The Anticancer Action of a Novel 1,2,4-Triazine Sulfonamide Derivative in Colon Cancer Cells
Abstract
1. Introduction
2. Results
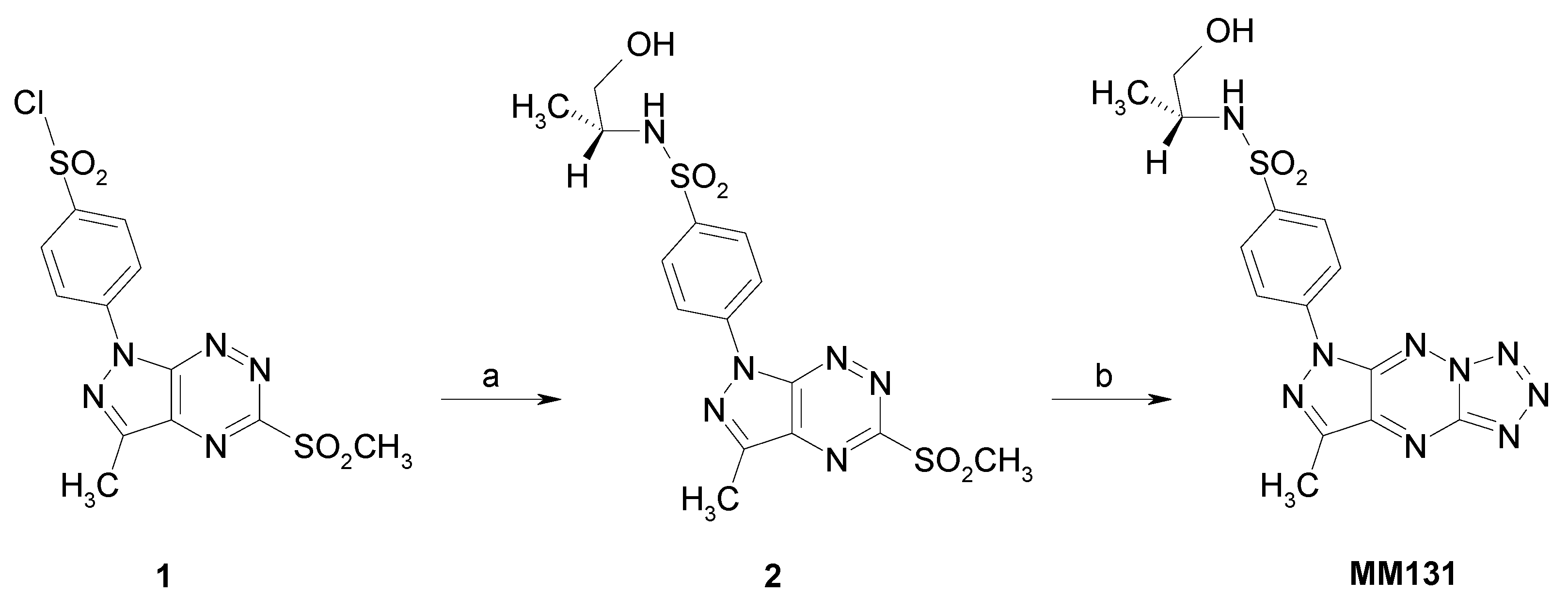
2.1. Chemistry
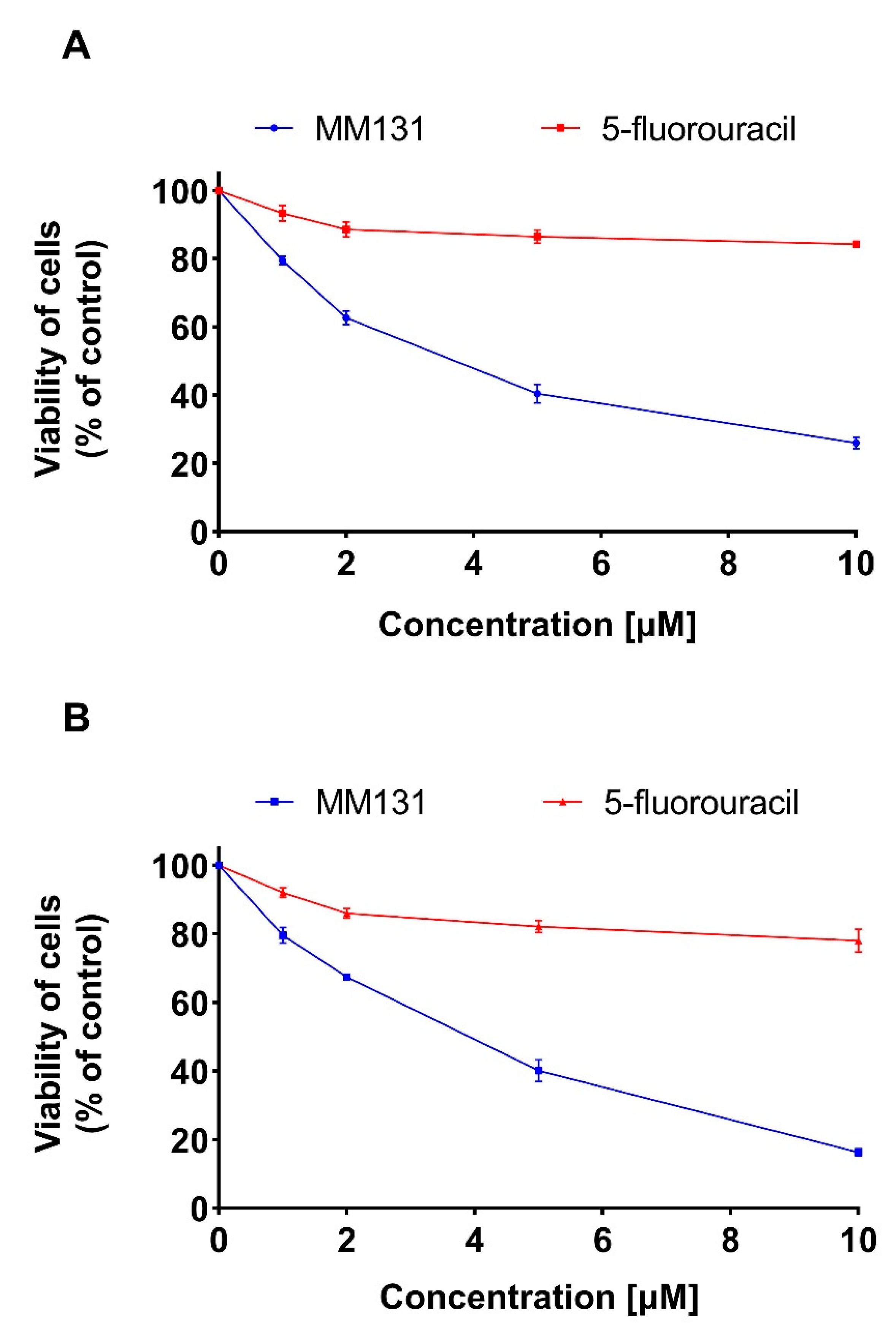
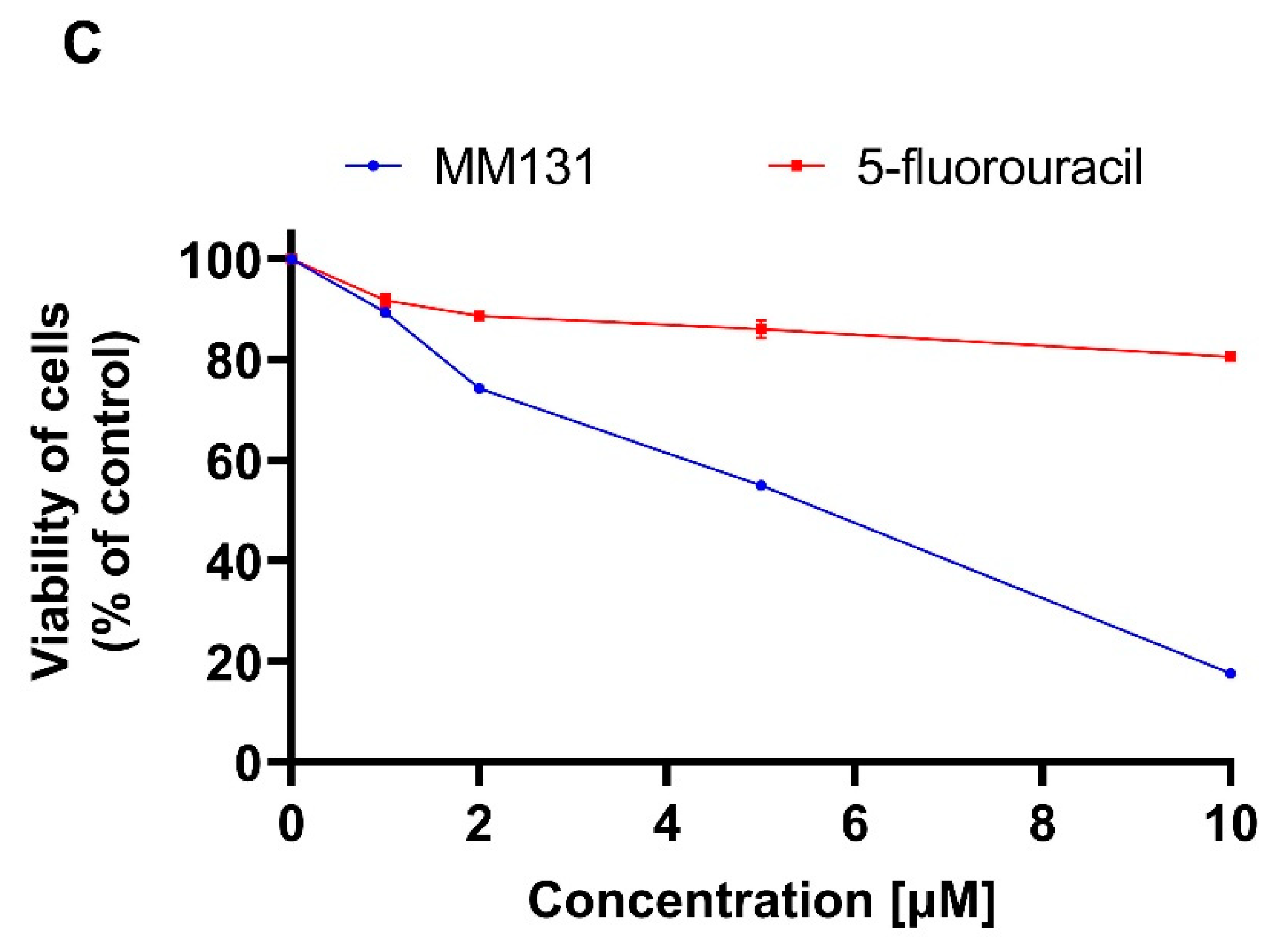
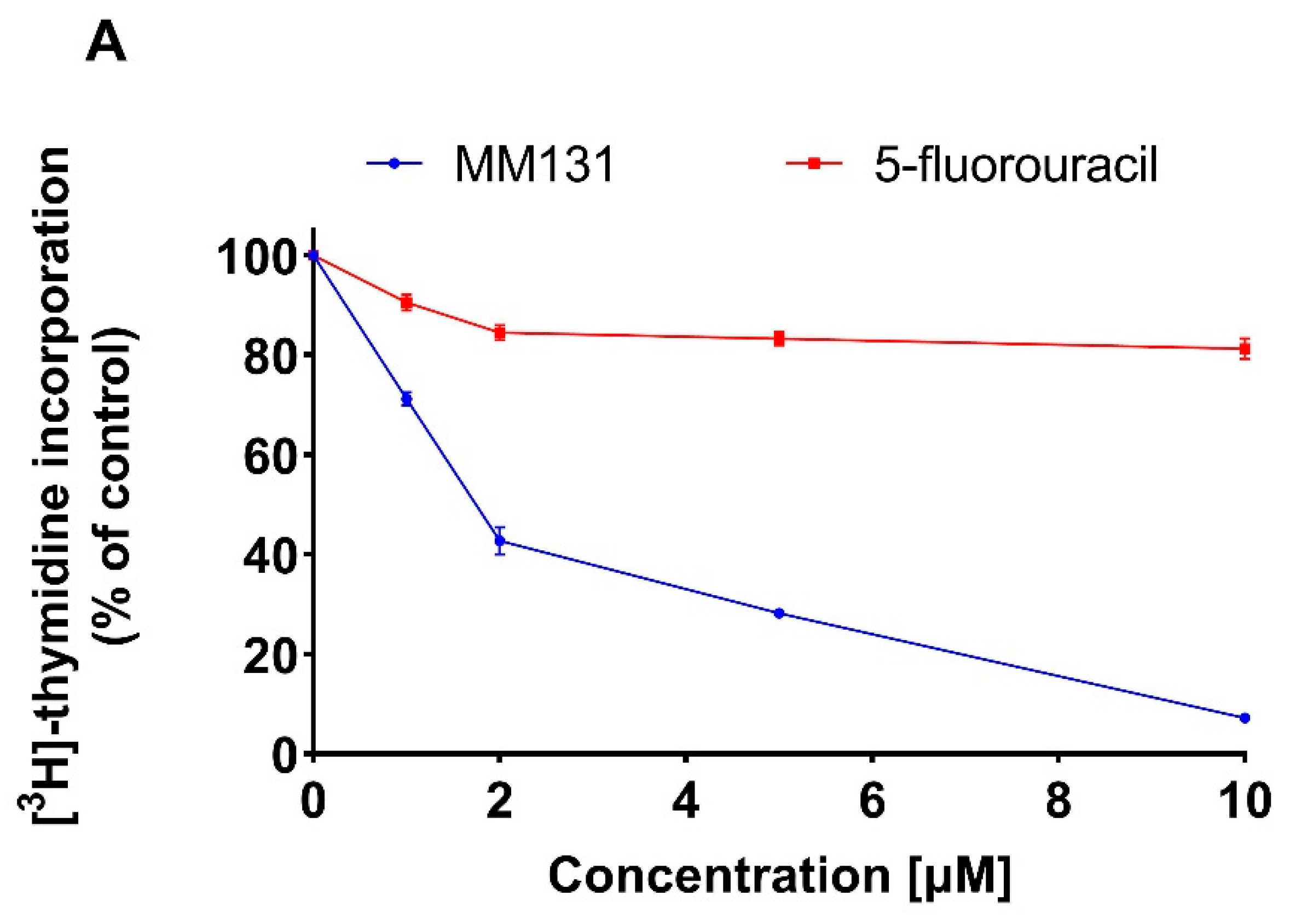
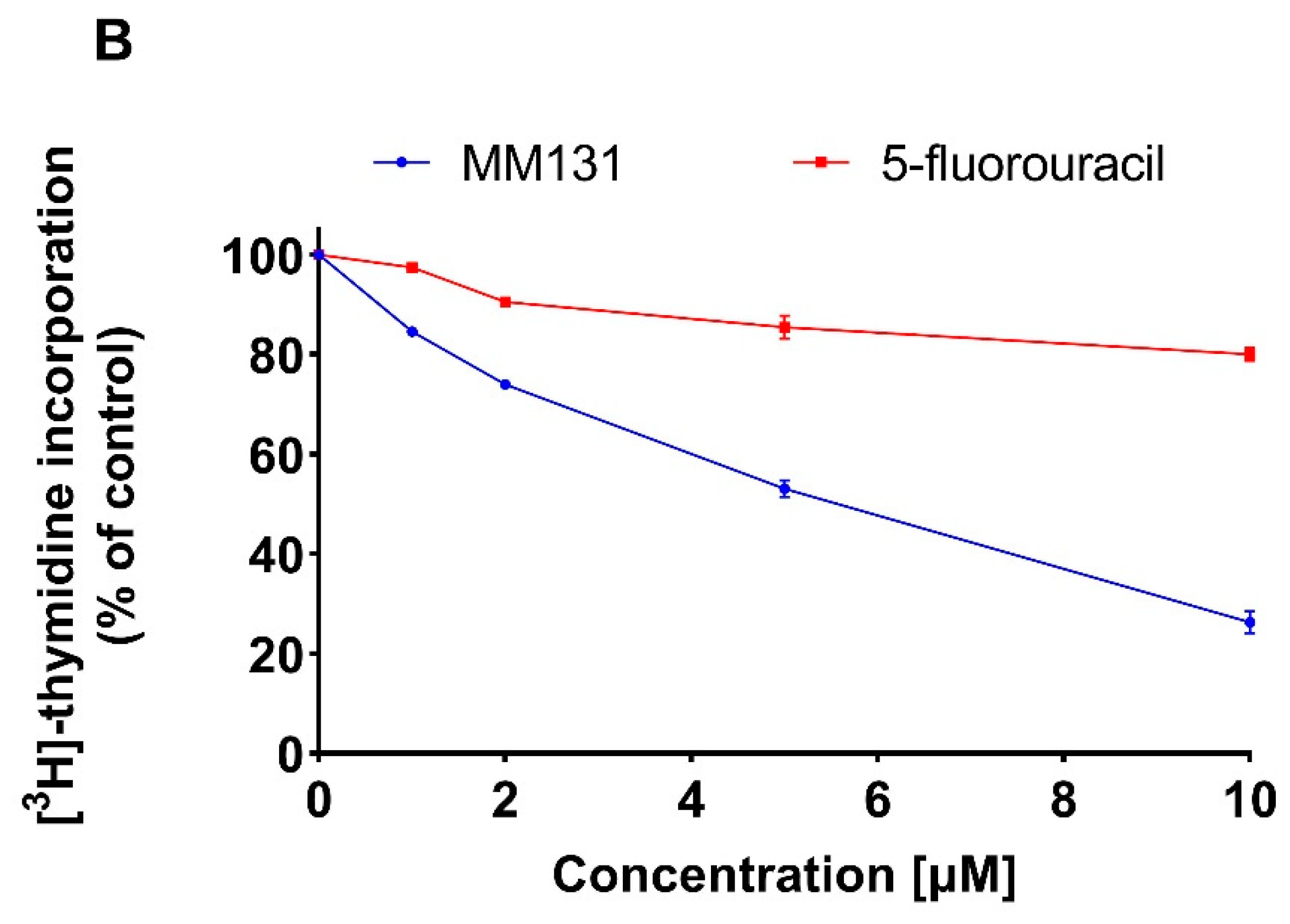
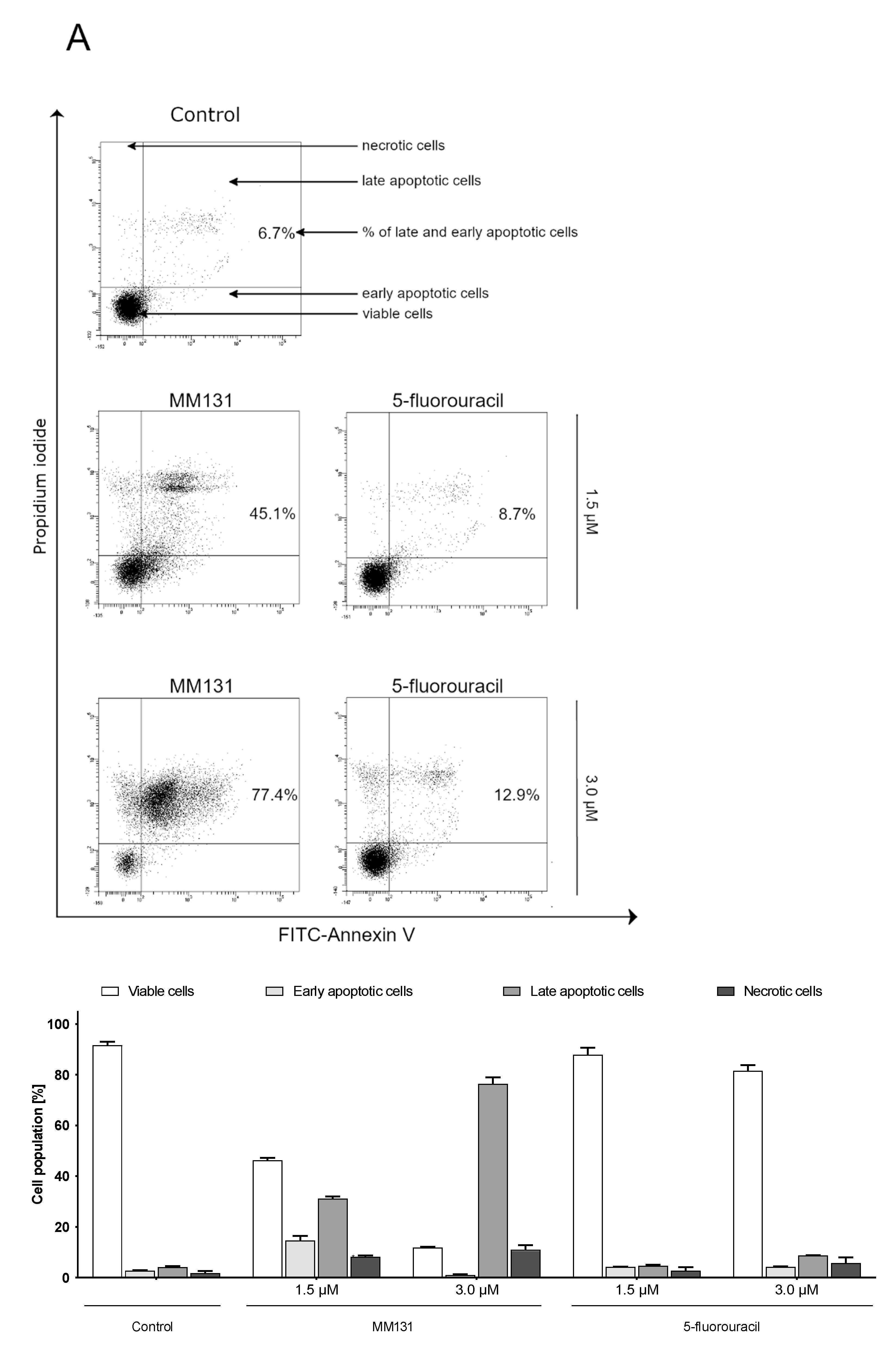
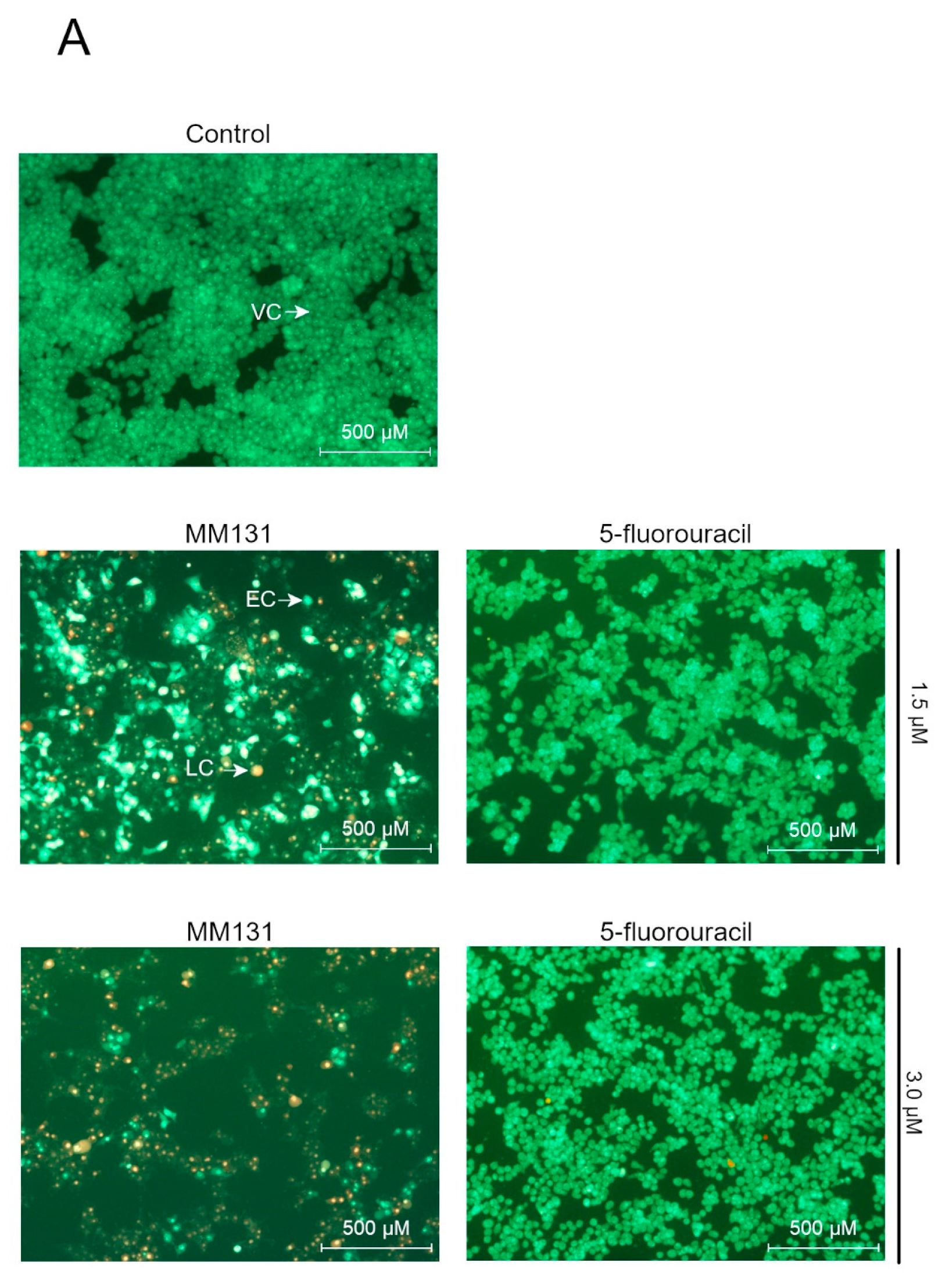
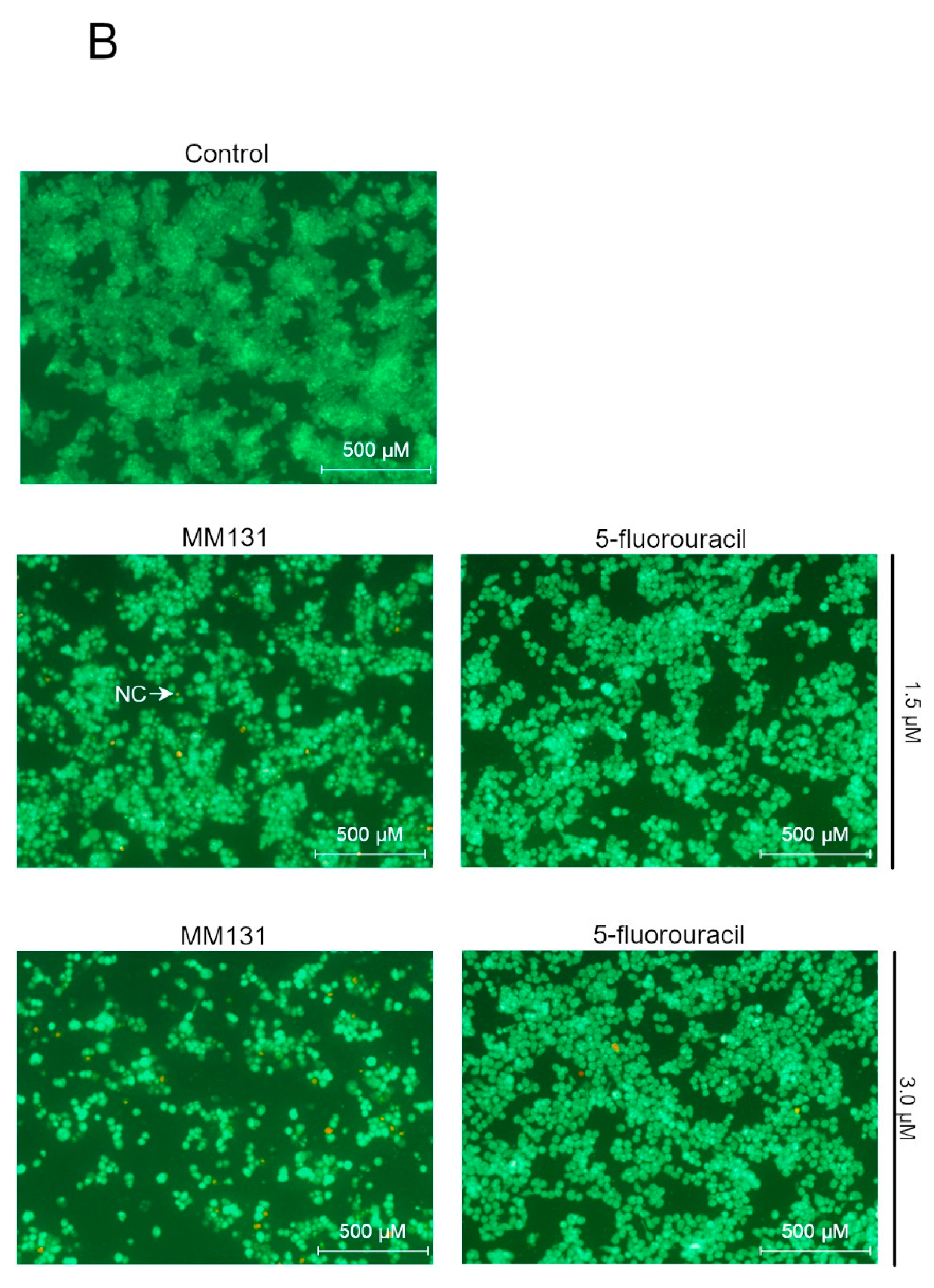
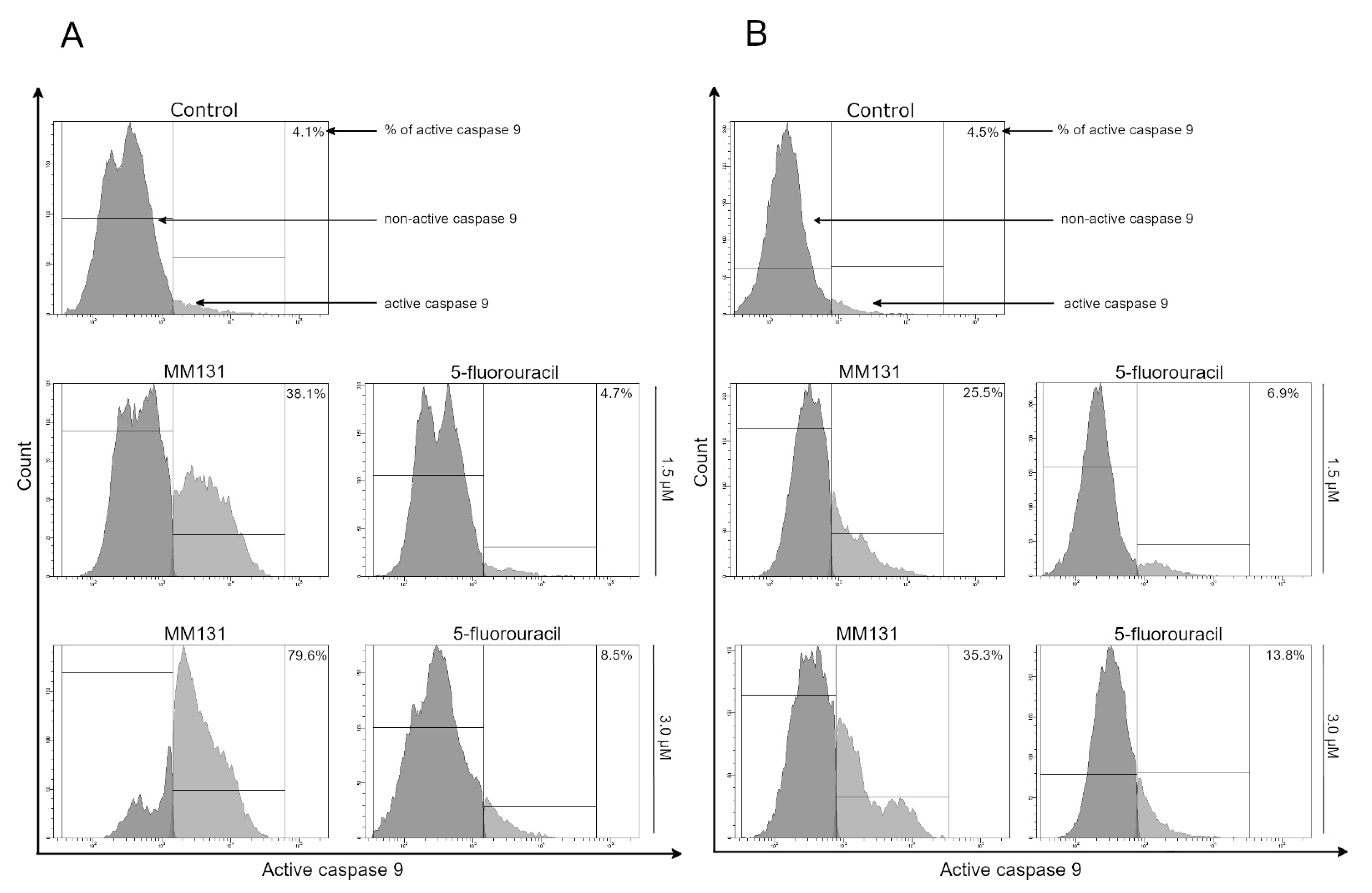
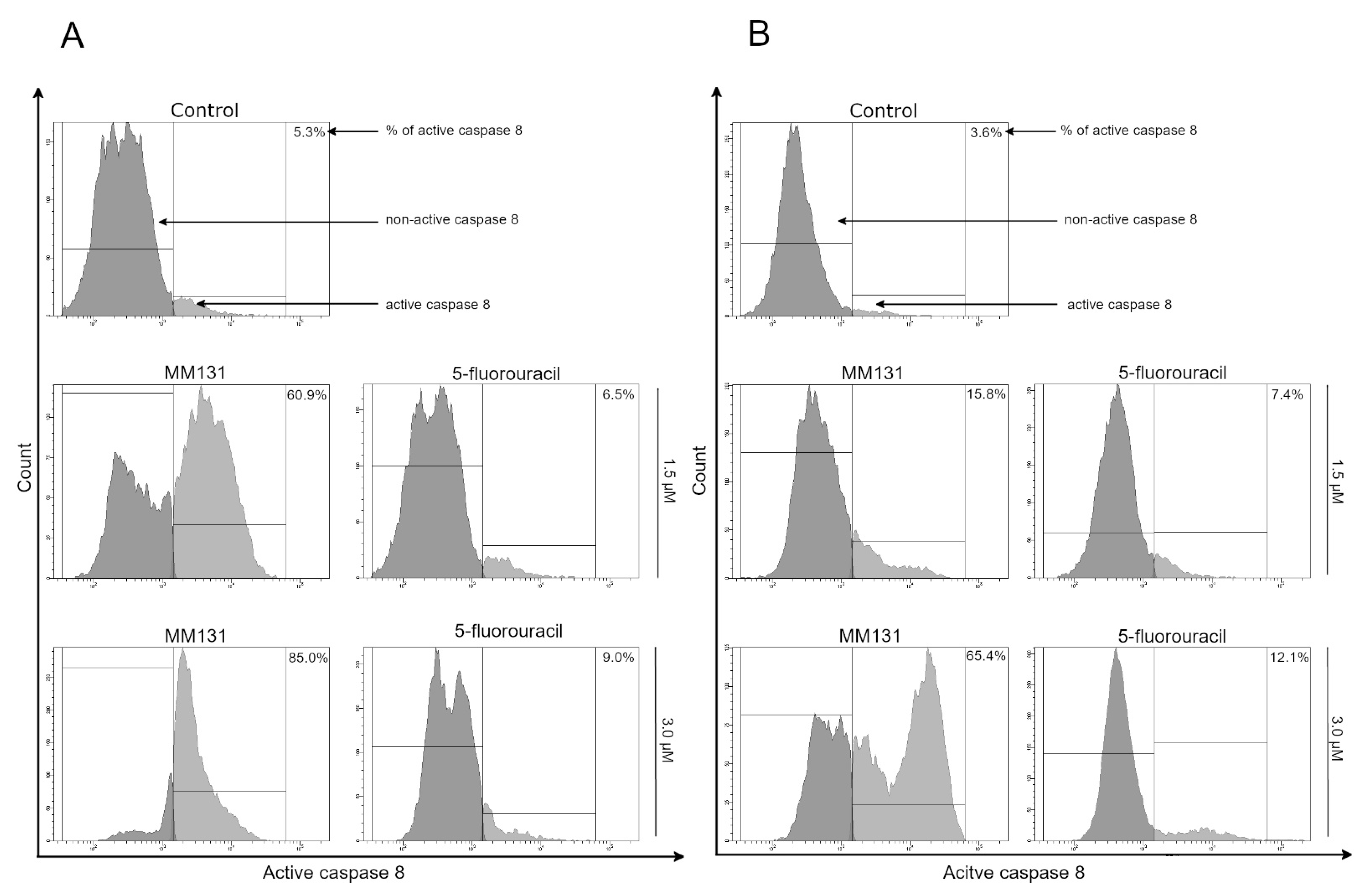
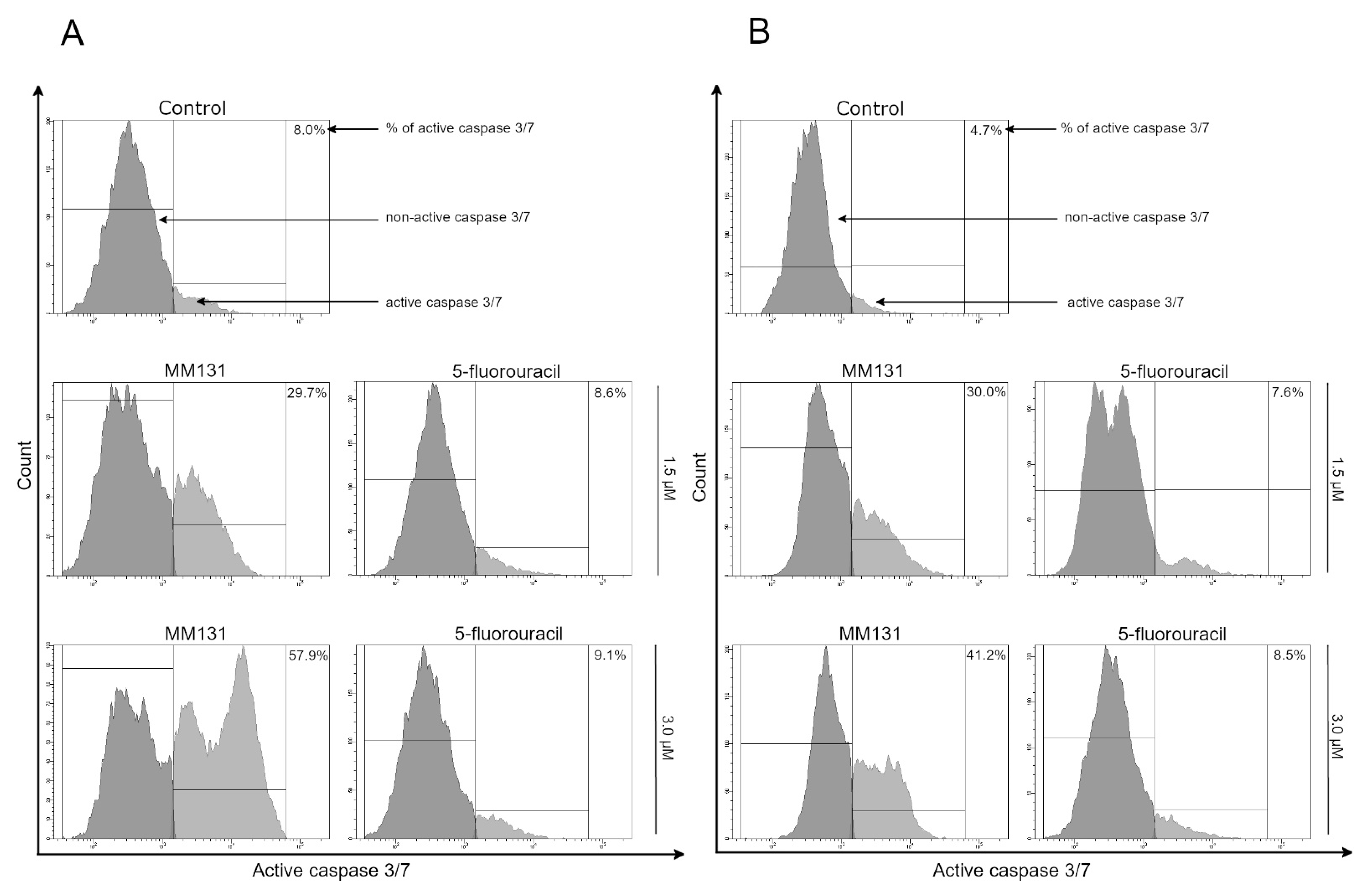
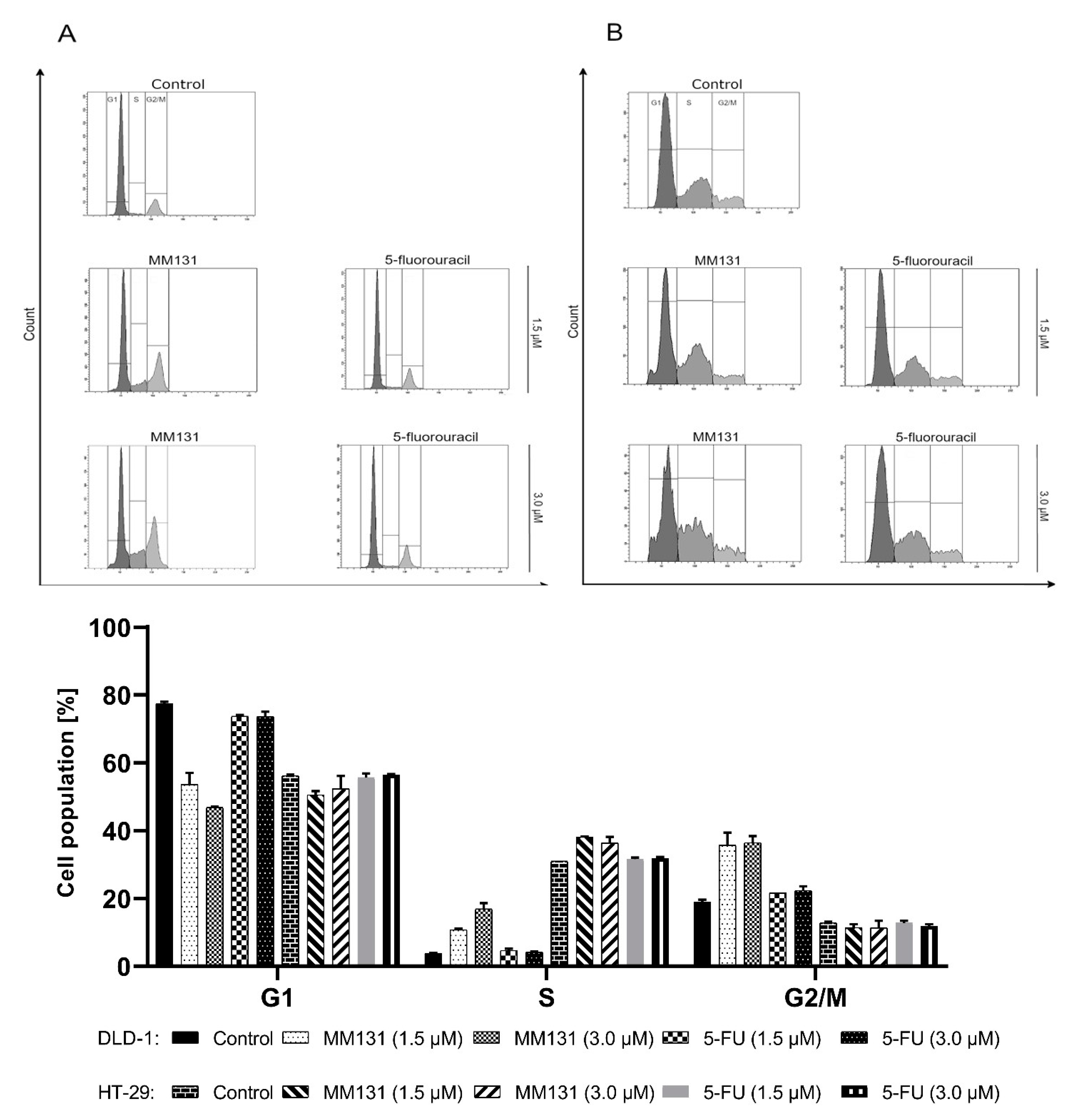
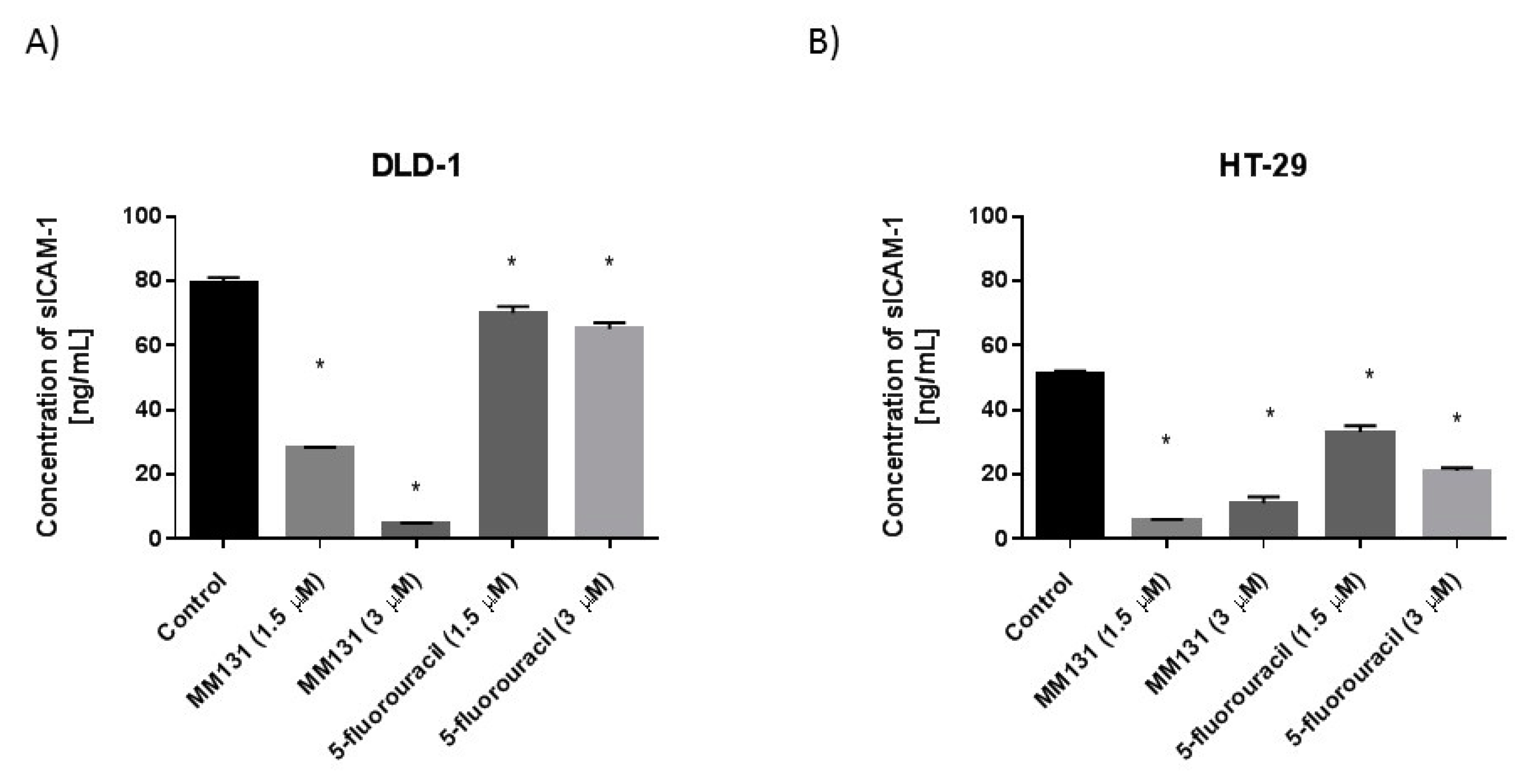
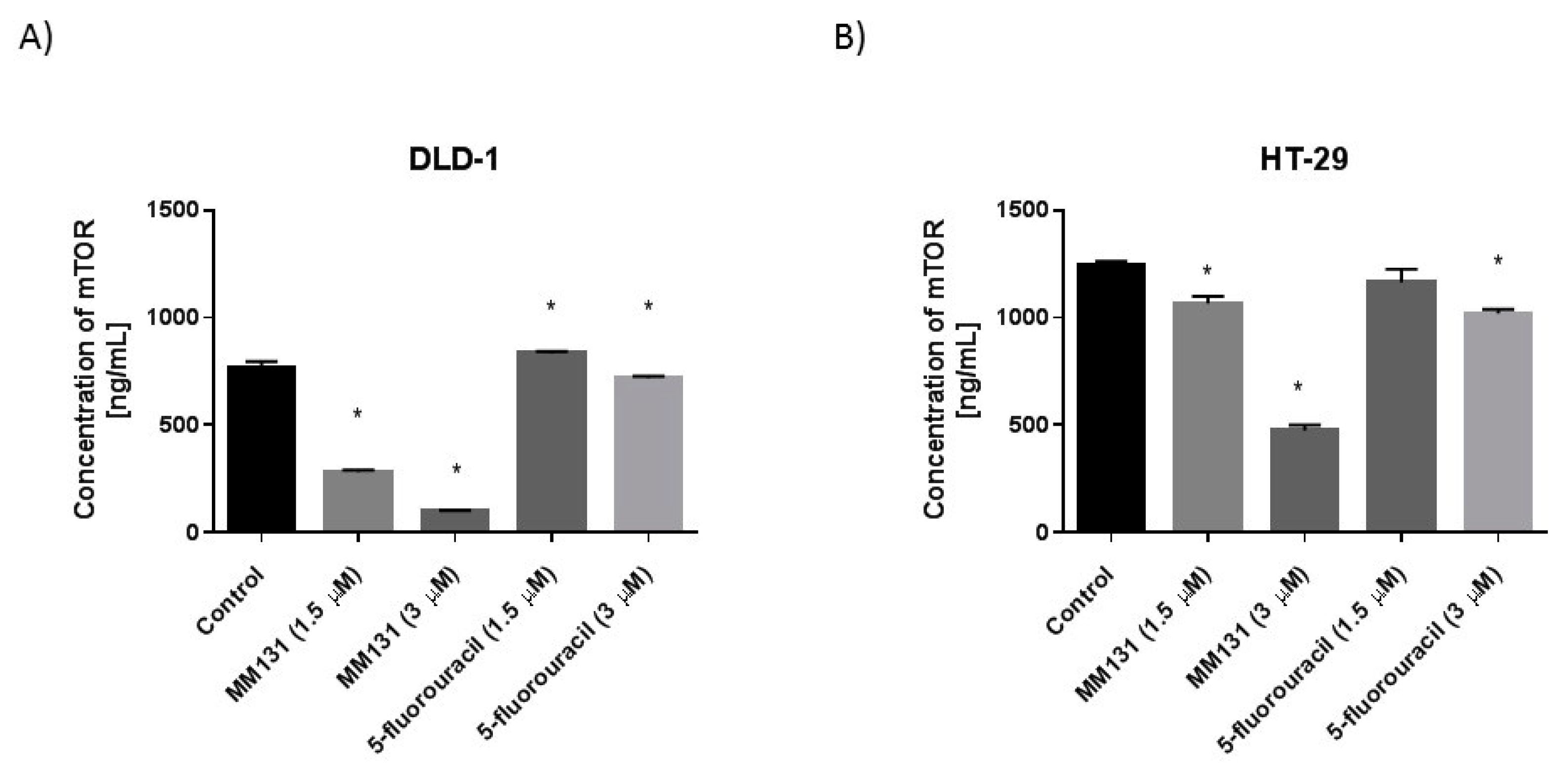
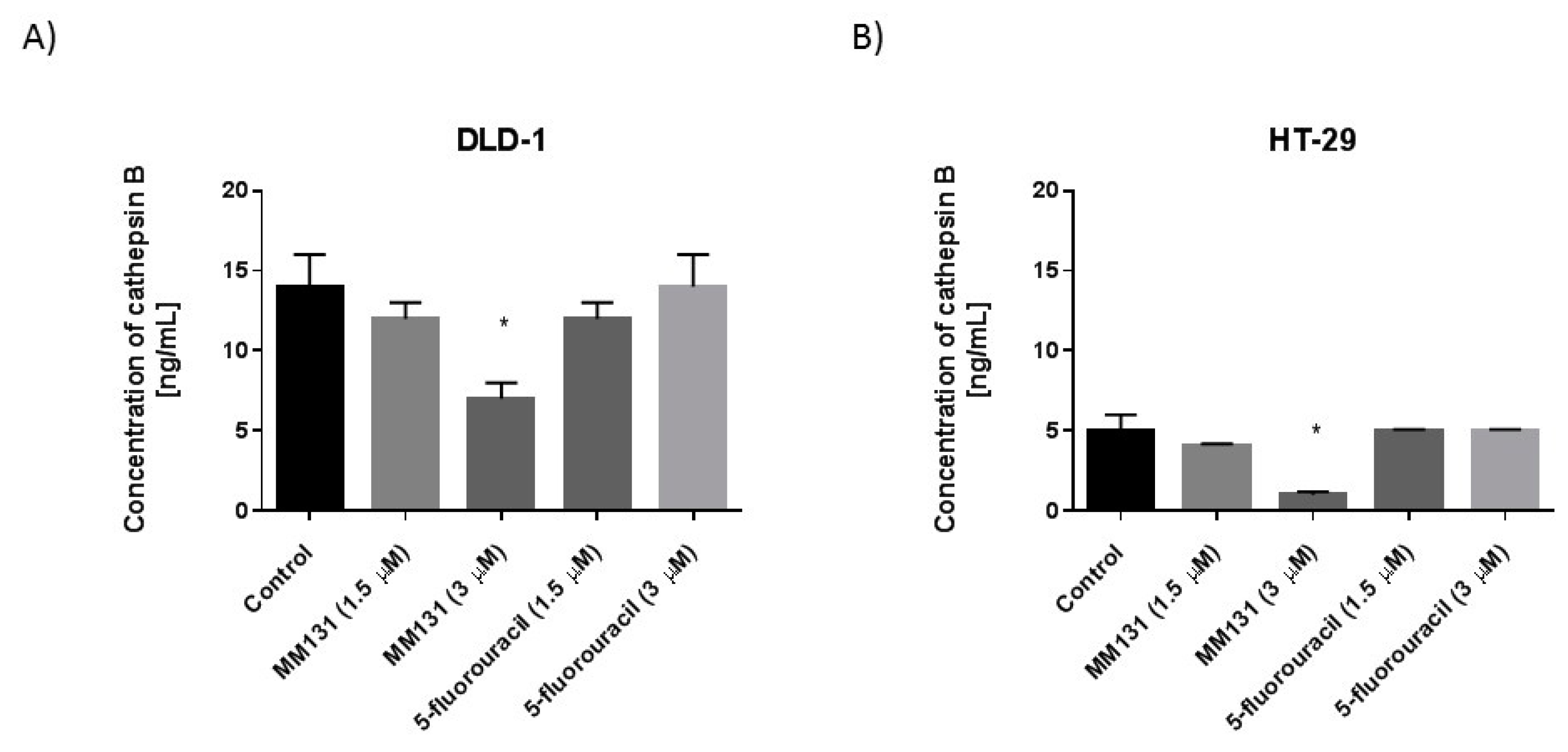
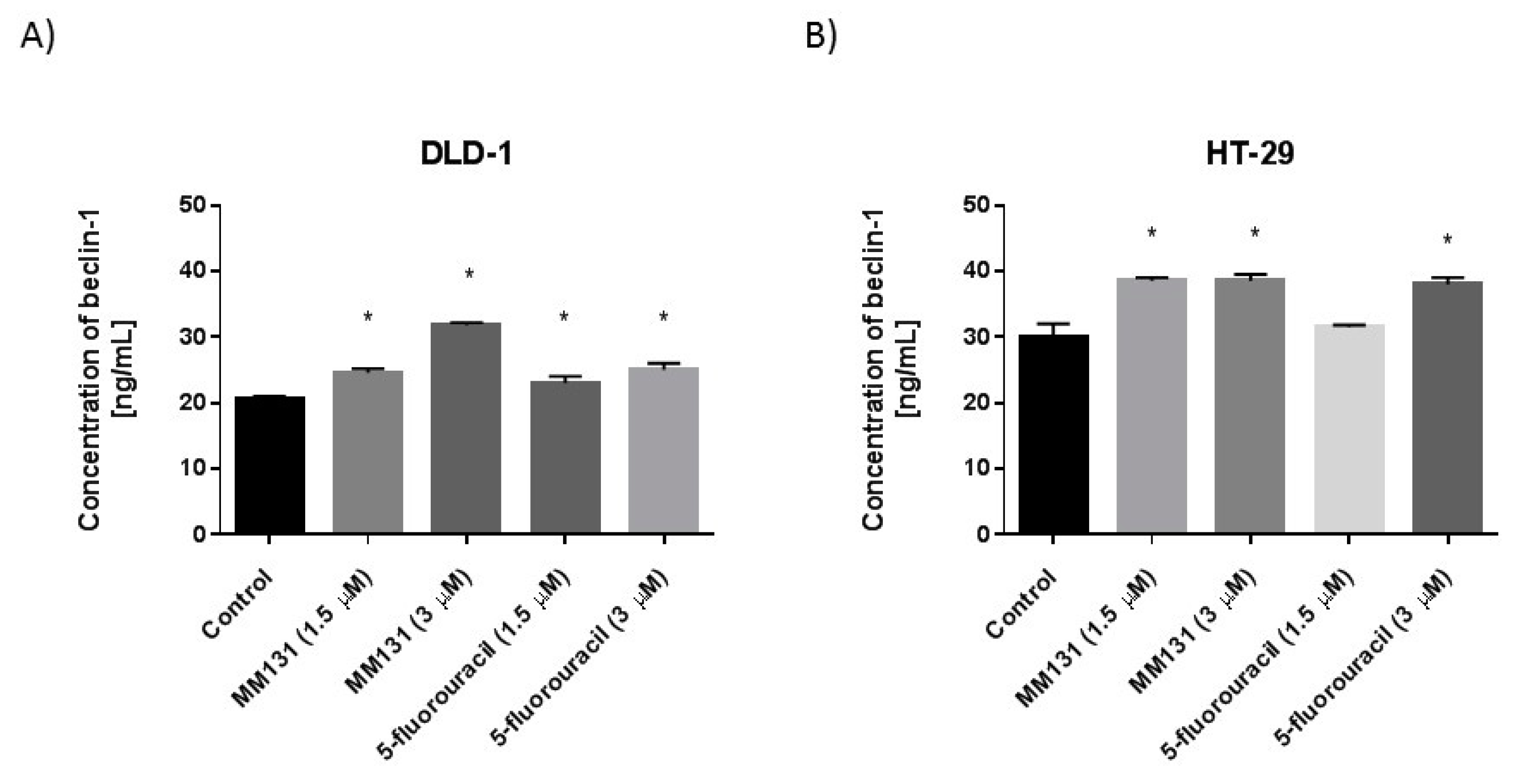
2.2. Biological Studies
3. Discussion
4. Materials and Methods
4.1. MM131 Synthesis
4.1.1. General
4.1.2. Synthesis of N-(R)-(1-hydroxypropan-2-yl)-4-(3-methyl-5-methylsulfonyl-1H-pyrazolo[4,3-e][1,2,4]triazyn-1-yl)benzenesulfonamide (2)
4.1.3. Synthesis of Tricyclic Sulfonamides (MM131)
4.2. Cell Culture of HT-29 and DLD-1 Cells
4.3. Cell Viability Assay
4.4. [3H]-Thymidine Incorporation Assay
4.5. Flow Cytometry Assessment of Annexin V Binding
4.6. Acridine Orange/ethidium Bromide Fluorescent Staining
