New Mononuclear and Binuclear Cu(II), Co(II), Ni(II), and Zn(II) Thiosemicarbazone Complexes with Potential Biological Activity: Antimicrobial and Molecular Docking Study
Abstract
1. Introduction
2. Materials and Methods
2.1. General Description of Materials and Methods
2.2. Synthetic Procedures and Analytic Data of Compounds
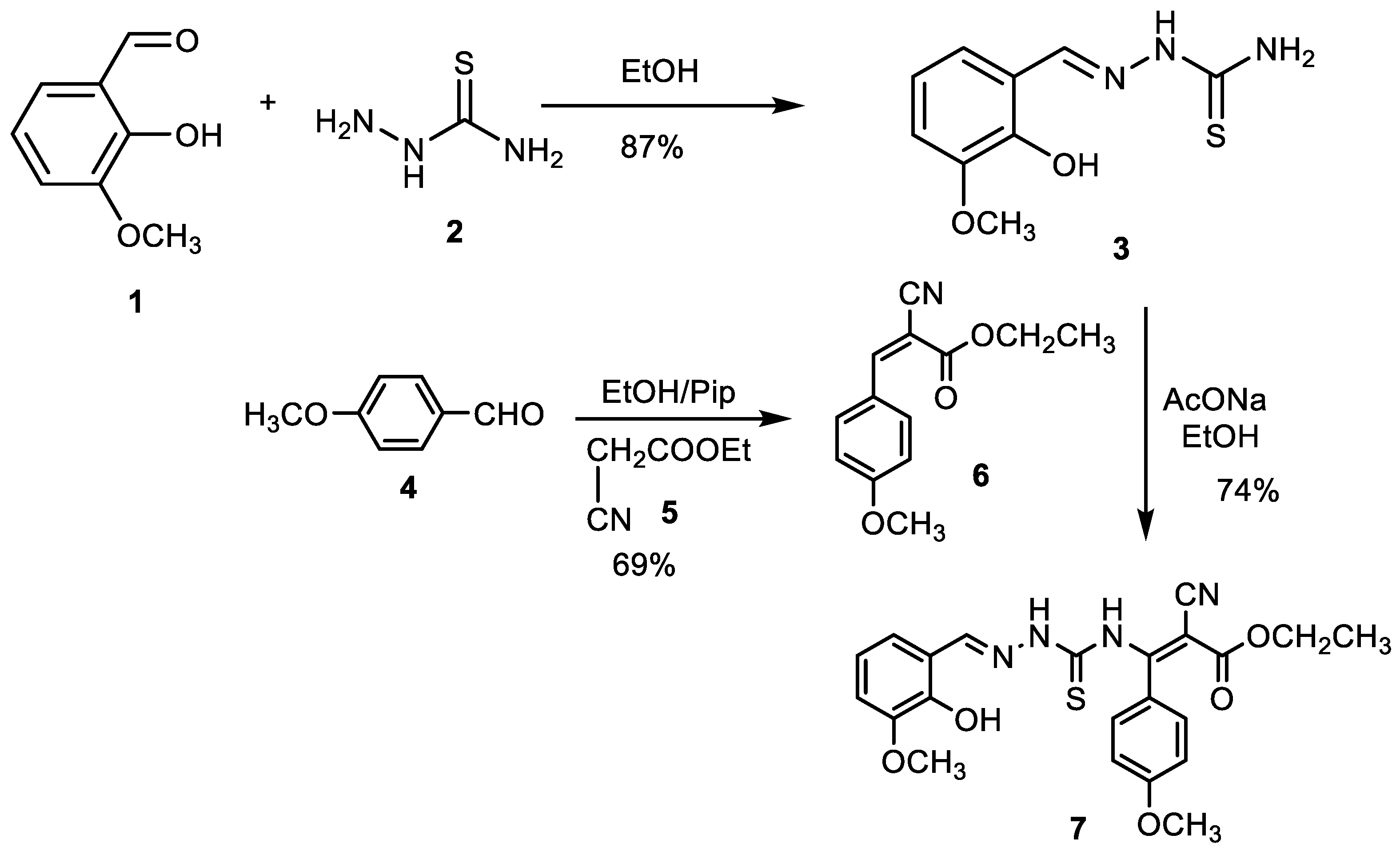
2.2.1. (E)-2-(2-hydroxy-3-methoxybenzylidene)hydrazine-1-carbothioamide Synthesis (3)
2.2.2. Ethyl-3-(4-methoxyphenyl-2-cyanoacrylate) Synthesis (6)
2.2.3. Ethyl (E)-2-Cyano-3-(2-((E)-2-hydroxy-3-methoxybenzylidene) Hydrazine-1-carbothioamido)-3-(4-methoxyhenyl) Acrylate (MTSC Ligand) Synthesis (7)
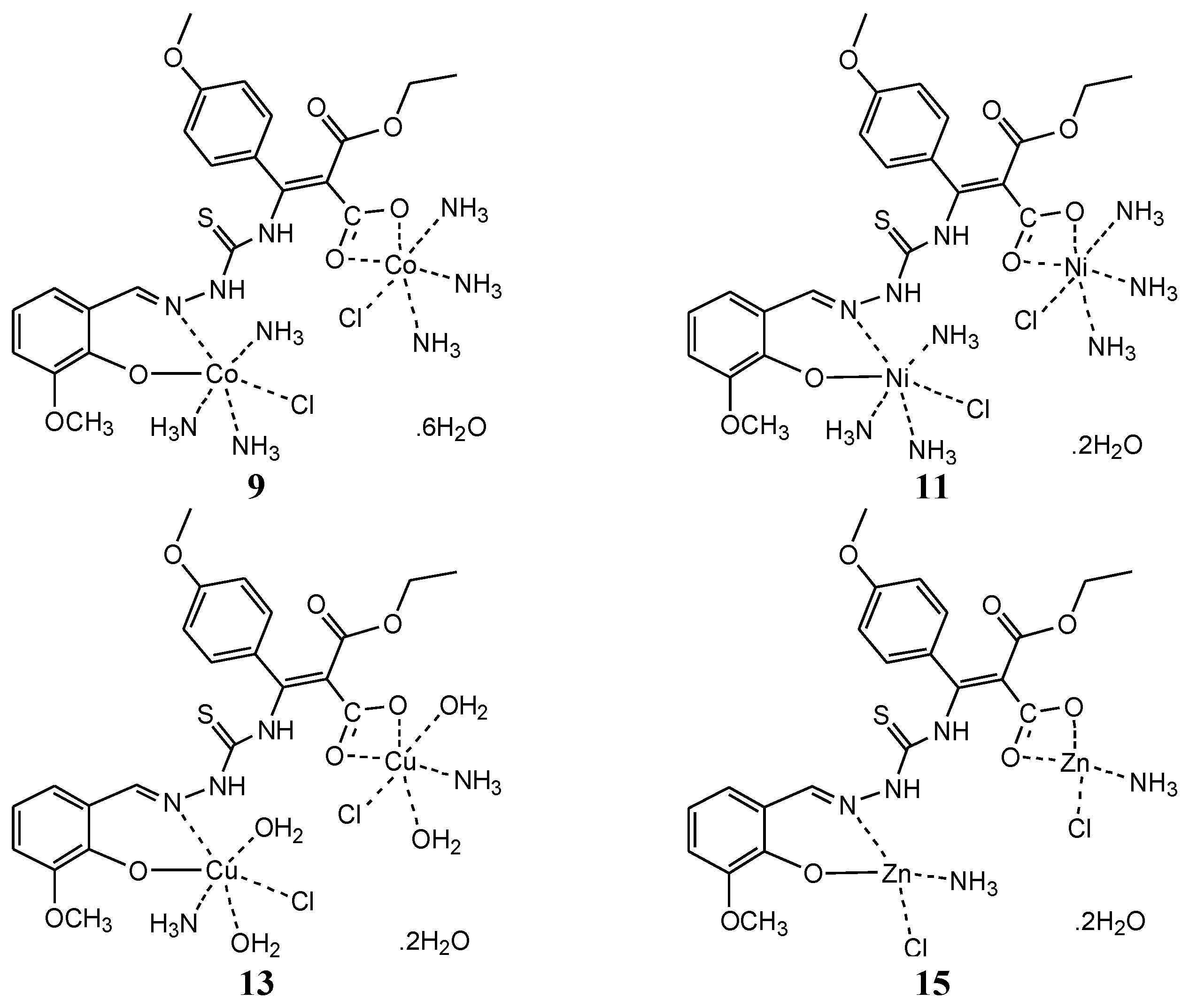
2.2.4. Synthesis of MTSC-Metal Complexes
- (1:1) Metal Complexes Synthesis
- (1:2) Metal Complexes Synthesis
2.3. Determination of the Antibacterial and Antifungal Activities
2.4. Molecular Docking Study
2.5. Statistical Analysis
3. Results and Discussion
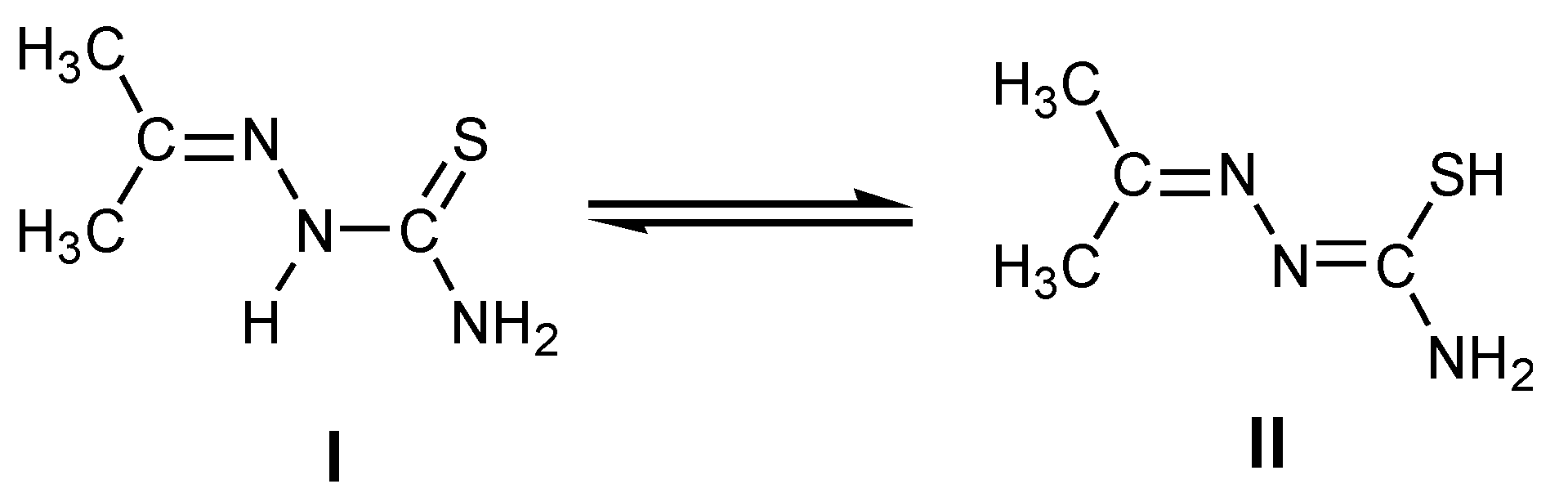
3.1. Synthesis of MTSC Ligand
3.2. Elemental Analyses and Physical Properties
3.3. Infrared Spectral Studies
3.4. UV-Vis and Magnetic Studies
3.5. 1H-NMR Spectra Studies
3.6. Thermo Gravimetric Studies
3.6.1. MTSC Ligand
3.6.2. MTSC-Metal Complexes in 1:1 Ratio (Metal:Ligand)
- Co (II) Complex 8
- Ni (II) Complex 10
- Copper (II) Complex 12
- Zinc (II) Complex 14
3.6.3. MTSC-Metal Complexes in 2:1 Ratio (Metal:Ligand)
- Cobalt (II) Complex 9
- Nickel (II) Complex 11
- Copper (II) Complex 13
- Zinc(II) Complex 15
3.7. Kinetic Thermodynamic Parameters
3.8. Proposed Structure of Synthesized Complexes
3.9. Biological Activity Evaluation
3.9.1. Antibacterial Assessments of MTSC Ligand and Its Metal Complexes
3.9.2. Antifungal Assessments of MTSC Ligand and Its Metal Complexes
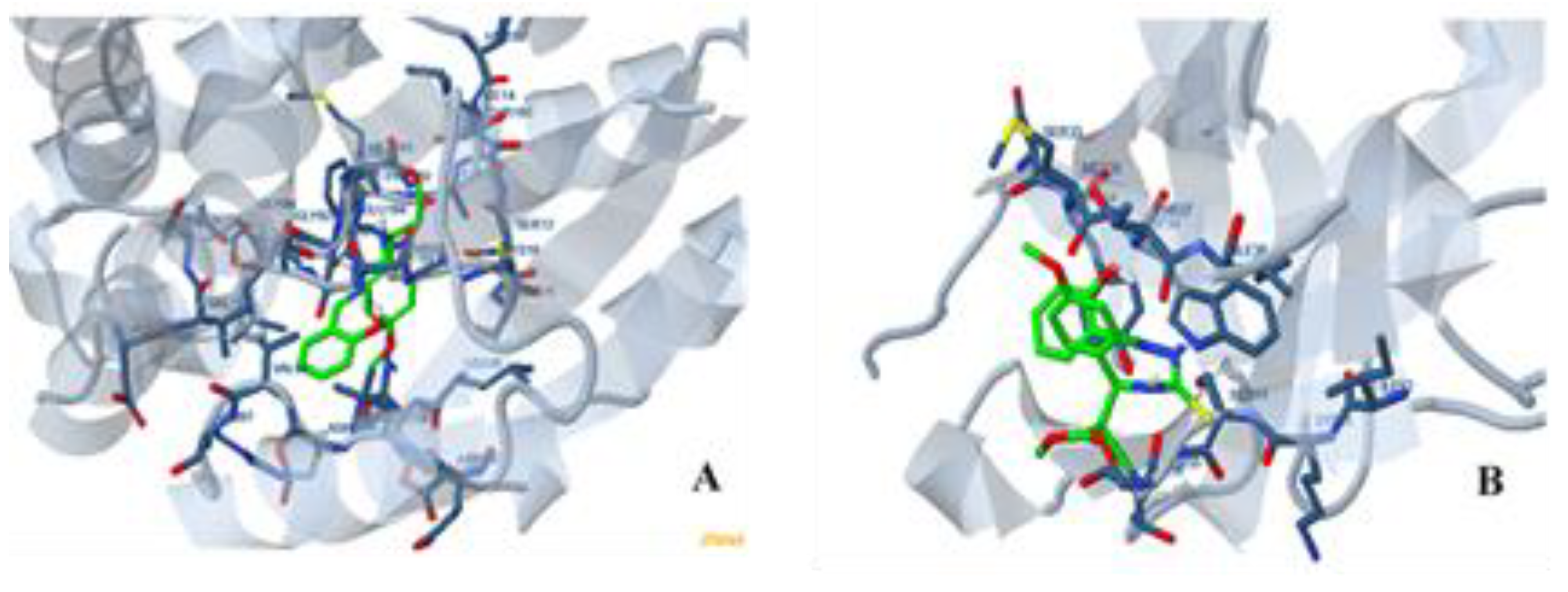
3.10. Molecular Docking
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balabanova, Y.; Gilsdorf, A.; Buda, S.; Burger, R.; Eckmanns, T.; Gaertner, B.; Gross, U.; Haas, W.; Hamouda, O.; Huebner, J.; et al. Communicable Diseases Prioritized for Surveillance and Epidemiological Research: Results of a Standardized Prioritization Procedure in Germany, 2011. PLoS ONE 2011, 6, e25691. [Google Scholar] [CrossRef]
- Augenbraun, M.; Bachmann, L.; Wallace, T.; Dubouchet, L.; McCormack, W.; Hook, E.W. Compliance with doxycycline therapy in sexually transmitted diseases clinics. Sex. Transm. Dis. 1998, 25, 1–4. [Google Scholar] [CrossRef][Green Version]
- Quiroga, A.G.; Ranninger, C.N. Contribution to the SAR field of metallated and coordination complexes: Studies of the palladium and platinum derivatives with selected thiosemicarbazones as antitumoral drugs. Coord. Chem. Rev. 2004, 248, 119–133. [Google Scholar] [CrossRef]
- Shn Moorthy, N.; Mfsa Cerqueira, N.; Ramos, M.J.; Fernandes, P.A. Aryl-and heteroaryl-thiosemicarbazone derivatives and their metal complexes: A pharmacological template. Recent Pat. Anti-Cancer Drug Discov. 2013, 8, 168–182. [Google Scholar] [CrossRef]
