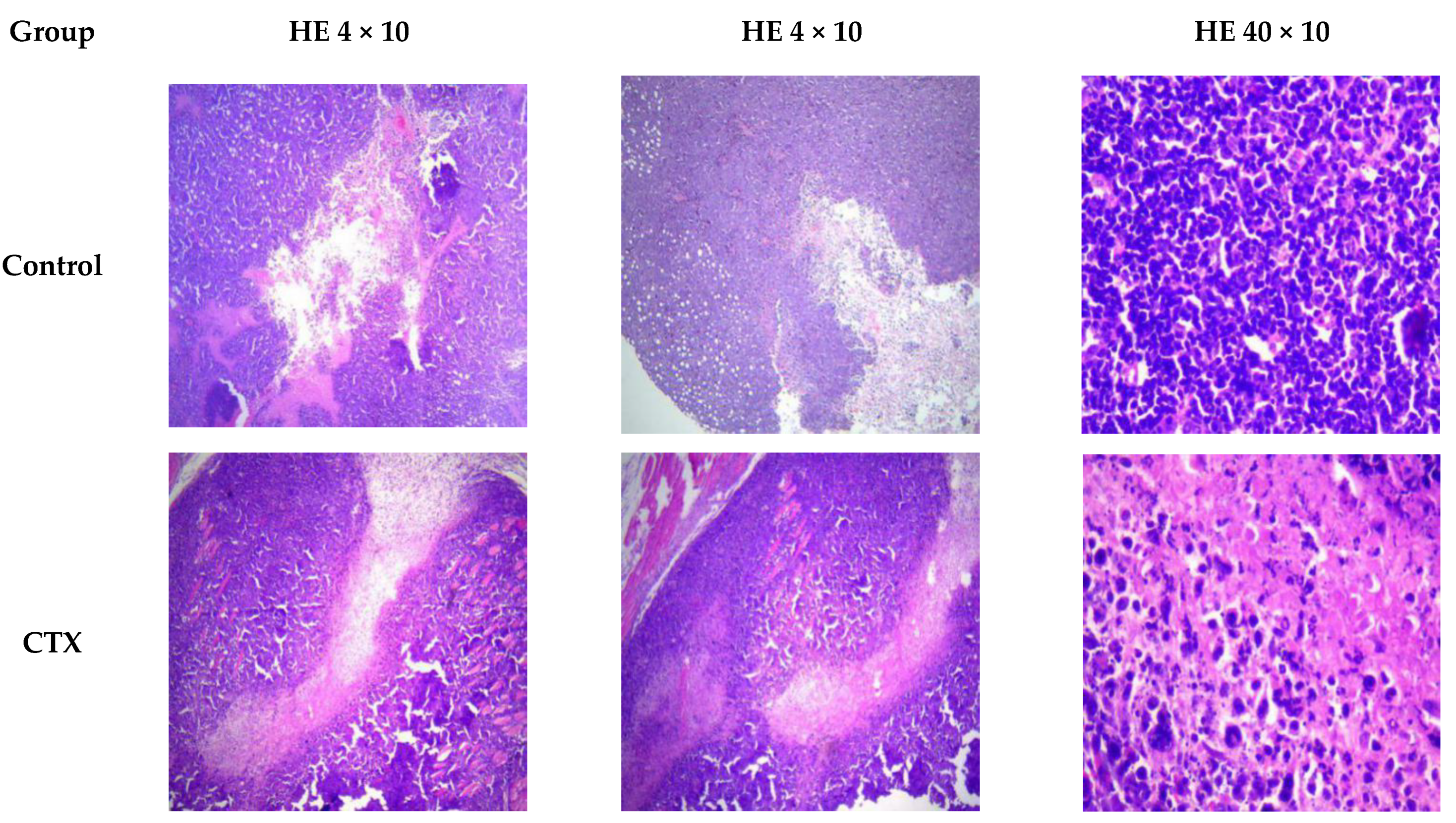
2.5. RHWPs Inhibited S180 Ascite Tumor Growth and Expanded the Necrotic Regions
2.6. RHWPs Increased Secretion of TNF-α, IFN-γ, and IL-2 in the Sera of Tumor-Bearing Mice
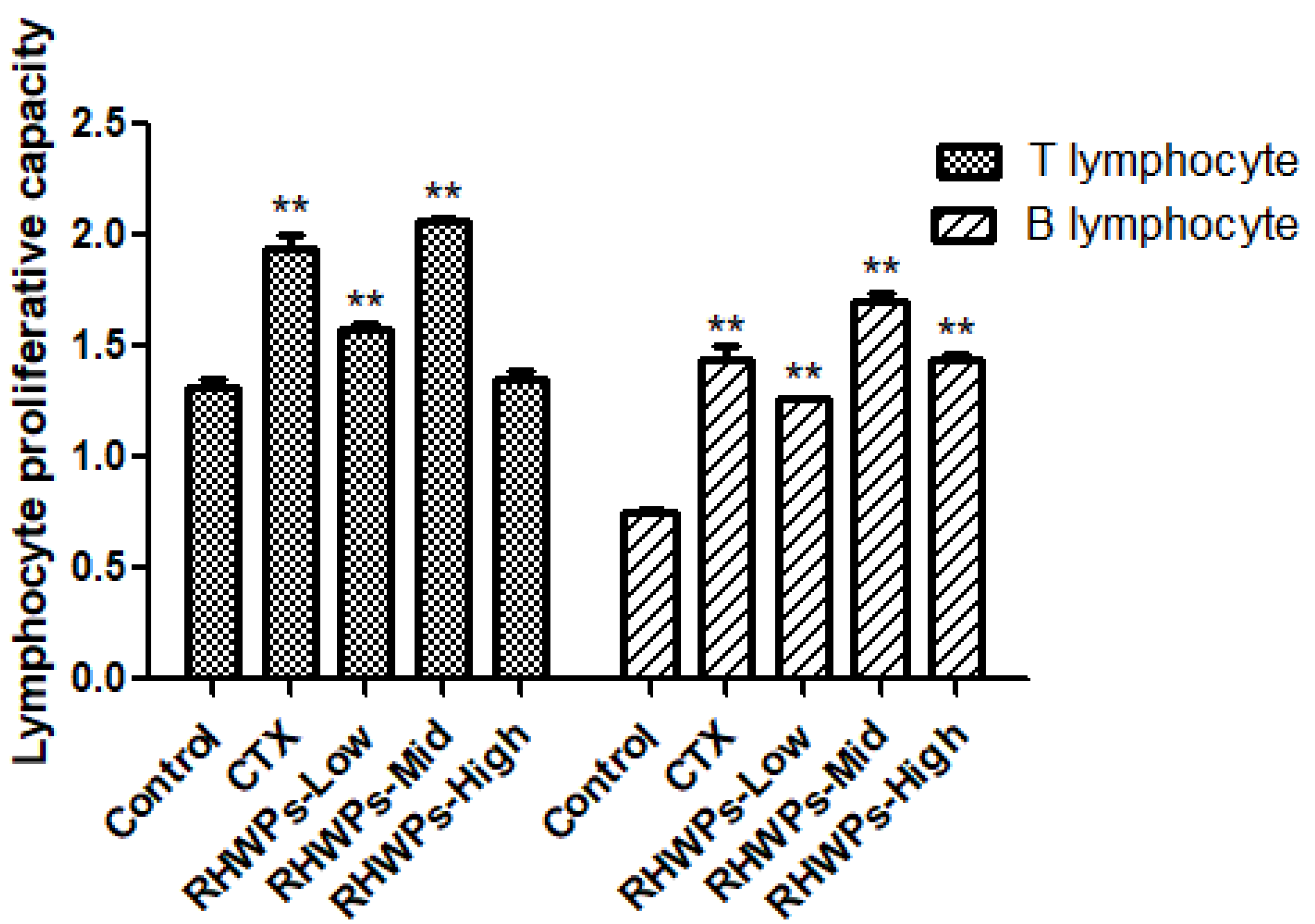
2.7. RHWPs Increased Lymphocyte Proliferation in the Tumor-Bearing Mice
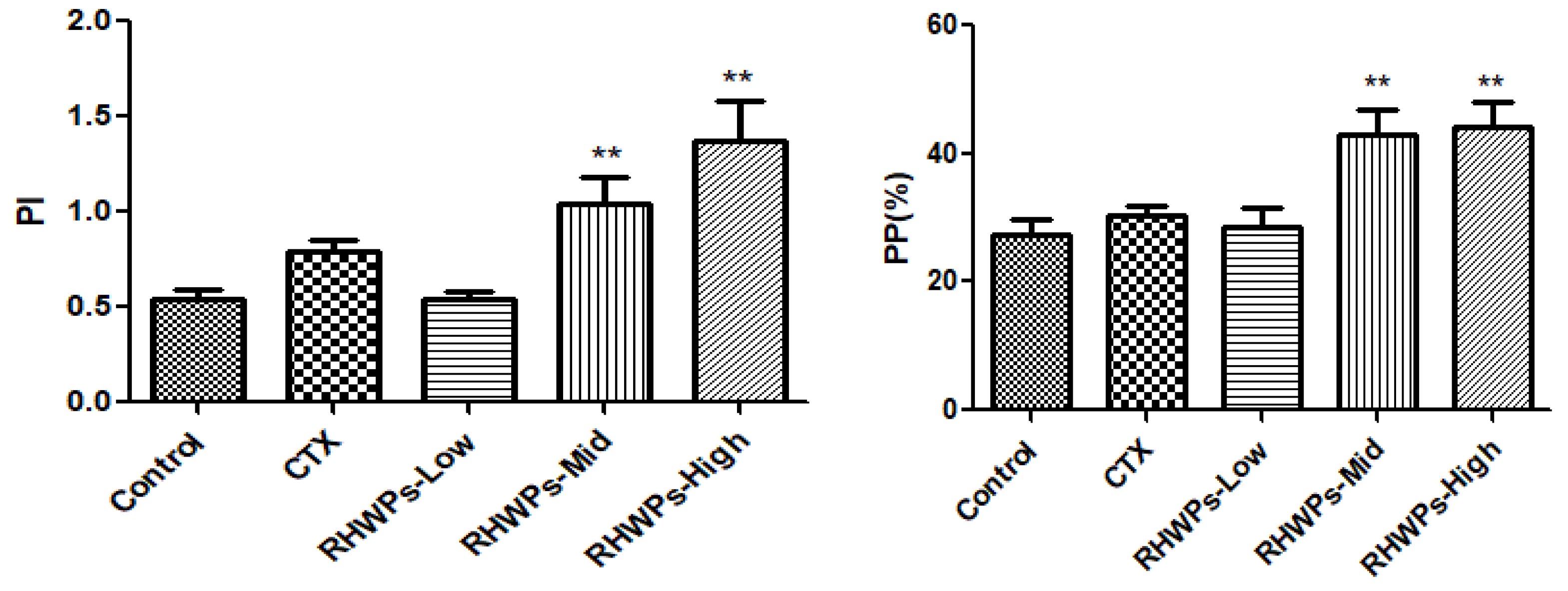
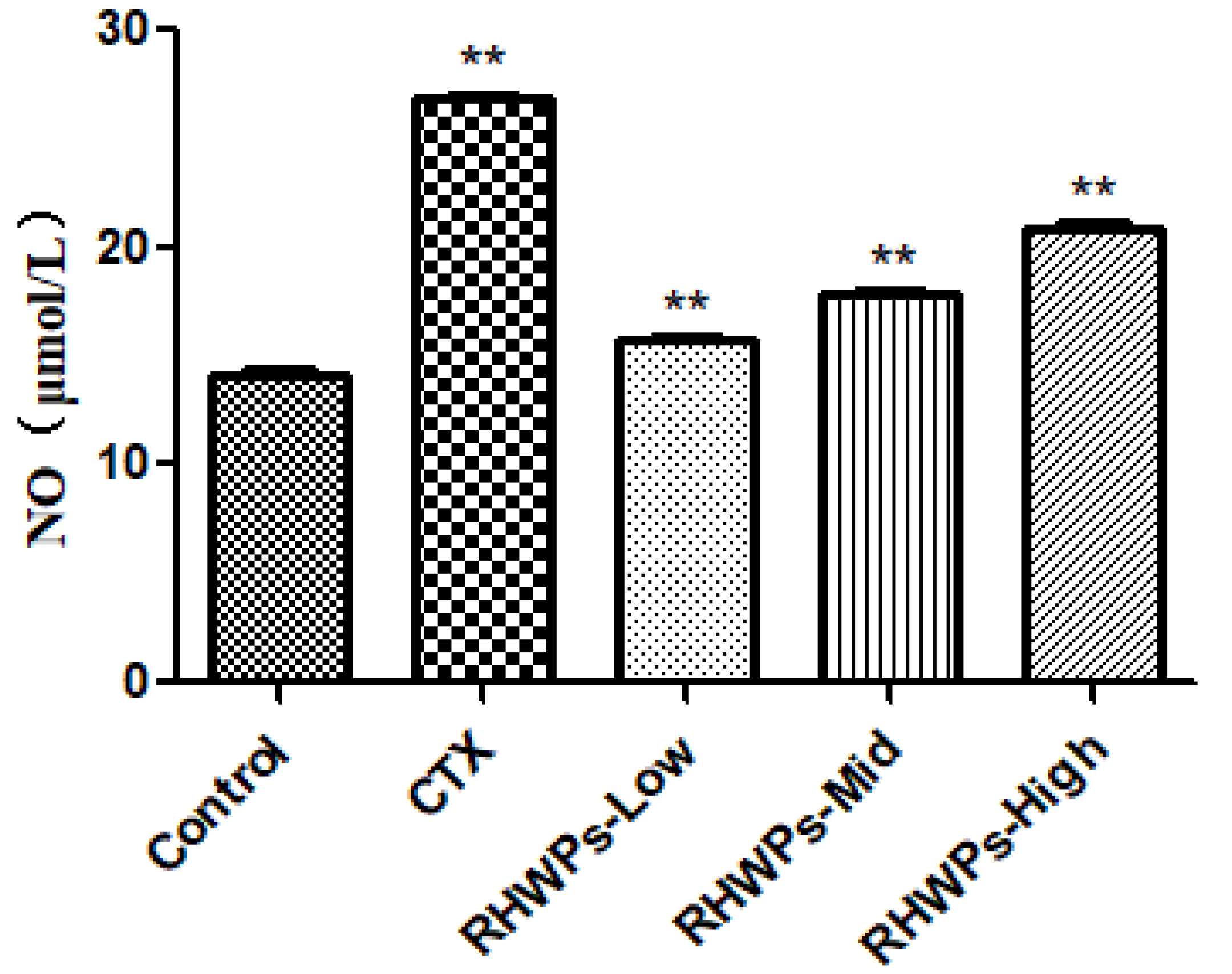
2.8. RHWPs Enhanced the Effector Functions of NK Cells and Peritoneal Macrophages In Vivo