4.7. Analysis of Caspase-8 Enzymatic Activity
4.8. Caspase-9 Enzymatic Activity Assay
4.9. Caspase-3/7 Enzymatic Activity Assay
4.10. Cell Cycle Analysis
4.11. Determination of sICAM-1, mTOR, Cathepsin B, Beclin-1
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| 13C-NMR | Carbon-13 Nuclear Magnetic Resonance |
| 1H-NMR | Hydrogen-1 Nuclear Magnetic Resonance |
| anti-EGFR | anti-epidermal growth factor receptor therapy |
| APAF1 | apoptotic protease activating factor 1 |
| ATCC | American Type Culture Collection |
| CK2 | ubiquitous serine/threonine protein kinase |
| COX-2 | cyclooxygenase-2 |
| CRC | colorectal cancer |
| DLD-1 | colorectal cancer cell line |
| DMSO | dimethyl sulfoxide |
| EDTA | ethylenediamine tetraacetic acid |
| EGFR | epidermal growth factor receptor |
| ELISA | Enzyme Immunosorbent Assay |
| ERK 1/2 | extracellular signal-regulated kinase 1/2 |
| EtOH | Ethanol |
| FADD | Fas-associated protein with death domain |
| FDA | Food and Drug Administration |
| FGFR | fibroblast growth factor receptor |
| GSK-3β | glycogen synthase kinase 3 β |
| H1299 | non-small cell lung carcinoma cell line |
| HRP | horseradish peroxidase |
| HT-29 | colorectal cancer cell line |
| IAP | inhibitors of apoptosis proteins |
| IC50 | half maximal inhibitory concentration |
| JAK | janus-activated kinases |
