Eugenol-Functionalized Magnetite Nanoparticles Modulate Virulence and Persistence in Pseudomonas aeruginosa Clinical Strains
Abstract
1. Introduction
2. Results and Discussion
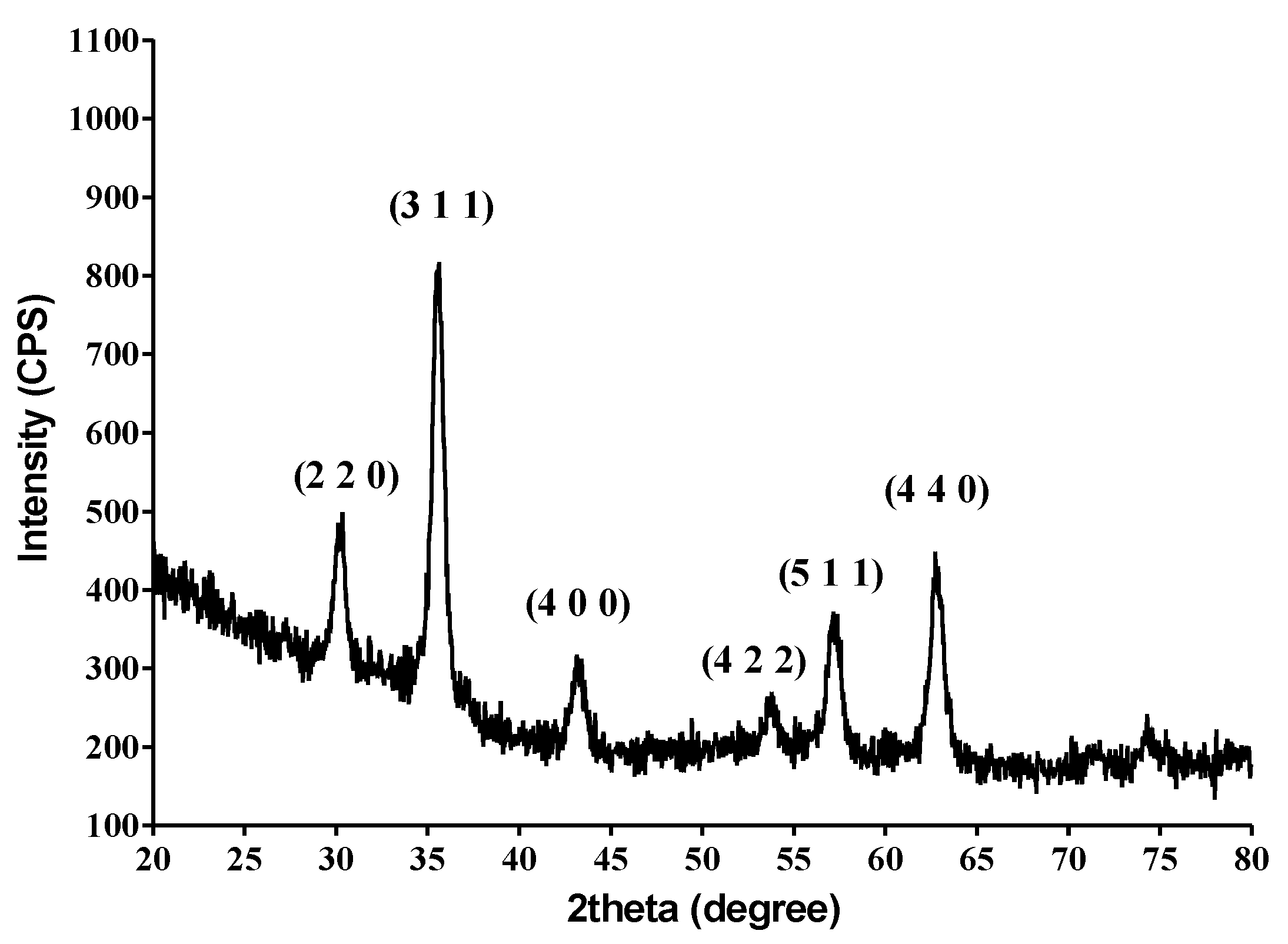
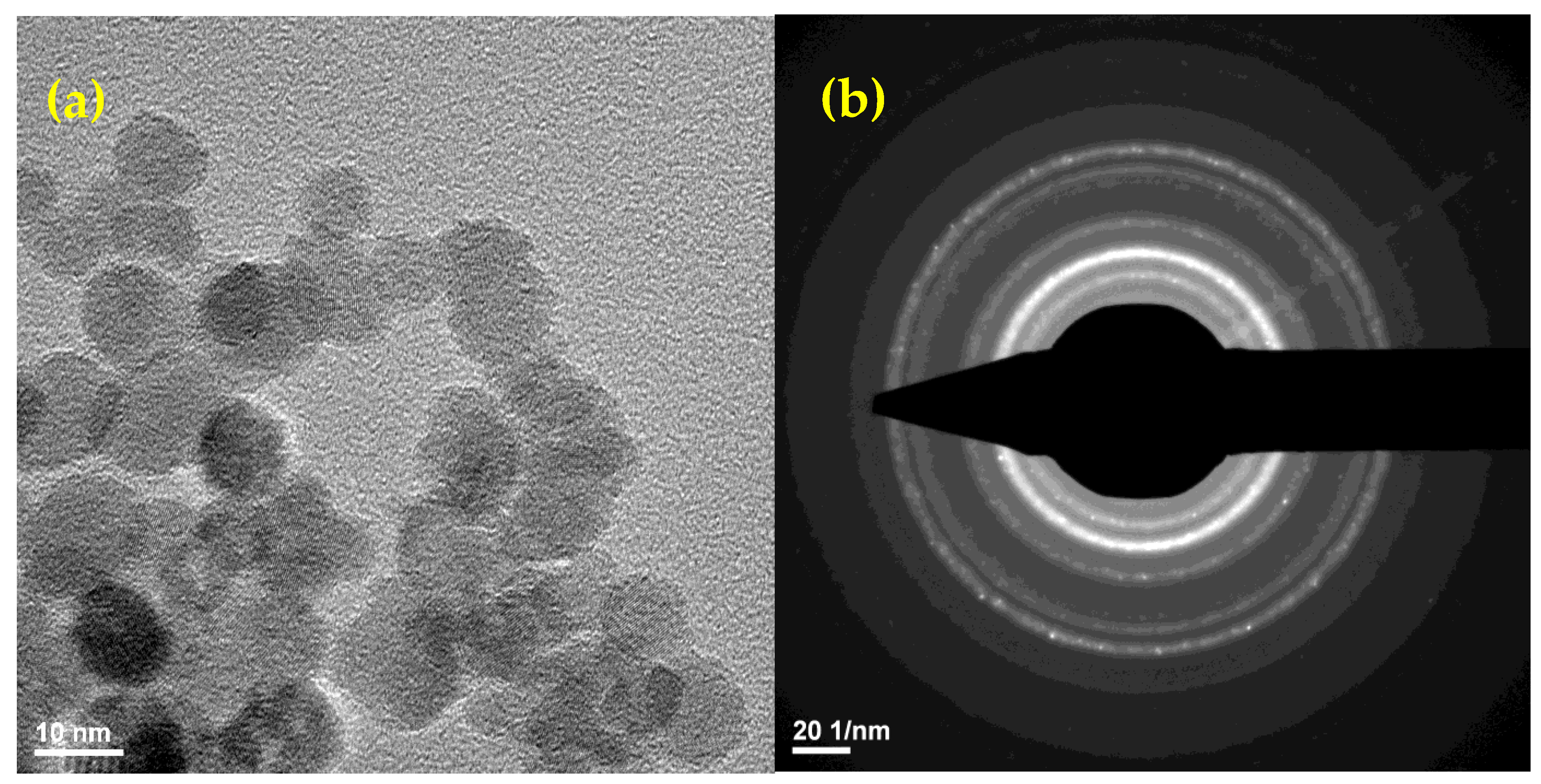
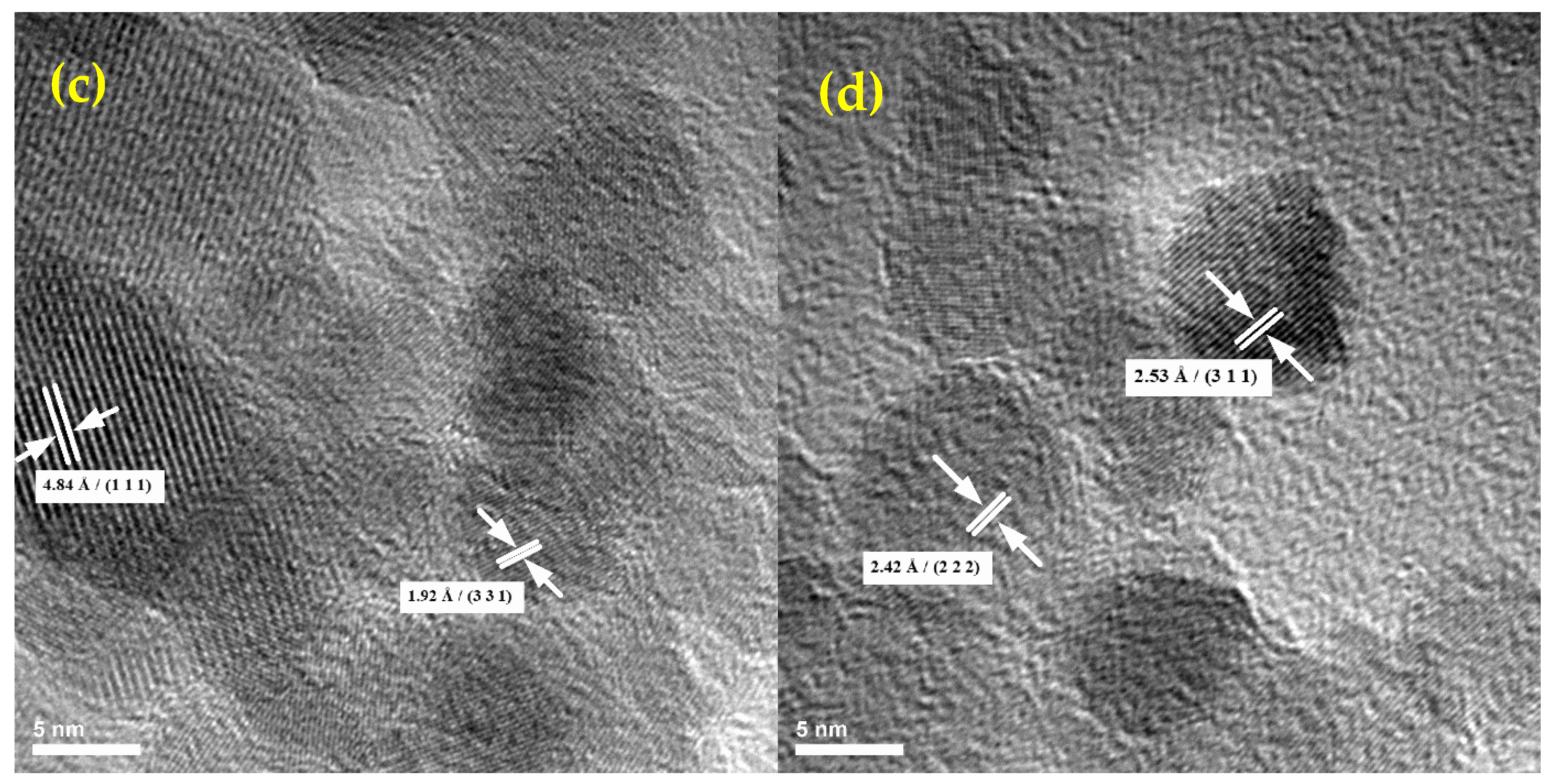
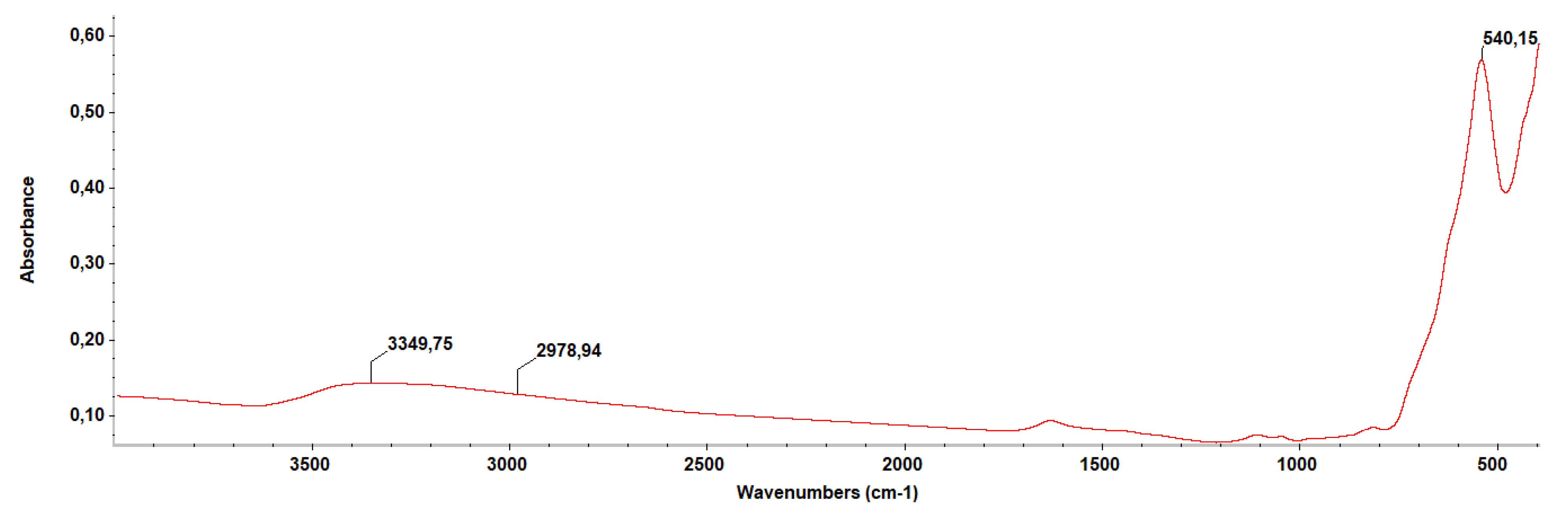
