Efficacy of Souroubea-Platanus Dietary Supplement Containing Triterpenes in Beagle Dogs Using a Thunderstorm Noise-Induced Model of Fear and Anxiety
Abstract
1. Introduction
2. Results
2.1. Tablet Stability
2.2. Activity/Inactivity Time Study during Simulated Thunderstorm with Five Consecutive Daily Doses
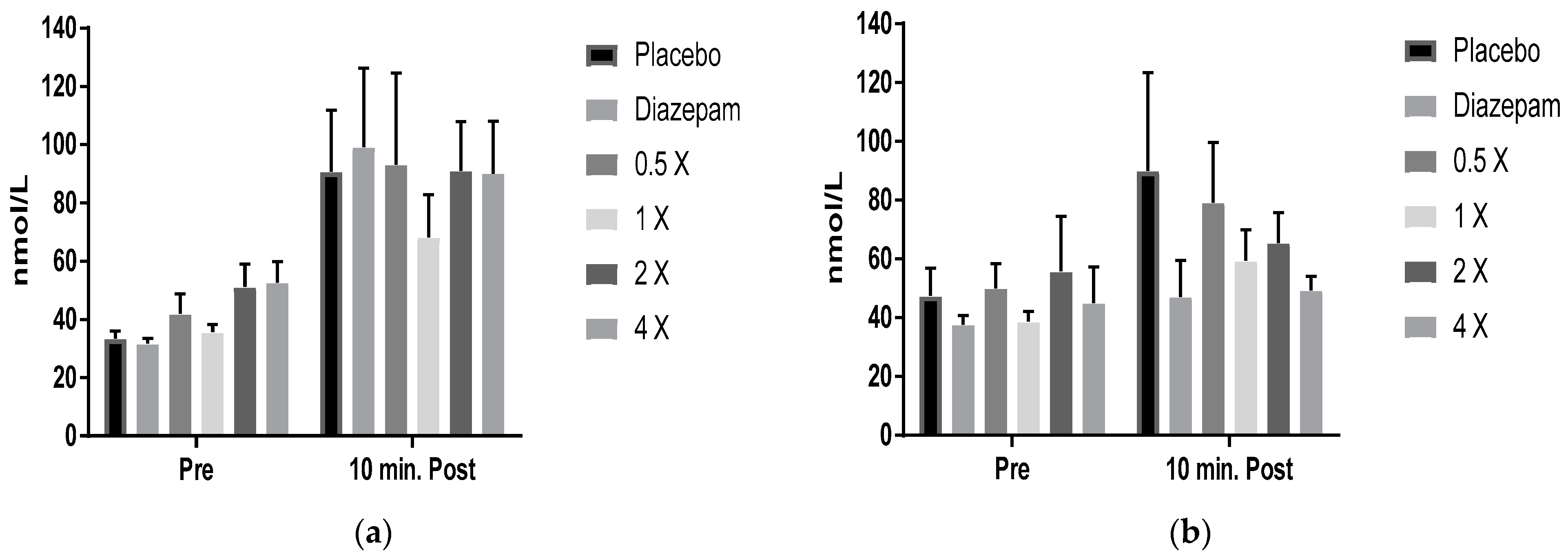
2.3. Cortisol Results
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Tablet Preparation and Analysis
4.3. Experimental Study Design
4.3.1. Animal Welfare
4.3.2. Blinding
4.3.3. Study Animals and Experimental Groups
4.3.4. Testing Procedure
4.3.5. Assessment of Efficacy
4.3.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Patents
References
- Mullally, M.; Kramp, K.; Cayer, C.; Saleem, A.; Ahmed, F.; McRae, C.; Baker, J.; Goulah, A.; Otorola, M.; Sanchez, P.; et al. Anxiolytic activity of a supercritical carbon dioxide extract of Souroubea sympetala (Marcgraviaceae). Phytother. Res. 2010, 25, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Puniani, E.; Cayer, C.; Kent, P.; Mullally, M.; Sánchez-Vindas, P.; Álvarez, L.P.; Durst, T. Ethnopharmacology of Souroubea sympetala and Souroubea gilgii (Marcgraviaceae) and identification of betulinic acid as an anxiolytic principle. Phytochemistry 2015, 113, 73–78. [Google Scholar] [CrossRef]
- Mullally, M.; Mimeault, C.; Rojas, M.O.; Vindas, P.S.; Garcia, M.; Alvarez, L.P.; Moon, T.W.; Gilmour, K.M.; Trudeau, V.L.; Arnason, J.T. A botanical extract of Souroubea sympetala and its active principle, betulinic acid, attenuate the cortisol response to a stressor in rainbow trout, Oncorhynchus mykiss. Aquaculture 2017, 468, 26–31. [Google Scholar] [CrossRef]
- Durst, T.; Baker, J.D.; Arnason, J.T.; Wade, J.M.; Merali, Z.; Mullally, M.; Cayer, C.; Alkemade, S.J.; Carballo, A.F.; Velji, I.; et al. Plant Compositions and Methods and Uses Thereof for Treating Elevated Glucocorticoid Related Disorders, and Anxiety. U.S. patent application US 10/004,773, 26 June 2018. [Google Scholar]
