Dynamics of Isomeric and Enantiomeric Fractions of Pinene in Essential Oil of Picea abies Annual Needles during Growing Season
Abstract
1. Introduction
| Species | α-Pinene, % | β-Pinene, % | Reference |
|---|---|---|---|
| Picea abies | 27.7 | 5.4 | [31] |
| Picea abies | 9 | 2 | [32] |
| Picea abies | 14.16 | 4.75 | [2] |
| Picea engelmanni | 5.77 | 0.87 | [2] |
| Picea glauca | 15.85 | – | [33] |
| Picea glauca | 5.2 | 1.0 | [29] |
| Picea mariana | 16.62 | – | [33] |
| Picea omorika | 3.8 a, 11.0 b, 5.1 c | 0.7 a, 0.9 b, 0.6 c | [34] |
| Picea pungens | 4.06 | – | [29] |
| Picea pungens | 7.3 | 1.1 | [30] |
| Picea rubens | 10.36 | 3.63 | [29] |
2. Results
2.1. Dynamics of Air-Dry Mass Yield and Quantitative Composition of Essential Oil in Annual Needles during the Growing Season
2.2. Dynamics of Percentages of Pinene Isomers in Annual Needles during the Growing Season
2.3. Dynamics of Percentages of Pinene Enantiomers in Annual Needles during the Growing Season
2.4. Correlations with Weather Conditions
3. Discussion
4. Materials and Methods
4.1. Plant Material and Habitats
4.2. Isolation and Analysis of Essential Oils
4.3. Meteorological Data
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caudullo, G.; Tinner, W.; de Rigo, D. Picea abies in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Durrant, T.H., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Kubeczka, K.-H.; Schultze, W. Biology and chemistry of conifer oils. Flavour Fragr. J. 1987, 2, 137–148. [Google Scholar] [CrossRef]
- Wajs, A.; Pranovich, A.; Reunanen, M.; Willför, S.; Holmbom, B. Characterisation of volatile organic compounds in stemwood using solid-phase microextraction. Phytochem. Anal. 2006, 17, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Wajs, A.; Pranovich, A.; Reunanen, M.; Willför, S.; Holmbom, B. Headspace-SPME analysis of the sapwood and heartwood of Picea abies, Pinus sylvestris and Larix decidua. J. Essent. Oil Res. 2007, 19, 125–133. [Google Scholar] [CrossRef]
- Salem, M.; Zeidler, A.; Böhm, M.; Mohamed, M.E.A.; Ali, H.M. GC/MS analysis of oil extractives from wood and bark of Pinus sylvestris, Abies alba, Picea abies, and Larix decidua. BioResources 2015, 10, 7725–7737. [Google Scholar] [CrossRef]
- Zule, J.; Tišler, V.; Žurej, A.; Torelli, N. Isolation and characterization of essential oils from the cones of Norway spruce (Picea abies Karst.), European larch (Larix decidua Mill.) and Scots pine (Pinus sylvestris L.). Zbornik Gozdarstva Lesar. 2003, 71, 159–172. [Google Scholar]