| MAPK | mitogen-activated protein kinase |
| MDA-MB-435 | melanoma cancer cell line |
| MeCN | acetonitrile |
| MMP-2 | matrix metalloproteinase 2 |
| MMP-9 | matrix metalloproteinase 9 |
| mTOR | mechanistic target of rapamycin |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NaN3 | Sodium azide |
| PBS | Phosphate Buffered Saline |
| PI | Propidium Iodide |
| PI3K | phosphoinositide 3-kinase |
| Pim-1 | proviral integration site for Moloney murine leukemia virus-1 kinase |
| rt | room temperature |
| SD | Standard Deviation |
| SDS | Sodium Dodecyl Sulfate |
| SEM | standard error of the mean |
| sICAM-1 | soluble intercellular adhesion molecule-1 |
| SYK | spleen tyrosine kinase |
| T-47D | breast cancer cell line |
| TCA | Trichloroacetic acid |
| Tie-2 | tyrosine kinase with immunoglobulin and epidermal growth factorhomology domains-2 |
| TLC | thin layer chromatography |
| TMB | Tetramethylbenzidine |
| TMS | tetramethylsilane |
| TNF | tumor necrosis factor |
| TRAIL | tumor necrosis factor (TNF)-related apoptosis-inducing ligand |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
References
- Favoriti, P.; Carbone, G.; Greco, M.; Pirozzi, F.; Pirozzi, R.E.M.; Corcione, F. Worldwide burden of colorectal cancer: A review. Updat. Surg. 2016, 68, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Kwong, T.N.; Wang, X.; Nakatsu, G.; Chow, T.C.; Tipoe, T.; Dai, R.Z.; Tsoi, K.K.; Wong, M.C.; Tse, G.; Chan, M.T.; et al. Association Between Bacteremia From Specific Microbes and Subsequent Diagnosis of Colorectal Cancer. Gastroenterology 2018, 155, 383–390.e8. [Google Scholar] [CrossRef] [PubMed]
- Yothers, G.; O’Connell, M.J.; Allegra, C.J.; Kuebler, J.P.; Colangelo, L.H.; Petrelli, N.J.; Wolmark, N. Oxaliplatin As Adjuvant Therapy for Colon Cancer: Updated Results of NSABP C-07 Trial, Including Survival and Subset Analyses. J. Clin. Oncol. 2011, 29, 3768–3774. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Huijberts, S.C.; Van Geel, R.M.; Bernards, R.; Beijnen, J.H.; Steeghs, N. Encorafenib, binimetinib and cetuximab combined therapy for patients with BRAFV600E mutant metastatic colorectal cancer. Futur. Oncol. 2020, 16, 161–173. [Google Scholar] [CrossRef]
- Sun, Z.; Qiu, Z.; Ma, B.; Wang, Z. Encorafenib enhances TRAIL-induced apoptosis of colorectal cancer cells dependent on p53/PUMA signaling. Cytotechnology 2020, 73, 1–8. [Google Scholar] [CrossRef]
- Bouchain, G.; Delorme, D. Novel hydroxamate and anilide derivatives as potent histone deacetylase inhibitors: Synthesis and antiproliferative evaluation. Curr. Med. Chem. 2003, 10, 2359–2372. [Google Scholar] [CrossRef]