2.1. Physico-Chemical Characterization of the NPs
2.2. In Vitro Antimicrobial Analysis and Virulence Modulation
2.2.1. Qualitative Antimicrobial Analysis—Growth Inhibition
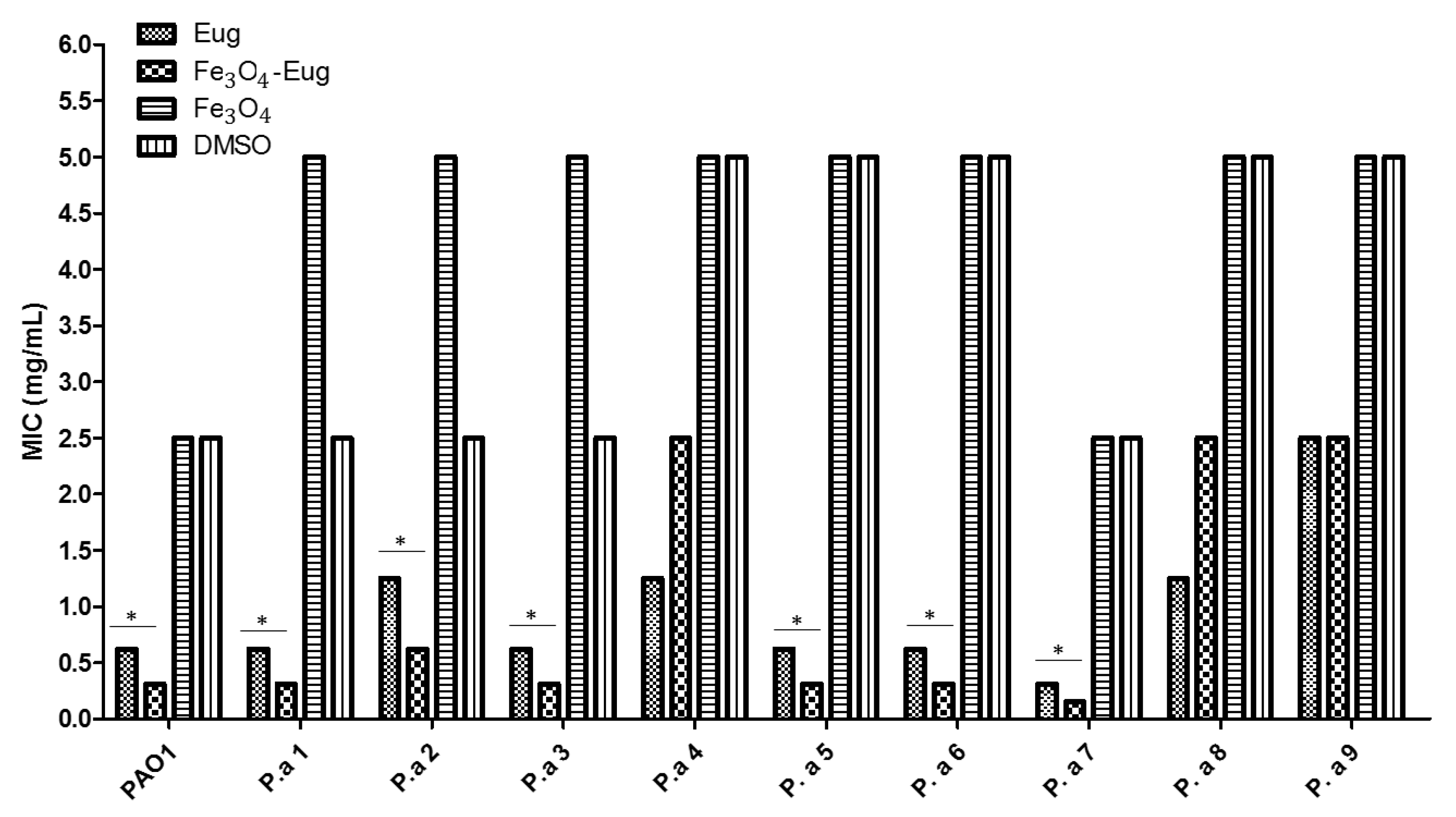
2.2.2. Quantitative Analysis of Antimicrobial Effect—MIC Values
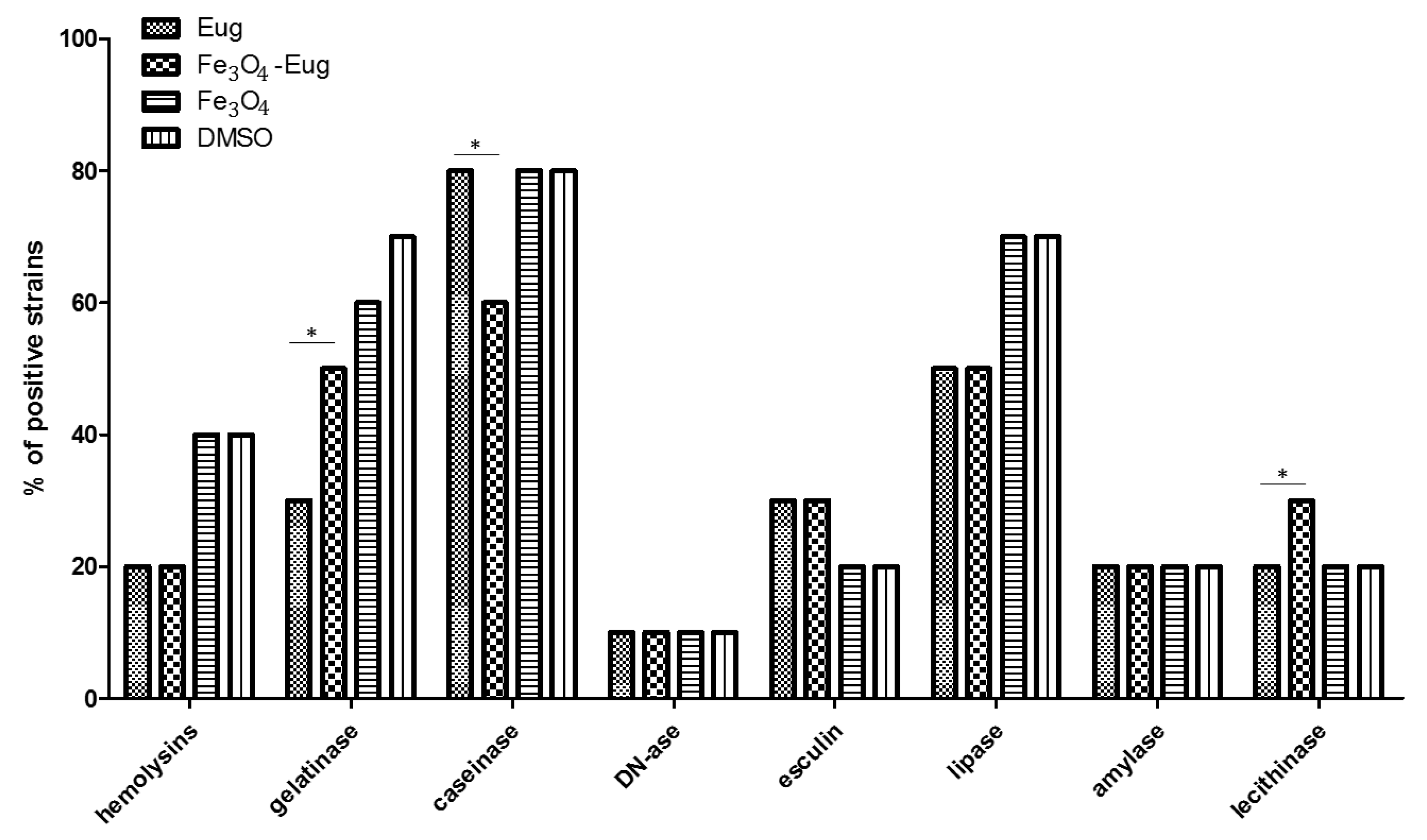
