Nanocomposite Polymer Electrolytes of Sodium Alginate and Montmorillonite Clay
Abstract
1. Introduction
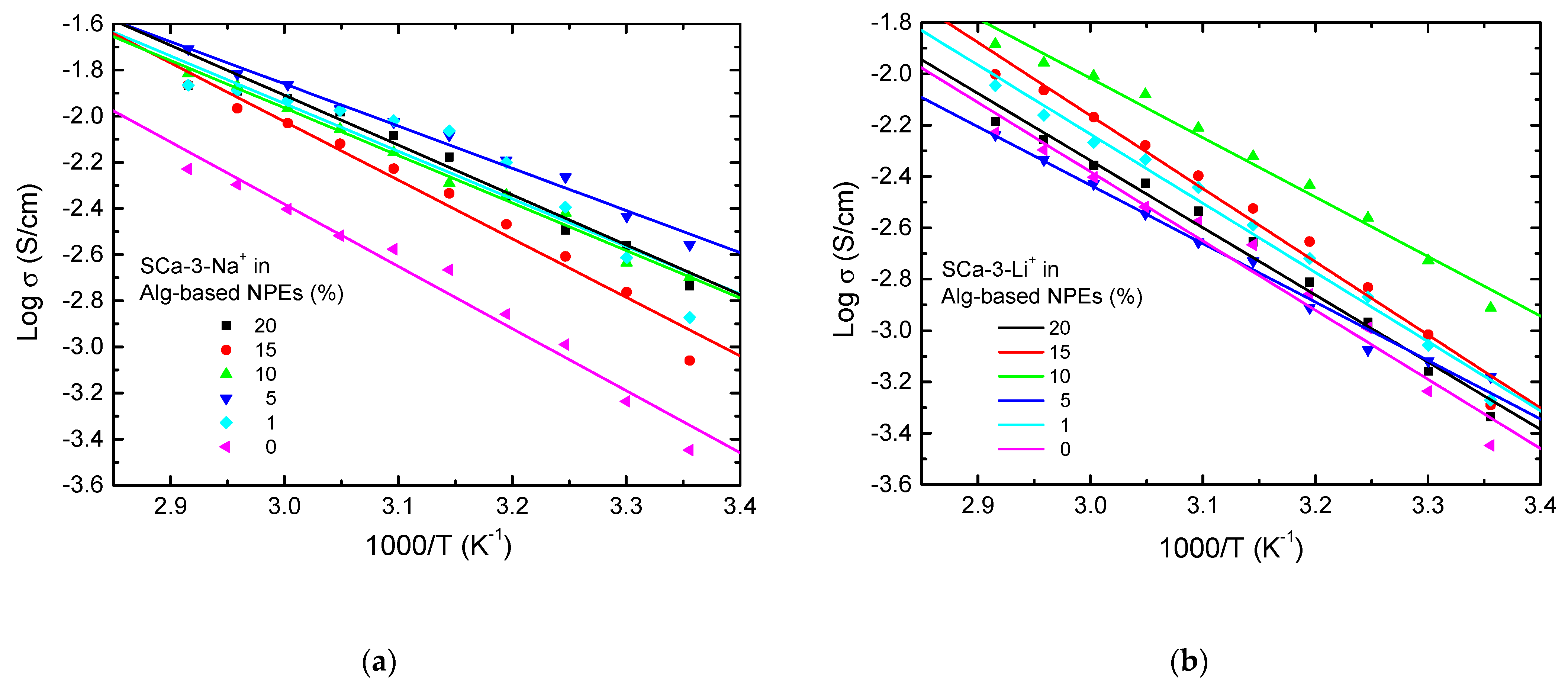
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gaaz, T.S.; Sulong, A.B.; Kadhum, A.A.H.; Al-Amiery, A.A.; Nassir, M.H.; Jaaz, A.H. The Impact of Halloysite on the Thermo-Mechanical Properties of Polymer Composites. Molecules 2017, 22, 838. [Google Scholar] [CrossRef]
- Lebedev, S.; Gefle, O.; Amitov, E.; Berchuk, D.Y.; Zhuravlev, D. Poly (lactic acid)-based polymer composites with high electric and thermal conductivity and their characterization. Polym. Test. 2017, 58, 241–248. [Google Scholar] [CrossRef]
- Thenepalli, T.; Jun, A.Y.; Han, C.; Ramakrishna, C.; Ahn, J.W. A strategy of precipitated calcium carbonate (CaCO 3) fillers for enhancing the mechanical properties of polypropylene polymers. Korean J. Chem Eng. 2015, 32, 1009–1022. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, C.-H.; Lin, C.-X.; Tong, D.-S.; Yu, W.-H. Synthesis of clay minerals. Appl. Clay Sci. 2010, 50, 1–11. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C. A review on polymer–layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Dhibar, S.; Kar, P.; Khatua, B. Preparation of highly exfoliated and transparent polycarbonate/clay nanocomposites by melt blending of polycarbonate and poly (methyl methacrylate)/clay nanocomposites. J. Appl. Polym. Sci. 2012, 125, E601–E609. [Google Scholar] [CrossRef]