- Holubova, V.; Hrdlička, P.; Kuban, V. Age and Space Distribution of Monoterpenes in Fresh Needles of Picea abies (L.) Karst. Determined by Gas Chromatography-Mass Spectrometry. Phytochem. Anal. 2001, 12, 243. [Google Scholar] [CrossRef]
- Merk, L.; Kloos, M.; Schonwitz, R.; Ziegler, H. Influence of various factors on quantitative composition of leaf monoterpenes of Picea abies (L.) Karst. Trees 1988, 2, 45–51. [Google Scholar] [CrossRef]
- Radulescu, V.; Saviuc, C.; Chifiriuc, C.; Oprea, E.; Ilies, D.C.; Marutescu, L.; Lazar, V. Chemical Composition and Antimicrobial Activity of Essential Oil from Shoots Spruce (Picea abies L.). Rev. Chim. 2011, 62, 69–74. [Google Scholar]
- Schönwitz, R.; Merk, L.; Ziegler, H. Variability in the Monoterpenes of Needles of Picea abies (L.) Karst. Flavour Fragr. J. 1989, 4, 149–153. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Review. Nat. Chem. Biol. 2007, 3, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications; Taylor & Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- Rivas Da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Kovac, J.; Simunovic, K.; Wu, Z.; Klancnik, A.; Bucar, F.; Zhang, Q.; Mozina, S.S. Antibiotic resistance modulation and modes of action of (-)-alpha-pinene in Campylobacter jejuni. PLoS ONE 2015, 10, e0122871. [Google Scholar] [CrossRef] [PubMed]
- Elanur, A.; Hasan, T.; Fatime, G. Antioxidative, anticancer and genotoxic properties of α-pinene on N2a neuroblastoma cells. Biologia 2013, 68, 1004–1009. [Google Scholar]
- Zhang, Z.; Guo, S.; Liu, X.; Gao, X. Synergistic antitumor effect of alpha-pinene and beta-pinene with paclitaxel against non-small-cell lung carcinoma (NSCLC). Drug Res. 2015, 65, 214–218. [Google Scholar]
- van Zyl, R.L.; Seatlholo, S.T.; van Vuuren, S.F. The biological activities of 20 nature identical essential oil constituents. J. Essent. Oil. Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Castells, A.A. The Role of Terpenes in the Defensive Responses of Conifers against Herbivores and Pathogens. Ph.D. Thesis, Universitat Autonoma de Barcelona, Cerdanyola del Vallès, Spain, 2015. [Google Scholar]
- Phillips, M.A.; Wildung, M.R.; Williams, D.C.; Hyatt, D.C.; Croteau, R. cDNA isolation, functional expression, and characterization of (+)-α-pinene synthase and (-)-α-pinene synthase from loblolly pine (Pinus taeda): Stereocontrol in pinene biosynthesis. Arch. Biochem. Biophys. 2003, 411, 267–276. [Google Scholar] [CrossRef]