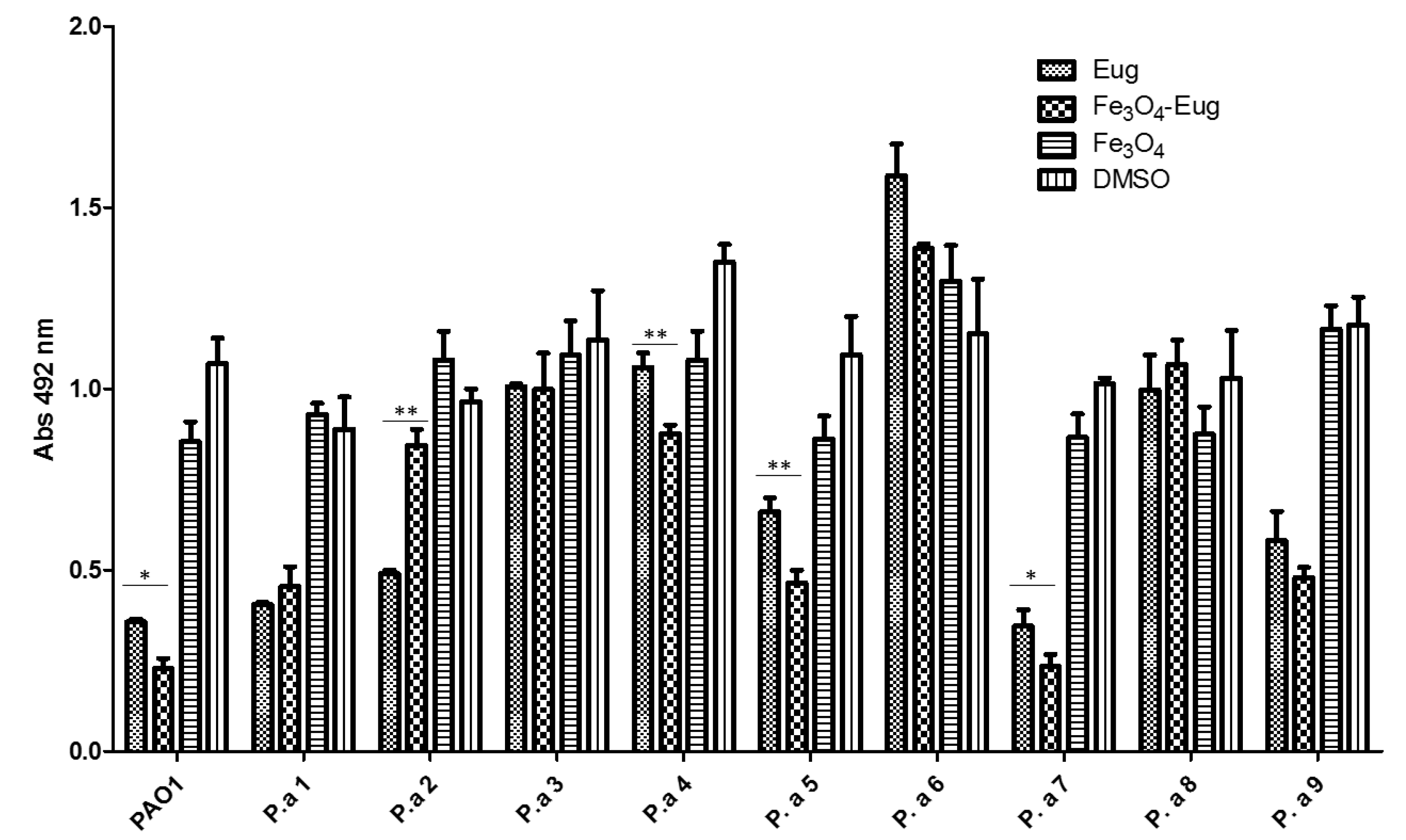
2.2.3. Evaluation of the Soluble Virulence Factors Production
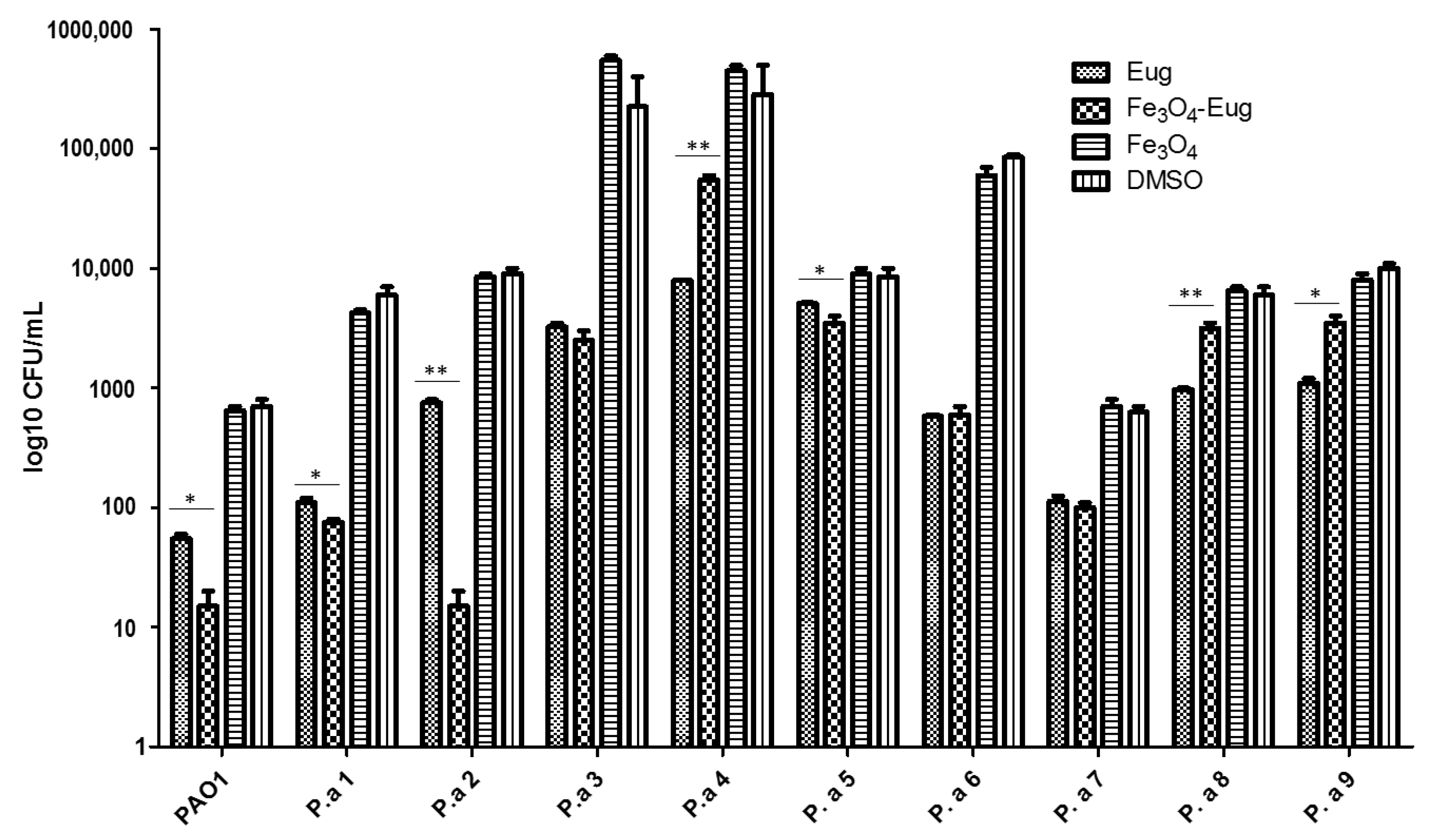
2.2.4. Influence of the Obtained Nanosystems on the Selection Rate of Persister Cells by Ciprofloxacin
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Physico-Chemical Characterization
4.2.1. TEM and SAED
4.2.2. X-ray Diffraction (XRD)
4.2.3. The Thermal Analysis TG-DSC
4.2.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
4.3. Antimicrobial Activity Testing
4.3.1. Bacterial Strains
4.3.2. Antimicrobial Qualitative Assessment
4.3.3. The MIC (Minimum Inhibitory Concentration) Assay
4.3.4. Evaluation of Soluble Virulence Factors Expression
4.3.5. Evaluation of Persister Cell Selection after Antibiotic Pressure in Planktonic Cultures and Biofilms
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death#:~:text=The%20top%20global%20causes%20of,birth%20asphyxia%20and%20birth%20trauma%2C (accessed on 6 January 2021).