- Chen, G.; Liu, S.; Chen, S.; Qi, Z. FTIR spectra, thermal properties, and dispersibility of a polystyrene/montmorillonite nanocomposite. Macromol. Chem. Phys. 2001, 202, 1189–1193. [Google Scholar] [CrossRef]
- Lu, J.; Wool, R.P. Additive toughening effects on new bio-based thermosetting resins from plant oils. Compos. Sci. Technol. 2008, 68, 1025–1033. [Google Scholar] [CrossRef]
- Yussuf, A.; Al-Saleh, M.; Al-Samhan, M.; Al-Enezi, S.; Al-Banna, A.; Abraham, G. Investigation of polypropylene-montmorillonite clay nanocomposite films containing a pro-degradant additive. J. Polym. Environ. 2018, 26, 275–290. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Wen, C.K.; Chuang, H.J.; Lin, M.J.; Ray, U. Effect of clay with different cation exchange capacity on the morphology and properties of poly (methyl methacrylate)/clay nanocomposites. Polym. Compos. 2009, 30, 1552–1561. [Google Scholar] [CrossRef]
- Oliveira, C.; Carastan, D.; Demarquette, N.; Fechine, G. Photooxidative behavior of polystyrene–montmorillonite nanocomposites. Polym. Eng. Sci. 2008, 48, 1511–1517. [Google Scholar] [CrossRef]