- Borg-Karlson, A.-K.; Lindstrom, M.; Norin, T.; Persson, M.; Valterova, I. Enantiomeric Composition of Monoterpene Hydrocarbons in Different Tissues of Norway Spruce, Picea abies (L.) Karst. A Multi-dimensional Gas Chromatography Study. Acta Chem. Scand. 1993, 47, 138–144. [Google Scholar] [CrossRef]
- Persson, M.; Borg-Karlson, A.-K.; Norin, T. Enantiomeric composition of six chiral monoterpene hydrocarbons in different tissues of Pica abies. Phytochemistry 1993, 33, 303–307. [Google Scholar] [CrossRef]
- Zhao, T.; Krokene, P.; Bjorklung, N.; Langstrom, B.; Solheim, H.; Christiansen, E.; Borg-Karlson, A.-K. The influence of Ceratocystis polonica inoculation and methyl jasmonate application on terpene chemistry of Norway spruce, Picea abies. Phytochemistry 2010, 71, 1332–1341. [Google Scholar] [CrossRef]
- Lindström, M.; Norin, T.; Birgersson, G.; Schlyter, F. Variation of enantiomeric composition of α-pinene in norway spruce, Picea abies, and its influence on production of verbenol isomers by Ips typographus in the field. J. Chem. Ecol. 1989, 15, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Wibe, A.; Borg-Karlson, A.-K.; Persson, M.; Norin, T.; Mustaparta, H. Enantiomeric composition of monoterpenes hydrocarbons in some conifers and receptor neuron discrimination of α-pinene and limonene enantiomers in the pine weevil. J. Chem. Ecol. 1998, 24, 273–287. [Google Scholar] [CrossRef]
- Lundborg, L.; Nordlander, G.; Björklund, N.; Nordenhem, H.; Borg-Karlson, A.-K. Methyl jasmonate-induced monoterpenes in Scots pine and Norway spruce tissues affect pine weevil orientation. J. Chem. Ecol. 2016, 42, 1237–1246. [Google Scholar] [CrossRef]
- Global Invasive Species Database. Species Profile: Tomicus piniperda. Available online: http://www.iucngisd.org/gisd/species.php?sc=1200 (accessed on 22 February 2021).
- Almquist, A.C.; Faldt, J.; Yart, A.; Chevet, Y.; Sauvard, D.; Lieutier, F.; Borg-Karlson, A.K. Host selection in Tomicus piniperda L.: Composition of monoterpene hydrocarbons in relation to attack frequency in shoot feeding phase. Z. Nat. C 2006, 61, 439–444. [Google Scholar] [CrossRef]
- von Rudloff, E. Seasonal variation in the composition of the volatile oil of the leaves, buds, and twigs of white spruce (Picea glauca). Can. J. Bot. 1972, 50, 1595–1603. [Google Scholar] [CrossRef]
- von Rudloff, E. Seasonal variation of the terpenes of the leaves, buds, and twigs of blue spruce (Picea pungens). Can. J. Bot. 1975, 53, 2978–2982. [Google Scholar] [CrossRef]
- Prieme, A.; Knudsen, T.B.; Glasius, M.; Christensen, S. Herbivory by the weevil, Strophosoma melanogrammum, causes severalfold increase in emission of monoterpenes from young Norway spruce (Picea abies). Atmos. Environ. 2000, 34, 711–718. [Google Scholar] [CrossRef]