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-Resistant Pseudomonas aeruginosa: Clinical Impact and Complex Regulation of Chromosomally Encoded Resistance Mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Jaiswal, S.; Sodhi, K.K.; Shree, P.; Singh, D.K.; Agrawal, P.K.; Shukla, P. Antibiotics bioremediation: Perspectives on its ecotoxicity and resistance. Environ. Int. 2019, 124, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob Resist Infect Control 2019, 8, 118. [Google Scholar] [CrossRef]
- Savoia, D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Saleem, M.; Nazir, M.; Ali, M.S.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: An update on future antibiotic drug candidates. Nat. Prod. Rep. 2010, 27, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A., Jr.; Ikryannikova, L.N. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef]
- Anghel, A.G.; Grumezescu, A.M.; Chirea, M.; Grumezescu, V.; Socol, G.; Iordache, F.; Oprea, A.E.; Anghel, I.; Holban, A.M. (APLE fabricated Fe3O4@Cinnamomum verum antimicrobial surfaces for improved gastrostomy tubes. Molecules 2014, 19, 8981–8994. [Google Scholar] [CrossRef]
- Holban, A.M.; Grumezescu, A.M.; Ficai, A.; Chifiriuc, M.C.; Lazar, V.; Radulescu, R. Fe3O4@C18- carvone to prevent Candida tropicalis biofilm development. Rom. J. Mat. 2013, 43, 300–305. [Google Scholar]
- Saviuc, C.; Cotar, A.I.; Holban, A.M.; Banu, O.; Grumezescu, A.M.; Chifiriuc, M.C. Phenotypic and molecular evaluation of Pseudomonas aeruginosa and Staphylococcus aureus virulence patterns in the presence of some essential oils and their major compounds. Lett. Appl. NanobioSci. 2013, 2, 91–96. [Google Scholar]
- Wagle, B.R.; Upadhyay, A.; Upadhyaya, I.; Shrestha, S.; Arsi, K.; Liyanage, R.; Venkitanarayanan, K.; Donoghue, D.J.; Donoghue, A.M. Trans-Cinnamaldehyde, Eugenol and Carvacrol Reduce Campylobacter jejuni Biofilms and Modulate Expression of Select Genes and Proteins. Front. Microbiol. 2019, 10, 1837. [Google Scholar] [CrossRef]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Khan, A.A.; Ahmed, I.; Musaddiq, M.; Ahmed, K.S.; Polasa, H.; Rao, L.V.; Habibullah, C.M.; Sechi, L.A.; Ahmed, N. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.; Grumezescu, A.M.; Holban, A.M. Magnetite nanostructures as novel strategies for anti-infectious therapy. Molecules 2014, 19, 12710–12726. [Google Scholar] [CrossRef] [PubMed]
- Rajendrachari, S.; Ceylan, K.B. The activation energy and antibacterial investigation of spherical Fe3O4 nanoparticles prepared by Crocus sativus (Saffron) flowers. Biointerface Res. Appl. Chem. 2020, 10, 5951–5959. [Google Scholar]
- Elazab, H.A.; El-Idreesy, T.T. Optimization of the catalytic performance of Pd/Fe3O4 nanoparticles prepared via microwave-assisted synthesis for pharmaceutical and catalysis applications. Biointerface Res. Appl. Chem. 2019, 9, 3794–3799. [Google Scholar]
- Mukherjee, S.; Liang, L.; Veiseh, O. Recent Advancements of Magnetic Nanomaterials in Cancer Therapy. Pharmaceutics 2020, 12, 147. [Google Scholar] [CrossRef]
- Hurley, J.C. Incidences of Pseudomonas aeruginosa-Associated Ventilator-Associated Pneumonia within Studies of Respiratory Tract Applications of Polymyxin: Testing the Stoutenbeek Concurrency Postulates. Antimicrob. Agents Chemother. 2018, 62, e00291-18. [Google Scholar] [CrossRef]
- Dolan, S.K. Current Knowledge and Future Directions in Developing Strategies to Combat Pseudomonas aeruginosa Infection. J. Mol. Biol. 2020, 432, 5509–5528. [Google Scholar] [CrossRef]
- López-de-la-Cruz, J.; Pérez-Aranda, M.; Alcudia, A.; Begines, B.; Caraballo, T.; Pajuelo, E.; Ginel, P.D. Dynamics and numerical simulations to predict empirical antibiotic treatment of multi-resistant Pseudomonas aeruginosa infection. Commun. Nonlinear Sci. Numer. Simulatio 2020, 91, 105418. [Google Scholar] [CrossRef]
- Todaka, Y.; Nakamura, M.; Hattor, S.; Tsuchiya, K.; Umemoto, M. Synthesis of Ferrite Nanoparticles by Mechanochemical Processing Using a Ball Mill. Mater. Trans. 2003, 44, 277–284. [Google Scholar] [CrossRef][Green Version]