- Mojzych, M.; Dolashki, A.; Voelter, W. Synthesis of pyrazolo[4,3- e ][1,2,4]triazine sulfonamides, novel Sildenafil analogs with tyrosinase inhibitory activity. Bioorganic Med. Chem. 2014, 22, 6616–6624. [Google Scholar] [CrossRef]
- Cheng, X.-C.; Wang, Q.; Fang, H.; Xu, W.-F. Role of sulfonamide group in matrix metalloproteinase inhibitors. Curr. Med. Chem. 2008, 15, 368–373. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Xiao, M.; Li, W.; Miller, D.D. An Overview of Tubulin Inhibitors That Interact with the Colchicine Binding Site. Pharm. Res. 2012, 29, 2943–2971. [Google Scholar] [CrossRef]
- Koyuncu, I.; Gonel, A.; Kocyigit, A.; Temiz, E.; Durgun, M.; Supuran, C.T. Selective inhibition of carbonic anhydrase-IX by sulphonamide derivatives induces pH and reactive oxygen species-mediated apoptosis in cervical cancer HeLa cells. J. Enzym. Inhib. Med. Chem. 2018, 33, 1137–1149. [Google Scholar] [CrossRef]
- Mboge, M.Y.; McKenna, R.; Frost, S.C.; Bentham Science Publisher Atta-ur-Rahman; Zaman, K. Advances in Anti-Cancer Drug Development Targeting Carbonic Anhydrase IX and XII. Top. Anti-Cancer Res. 2016, 5, 3–42. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kakutani, S.; Sato, Y.; Hanashi, A.; Kinoshita, Y.; Ishikawa, A. Drug review: Pazopanib. Jpn. J. Clin. Oncol. 