- Schidler, T.; Kotzias, D. Comparison monoterpene volatilization and leaf-oil composition of conifers. Naturwissenschaften 1989, 76, 475–476. [Google Scholar] [CrossRef]
- Kocak, A.; Kilic, O. Identification of essetial oil composition of four Picea Mill. (Pinaceae) species from Canada. J. Agric. Sci. Technol. B 2014, 4, 209–214. [Google Scholar]
- Janczic, T.S.; Isajev, V.; Lange, W. Needle oil terpenes of Serbian spruce from 3 localities. Holz Roh Werkst. 1993, 51, 283–286. [Google Scholar] [CrossRef]
- Kairiūkštis, L. Medžių augimas vegetacijos metu. LMŪMTI Darb. 1963, 7, 3–38. [Google Scholar]
- Navasaitis, M.; Ozolinčius, R.; Smaliukas, D.; Balevičienė, J. Lietuvos Dendroflora; Lututė: Kaunas, Lithuania, 2003. [Google Scholar]
- Korica, A.; Polis, O.; Spalvis, K. Seasonal variations in essential oil content and quality of Scots pine and Norway spruce foliage. Mežzinātne 2011, 24, 93–104. [Google Scholar]
- Korica, A.; Polis, O.; Spalvis, K.; Bartkevics, V. Quantitative and qualitative seasonal changes of Scots pine and Norway spruce foliage essential oils in Latvia, and the extraction dynamics thereof. Balt. For. 2015, 21, 51–58. [Google Scholar]
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of essential oil production in plant. J. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- Kännaste, A.; Zhao, T.; Lindström, A.; Stattin, E.; Långström, B.; Borg-Karlson, A.-K. Odors of Norway spruce (Picea abies L.) seedlings: Differences due to age and chemotype. Trees 2013, 27, 149–159. [Google Scholar] [CrossRef]
- Baath, M.H.; Burzo, I. Quantitative and qualitative seasonal variation of volatile oil from 16 conifer species. An. Stiintifice Univ. Al. I. Cuza Iasi 2009, 55, 103. [Google Scholar]
- Vaičiulytė, V.; Ložienė, K. Variation of chemical and morphological characters of leaves and unripe cones in Juniperus Communis. Bot. Lith. 2013, 19, 37–47. [Google Scholar] [CrossRef]
- Brooks, J.E.; Borden, J.H.; Pierce, H.D.; Lister, G.R. Seasonal variation in foliar and bud monoterpenes in Sitka spruce. Canad. J. Bot. 1987, 65, 1249–1252. [Google Scholar] [CrossRef]
- Ozolinčius, R. Lietuvos Spygliuočiai: Morfologinės Struktūros Transformacijos bei jas Indukuojantys Veiksniai; Lietuvos Miškų Institutas: Kaunas, Lithuania, 1998; p. 300. [Google Scholar]