- Dyartanti, E.R.; Purwanto, A.; Widiasa, I.N.; Susanto, H. Ionic Conductivity and Cycling Stability Improvement of PVDF/Nano-Clay Using PVP as Polymer Electrolyte Membranes for LiFePO4 Batteries. Membranes 2018, 8, 36. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. Surface modification of clay minerals. Appl. Clay Sci. 2001, 1, 1–3. [Google Scholar] [CrossRef]
- Kozak, M.; Domka, L. Adsorption of the quaternary ammonium salts on montmorillonite. J. Phys. Chem. Solids 2004, 65, 441–445. [Google Scholar] [CrossRef]
- Iwaki, Y.; Escalona, M.H.; Briones, J.; Pawlicka, A. Sodium alginate-based ionic conducting membranes. Mol. Cryst. Liq. Cryst. 2012, 554, 221–231. [Google Scholar] [CrossRef]
- Ponez, L.; Sentanin, F.; Majid, S.R.; Arof, A.K.; Pawlicka, A. Ion-Conducting Membranes Based on Gelatin and Containing LiI/I2 for Electrochromic Devices. Mol. Cryst. Liq. Cryst. 2012, 554, 239–251. [Google Scholar] [CrossRef]
- Leones, R.; Sabadini, R.C.; Sentanin, F.C.; Esperança, J.M.S.S.; Pawlicka, A.; Silva, M.M. Polymer electrolytes for electrochromic devices through solvent casting and sol-gel routes. Sol. Energ. Mat. Sol. Cells 2017, 169, 98–106. [Google Scholar] [CrossRef]
- Lu, W.; Fadeev, A.G.; Qi, B.; Smela, E.; Mattes, B.R.; Ding, J.; Spinks, G.M.; Mazurkiewicz, J.; Zhou, D.; Wallace, G.G. Use of ionic liquids for π-conjugated polymer electrochemical devices. Science 2002, 297, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Wungu, T.D.K.; Rusydi, F.; Dipojono, H.K.; Kasai, H. A density functional theory study on the origin of lithium-montmorillonite’s conductivity at low water content: A first investigation. Solid State Commun. 2012, 152, 1862–1866. [Google Scholar] [CrossRef]
- Sentanin, F.; Sabadini, R.; Barros, S.; Caliman, W.; Cavalheiro, C.; Kanicki, J.; Donoso, J.P.; Magon, C.J.; Silva, I.; Silva, M. Study of ionically conducting nanocomposites for reflective electrochromic devices. Electrochim. Acta 2019, 301, 174–182. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Kehoe, T.; Latorre, M.; Serrano, C.; Philippe, S.; Schmid, M. Recent prospects in the inline monitoring of nanocomposites and nanocoatings by optical technologies. Nanomaterials 2016, 6, 150. [Google Scholar] [CrossRef] [PubMed]
- Aprilliza, M. Characterization and properties of sodium alginate from brown algae used as an ecofriendly superabsorbent. IOP Publ. 2017, 188, 012019. [Google Scholar]
- Li, Y.; Zhao, B.; Xie, S.; Zhang, S. Synthesis and properties of poly (methyl methacrylate)/montmorillonite (PMMA/MMT) nanocomposites. Polym. Intern. 2003, 52, 892–898. [Google Scholar] [CrossRef]