2018, 48, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Gimsing, P. Belinostat: A new broad acting antineoplastic histone deacetylase inhibitor. Expert Opin. Investig. Drugs 2009, 18, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Z.; Kwitkowski, V.E.; Del Valle, P.L.; Ricci, M.S.; Saber, H.; Habtemariam, B.A.; Bullock, J.; Bloomquist, E.; Shen, Y.L.; Chen, X.-H.; et al. FDA Approval: Belinostat for the Treatment of Patients with Relapsed or Refractory Peripheral T-cell Lymphoma. Clin. Cancer Res. 2015, 21, 2666–2670. [Google Scholar] [CrossRef] [PubMed]
- Medina, T.; Amaria, M.N.; Jimeno, A. Dabrafenib in the treatment of advanced melanoma. Drugs Today 2013, 49, 377–385. [Google Scholar]
- Swaika, A.; Crozier, J.A.; Joseph, R.W. Vemurafenib: An evidence-based review of its clinical utility in the treatment of metastatic melanoma. Drug. Des. Devel. Ther. 2014, 8, 775–787. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Jin, S.; Abraham, V.; Huang, X.; Liu, B.; Mitten, M.J.; Nimmer, P.; Lin, X.; Smith, M.L.; Shen, Y.; et al. The Bcl-2/Bcl-XL/Bcl-w Inhibitor, Navitoclax, Enhances the Activity of Chemotherapeutic Agents In Vitro and In Vivo. Mol. Cancer Ther. 2011, 10, 2340–2349. [Google Scholar] [CrossRef]