- Melha, K.S.A. In-vitro antibacterial, antifungal activity of some transition metal complexes of thiosemicarbazone Schiff base (HL) derived from N4-(70-chloroquinolin-40-ylamino) thiosemicarbazide. J. Enzym. Inhib. Med. Chem. 2008, 23, 493–503. [Google Scholar] [CrossRef]
- Elsayed, S.A.; El-Hendawy, A.M.; Mostafa, S.I.; Jean-Claude, B.J.; Todorova, M.; Butler, I.S. Antineoplastic activity of new transition metal complexes of 6-methylpyridine-2-carbaldehyde-n(4)-ethylthiosemicarbazone: X-Ray crystal structures of [VO2 (mpETSC)] and [Pt (mpETSC) Cl]. Bioinorg. Chem. Appl. 2010, 2010, 149149. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in copper complexes as anticancer agents. Chem. Rev. 2013, 114, 815–862. [Google Scholar] [CrossRef]
- Glisoni, R.J.; Cuestas, M.L.; Mathet, V.L.; Oubiña, J.R.; Moglioni, A.G.; Sosnik, A. Antiviral activity against the hepatitis C virus (HCV) of 1-indanone thiosemicarbazones and their inclusion complexes with hydroxypropyl-β-cyclodextrin. Eur. J. Pharm. Sci. 2012, 47, 596–603. [Google Scholar] [CrossRef]
- Lessa, J.A.; Soares, M.A.; Dos Santos, R.G.; Mendes, I.C.; Salum, L.B.; Daghestani, H.N.; Andricopulo, A.D.; Day, B.W.; Vogt, A.; Beraldo, H. Gallium (III) complexes with 2-acetylpyridine-derived thiosemicarbazones: Antimicrobial and cytotoxic effects and investigation on the interactions with tubulin. Biometals 2013, 26, 151–165. [Google Scholar] [CrossRef]
- Kalaivani, P.; Prabhakaran, R.; Ramachandran, E.; Dallemer, F.; Paramaguru, G.; Renganathan, R.; Poornima, P.; Padma, V.V.; Natarajan, K. Influence of terminal substitution on structural, DNA, protein binding, anticancer and antibacterial activities of palladium (II) complexes containing 3-methoxy salicylaldehyde-4 (N) substituted thiosemicarbazones. Dalton Trans. 2012, 41, 2486–2499. [Google Scholar] [CrossRef]
- Li, M.X.; Chen, C.L.; Zhang, D.; Niu, J.Y.; Ji, B.S. Mn (II), Co (II) and Zn (II) complexes with heterocyclic substituted thiosemicarbazones: Synthesis, characterization, X-ray crystal structures and antitumor comparison. Eur. J. Med. Chem. 2010, 45, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Abdalla1, O.; Farina1, Y.; Ibrahim, N. Synthesis characterization and antibacterial study of copper (ii) complexes of thiosemicarbazones. Malays. J. Anal. Sci. 2015, 19, 1171–1178. [Google Scholar]
- Gingras, B.A.; Somorjai, R.L.; Bayley, C.H. The preparation of some thiosemicarbazones and their copper complexes. Can. J. Chem. 1961, 39, 973–985. [Google Scholar] [CrossRef]
- Poddar, S.N.; Saha, N. Metal chelate complexes of thiosemicarbazone of pyruvic acid. J. Indian Chem. Soc. 1975, 52, 57. [Google Scholar]
- Chandra, S.; Pandeya, K.B.; Singh, R.B. Stereochemical versatility of nickel(II). Nickel(II) complexes of cyclohexanone thiosemicarbazone. Transit. Met. Chem. 1980, 5, 257–259. [Google Scholar] [CrossRef]
- Brockman, R.W.; Thomson, J.R.; Bell, M.J.; Skipper, H.E. Observations on the antileukemic activity of pyridine-2-carboxaldehyde thiosemicarbazone and thiocarbohydrazone. Cancer Res. 1956, 16, 167–170. [Google Scholar]
- Kune, G.A. Today’s drugs: Methisazone. Br. Med. J. 1964, 2, 621. [Google Scholar]