- Rötzer, T.; Grote, R.; Pretzsch, H. The timing of bud burst and its effect on tree growth. Int. J. Biometeorol. 2004, 48, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, A.M.; Linderson, M.L.; Stjernquist, P.S.; Bärring, L. Climate change and the effect of temperature backlashes causing frost damage in Picea abies. Glob. Planet. Chang. 2004, 44, 195–207. [Google Scholar] [CrossRef]
- Schleip, C.; Menzel, A.; Dose, V. Norway spruce (Picea abies): Bayesian analysis of the relationship between temperature and bud burst. Agric. For. Meteorol. 2008, 148, 631–643. [Google Scholar] [CrossRef]
- Sutinen, S.; Partanen, J.; Viherä-Aarnio, A.; Häkkinen, R. Development and growth of primordial shoots in Norway spruce buds before visible bud burst in relation to time and temperature in the field. Tree Physiol. 2012, 32, 987–997. [Google Scholar] [CrossRef]
- Marquis, B.; Bergeron, Y.; Simard, M.; Tremblay, F. Probability of spring frosts, not growing degree-days, drives onset of spruce bud burst in plantations at the boreal-temperate forest ecotone. Front. Plant. Sci. 2020, 11, 1031. [Google Scholar] [CrossRef]
- Jonaitis, V.; Ivinskis, P. Some situation that determine un- or sustainable development of plant-insect systems in Lithuania. Environ. Res. Eng. Manag. 2004, 1, 34–39. [Google Scholar]
- Jonaitis, V.; Zajančkauskas, P. The predators of small spruce sawfly (Lygaeonematus abietinus Christ) and their significance in the reduction of the pest populations. Acta Entomol. Lit. 1973, 2, 115–125. [Google Scholar]
- Pileckis, S.; Monsevičius, V. Lietuvos Fauna. Vabalai; Publishing House of Science and Encyclopaedia: Vilnius, Lithuania, 1995; p. 304. [Google Scholar]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2009, 101, 372–378. [Google Scholar] [CrossRef]
- Lucia, A.; Audino, G.P.; Seccacini, E.; Licastro, S.; Zerba, E.; Masuh, H. Larvicidal effect of Eucalyptus grandis essential oil and turpentine and their major components on Aedes aegypti larvae. J. Am. Mosq. Control. Assoc. 2007, 23, 299–303. [Google Scholar] [CrossRef]
- Wang, Z.; Song, J.; Chen, J.; Song, Z.; Shang, S.; Jiang, Z.; Han, Z. QSAR study of mosquito repellents from terpenoid with a six-member-ring. Bioorg. Med. Chem. Lett. 2008, 18, 2854–2859. [Google Scholar] [CrossRef]
- Haselton, A.T.; Acevedo, A.; Kuruvilla, J.; Werner, E.; Kiernan, J.; Dhar, P. Repellency of α-pinene against the house fly, Musca domestica. Phytochemistry 2015, 117, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, N.; Zhao, T.; Swedjemark, G.; Ashitani, T.; Takahashi, K.; Borgkarlson, A.K. Antifungal properties of terpenoids in Picea abies against Heterobasidion parviporum. For. Path. 2014, 44, 353–361. [Google Scholar] [CrossRef]