- Rudin, C.M.; Hann, C.L.; Garon, E.B.; De Oliveira, M.R.; Bonomi, P.D.; Camidge, D.R.; Chu, Q.; Giaccone, G.; Khaira, D.; Ramalingam, S.S.; et al. Phase II Study of Single-Agent Navitoclax (ABT-263) and Biomarker Correlates in Patients with Relapsed Small Cell Lung Cancer. Clin. Cancer Res. 2012, 18, 3163–3169. [Google Scholar] [CrossRef]
- Kipps, T.J.; Eradat, H.; Grosicki, S.; Catalano, J.; Cosolo, W.; Dyagil, I.S.; Yalamanchili, S.; Chai, A.; Sahasranaman, S.; Punnoose, E.; et al. A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leuk. Lymphoma 2015, 56, 2826–2833. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Parrino, B.; Spanò, V.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G. Synthesis and antitumor activities of 1,2,3-triazines and their benzo- and heterofused derivatives. Eur. J. Med. Chem. 2017, 142, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Parrino, B.; Spanò, V.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G. An overview on the recent developments of 1,2,4-triazine derivatives as anticancer compounds. Eur. J. Med. Chem. 2017, 142, 328–375. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Parrino, B.; Spanò, V.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G. 1,3,5-Triazines: A promising scaffold for anticancer drugs development. Eur. J. Med. Chem. 2017, 142, 523–549. [Google Scholar] [CrossRef]