- Nutting, C.M.; Van Herpen, C.M.L.; Miah, A.B.; Bhide, S.A.; Machiels, J.P.; Buter, J.; Kelly, C.; De Raucourt, D.; Harrington, K.J. Phase II study of 3-AP Triapine in patients with recurrent or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 2009, 20, 1275–1279. [Google Scholar] [CrossRef]
- Ma, B.; Goh, B.C.; Tan, E.H.; Lam, K.C.; Soo, R.; Leong, S.S.; Wang, L.Z.; Mo, F.; Chan, A.T.; Zee, B.; et al. A multicenter phase II trial of 3- aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine) and gemcitabine in advanced non-small-cell lung cancer with pharmacokinetic evaluation using peripheral blood mononuclear cells. Invest. New Drugs 2008, 26, 169–173. [Google Scholar] [CrossRef]
- Jansson, P.J.; Kalinowski, D.S.; Lane, D.J.R.; Kovacevic, Z.; Seebacher, N.A.; Fouani, L.; Sahni, S.; Merlot, A.M.; Richardson, D.R. The renaissance of polypharmacology in the development of anti-cancer therapeutics: Inhibition of the “Triad of Death” in cancer by Di-2-pyridylketone thiosemicarbazones. Pharmacol. Res. 2015, 100, 255–260. [Google Scholar] [CrossRef]
- Salim, K.Y.; Danter, W.R.; Maleki, V.S.; Koropatnick, J. COTI-2, a novel small molecule that is active against multiple human cancer cell lines in vitro and in vivo. Oncotarget 2016, 7, 41363–41379. [Google Scholar] [CrossRef]
- Garcia-Tojal, J.; Gil-Garcia, R.; Gomez-Saiz, P.; Ugalde, M. Pyridine-2-Carbaldehyde Thiosemicarbazonecopper System: Extending Some Findings to Other Thiosemicarbazone and Coordination Compounds. Curr. Inorg. Chem. 2011, 1, 189–210. [Google Scholar] [CrossRef]
- Aly, M.M.; Mohamed, Y.A.; El-Bayouki, K.A.; Basyouni, W.M.; Abbas, S.Y. Synthesis of Some New 4(3h)-Quinazolinone-2-Carboxaldehyde Thiosemicarbazones and Their Metal Complexes and a Study on Their Anticonvulsant, Analgesic, Cytotoxic and Antimicrobial Activities—Part-1. Eur. J. Med. Chem. 2010, 45, 3365–3373. [Google Scholar] [CrossRef]
- Vinuelas-Zahinos, E.; Luna-Giles, F.; Torres-Garcia, P.; Fernandez-Calderon, M.C. C(III), N(II), Z(II) and C(II) Complexes with 2-Acetyl-2-Thiazoline Thiosemicarbazone: Synthesis, Characterization, XRay Structures and Antibacterial Activity. Eur. J. Med. Chem. 2011, 46, 150–159. [Google Scholar] [CrossRef]
- Halder, S.; Paul, P.; Peng, S.M.; Lee, G.-H.; Mukherjee, A.; Dutta, S.; Sanyal, U.; Bhattacharya, S. Benzaldehyde Thiosemicarbazone Complexes of Platinum: Syntheses, Structures and Cytotoxic Properties. Polyhedron 2012, 45, 177–184. [Google Scholar] [CrossRef]
- Liu, Z.C.; Wang, B.D.; Yang, Z.Y.; Li, Y.; Qin, D.D.; Li, T.R. Synthesis, Crystal Structure, DNA Interaction and Antioxidant Activities of Two Novel Water-Soluble Cu2+ Complexes Derivated from 2-Oxo-Quinoline-3-Carbaldehyde Schiff-Bases. Eur. J. Med. Chem. 2009, 44, 4477–4484. [Google Scholar] [CrossRef] [PubMed]
- Sampath, K.; Sathiyaraj, S.; Raja, G.; Jayabalakrishnan, C. Mixed Ligand Ruthenium (III) Complexes of Benzaldehyde 4-Methyl-3-Thiosemicarbazones with Triphenylphosphine/Triphenylarsine Co-Ligands: Synthesis, DNA Binding, DNA Cleavage, Antioxidative and Cytotoxic Activity. J. Mol. Struct. 2013, 1046, 82–91. [Google Scholar] [CrossRef]