- Persson, M.; Sjodin, K.; BorgKarlson, A.K.; Norin, T.; Ekberg, I. Relative amounts and enantiomeric compositions of monoterpene hydrocarbons in xylem and needles of Picea abies. Phytochemistry 1996, 42, 1289–1297. [Google Scholar] [CrossRef]
- Sjodin, K.; Persson, M.; Faldt, J.; Ekberg, I.; Borg-Karlson, A.K. Occurrence and correlations of monoterpene hydrocarbon enantiomers in Pinus sylvestris and Picea abies. J. Chem. Ecol. 2000, 26, 1701–1720. [Google Scholar] [CrossRef]
- Yassaa, N.; Song, W.; Lelieveld, J.; Vanhatalo, A.; Bäck, J.; Williams, J. Diel cycles of isoprenoids in the emissions of Norway spruce, four Scots pine chemotypes, and in Boreal forest ambient air during HUMPPA-COPEC-2010. Atmos. Chem. Phys. 2012, 12, 7215–7229. [Google Scholar] [CrossRef]
- Ložienė, K.; Švedienė, J.; Paškevičius, A.; Raudonienė, V.; Sytar, O.; Kosyan, A. Influence of plant origin natural α-pinene with different enantiomeric composition on bacteria, yeasts and fungi. Fitoterapia 2018, 127, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.M.; Faldt, J.; And Bohlmann, J. Functional characterization of nine Norway spruce TPS genes and evolution of gymnosperm terpene synthases of the TPS-d subfamily. Plant. Physiol. 2004, 135, 1908–1927. [Google Scholar] [CrossRef]
- Pinto, L.; Bonifacio, M.A.; De Giglio, E.; Cometa, S.; Logrieco, A.F.; Baruzzi, F. Unravelling the Antifungal Effect of Red Thyme Oil (Thymus vulgaris L.) Compounds in Vapor Phase. Molecules 2020, 25, 4761. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.; Cefola, M.; Bonifacio, M.; Cometa, S.; Bocchino, C.; Pace, B.; De Giglio, E.; Palumbo, M.; Sada, A.; Logrieco, A.; et al. Effect of red thyme oil (Thymus vulgaris L.) vapours on fungal decay, quality parameters and shelf-life of oranges during cold storage. Food Chem. 2021, 336, 127590. [Google Scholar] [CrossRef]
- Dziedzinski, M.; Kobus-Cisowska, J.; Szymanowska, D.; Stuper-Szablewska, K.; Baranowska, M. Identification of Polyphenolsfrom Coniferous Shoots as Natural Antioxidants and Antimicrobial Compounds. Molecules 2020, 25, 3527. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Directorate for the Quality of Medicines and HealthCare of the Council of Europe (EDQM), 10th ed.; Directorate for the Quality of Medicines and Healthcare of the Council of Europe (EDQM): Strasbourg, France, 2008; Volume 1, pp. 307–308. [Google Scholar]
- Past Weather in Vilnius, Lithuania—September 2015. Available online: https://www.timeanddate.com/weather/lithuania/vilnius/historic?month=9&year=2015 (accessed on 15 March 2021).