- Mullally, M.; Cayer, C.; Kramp, K.; Rojas, M.O.; Vindas, P.S.; Garcia, M.; Alvarez, L.P.; Durst, T.; Merali, Z.; Trudeau, V.L.; et al. Souroubea sympetala (Marcgraviaceae): A medicinal plant that exerts anxiolysis through interaction with the GABAA benzodiazepine receptor. Can. J. Physiol. Pharmacol. 2014, 92, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Carballo-Arce, A.-F.; Singh, R.; Saleem, A.; Rocha, M.; Mullally, M.; Otarola-Rojas, M.; Alvarrez, L.P.; Sanchez-Vindas, P.; Garcia, M.; et al. A Selective Ion HPLC-APCI-MS Method for the Quantification of Pentacyclic Triterpenes in an Anxiolytic Botanical Dietary Supplement for the Animal Health Market. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Villalobos, P.; Baker, J.; Sanchez Vindas, P.; Durst, T.; Masic, A.; Arnason, J.T. Clinicalobservations and safety profile of oral herbal products, Souroubea and Platanus spp: A pilot toxicology study in dogs. Acta Vet. 2014, 64, 269–275. [Google Scholar] [CrossRef]
- Masic, A.; Liu, R.; Simkus, K.; Wilson, J.; Baker, J.; Sanchez, P.; Saleem, A.; Harris, C.C.; Durst, T.; Arnason, J.T. Safety evaluation of a new anxiolytic product containing botanicals Souroubea spp. and Platanus spp. in dogs. Can. J. Veter Res. Rev. Can. Rech. Veter- 2018, 82, 3–11. [Google Scholar]
- Vargas, A.R.; Gomez, A.H.; Liu, R.; Rojas, M.O.; Vindas, P.S.; Durst, T.; Arnason, J.T. In Vitro Culture of the New Anxiolytic Plant, Souroubea Sympetala. J. Nat. Heal. Prod. Res. 2020, 2, 1–10. [Google Scholar] [CrossRef][Green Version]
- Riemer, S. Effectiveness of treatments for firework fears in dogs. J. Veter Behav. 2020, 37, 61–70. [Google Scholar] [CrossRef]
- Araujo, J.A.; De Rivera, C.; Landsberg, G.M.; Adams, P.E.; Milgram, N.W. Development and validation of a novel laboratory model of sound-induced fear and anxiety in Beagle dogs. J. Veter Behav. 2013, 8, 204–212. [Google Scholar] [CrossRef]
- Carballo, A.F. Phytochemical Investigations of Costa Rican Marcgraviaceae and Development of Insecticide Synergists. Ph.D. Thesis, Faculty of Science, University of Ottawa, Ottawa, ON, Canada, 2013. [Google Scholar]
- Murkar, A.; Cayer, C.; James, J.; Durst, T.; Arnason, J.T.; Sanchez-Vindas, P.E.; Otarola Rojas, M.; Merali, Z. Extract and Active Principal of the Neotropical Vine Souroubea sympetala Gilg. Block Fear Memory Reconsolidation. Front. Pharmacol. 2019, 10, 1496. [Google Scholar] [CrossRef] [PubMed]
- Mastinu, A.; Bonini, S.; Premoli, M.; Maccarinelli, G.; Mac Sweeney, E.; Zhang, L.; Lucini, L.; Memo, M. Protective Effects of Gynostemma pentaphyllum (var. Ginpent) against Lipopolysaccharide-Induced Inflammation and Motor Alteration in Mice. Molecules 2021, 26, 570. [Google Scholar] [CrossRef] [PubMed]