- Chandra, S.; Bargujar, S.; Nirwal, R.; Yadav, N. Synthesis, Spectral Characterization and Biological Evaluation of Copper (II) and Nickel (II) Complexes with Thiosemicarbazones Derived from a Bidentate Schiff Base. Spectrochim. Acta A Mol. Biomol. 2013, 106, 91–98. [Google Scholar] [CrossRef]
- Gaber, A.; Alsanie, W.F.; Kumar, D.N.; Refat, M.S.; Saied, E.M. Novel Papaverine Metal Complexes with Potential Anticancer Activities. Molecules 2020, 25, 5447. [Google Scholar] [CrossRef]
- Refat, M.S.; Ibrahim, H.K.; Sowellim, S.Z.A.; Soliman, M.H.; Saeed, E.M. Spectroscopic and Thermal Studies of Mn(II), Fe(III), Cr(III) and Zn(II) Complexes Derived from the Ligand Resulted by the Reaction between 4-Acetyl Pyridine and Thiosemicarbazide. J. Inorg. Organomet. Polym. Mater. 2009, 19, 521–531. [Google Scholar] [CrossRef]
- Refat, M.S.; Altalhi, T.; Hassan, R.F. Synthesis, spectroscopic, structural and morphological characterizations of magnesium(II), calcium(II), strontium(II) and barium(II) folate complexes. J. Mol. Struct. 2021, 1227, 129519. [Google Scholar] [CrossRef]
- Matar, M.J.; Ostrosky-Zeichner, L.; Paetznick, V.L.; Rodriguez, J.R.; Chen, E.; Rex, J.H. Correlation between E-test, disk diffusion, andmicrodilution methods for antifungal susceptibility testing of fluconazole andvoriconazole. Antimicrob. Agents Chemother. 2003, 47, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Saxena, R.K.; Chatarvedi, P.; Virdi, J.S. Chitinase production by Streptomyces viridificans: Its potential in fungal cell wall lysis. J. Appl. Bacteriol. 1995, 78, 378. [Google Scholar] [CrossRef]
- Bikadi, Z.; Hazai, E. Application of the PM6 semi-empirical method to modeling proteins enhances docking accuracy of AutoDock. J. Cheminf. 2009, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parametrization, and performance of MMFF94. J. Comput. Chem. 1998, 17, 490–519. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Solis, F.J.; Wets, R.J.B. Minimization by Random Search Techniques. Math. Oper. Res. 1981, 6, 19–30. [Google Scholar] [CrossRef]
- El-Habeeb, A.A.; Refat, M.S. Synthesis, structure interpretation, antimicrobial and anticancer studies of tranexamic acid complexes towards Ga(III), W(VI), Y(III) and Si(IV) metal ions. J. Mol. Struct. 2019, 1175, 65–72. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 4th ed.; Elsevier: London, UK, 1980; p. 481. [Google Scholar]
- Chandra, S.; Gupta, K. Twelve-, fourteen- and sixteenmembered macrocyclic ligands and a study of the effect of ring size on ligand field strength. Transit. Met. Chem. 2002, 27, 329–332. [Google Scholar] [CrossRef]
- Lever, A.B.P. Crystal Field Spectra. Inorganic Electronic Spectroscopy, 1st ed.; Academic Press: Amsterdam, The Netherlands, 1968. [Google Scholar]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Coats, A.W.; Redfern, J.P. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68. [Google Scholar] [CrossRef]
- Horowitz, H.W.; Metzger, G. A new analysis of thermogravimetric traces. Anal. Chem. 1963, 35, 1464. [Google Scholar] [CrossRef]
- Freeman, E.S.; Carroll, B. The Application of Thermoanalytical Techniques to Reaction Kinetics: The Thermogravimetric Evaluation of the Kinetics of the Decomposition of Calcium Oxalate Monohydrate. J. Phys. Chem. 1958, 62, 394. [Google Scholar] [CrossRef]