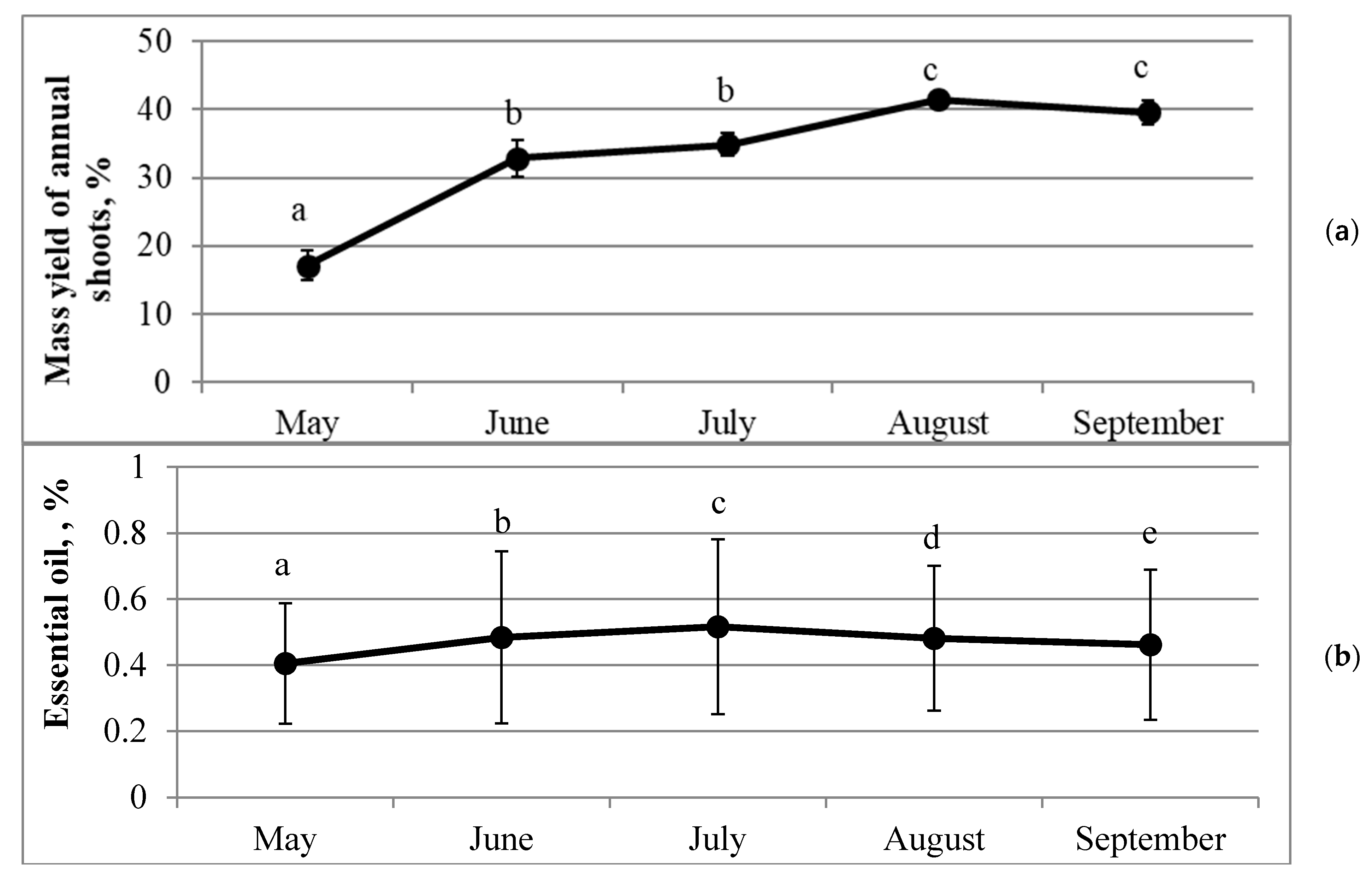

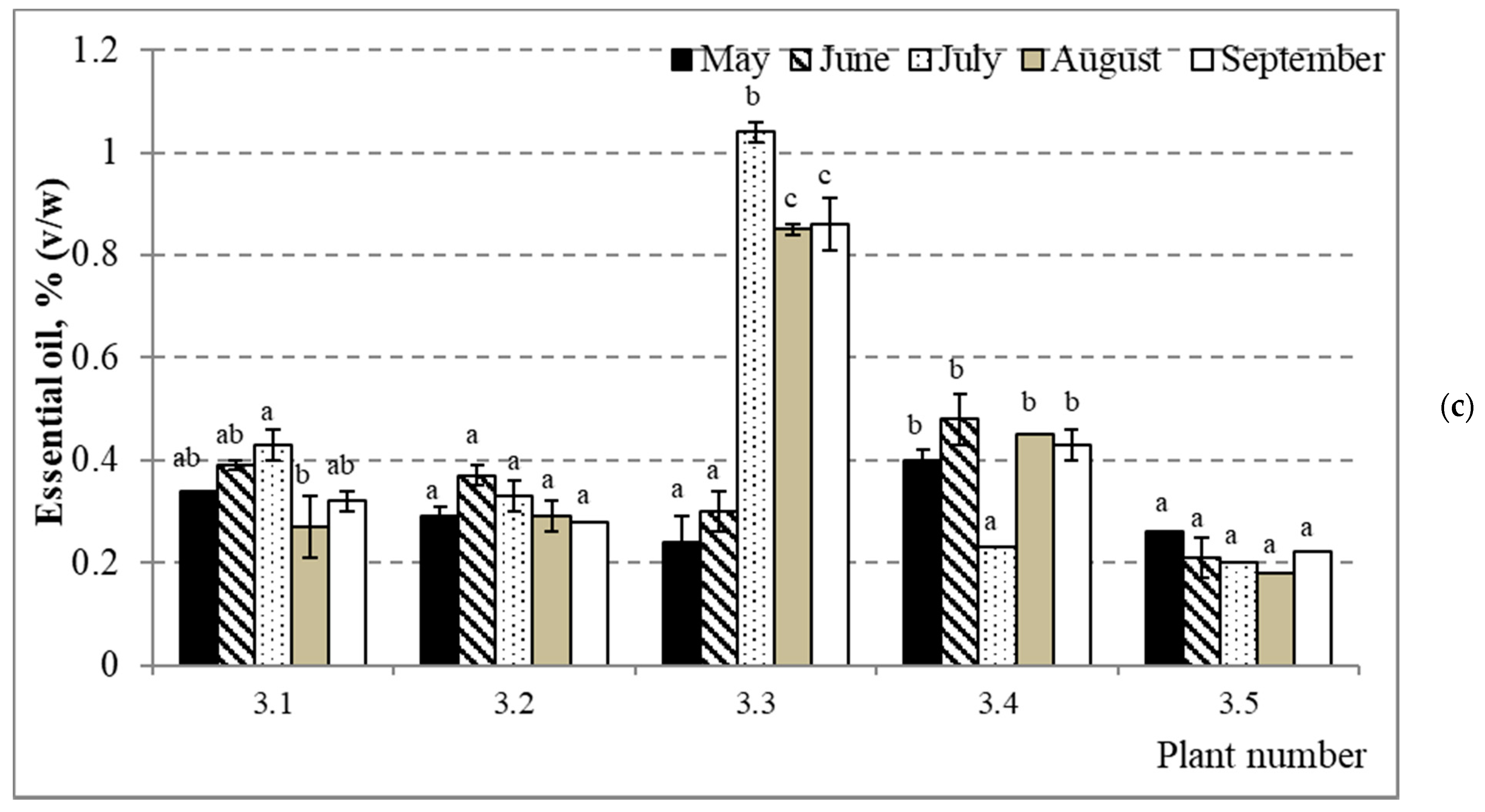
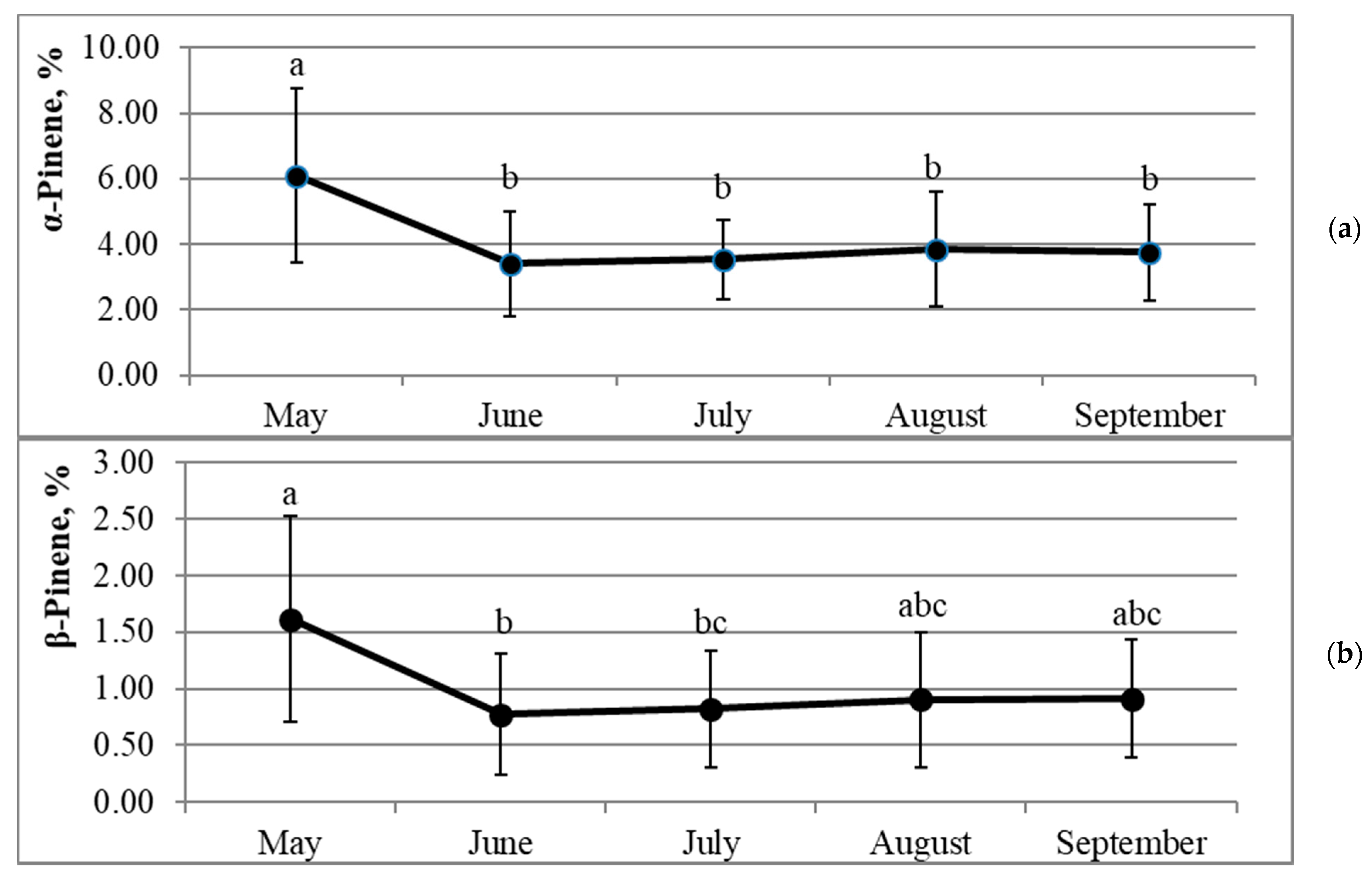
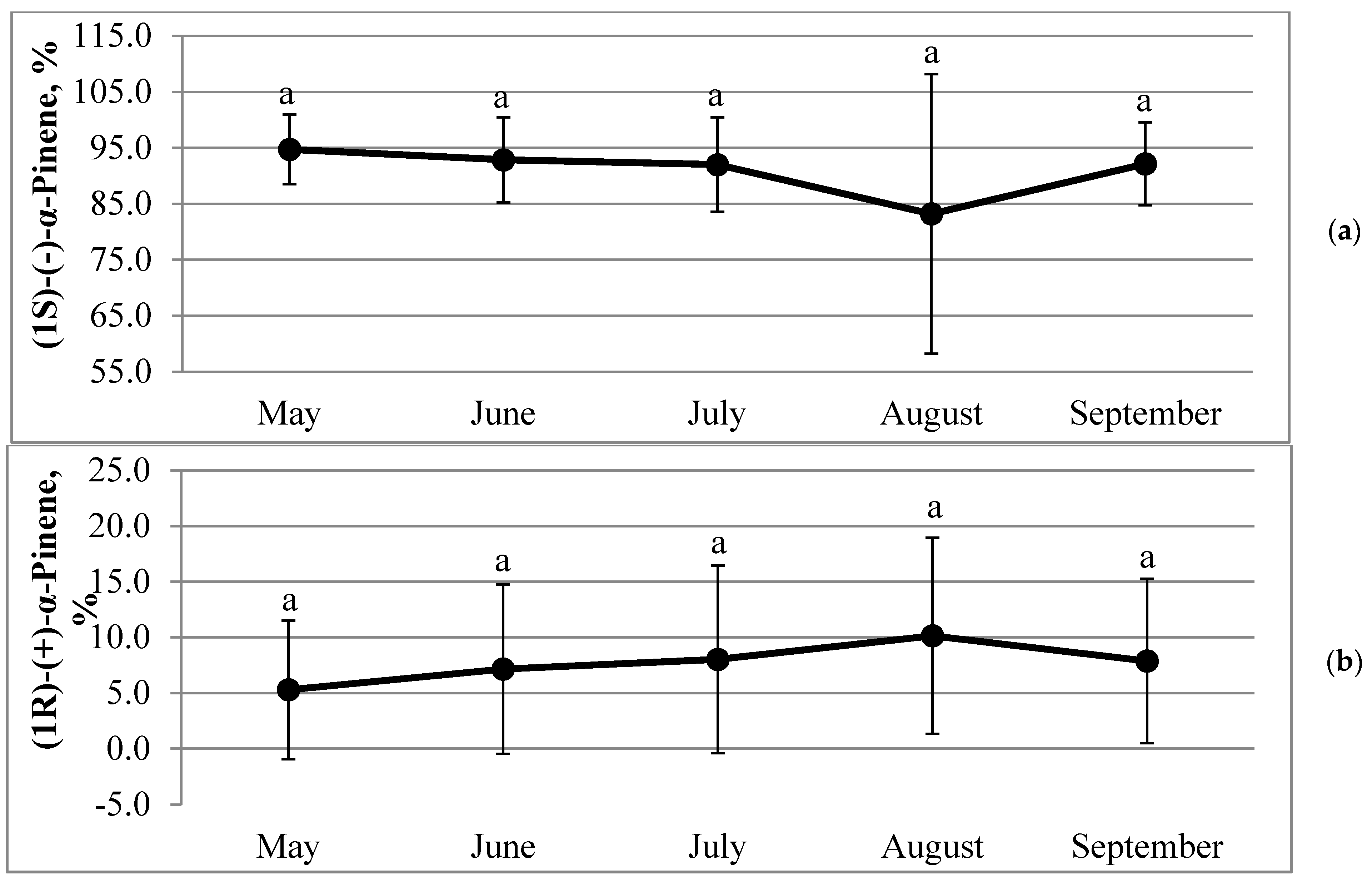
| Property | Correlation Coefficient R | Significance p | Sample Size N |
|---|---|---|---|
| Air-dry mass yield, g: | |||
| all habitats (by all trees) | 0.773 ** | <0.01 | 75 |
| all habitats (by avg. tree) | 0.786 ** | <0.01 | 15 |
| habitat 1 (by all trees) | 0.733 ** | <0.01 | 25 |
| habitat 2 (by all trees) | 0.817 ** | <0.01 | 25 |
| habitat 3 (by all trees) | 0.782 ** | <0.01 | 25 |
| habitat 1 (by avg. tree) | 0.751 ns | 0.144 | 5 |
| habitat 2 (by avg. tree) | 0.827 ns | 0.084 | 5 |
| habitat 3 (by avg. tree) | 0.792 ns | 0.110 | 5 |
| all habitats jointly | 0.791 ns | 0.111 | 5 |
| Essential oil, %: | |||
| all habitats (by all trees) | 0.126 ns | 0.282 | 75 |
| all habitats (by avg. tree) | 0.311 ns | 0.259 | 15 |
| habitat 1 (by all trees) | 0.129 ns | 0.540 | 25 |
| habitat 2 (by all trees) | 0.133 ns | 0.526 | 25 |
| habitat 3 (by all trees) | 0.148 ns | 0.479 | 25 |
| habitat 1 (by avg. tree) | 0.560 ns | 0.326 | 5 |
| habitat 2 (by avg. tree) | 0.612 ns | 0.273 | 5 |
| habitat 3 (by avg. tree) | 0.620 ns | 0.264 | 5 |
| all habitats jointly | 0.762 ns | 0.134 | 5 |
| α-Pinene, %: | |||
| all habitats (by all trees) | −0.355 ** | 0.002 | 75 |
| all habitats (by avg. trees) | −0.544 * | 0.036 | 15 |
| habitat 1 (by all trees) | −0.573 ** | 0.003 | 25 |
| habitat 2 (by all trees) | −0.367 ns | 0.071 | 25 |
| *habitat 3 (by all trees) | −0.233 ns | 0.262 | 25 |
| habitat 1 (by avg. tree) | −0.700 ns | 0.188 | 5 |
| habitat 2 (by avg. tree) | −0.726 ns | 0.165 | 5 |
| habitat 3 (by avg. tree) | −0.622 ns | 0.263 | 5 |
| all habitats jointly | −0.720 ns | 0.169 | 5 |
| β-Pinene, %: | |||
| all habitats (by all trees) | −0.334 ** | 0.003 | 75 |
| all habitats (by avg. trees) | −0.619 * | 0.014 | 15 |
| habitat 1 (by all trees) | −0.318 ns | 0.122 | 25 |
| habitat 2 (by all trees) | −0.321 ns | 0.117 | 25 |
| habitat 3 (by all trees) | −0.413 * | 0.040 | 25 |
| habitat 1 (by avg. tree) | −0.667 ns | 0.219 | 5 |
| habitat 2 (by avg. tree) | −0.762 ns | 0.134 | 5 |
| habitat 3 (by avg. tree) | −0.760 ns | 0.136 | 5 |
| all habitats jointly | −0.740 ns | 0.156 | 5 |
| (1S)-(–)-α-Pinene, %: | |||
| all habitats (by all trees) | −0.261 * | 0.024 | 75 |
| all habitats (by avg. tree) | −0.547 * | 0.035 | 15 |
| habitat 1 (by all trees) | 0.058 ns | 0.784 | 25 |
| habitat 2 (by all trees) | −0.297 ns | 0.149 | 25 |