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Extraction and Characterization of Polysaccharides
3.2.1. Extraction and Purification
3.2.2. Quantification of RHWPs
3.2.3. Uniform Design Experimentation
3.2.4. Purification of RHWPs
3.2.5. FTIR Spectrometry
3.2.6. GPC Analysis
3.2.7. Analysis of Monosaccharide Compositions
3.3. In Vitro Antitumor Activity
3.3.1. CCK-8 Assay
3.3.2. Florescence Microscopy
3.4. In Vivo Anti-Tumor Activity
3.4.1. Establishment of Tumor Model and Treatment Regimen
3.4.2. Gross and Histological Examination of Tumors
3.4.3. ELISA
3.4.4. Lymphocyte Proliferation Assay
3.4.5. NK Cell Killing Assay
3.4.6. Phagocytosis Assay
Phagocytic index (PI) = total number of chicken RBCs engulfed by 100 phagocytic cells/100
3.4.7. NO production Assay
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| RHWPs | polysaccharides from the root of Henry wood betony |
| FACS | fluorescence activated cell sorting |
| FITC | fluorescein isothiocyanate |
| NK | natural killer cells |
| PP | phagocytosis rate |
| PI | phagocytic index |
| IL-2 | interleukin-2 |
| TNF-α | tumor necrosis factor alpha |
| IFN-γ | interferon-γ |
| LPS | lipopolysaccharide |
| ConA | concanavalin A |
| IR | infrared |
| PM | peritoneal macrophages |
References
- World Health Organization. Cancer. Available online: https://www.who.int/en/news-room/fact-sheets/detail/cancer (accessed on 3 March 2018).