- Sestak, J.; Satava, V.; Wendlandt, W.W. The study of heterogeneous processes by TA. Thermochim. Acta 1973, 7, 333. [Google Scholar] [CrossRef]
- Brown, M.E. Introduction to Thermal Analysis—Techniques and Applications; Chapman and Hall: London, UK, 1988; pp. 7–21. [Google Scholar]
- Osawa, T. Thermal Analysis Proc; Kodanska and Halsted: Tokyo, Japan, 1974; p. 155. [Google Scholar]
- Wendlandt, W.W. Thermal Methods of Analysis; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Adonyi, Z.; Körösi, G. Thermal Analysis Proc; Heyden & Sons: London, UK, 1977; p. 477. [Google Scholar]
- Pahontu, E.; Fala, V.; Gulea, A.; Poirier, D.; Tapcov, V.; Rosu, T. Synthesis and characterization of some new Cu(II), Ni(II) and Zn(II) complexes with salicylidene thiosemicarbazones: Antibacterial, antifungal and in vitro antileukemia activity. Molecules 2013, 18, 8812–8836. [Google Scholar] [CrossRef] [PubMed]
- Esimone, C.O.; Okoye, F.B.; Nworu, C.S.; Agubata, C.O. In vitro interaction between caffeine and some penicillin antibiotics against Staphylococcus aureus. Tropical J. Pharm. Res. 2008, 7, 969–974. [Google Scholar] [CrossRef]
- Refat, M.S.; BelalI, A.A.M.; El-Deen, M.; Hassan, N.; Zakaria, R. Synthesis, spectroscopic, thermal and antimicrobial investigations of new mono and binuclear Cu (II), Co (II), Ni (II), and Zn (II) thiosemicarbazide complexes. J. Mol. Struct. 2020, 1218, 128516. [Google Scholar] [CrossRef]
- El-Bindary, A.A.; El-Sonbatia, A.Z.; Diaba, M.A.; El-Ghamazb, N.A.; Shoaira, A.F.; Nozha, S.G. Potentiometric studies and molecular docking of quinoline Schiff base and its metal complexes. J. Mater. Environ. Sci. 2016, 7, 1934–1947. [Google Scholar]

| Compounds | Color | %Found (% Calcd.) | |||||
|---|---|---|---|---|---|---|---|
| C% | H% | N% | Cl% | M% | S% | ||
| MTSC | Yellow | 58.03(58.14) | 4.61(4.88) | 12.12(12.33) | - | - | 7.39(7.06) |
| I | Brown | 38.11(38.29) | 5.59(5.70) | 12.09(12.18) | 5.09(5.14) | 8.43(8.54) | 4.60(4.65) |
| II | Dark brown | 30.21(30.35) | 5.80(5.91) | 14.31(14.48) | 8.10(8.15) | 13.43(13.54) | 3.61(3.68) |
| III | Orange brown | 40.33(40.42) | 5.23(5.40) | 12.77(12.85) | 5.33(5.42) | 8.78(8.98) | 4.88(4.90) |
| IV | Reddish brown | 33.09(33.11) | 5.30(5.43) | 15.76(15.80) | 8.81(8.89) | 14.65(14.71) | 3.89(4.02) |
| V | Brown | 39.01(39.05) | 5.45(5.51) | 12.33(12.42) | 5.19(5.24) | 9.29(9.39) | 4.70(4.74) |
| VI | Green | 32.44(32.56) | 4.80(4.84) | 8.56(8.63) | 8.65(8.74) | 15.56(15.66) | 3.91(3.95) |
| VII | Yellow | 42.09(42.18) | 4.45(4.67) | 8.91(8.94) | 5.60(5.66) | 10.34(10.44) | 5.10(5.12) |
| VIII | Yellow | 35.23(35.55) | 4.11(4.20) | 9.33(9.42) | 9.41(9.54) | 17.54(17.60) | 4.22(4.31) |
| Compounds | ν(O-H) | ν(N-H) | ν(COCH3) | ν(C=N) | νas(COO) | νs(COO) | ν(C–O) | ν(C=S) | ν(M–O) | ν(M–N) |
|---|---|---|---|---|---|---|---|---|---|---|
| MTSC | 3340 | - | 2843 | 1589 | - | - | 1211 | 1018 | - | - |
| I | 3313 | 3174 | 2835 | 1508 | 1600 | 1438 | 1172 | 968 | 551, 516 | 443, 420 |
| II | 3332 | 3170 | 2835 | 1512 | 1589 | 1435 | 1180 | 1018 | 555, 516 | 443, 421 |