| habitat 3 (by all trees) | −0.397 * | 0.049 | 25 |
| habitat 1 (by avg. tree) | 0.327 ns | 0.592 | 5 |
| habitat 2 (by avg. tree) | −0.880 * | 0.049 | 5 |
| habitat 3 (by avg. tree) | −0.789 ns | 0.113 | 5 |
| all habitats jointly | −0.641 ns | 0.244 | 5 |
| (1R)-(+)-α-Pinene, % | |||
| all habitats (by all trees) | 0.190 ns | 0.103 | 75 |
| all habitats (by avg. tree) | 0.391 ns | 0.150 | 15 |
| habitat 1 (by all trees) | −0.058 ns | 0.784 | 25 |
| habitat 2 (by all trees) | 0.297 ns | 0.149 | 25 |
| habitat 3 (by all trees) | 0.323 ns | 0.116 | 25 |
| habitat 1 (by avg. tree) | −0.327 ns | 0.592 | 5 |
| habitat 2 (by avg. tree) | 0.880 * | 0.049 | 5 |
| habitat 3 (by avg. tree) | 0.858 ns | 0.063 | 5 |
| all habitats jointly | 0.928 * | 0.023 | 5 |
| Indicator | May | June | July | August | September |
|---|---|---|---|---|---|
| Temperature, °C | 11 | 16 | 17 | 20 | 14 |
| Sum of effective temperatures (≥5 °C) * | 295 | 611 | 993 | 1454 | 1717 |
| Sunshine duration, h | 220.0 | 291.1 | 235.9 | 350.0 | 146.3 |
| Humidity, % | 73 | 65 | 71 | 60 | 81 |
| Pressure, mbar | 1014 | 1017 | 1012 | 1020 | 1018 |
| Precipitation, mm | 79.6 | 16.1 | 67.9 | 25.2 | 55.0 |
| Habitat No. | Habitat Type * | Age of Trees (Years) | Locality | WGS–84 Coordinates |
|---|---|---|---|---|
| 1 | Ndp | 15 | Sužionys | 54.977820 N 25.469426 E |
| 2 | Ncl | 15 | Žemaitėliai | 54.954513 N 25.327365 E |
| 3 | Ncl | 17 | Adomciškės | 54.810057 N 24.987477 E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamaitytė-Bukelskienė, L.; Ložienė, K.; Labokas, J. Dynamics of Isomeric and Enantiomeric Fractions of Pinene in Essential Oil of Picea abies Annual Needles during Growing Season. Molecules 2021, 26, 2138. https://doi.org/10.3390/molecules26082138
Kamaitytė-Bukelskienė L, Ložienė K, Labokas J. Dynamics of Isomeric and Enantiomeric Fractions of Pinene in Essential Oil of Picea abies Annual Needles during Growing Season. Molecules. 2021; 26(8):2138. https://doi.org/10.3390/molecules26082138
Chicago/Turabian StyleKamaitytė-Bukelskienė, Liucija, Kristina Ložienė, and Juozas Labokas. 2021. "Dynamics of Isomeric and Enantiomeric Fractions of Pinene in Essential Oil of Picea abies Annual Needles during Growing Season" Molecules 26, no. 8: 2138. https://doi.org/10.3390/molecules26082138
APA StyleKamaitytė-Bukelskienė, L., Ložienė, K., & Labokas, J. (2021). Dynamics of Isomeric and Enantiomeric Fractions of Pinene in Essential Oil of Picea abies Annual Needles during Growing Season. Molecules, 26(8), 2138. https://doi.org/10.3390/molecules26082138