- Wang, R.J.; Wang, S.; Xia, Y.J.; Tu, M.W.L.J.; Zhang, L.J.; Wang, Y.M. Antitumor effects and immune regulation activities of a purified polysaccharide extracted from Juglan regia. Int. J. Biol. Macromol. 2015, 72, 771–775. [Google Scholar]
- He, Y.L.; Zhang, L.J.; Wang, H. The biological activities of the antitumor drug Grifola frondosa polysaccharide. Prog. Mol. Biol. Transl. Sci. 2019, 163, 221–261. [Google Scholar]
- Yin, Y.; Gao, W.H.; Yu, S.J. Research progress in polysaccharide extraction technology. Sci. Technol. Food Ind. 2007, 2, 247–250. [Google Scholar]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Ikekawa, T. Beneficial effects of edible and medicinal mushrooms in health care. Int. J. Med. Mushrooms 2001, 3, 291–298. [Google Scholar]
- Cui, W.; Wu, G.X.; Zhang, Z.L. Effects on achyranthis bidentata polysaccharides on enhancing immune function. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 141–143. [Google Scholar]
- Wang, H.W.; Jiang, M.J.; Zhao, H.X. Immunomodulatory effects of saponin-polysaccharide and Panax japonicus composition on cyclophosphamide-induced immunosuppressed mice. Guangdong Med. J. 2010, 31, 2620–2622. [Google Scholar]
- Joseph, M.M.; Aravind, S.R.; Varghese, S.; Mini, S.; Sreelekha, T.T. Evaluation of antioxidant, antitumor and immunomodulatory properties of polysaccharide isolated from fruit rind of Punica granatum. Mol. Med. Rep. 2012, 5, 489–496. [Google Scholar] [PubMed]
- Rong, L.X.; Lu, S.; Liu, Y.M. Assessment of preliminary processing and its experimental study on the immunoregulation function of Radix Glehniae polysaccharide. Chin. J. Basic Med. Tradit. Chin. Med. 2013, 19, 1090–1091. [Google Scholar]
- Ni, W.H.; Zhang, X.; Wang, B.; Chen, Y.; Han, H.; Fan, Y.Y.; Zhou, Y.F.; Tai, G.H. Antitumor activities and immunomodulatory effects of ginseng neutral polysaccharides in combination with 5-fluorouracil. J. Med. Food 2010, 13, 270–277. [Google Scholar] [CrossRef]
- Bai, R.B.; Li, W.Y.; Li, Y.D.; Ma, M.; Wang, Y.P.; Zhang, J.; Hu, F.D. Cytotoxicity of two water-soluble polysaccharides from Codonopsis pilosula Nannf. var. modesta (Nannf.) L.T. Shen against human hepatocellular carcinoma HepG2 cells and its mechanism. Int. J. Biol. Macromol. 2018, 120, 1544–1550. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Khoo, K.H.; Chen, S.T.; Lin, C.C.; Wong, C.H.; Lin, C.H. Studies on the immuno-modulating and antitumor activities of Ganoderma lucidum (Reishi) polysaccharides: Functional and proteomic analyses of a fucose-containing glycoprotein fraction responsible for the activities. Bioorganic Med. Chem. 2002, 10, 1057–1062. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chang, H.M. Antiproliferative and differentiating effects of polysaccharide fraction from fu-ling (Poria cocos) on human leukemic U937 and HL-60 cells. Food Chem. Toxicol. 2004, 42, 759–769. [Google Scholar] [CrossRef]
- Li, G.; Kim, D.-H.; Kim, T.-D.; Park, B.-J.; Park, H.-D.; Park, J.-I.; Na, M.-K.; Kim, H.-C.; Hong, N.-D.; Lim, K.; et al. Protein-bound polysaccharide from Phellinus linteus induces G2/M phase arrest and apoptosis in SW480 human colon cancer cells. Cancer Lett. 2004, 216, 175–181. [Google Scholar] [CrossRef]