- Kazim, S.; Ahmad, S.; Pfleger, J.; Plestil, J.; Joshi, Y.M. Polyaniline–sodium montmorillonite clay nanocomposites: Effect of clay concentration on thermal, structural, and electrical properties. J. Mater. Sci. 2012, 47, 420–428. [Google Scholar] [CrossRef]
- Sakugawa, K.; Ikeda, A.; Takemura, A.; Ono, H. Simplified method for estimation of composition of alginates by FTIR. J. Appl. Polym. Sci. 2004, 93, 1372–1377. [Google Scholar] [CrossRef]
- Pathak, T.S.; San Kim, J.; Lee, S.-J.; Baek, D.-J.; Paeng, K.-J. Preparation of alginic acid and metal alginate from algae and their comparative study. J. Polym. Environ. 2008, 16, 198–204. [Google Scholar] [CrossRef]
- Pawlicka, A. Development of electrochromic devices. Recent Pat. Nanotech. 2009, 3, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, I.; Lüdtke, A.; Tavares, F.; Cholant, C.; Balboni, R.; Flores, W.H.; Galio, A.; Pawlicka, A.; Avellaneda, C.O. Ecologically friendly xanthan gum-PVA matrix for solid polymeric electrolytes. Ionics 2018, 24, 413–420. [Google Scholar] [CrossRef]
- Naderi-Samani, H.; Loghman-Estarki, M.; Razavi, R.S.; Ramazani, M. The Effects of organoclay on the morphology, thermal stability, transparence and hydrophobicity properties of polyamide− imide/nanoclay nanocomposite coatings. Prog. Org. Coat. 2017, 112, 162–168. [Google Scholar] [CrossRef]