| III | 3344 | 3190 | 2835 | 1531 | 1604 | 1454 | 1172 | 972 | 594, 570 | 455, 432 |
| IV | 3317 | 3232 | 2831 | 1527 | 1604 | 1458 | 1176 | 968 | 594, 524 | 447, 416 |
| V | 3321 | 3194 | 2835 | 1504 | 1604 | 1454 | 1172 | 968 | 586, 516 | 470, 439 |
| VI | 3336 | 3012 | 2860 | 1508 | 1604 | 1450 | 1172 | 972 | 558, 516 | 470, 447 |
| VII | 3309 | 3116 | 2897 | 1573 | 1600 | 1384 | 1161 | 1080 | 597, 551 | 455, 420 |
| VIII | 3236 | 3132 | 2831 | 1512 | 1612 | 1562 | 1172 | 964 | 516, 551 | 495, 430 |
| Complex | Steps | Temp Range/°C | DTG Peak/°C | Decomposed Assignments | Weight Loss Found (Calc.%) |
|---|---|---|---|---|---|
| MTSC | 1st | 20–295 | 195 495 | 10C2H2 + CO + N2 | 69(69.7) |
| 2nd | 295–530 | 2NO2 | 21(20) | ||
| residue | 530–800 | H2S + carbon residue | 10(10.1) | ||
| 8 | 1st | 35–203 203–460 460–620 | 60 275 515 | 3H2O | 8(7.8) |
| 2nd | 3H2O + 2H2 + 6C2H2 + 3NH3 + H2S + N2 + ½Cl2 | 52(52.5) | |||
| 3rd | CO2 + NO2 | 13(13.1) | |||
| residue | CoO + carbon residue | 27(26.4) | |||
| 9 | 1st | 35–180 | 52 130,220 450 | 6H2O | 13(12.4) |
| 2nd | 180–420 | H2 + 4NH3 + 9C2H2 + 4CO + H2S | 52(51.7) | ||
| 3rd | 420–547 | Cl2 + 2N2 + NH3 + H2O | 17(18.5) | ||
| residue | 547–800 | 2CoO | 17(17.2) | ||
| 10 | 1st | 35–249 | 45 300 445 | 2H2O | 5(5.5) |
| 2nd | 249–370 | 3NH3 + H2O + ½Cl2 + H2S + 4H2 | 23(22.4) | ||
| 3rd | 370–585 | H2O + N2 + NO + 3CO + 4C2H2 | 40.5(40.5) | ||
| residue | 585–800 | NiO + carbon residue | 31.5(31.4) | ||
| 11 | 1st | 25–230 | 145 296 445 | 2H2O + 3NH3 | 11(10.9) |
| 2nd | 230–375 | 2NH3 + Cl2 + H2S + 4H2 | 19(18.5) | ||
| 3rd | 375–551 | 5C2H2 + 2NO + H2O + N2 | 34(33.9) | ||
| residue | 551–800 | 2NiO + carbon residue | 36(36.7) | ||
| 12 | 1st | 20–175 | 90 210,255,330 504 650 | 3H2O | 8(8) |
| 2nd | 175–405 | 3NH3 + 5C2H4 | 27(26.76) | ||
| 3rd | 405–605 | 2H2O + 2NH3 + H2S + NO2 + ½Cl2 | 28(27.54) | ||
| 4th | 605–710 | CO2 | 6(6.5) | ||
| residue | 710–800 | CuO+ carbon residue | 31(31.6) | ||
| 13 | 1st | 20–155 | 40 215,305,359 655 | 2H2O | 4(4.4) |
| 2nd | 155–525 | 6H2O + NH3 + Cl2 + H2S + N2 + NO + 9H2 | 38(37.8) | ||
| 3rd | 525–740 | NO2 | 6(5.7) | ||
| residue | 740–800 | 2CuO + carbon residue | 52(52.4) | ||
| 14 | 1st | 30–180 | 120,140 255 530,570 | H2O | 3(2.9) |
| 2nd | 180–450 | H2O + NO2 + 6C2H2 | 35(35.1) | ||
| 3rd | 450–630 | ½Cl2 + NH3 + N2 + H2S + H2O + H2 + CO2 | 30(32) | ||
| residue | 630–800 | ZnO + carbon residue | 31.5(30) | ||
| 15 | 1st | 25–345 | 160,245 540 | 2H2O + 2NO2 + N2 + 7C2H2 | 46(45.5) |
| 2nd | 345–545 | Cl2 + H2S + CH4 + H2O + H2 + NH3 | 21(21.4) | ||
| residue | 545–800 | 2ZnO + carbon residue | 33(33.1) |
| Compound | Stage | Method | Parameter | r | ||||
|---|---|---|---|---|---|---|---|---|
| E* | A | ΔS | ΔH | ΔG | ||||
| (J mol−1) | (s−1) | (J mol−1 K−1) | (J mol−1) | (J mol−1) | ||||
| MTSC | 1st 2nd | CR | 3.32 × 10−1 | 5.15 × 10−9 | −4.07 × 102 | −3.77 × 103 | 1.81 × 105 | 0.9026 |
| HM | 7.96 × 102 | 9.60 × 10−5 | −3.25 × 102 | −2.97 × 103 | 1.44 × 105 | 0.9205 | ||
| CR | 9.31 × 10−2 | 1.40 × 10−9 | −4.21 × 102 | −5.76 × 103 | 2.86 × 105 | 0.991 | ||