- Lin, X.; Cai, Y.; Li, Z.; Chen, Q.; Liu, Z.; Wang, R. Structure determination, apoptosis induction, and telomerase inhibition of CEP-2, a novel lichenin from Cladonia furcata. Biochim. Biophys. Acta. 2003, 1622, 99–108. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Huang, J.; Li, Z.; Zhu, C.P.; Zhang, S.H. Effect of lycium barbarum polysaccharide on human hepatoma QGY7703 cells: Inhibition of proliferation and induction of apoptosis. Life Sci. 2005, 76, 2115–2124. [Google Scholar] [CrossRef]
- Ge, Y.; Duan, Y.; Fang, G.; Zhang, Y.; Wang, S. Polysaccharides from fruit calyx of Physalis alkekengi var. francheti: Isolation, purification, structural features and antioxidant activities. Carbohyd. Polym. 2019, 77, 188–193. [Google Scholar] [CrossRef]
- Du, Y.Q.; Liu, Y.; Wang, J.H. Polysaccharides from Umbilicaria esculenta cultivated in Huangshan Mountain and immunomodulatory activity. Int. J. Biol. Macromol. 2015, 72, 1272–1276. [Google Scholar] [CrossRef]
- Jia, X.; Ding, C.; Yuan, S.; Zhang, Z.; Chen, Y.; Du, L.; Yuan, M. Extraction, purification and characterization of polysaccharides from hawk tea. Carbohyd. Polym. 2014, 99, 319–324. [Google Scholar] [CrossRef]
- Jing, F.; Feng, H.B.; Yu, Y.; Sun, M.X.; Liu, Y.R.; Li, T.Z.; Xin, S.; Liu, S.J.; Sun, M.D. Antioxidant activities of the polysaccharides of Chuanminshen violaceum. Carbohyd. Polym. 2016, 157, 629–636. [Google Scholar]
- Wang, Y.; Wang, F.; Ma, X.; Sun, S.; Leng, F.; Zhang, W.; Wang, X. Extraction, purification, characterization and antioxidant activity of polysaccharides from piteguo fruit. Ind. Crop. Prod. 2015, 77, 467–475. [Google Scholar] [CrossRef]
- Wei, Q.; Ren, D.M.; Li, S.C.; Sun, T.; Lv, L.H. Extraction and purification of polysaccharides from stems and leaves of Taxus grown in Mountain areas in Southern Anhui Province and their monosaccharide composition. Food Sci. 2017, 38, 1–10. [Google Scholar]
- Dou, X.X. Study of Polysaccharides from Phellinus linteus: Extraction, Purification, Characterization, Activity and Tablets Preparation; Institute of Microbiology and Epidemiology, Academy Medical Science: Beijing, China, 2008; pp. 106–111. [Google Scholar]
- Veta, M.; Pluim, J.P.; Van Diest, P.J.; Viergever, M.A. Breast cancer histopathology image analysis: A review. IEEE Trans. Biomed. Eng. 2014, 61, 1400–1411. [Google Scholar] [CrossRef]
- Zeng, W.-C.; Zhang, Z.; Gao, H.; Jia, L.-R.; Chen, W.-Y. Characterization of antioxidant polysaccharides from Auricularia auricular using microwave-assisted extraction. Carbohydr. Polym. 2012, 89, 694–700. [Google Scholar] [CrossRef]
- Chen, G.C.; Zhang, P.Y.; Huang, T.T.; Yu, W.Q.; Lin, J.; Li, P.; Chen, K.S. Polysaccharides from Rhizopus nigricans mycelia induced apoptosis and G2/M arrest in BGC-823 cells. Carbohydr. Polym. 2013, 97, 800–808. [Google Scholar] [CrossRef]
- Liu, L.L.; Knag, Z.L.; Zhu, Y.K. Cytokines and tumor immunity. Shanxi Med. J. 2013, 42, 1250–1252. [Google Scholar]
- Beatty, G.; Paterson, Y. IFN-gamma-dependent inhibition of tumor angiogenesis by tumor-infiltrating CD4+ T cells requires tumor responsiveness to IFN-gamma. J. Immunol. 2001, 166, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Takeda, K.; Yagita, H.; Smyth, M.J.; Kaer, L.V.; Okumura, K.; Saiki, I. IFN-gamma-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, alpha-galactosylceramide. Blood 2002, 100, 1728–1733. [Google Scholar] [PubMed]
- Kammertoens, T.; Friese, C.; Arina, A.; Idel, C.; Briesemeister, D.; Rothe, M.; Ivanov, A.; Szymborska, A.; Patone, G.; Kunz, S.; et al. Tumour ischaemia by interferon-gamma resembles physiological blood vessel regression. Nature 2017, 545, 98–102. [Google Scholar] [CrossRef]