| Date of Analysis | Sample | Mean (SE) Tablet Weight (g) | Mean (SE) Concentration (mg/Tablet) | |
|---|---|---|---|---|
| Betulinic Acid | α-Amyrin | |||
| 14 March 2016 | Tablet | 3.499 (0.021) | 11.92 (0.472) | 0.376 (0.029) |
| 14 October 2018 | Tablet | 3.561 (0.012) | 11.68 (0.345) | 0.369 (0.024) |
| Dose | Inactivity LSMean | Change in Inactivity | p-Value | Activity LSMean | Change in Activity | p-Value |
|---|---|---|---|---|---|---|
| Placebo | 114.48 | - | - | 24.46 | - | - |
| 0.5× * | 106.48 | 8.01 | 0.6162 | 24.21 | 0.244 | 0.9783 |
| 1× | 72.61 | 41.87 | 0.0111 a | 44.60 | −20.14 | 0.0299 a |
| 2× | 70.14 | 44.34 | 0.0084 a | 39.92 | −15.47 | 0.0942 b |
| 4× | 90.41 | 24.08 | 0.1358 | 45.93 | −21.48 | 0.0199 a |
| Diazepam | 61.31 | 53.17 | 0.0016 a | 58.99 | −34.53 | 0.0003 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masic, A.; Landsberg, G.; Milgram, B.; Merali, Z.; Durst, T.; Sanchez Vindas, P.; Garcia, M.; Baker, J.; Liu, R.; Arnason, J. Efficacy of Souroubea-Platanus Dietary Supplement Containing Triterpenes in Beagle Dogs Using a Thunderstorm Noise-Induced Model of Fear and Anxiety. Molecules 2021, 26, 2049. https://doi.org/10.3390/molecules26072049
Masic A, Landsberg G, Milgram B, Merali Z, Durst T, Sanchez Vindas P, Garcia M, Baker J, Liu R, Arnason J. Efficacy of Souroubea-Platanus Dietary Supplement Containing Triterpenes in Beagle Dogs Using a Thunderstorm Noise-Induced Model of Fear and Anxiety. Molecules. 2021; 26(7):2049. https://doi.org/10.3390/molecules26072049
Chicago/Turabian StyleMasic, Aleksandar, Gary Landsberg, Bill Milgram, Zul Merali, Tony Durst, Pablo Sanchez Vindas, Mario Garcia, John Baker, Rui Liu, and John Arnason. 2021. "Efficacy of Souroubea-Platanus Dietary Supplement Containing Triterpenes in Beagle Dogs Using a Thunderstorm Noise-Induced Model of Fear and Anxiety" Molecules 26, no. 7: 2049. https://doi.org/10.3390/molecules26072049
APA StyleMasic, A., Landsberg, G., Milgram, B., Merali, Z., Durst, T., Sanchez Vindas, P., Garcia, M., Baker, J., Liu, R., & Arnason, J. (2021). Efficacy of Souroubea-Platanus Dietary Supplement Containing Triterpenes in Beagle Dogs Using a Thunderstorm Noise-Induced Model of Fear and Anxiety. Molecules, 26(7), 2049. https://doi.org/10.3390/molecules26072049