- Grumezescu, A.M.; Gestal, M.C.; Holban, A.M.; Grumezescu, V.; Vasile, B.S.; Mogoantă, L.; Iordache, F.; Bleotu, C.; Mogoșanu, G.D. Biocompatible Fe3O4 Increases the Efficacy of Amoxicillin Delivery against Gram-Positive and Gram-Negative Bacteria. Molecules 2014, 19, 5013–5027. [Google Scholar] [CrossRef] [PubMed]
- Bridget, G.; Grzegorz, G.; Charlotte, M.; Sophie, H. Bacterial Persisters and Infection: Past, Present, and Progressing. Annu. Rev. Microbiol. 2019, 73, 359–385. [Google Scholar]
- Wang, G.; Chang, Y.; Wang, L.; Wei, Z.; Kang, J.; Sang, L.; Dong, X.; Chen, G.; Wang, H.; Qi, M. Preparation and characterization of PVPI-coated Fe3O4 nanoparticles as an MRI contrast agent. J. Magn. Magn. Mater. 2013, 340, 57–60. [Google Scholar] [CrossRef]
- Georgescu, M.; Gheorghe, I.; Curutiu, C.; Lazar, V.; Bleotu, C.; Chifiriuc, M.C. Virulence and resistance features of Pseudomonas aeruginosa strains isolated from chronic leg ulcers. BMC Infect. Dis. 2016, 16 (Suppl. 1), 92. [Google Scholar] [CrossRef]
- Holban, A.M.; Chifiriuc, M.C.; Cotar, A.I.; Bleotu, C.; Grumezescu, A.M.; Banu, O.; Lazar, V. Virulence markers in Pseudomonas aeruginosa isolates from hospital acquired infections occurred in patients with underlying cardiovascular disease. Rom. Biotechnol. Lett. 2013, 18, 8843–8854. [Google Scholar]
- Grassi, L.; Di Luca, M.; Maisetta, G.; Rinaldi, A.C.; Esin, S.; Trampuz, A.; Batoni, G. Generation of Persister Cells of Pseudomonas aeruginosa and Staphylococcus aureus by Chemical Treatment and Evaluation of Their Susceptibility to Membrane-Targeting Agents. Front. Microbiol. 2017, 8, 1917. [Google Scholar] [CrossRef]
- Pircalabioru, G.G.; Chifiriuc, M.C. Nanoparticulate drug-delivery systems for fighting microbial biofilms: From bench to bedside. Future Microbiol. 2020, 15, 679–698. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Satishchandra., B.O.; Thirumalai, V.V.; Mark, G.B. Functional Metal Oxides: New Science and Novel Applications, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; ISBN-13 978-3527331796. [Google Scholar]
- Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. Control of biofilm-associated infections by signaling molecules and nanoparticles. Int. J. Pharm. 2016, 10, 409–418. [Google Scholar] [CrossRef]
- Pinheiro, P.F.; Menini, L.A.P.; Bernardes, P.C.; Saraiva, S.H.; Carneiro, J.W.M.; Costa, A.V.; Arruda, T.R.; Lage, M.R.; Gonçalves, P.M.; Bernardes, C.O.; et al. Semisynthetic Phenol Derivatives Obtained from Natural Phenols: Antimicrobial Activity and Molecular Properties. J. Agric. Food Chem. 2018, 66, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Hemaiswarya, S.; Doble, M. Synergistic interaction of eugenol with antibiotics against Gram negative bacteria. Phytomedicine 2009, 16, 997–1005. [Google Scholar] [CrossRef]
- Rathinam, P.; Vijay Kumar, H.S.; Viswanathan, P. Eugenol exhibits anti-virulence properties by competitively binding to quorum sensing receptors. Biofouling 2017, 33, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Letsididi, K.S.; Yu, F.; Pei, Z.; Wang, H.; Letsididi, R. Inhibitive Effect of Eugenol and Its Nanoemulsion on Quorum Sensing-Mediated Virulence Factors and Biofilm Formation by Pseudomonas aeruginosa. J. Food Prot. 2019, 82, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Dubnau, D.; Losick, R. Bistability in bacteria. Mol. Microbiol. 2006, 61, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Veening, J.W.; Smits, W.K.; Kuipers, O.P. Bistability, epigenetics, and bet-hedging in bacteria. Annu. Rev. Microbiol. 2008, 62, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, N.; Saar Dover, R.; Titon, E.; Shai, Y.; Rom-Kedar, V. Bistable Bacterial Growth Dynamics in the Presence of Antimicrobial Agents. Antibiotics 2021, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z. Persisters, persistent infections and the Yin–Yang model. Emerg. Microbes Infect. 2014, 3, e3. [Google Scholar]
- Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. New molecular strategies for reducing implantable medical devices associated infections. Curr. Med. Chem. 2014, 21, 3375–3382. [Google Scholar] [CrossRef]
- Malka, R.; Shochat, E.; Rom-Kedar, V. Bistability and Bacterial Infections. PLoS ONE 2010, 5, e10010. [Google Scholar] [CrossRef]