- Pawlicka, A.; Sentanin, F.; Firmino, A.; Grote, J.G.; Kajzar, F.; Rau, I. Ionically conducting DNA-based membranes for eletrochromic devices. Synth. Met. 2011, 161, 2329–2334. [Google Scholar] [CrossRef]
- Pawlicka, A.; Vieira, D.; Sabadini, R.C. Gelatin-HCl biomembranes with ionic-conducting properties. Ionics 2013, 19, 1723–1731. [Google Scholar] [CrossRef]
- Mattos, R.I.; Pawlicka, A.; Lima, J.F.; Tambelli, C.E.; Magon, C.J.; Donoso, J.P. Magnetic resonance and conductivity study of gelatin-based proton conductor polymer electrolytes. Electrochim. Acta 2010, 55, 1396–1400. [Google Scholar] [CrossRef]
- Alves, R.; Sentanin, F.; Sabadini, R.C.; Pawlicka, A.; Silva, M.M. Solid polymer electrolytes based on chitosan and Dy(CF3SO3)3 for electrochromic devices. Solid State Ion. 2017, 310, 112–120. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, E.-J.; Jung, Y.; Han, M.; Park, S.-J. Ionic conductivity of polymeric nanocomposite electrolytes based on poly (ethylene oxide) and organo-clay materials. Colloid Surf. A 2008, 313, 216–219. [Google Scholar] [CrossRef]
- Sheela, T.; Bhajantri, R.; Nambissan, P.; Ravindrachary, V.; Lobo, B.; Naik, J.; Rathod, S.G. Ionic conductivity and free volume related microstructural properties of LiClO4/PVA/NaAlg polymer composites: Positron annihilation spectroscopic studies. J. Non-Cryst. Solids 2016, 454, 19–30. [Google Scholar] [CrossRef]