| HM | 1.52 × 103 | 8.28 × 10−5 | −3.30 × 102 | −4.24 × 103 | 2.25 × 105 | 0.983 | ||
| 8 | 1st 2nd | CR | 1.17 × 10−1 | 2.23 × 10−9 | −4.15 × 102 | −4.27 × 103 | 2.09 × 105 | 0.9965 |
| HM | 1.01 × 103 | 9.79 × 10−5 | −3.26 × 102 | −3.25 × 103 | 1.64 × 105 | 0.9272 | ||
| CR | 1.56 × 10−1 | 4.41 × 10−9 | −4.13 × 102 | −6.26 × 103 | 3.04 × 105 | 0.9939 | ||
| HM | 1.52 × 103 | 6.82 × 10−5 | −3.32 × 102 | −4.75 × 103 | 2.46 × 105 | 0.996 | ||
| 9 | 1st 2nd | CR | 1.97 × 10−1 | 6.93 × 10−9 | −4.05 × 102 | −3.85 × 103 | 1.84 × 105 | 0.9939 |
| HM | 8.51 × 102 | 9.91 × 10−5 | −3.25 × 102 | −3.00 × 103 | 1.48 × 105 | 0.9217 | ||
| CR | 1.57 × 10−1 | 8.26 × 10−9 | −4.07 × 102 | −6.05 × 103 | 2.90 × 105 | 0.9834 | ||
| HM | 1.62 × 103 | 7.97 × 10−5 | −3.31 × 102 | −4.44 × 103 | 2.36 × 105 | 0.9877 | ||
| 10 | 1st 2nd | CR | 4.57 × 10−2 | 2.10 × 10−9 | −4.15 × 102 | −4.18 × 103 | 2.05 × 105 | 0.9113 |
| HM | 6.45 × 102 | 5.96 × 10−5 | −3.30 × 102 | −3.54 × 103 | 1.63 × 105 | 0.9949 | ||
| CR | 2.88 × 10−1 | 1.29 × 10−8 | −4.04 × 102 | −6.30 × 103 | 3.00 × 105 | 0.9009 | ||
| HM | 2.17 × 103 | 1.07 × 10−4 | −3.29 × 102 | −4.14 × 103 | 2.45 × 105 | 0.9868 | ||
| 11 | 1st 2nd 3rd | CR | 2.44 × 10−1 | 1.35 × 10−8 | −3.99 × 102 | −3.73 × 103 | 1.75 × 105 | 0.9798 |
| HM | 5.90 × 102 | 6.90 × 10−5 | −3.28 × 102 | −3.14 × 103 | 1.44 × 105 | 0.9882 | ||
| CR | 3.74 × 10−1 | 3.00 × 10−9 | −4.14 × 102 | −4.77 × 103 | 2.32 × 105 | 0.9838 | ||
| HM | 1.49 × 103 | 1.24 × 10−4 | −3.25 × 102 | −3.28 × 103 | 1.83 × 105 | 0.9715 | ||
| CR | 3.88 × 10−1 | 1.16 × 10−8 | −4.04 × 102 | −6.10 × 103 | 2.90 × 105 | 0.9814 | ||
| HM | 1.72 × 103 | 8.53 × 10−5 | −3.30 × 102 | −4.37 × 103 | 2.38 × 105 | 0.9735 | ||
| 12 | 1st 2nd | CR | 1.07 × 10−1 | 3.88 × 10−9 | −4.10 × 102 | −4.14 × 103 | 2.00 × 105 | 0.9756 |
| HM | 8.29 × 102 | 8.18 × 10−5 | −3.27 × 102 | −3.31 × 103 | 1.60 × 105 | 0.9654 | ||
| CR | 1.47 × 10−1 | 5.18 × 10−9 | −4.12 × 102 | −6.64 × 103 | 3.22 × 105 | 0.9118 | ||
| HM | 1.90 × 103 | 7.96 × 10−5 | −3.32 × 102 | −4.74 × 103 | 2.60 × 105 | 0.9936 | ||
| 13 | 1st 2nd | CR | 1.71 × 10−1 | 4.42 × 10−9 | −4.08 × 102 | −3.73 × 103 | 1.79 × 105 | 0.9883 |
| HM | 7.71 × 102 | 9.46 × 10−5 | −3.25 × 102 | −2.96 × 103 | 1.43 × 105 | 0.956 | ||
| CR | 1.41 × 10−1 | 1.02 × 10−8 | −4.05 × 102 | −5.68 × 103 | 2.71 × 105 | 0.9632 | ||
| HM | 1.28 × 103 | 6.87 × 10−5 | −3.32 × 102 | −4.40 × 103 | 2.22 × 105 | 0.9936 | ||
| 14 | 1st 2nd | CR | 1.17 × 10−1 | 2.90 × 10−9 | −4.14 × 102 | −4.60 × 103 | 2.24 × 105 | 0.9798 |
| HM | 9.65 × 102 | 7.80 × 10−5 | −3.29 × 102 | −3.63 × 103 | 1.78 × 105 | 0.9709 | ||
| CR | 1.32 × 10−1 | 3.59 × 10−9 | −4.15 × 102 | −7.09 × 103 | 3.47 × 105 | 0.9987 | ||
| HM | 2.67 × 103 | 1.07 × 10−4 | −3.30 × 102 | −4.43 × 103 | 2.77 × 105 | 0.9782 | ||
| 15 | 1st 2nd | CR | 5.70 × 10−1 | 8.61 × 10−9 | −4.02 × 102 | −3.60 × 103 | 1.71 × 105 | 0.9109 |
| HM | 9.65 × 102 | 7.80 × 10−5 | −3.29 × 102 | −3.63 × 103 | 1.78 × 105 | 0.9994 | ||
| CR | 5.15 × 10−2 | 1.51 × 10−9 | −4.20 × 102 | −5.51 × 103 | 2.73 × 105 | 0.9546 | ||
| HM | 1.24 × 103 | 7.10 × 10−5 | −3.31 × 102 | −4.27 × 103 | 2.15 × 105 | 0.9895 | ||