- Baldry, M.; Bojer, M.S.; Najarzadeh, Z.; Vestergaard, M.; Meyer Rikke, L.; Otzen Daniel, E.; Ingmer, H. Phenol-Soluble Modulins Modulate Persister Cell Formation in Staphylococcus aureus. Front. Microbiol. 2020, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.R.; López-Abarrategui, C.; de la Serna Gómez, I.; Campos Dias, S.; Otero-González, A.J.; Franco, O.L. Antimicrobial magnetic nanoparticles based-therapies for controlling infectious diseases. Int. J. Pharm. 2019, 555, 356–367. [Google Scholar] [CrossRef]
- Lynch, S.V.; Wiener-Kronish, J.P. Novel strategies to combat bacterial virulence. Curr. Opin. Crit. Care 2008, 14, 593–599. [Google Scholar] [CrossRef]
- Xiong, W.; Sun, Y.; Ding, X.; Wang, M.; Zeng, Z. Selective pressure of antibiotics on ARGs and bacterial communities in manure-polluted freshwater-sediment microcosms. Front. Microbiol. 2015, 6, 194. [Google Scholar] [CrossRef]
- Klümper, U.; Recker, M.; Zhang, L.; Yin, X.; Zhang, T.; Buckling, A.; Gaze, W.H. Selection for antimicrobial resistance is reduced when embedded in a natural microbial community. ISME J. 2019, 13, 2927–2937. [Google Scholar] [CrossRef]
- Rasko, D.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Maia, N.L.; de Barros, M.; de Oliveira, L.L.; Cardoso, S.A.; Dos Santos, M.H.; Pieri, F.A.; Ramalho, T.C.; da Cunha, E.; Moreira, M. Synergism of Plant Compound With Traditional Antimicrobials Against Streptococcus spp. Isolated From Bovine Mastitis. Front. Microbiol. 2018, 9, 1203. [Google Scholar] [CrossRef] [PubMed]
- Akinyele, T.A.; Igbinosa, E.O.; Akinpelu, D.A.; Okoh, A.I. In vitro assessment of the synergism between extracts of Cocos nucifera husk and some standard antibiotics. Asian Pac. J. Trop. Biomed. 2017, 7, 306–313. [Google Scholar] [CrossRef]
- Krychowiak, M.; Kawiak, A.; Narajczy, M.; Borowi, A.; Królicka, A. Silver Nanoparticles Combined With Naphthoquinones as an Effective Synergistic Strategy Against Staphylococcus aureus. Front. Pharmacol. 2017, 9, 816. [Google Scholar] [CrossRef]
- Abo-Shama, U.H.; El-Gendy, H.; Mousa, W.S.; Hamouda, R.A.; Yousuf, W.E.; Hetta, H.F.; Abdeen, E.E. Synergistic and Antagonistic Effects of Metal Nanoparticles in Combination with Antibiotics Against Some Reference Strains of Pathogenic Microorganisms. Infect. Drug Resist. 2020, 13, 351–362. [Google Scholar] [CrossRef]
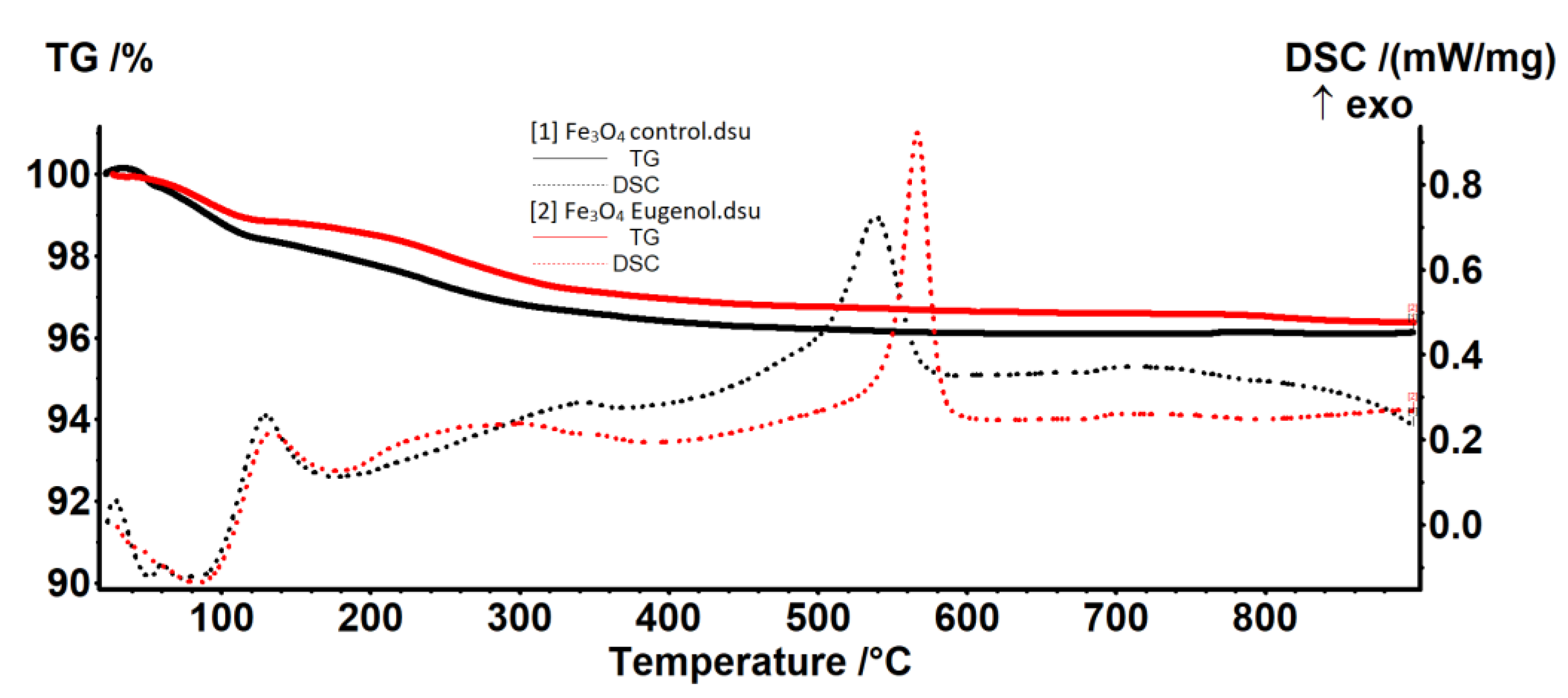


| Sample | RT-120 °C | 120–400 °C | 400–900 °C | Residual Mass % | Endo I Weak Bonded Molecules Elimination | Exo II Magnetite to Maghemite | Exo III Maghemite to Hematite |
|---|---|---|---|---|---|---|---|
| Fe3O4-control | 1.54% | 2.07% | 0.26% | 96.12% | 52.3/73.1 | 345.4 | 538.8 |
| Fe3O4-eugenol | 1.11% | 1.95% | 0.56% | 96.37% | 83.7 | 299.0 | 566.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, H.B.; Rayyif, S.M.I.; Curutiu, C.; Birca, A.C.; Oprea, O.-C.; Grumezescu, A.M.; Ditu, L.-M.; Gheorghe, I.; Chifiriuc, M.C.; Mihaescu, G.; et al. Eugenol-Functionalized Magnetite Nanoparticles Modulate Virulence and Persistence in Pseudomonas aeruginosa Clinical Strains. Molecules 2021, 26, 2189. https://doi.org/10.3390/molecules26082189
Mohammed HB, Rayyif SMI, Curutiu C, Birca AC, Oprea O-C, Grumezescu AM, Ditu L-M, Gheorghe I, Chifiriuc MC, Mihaescu G, et al. Eugenol-Functionalized Magnetite Nanoparticles Modulate Virulence and Persistence in Pseudomonas aeruginosa Clinical Strains. Molecules. 2021; 26(8):2189. https://doi.org/10.3390/molecules26082189
Chicago/Turabian StyleMohammed, Hamzah Basil, Sajjad Mohsin I. Rayyif, Carmen Curutiu, Alexandra Catalina Birca, Ovidiu-Cristian Oprea, Alexandru Mihai Grumezescu, Lia-Mara Ditu, Irina Gheorghe, Mariana Carmen Chifiriuc, Grigore Mihaescu, and et al. 2021. "Eugenol-Functionalized Magnetite Nanoparticles Modulate Virulence and Persistence in Pseudomonas aeruginosa Clinical Strains" Molecules 26, no. 8: 2189. https://doi.org/10.3390/molecules26082189
APA StyleMohammed, H. B., Rayyif, S. M. I., Curutiu, C., Birca, A. C., Oprea, O.-C., Grumezescu, A. M., Ditu, L.-M., Gheorghe, I., Chifiriuc, M. C., Mihaescu, G., & Holban, A.-M. (2021). Eugenol-Functionalized Magnetite Nanoparticles Modulate Virulence and Persistence in Pseudomonas aeruginosa Clinical Strains. Molecules, 26(8), 2189. https://doi.org/10.3390/molecules26082189