- Meneghetti, P.; Qutubuddin, S. Synthesis, thermal properties and applications of polymer-clay nanocomposites. Thermochim. Acta 2006, 442, 74–77. [Google Scholar] [CrossRef]
- Pawlicka, A.; Danczuk, M.; Wieczorek, W.; Zygadlo-Monikowska, E. Influence of plasticizer type on the properties of polymer electrolytes based on chitosan. J. Phys. Chem A 2008, 112, 8888–8895. [Google Scholar] [CrossRef]
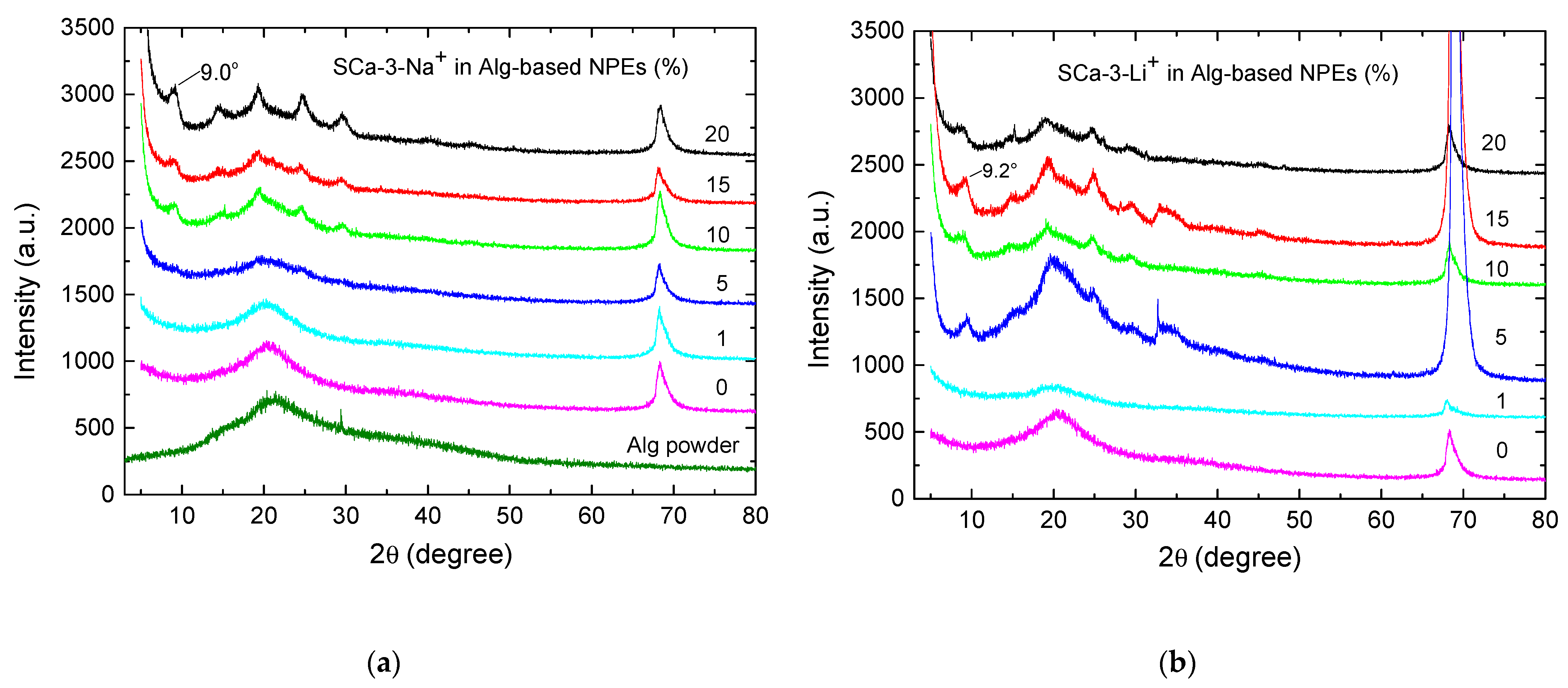
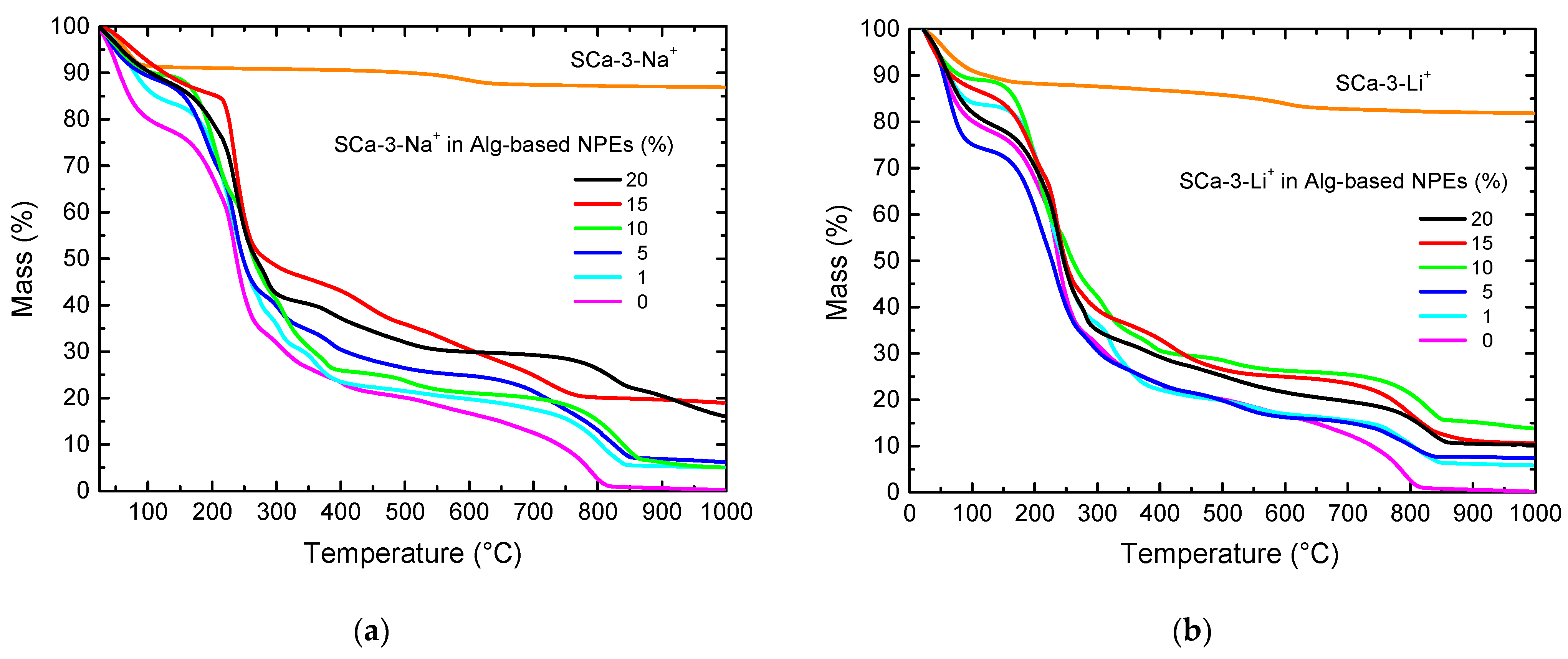
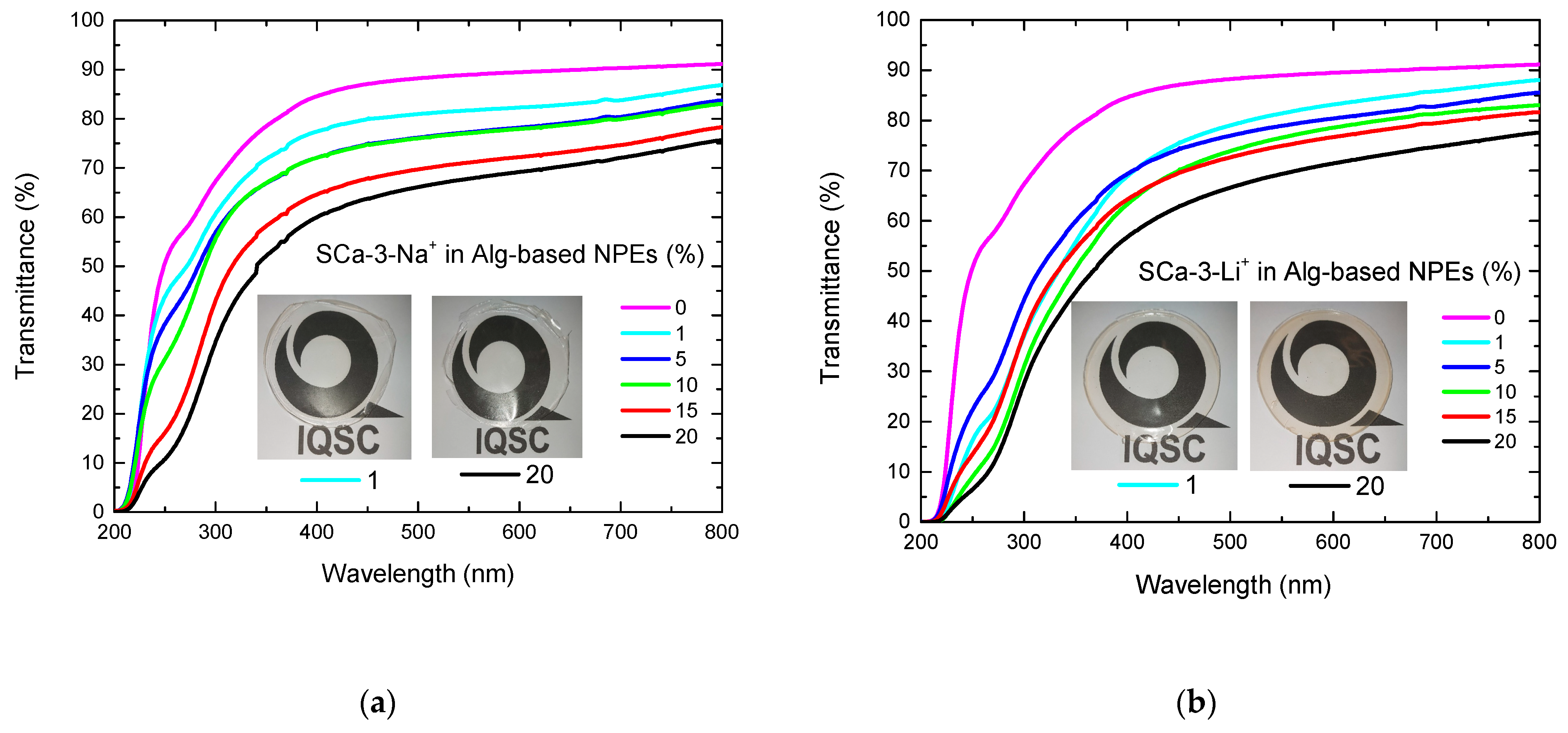
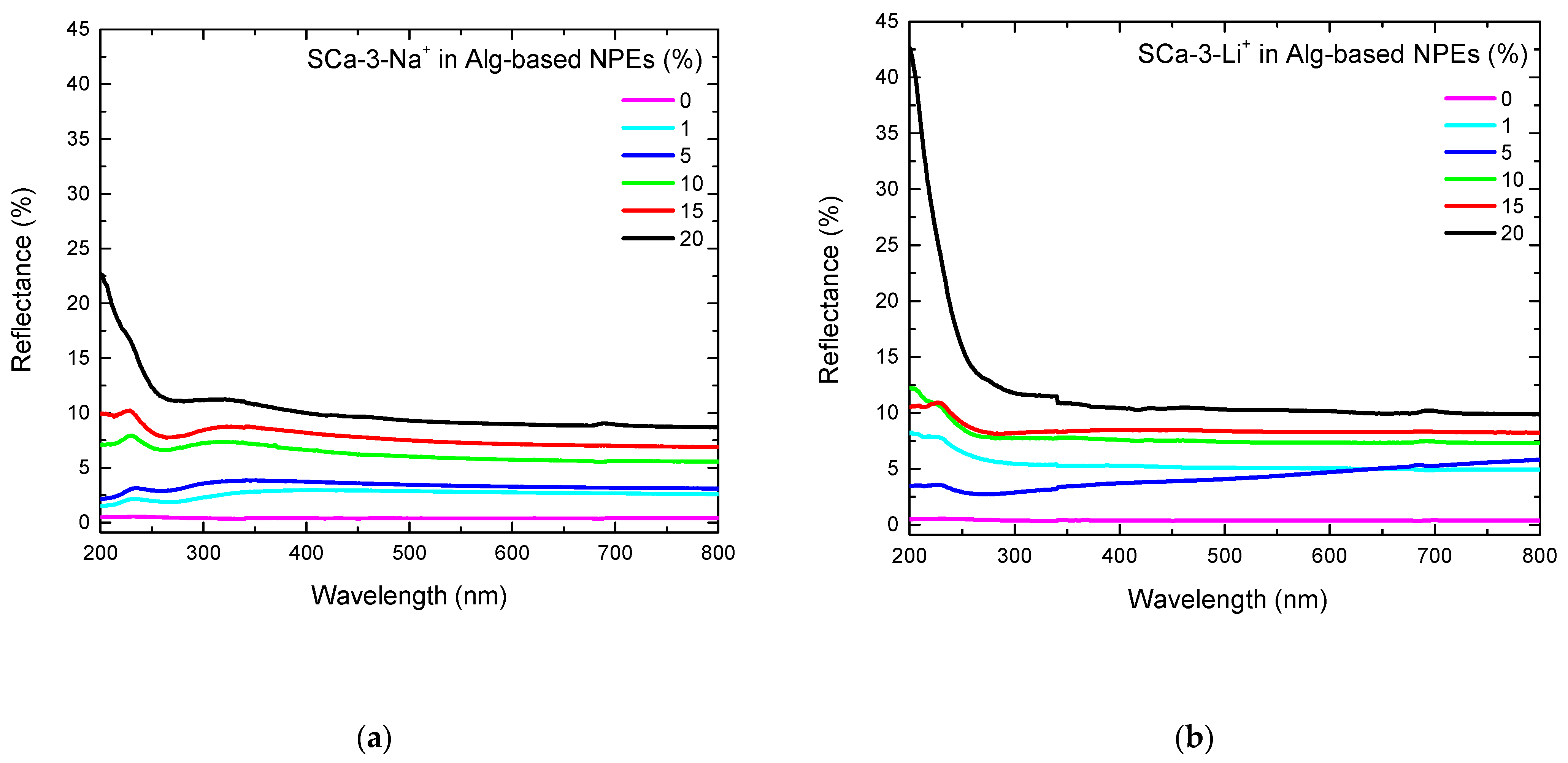
| Clay (wt%) | Clay Mass (g) Dissolved in 20 mL of H2O | Alg-Based NPE with Clay Thickness (mm) | |
|---|---|---|---|
| SCa-3-Na+ | SCa-3-Li+ | ||
| 0 | 0.000 | 0.04 | 0.04 |
| 1 | 0.006 | 0.05 | 0.09 |
| 5 | 0.030 | 0.06 | 0.08 |
| 10 | 0.060 | 0.06 | 0.05 |
| 15 | 0.090 | 0.07 | 0.06 |
| 20 | 0.120 | 0.10 | 0.08 |
| Alg-Based NPE with Clay (wt%) | σ (S/cm2) at 25 °C | σ (S/cm2) at 70 °C | Ea (eV) | |
|---|---|---|---|---|
| SCa-3-Na+ | 0 | 3.57 × 10−4 | 5.90 × 10−3 | 0.23 |
| 1 | 1.34 × 10−3 | 1.36 × 10−2 | 0.19 | |
| 5 | 2.77 × 10−3 | 1.96 × 10−2 | 0.16 | |
| 10 | 2.00 × 10−3 | 1.53 × 10−2 | 0.18 | |
| 15 | 8.73 × 10−4 | 1.36 × 10−2 | 0.22 | |
| 20 | 1.84 × 10−3 | 1.36 × 10−2 | 0.18 | |
| SCa-3-Li+ | 1 | 5.36 × 10−4 | 9.02 × 10−3 | 0.23 |