| Ligand/Complex | Gram-Positive Bacteria | Gram-Negative Bacteria | ||
|---|---|---|---|---|
| Bacillussubtilis | Staphylococcus aureus | E. coli | Proteus vulgaris | |
| MTSC | 15 ± 1.2 d | NA | 10 ± 0.7 e | 9 ± 0.3 e |
| 8 | 15 ± 0.9 d | 10 ± 0.8 d | 13 ± 0.8 d | 14 ± 0.7 c,d |
| 9 | 16 ± 1.1 d | 12 ± 0.9 d | 16 ± 0.9 c | 15 ± 0.5 c |
| 10 | 21 ± 0.7 c | 15 ± 1.1 c | 19 ± 1.1 b | 15 ± 0.8 c |
| 11 | 22 ± 0.9 c | 18 ± 1.3 b | 21 ± 1.2 b | 19 ± 1.1 b |
| 12 | 33 ± 1.8 a | 18 ± 0.8 b | 22 ± 1.1 b | 25 ± 1.2 a |
| 13 | 33 ± 1.2 a | 17 ± 1.2 b | 20 ± 0.9 b | 26 ± 1.5 a |
| 14 | 17 ± 1.3 d | 12 ± 0.8 d | 11 ± 0.3 e | 12 ± 0.8 d |
| 15 | 21 ± 0.5 c | 14 ± 0.4 c | 16 ± 0.6 c | 14 ± 0.4 c,d |
| Gentamicin | 26 ± 1.7 b | 24 ± 1.8 a | 30 ± 2.3 a | 25 ± 1.9 a |
| Ligand/Complex | Aspergillus flavus | Candida albicans |
|---|---|---|
| MTSC | 10 ± 0.4 b | 11 ± 0.8 b |
| 8 | NA | NA |
| 9 | NA | NA |
| 10 | NA | 9 ± 0.4 c |
| 11 | NA | 8 ± 0.2 c |
| 12 | NA | 12 ± 0.7 b |
| 13 | NA | 8 ± 0.3 c |
| 14 | NA | 8 ± 0.2 c |
| 15 | NA | 9 ± 0.3 c |
| Ketoconazole | 16 ± 0.7 a | 20 ± 1.1 a |
| Est. Free Energy of Binding | Est. Inhibition Constant, Ki | vdW+Hbond+Desolv Energy | Electrostatic Energy | Total Intermolec. Energy | Interact. Surface | |
|---|---|---|---|---|---|---|
| Oxidoreductase protein | −5.83 kcal/mol | 53.71 μM | −8.96 kcal/mol | −0.30 kcal/mol | −9.27 kcal/mol | 1005.512 |
| SPOP protein | 312.12 kcal/mol | - | 231.33 kcal/mol | −0.15 kcal/mol | 231.18 kcal/mol | 543.026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaber, A.; Refat, M.S.; Belal, A.A.M.; El-Deen, I.M.; Hassan, N.; Zakaria, R.; Alhomrani, M.; Alamri, A.S.; Alsanie, W.F.; M. Saied, E. New Mononuclear and Binuclear Cu(II), Co(II), Ni(II), and Zn(II) Thiosemicarbazone Complexes with Potential Biological Activity: Antimicrobial and Molecular Docking Study. Molecules 2021, 26, 2288. https://doi.org/10.3390/molecules26082288
Gaber A, Refat MS, Belal AAM, El-Deen IM, Hassan N, Zakaria R, Alhomrani M, Alamri AS, Alsanie WF, M. Saied E. New Mononuclear and Binuclear Cu(II), Co(II), Ni(II), and Zn(II) Thiosemicarbazone Complexes with Potential Biological Activity: Antimicrobial and Molecular Docking Study. Molecules. 2021; 26(8):2288. https://doi.org/10.3390/molecules26082288
Chicago/Turabian StyleGaber, Ahmed, Moamen S. Refat, Arafa A.M. Belal, Ibrahim M. El-Deen, Nader Hassan, Rozan Zakaria, Majid Alhomrani, Abdulhakeem S. Alamri, Walaa F. Alsanie, and Essa M. Saied. 2021. "New Mononuclear and Binuclear Cu(II), Co(II), Ni(II), and Zn(II) Thiosemicarbazone Complexes with Potential Biological Activity: Antimicrobial and Molecular Docking Study" Molecules 26, no. 8: 2288. https://doi.org/10.3390/molecules26082288
APA StyleGaber, A., Refat, M. S., Belal, A. A. M., El-Deen, I. M., Hassan, N., Zakaria, R., Alhomrani, M., Alamri, A. S., Alsanie, W. F., & M. Saied, E. (2021). New Mononuclear and Binuclear Cu(II), Co(II), Ni(II), and Zn(II) Thiosemicarbazone Complexes with Potential Biological Activity: Antimicrobial and Molecular Docking Study. Molecules, 26(8), 2288. https://doi.org/10.3390/molecules26082288