- Carolina, V.; Mariángeles, C.; Delia, W.; Koncurat, M.A.; Santa-Coloma, T.A.; Claudio, B. IL-1β, IL-2 and IL-4 concentration during porcine gestation. Theriogenology 2019, 128, 133–139. [Google Scholar]
- Hou, F.X.; Yang, H.F.; Yu, T. Feasibility of test procedures of lipopolysaccharide-induced and concanavalin A-induced rat splenocyte proliferation in assessment of immunotoxicity. Ind. Hyg. Occup. Dis. 2007, 33, 336–339. [Google Scholar]
- Maccalli, C.; Scaramuzza, S.; Parmiani, G. TNK cells (NKG2D+ CD8+ or CD4+ T lymphocytes) in the control of human tumors. Cancer Immunol. Immunother. 2009, 58, 801–808. [Google Scholar] [CrossRef]
- Segal, A.W.; Abo, A. The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem. Sci. 1993, 18, 43–47. [Google Scholar] [CrossRef]
- Song, J.Y.; Han, S.K.; Son, E.H.; Pyo, S.N.; Yun, Y.S.; Yi, S.Y. Induction of secretory and tumoricidal activities in peritoneal macrophages by ginsan. Int. Immunopharmacol. 2002, 2, 857–865. [Google Scholar] [CrossRef]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef]
- Avni, D.; Goldsmith, M.; Ernst, O.; Mashiach, R.; Tuntland, T.; Meijler, M.M.; Gray, N.S.; Rosen, H.; Zor, T. Modulation of TNF alpha, IL-10 and IL-12p40 levels by a ceramide-1-phosphate analog, PCERA-1, in vivo and ex vivo in primary macrophages. Immunol. Lett. 2009, 123, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Staub, A.M. Removal of proteins: Sevag method. Methods Carbohydr. Chem. 1965, 5, 5–6. [Google Scholar]
- He, F.L.; Tan, M.Y.; Li, C.J.; Zhan, L.Z. Optimization of extraction process for Polysaccharides from Bletilla striata using uniform design. Hunan J. Tradit. Chin. Med. 2015, 31, 156–157. [Google Scholar]
- Yang, J.; Lin, Y.; Yang, C.F.; Qiu, L. Optimization of extraction process of total polysaccharide from konjac. Asia-Pac. Tradit. Med. 2018, 98, 60–62. [Google Scholar]
- Gao, X.; Guo, Y.M.; Zhou, J.; Liu, J.W. Study on the determination of Polysaccharide contents of Red Ginseng by phenol-sulfuric acid method. Lab. Sci. 2018, 21, 28–30, 33. [Google Scholar]
- Ma, X.H.; Li, C.C. Determination and analysis of different species of dendrobium candidumand its Polysaccharide by IR spectra. Heilongjiang Agric. Sci. 2015, 9, 116–118. [Google Scholar]
- Li, H.; Cao, K.; Cong, P.; Liu, Y.; Cui, H.; Xue, C. Structure characterization and antitumor activity of the extracellular polysaccharide from the marine fungus Hansfordia sinuosae. Carbohydr. Polym. 2018, 190, 87–94. [Google Scholar] [CrossRef]
- Kang, H.Q. Studies on preparation, components analysis and antitumor activities of polysaccharides from the fruit shell of Camellia Oleifera abel. Zhejiang Univ. 2010, 7, 95–105. [Google Scholar]
- Gao, Z.Z.; Liu, K.H.; Tian, W.J.; Wang, H.C.; Liu, Z.G.; Li, Y.Y.; Li, E.T.; Liu, C.; Li, X.; Hou, R.R.; et al. Effects of selenizing angelica polysaccharide and selenizing garlic polysaccharide on immune function of murine peritoneal macrophage. Int. Immunopharmacol. 2015, 27, 104–109. [Google Scholar] [CrossRef]





| Number | Extraction Temperature (°C) | Extraction Time (min) | Liquid/Solid Ratio (mL/g) | Soaking Time (min) | Extraction Rate (%) |
|---|---|---|---|---|---|
| 1 | 1 (50) | 2 (50) | 3 (40) | 6 (150) | 33.95 |
| 2 | 2 (60) | 4 (70) | 6 (55) | 5 (140) | 32.89 |
| 3 | 3 (70) | 6 (90) | 2 (35) | 4 (130) | 35.08 |