| 5 | 6.61 × 10−4 | 5.80 × 10−3 | 0.19 | |
| 10 | 1.22 × 10−3 | 1.30 × 10−2 | 0.23 | |
| 15 | 5.13 × 10−4 | 1.00 × 10−2 | 0.24 | |
| 20 | 4.62 × 10−4 | 6.52 × 10−3 | 0.22 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sentanin, F.C.; Caliman, W.R.; Sabadini, R.C.; Cavalheiro, C.C.S.; Pereira, R.F.P.; Silva, M.M.; Pawlicka, A. Nanocomposite Polymer Electrolytes of Sodium Alginate and Montmorillonite Clay. Molecules 2021, 26, 2139. https://doi.org/10.3390/molecules26082139
Sentanin FC, Caliman WR, Sabadini RC, Cavalheiro CCS, Pereira RFP, Silva MM, Pawlicka A. Nanocomposite Polymer Electrolytes of Sodium Alginate and Montmorillonite Clay. Molecules. 2021; 26(8):2139. https://doi.org/10.3390/molecules26082139
Chicago/Turabian StyleSentanin, Franciani C., Willian R. Caliman, Rodrigo C. Sabadini, Carla C. S. Cavalheiro, Rui F. P. Pereira, Maria M. Silva, and Agnieszka Pawlicka. 2021. "Nanocomposite Polymer Electrolytes of Sodium Alginate and Montmorillonite Clay" Molecules 26, no. 8: 2139. https://doi.org/10.3390/molecules26082139
APA StyleSentanin, F. C., Caliman, W. R., Sabadini, R. C., Cavalheiro, C. C. S., Pereira, R. F. P., Silva, M. M., & Pawlicka, A. (2021). Nanocomposite Polymer Electrolytes of Sodium Alginate and Montmorillonite Clay. Molecules, 26(8), 2139. https://doi.org/10.3390/molecules26082139