| 4 | 4 (80) | 1 (40) | 5 (50) | 3 (120) | 37.68 |
| 5 | 5 (90) | 3 (60) | 1 (30) | 2 (110) | 38.97 |
| 6 | 6 (100) | 5 (80) | 4 (45) | 1 (100) | 37.85 |
| Project | Sum of Square | Degree of Freedom | Mean Square | F | P |
|---|---|---|---|---|---|
| Return Residual Total | 117.871 1.150 119.021 | 3 2 5 | 39.290 0.575 | 68.340 | 0.014 |
| Analysis Item | Index | Peak 1 | Peak 2 |
|---|---|---|---|
| Molecular weight | PD (Mw/Mn) | 3.929 | 1.083 |
| Mn (kDa) | 58.19 | 4.619 | |
| Mw (kDa) | 2.286 × 102 | 5.001 |
| Monosaccharide | Mass Ratio | Molar Ratio |
|---|---|---|
| Mannose | 686.68 | 3.81 |
| Ribose | 57.07 | 0.38 |
| l-Rhamnose monohydrate | 318.68 | 1.94 |
| Glucuronic acid | 242.65 | 1.25 |
| Galacturonic acid | 598.00 | 3.08 |
| Glucose | 27,755.21 | 154.06 |
| Galactose | 6108.74 | 33.91 |
| Xylose | 98.58 | 0.66 |
| Arabinose | 1385.43 | 9.23 |
| Fucose | 161.74 | 0.99 |
| Concentration (mg/mL) | OD | Inhibition Rate (%) |
|---|---|---|
| 0 | 1.109 ± 0.002 | - |
| 0.125 | 0.979 ± 0.026 ** | 11.74 |
| 0.25 | 0.934 ± 0.011 ** | 15.78 |
| 0.5 | 0.731 ± 0.009 ** | 34.12 |
| 1 | 0.699 ± 0.014 ** | 36.96 |
| 2 | 0.673 ± 0.004 ** | 39.37 |
| 4 | 0.585 ± 0.058 ** | 47.31 |
| 6 | 0.440 ± 0.036 ** | 60.33 |
| 8 | 0.401 ± 0.026 ** | 63.85 |
| Group | Dose (mg/kg) | Tumor Weight (g) | Inhibition Rate (%) |
|---|---|---|---|
| Control | - | 0.4227 ± 0.1332 | - |
| CTX | 50 | 0.1150 ± 0.0471 ** | 72.79 |
| RHWPs-Low | 50 | 0.3053 ± 0.1059 * | 27.77 |
| RHWPs-Mid | 150 | 0.1537 ± 0.0573 ** | 63.63 |
| RHWPs-High | 450 | 0.2241 ± 0.1336 ** | 46.98 |
| Group | Dose (mg/kg) | Natural Release Well (OD) | Reaction Well (OD) | Maximum Release Well (OD) | Killing Activity (%) |
|---|---|---|---|---|---|
| Control | - | 0.244 ± 0.010 | 0.254 ± 0.004 | 0.518 ± 0.008 | 3.65 |
| CTX | 50 | 0.162 ± 0.026 | 0.270 ± 0.002 | 0.434 ± 0.002 | 39.71 |
| RHWPs-Low | 50 | 0.090 ± 0.001 | 0.153 ± 0.027 | 0.675 ± 0.022 | 10.77 |
| RHWPs-Mid | 150 | 0.097 ± 0.006 | 0.170 ± 0.001 | 0.427 ± 0.085 | 22.12 |
| RHWPs-High | 450 | 0.024 ± 0.004 | 0.172 ± 0.001 | 0.521 ± 0.012 | 29.78 |
| Number | Extraction Temperature (°C) | Extraction Time (min) | Liquid/Solid Ratio (mL/g) | Soaking Time (min) |
|---|---|---|---|---|
| 1 | 1 (50) | 2 (50) | 3 (40) | 6 (150) |
| 2 | 2 (60) | 4 (70) | 6 (55) | 5 (140) |
| 3 | 3 (70) | 6 (90) | 2 (35) | 4 (130) |
| 4 | 4 (80) | 1 (40) | 5 (50) | 3 (120) |
| 5 | 5 (90) | 3 (60) | 1 (30) | 2 (110) |
| 6 | 6 (100) | 5 (80) | 4 (45) | 1 (100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Tian, L. Study on Extraction Process of Root of Henry Wood Betony Polysaccharides and Their Antitumor Activity against S180. Molecules 2021, 26, 2359. https://doi.org/10.3390/molecules26082359
Feng H, Tian L. Study on Extraction Process of Root of Henry Wood Betony Polysaccharides and Their Antitumor Activity against S180. Molecules. 2021; 26(8):2359. https://doi.org/10.3390/molecules26082359
Chicago/Turabian StyleFeng, Haibo, and Lan Tian. 2021. "Study on Extraction Process of Root of Henry Wood Betony Polysaccharides and Their Antitumor Activity against S180" Molecules 26, no. 8: 2359. https://doi.org/10.3390/molecules26082359
APA StyleFeng, H., & Tian, L. (2021). Study on Extraction Process of Root of Henry Wood Betony Polysaccharides and Their Antitumor Activity against S180. Molecules, 26(8), 2359. https://doi.org/10.3390/molecules26082359