- Gornowicz, A.; Szymanowska, A.; Mojzych, M.; Bielawski, K.; Bielawska, A. The Effect of Novel 7-methyl-5-phenyl-pyrazolo[4,3-e]tetrazolo[4,5-b][1,2,4]triazine Sulfonamide Derivatives on Apoptosis and Autophagy in DLD-1 and HT-29 Colon Cancer Cells. Int. J. Mol. Sci. 2020, 21, 5221. [Google Scholar] [CrossRef] [PubMed]
- Hermanowicz, J.M.; Szymanowska, A.; Sieklucka, B.; Czarnomysy, R.; Pawlak, K.; Bielawska, A.; Bielawski, K.; Kalafut, J.; Przybyszewska, A.; Surażyński, A.; et al. Exploration of novel heterofused 1,2,4-triazine derivative in colorectal cancer. J. Enzyme Inhib. 2021, 36, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Rykowski, A.; Mojzych, M.; Karczmarzyk, Z. A New Synthesis of Pyrazolo- [4,3-e][1,2,4]triazines via Acid Promoted Ring Closure of the Phenylhydrazones of 5-Acyl-1,2,4-triazines. Heterocycles 2000, 53, 2175. [Google Scholar] [CrossRef]
- Rykowski, A.; Mojzych, M. Synthesis of Functionalized 1H-Pyrazolo[4,3-e][1,2,4]triazines and Their Fused Derivatives via Ipso-Substitution of Methylsulfonyl Group with O-, N-, S- and C-Nucleophiles. Heterocycles 2004, 63, 1829. [Google Scholar] [CrossRef]
- Mojzych, M.; Rykowski, A. Transformations of phenylhydrazones of 5-acyl-1,2,4-triazines to pyrazolo[4,3-e][1,2,4]triazines or 4-cyanopyrazole. J. Heterocycl. Chem. 2007, 44, 1003–1007. [Google Scholar] [CrossRef]
- Mojzych, M.; Ceruso, M.; Bielawska, A.; Bielawski, K.; Fornal, E.; Supuran, C.T. New pyrazolo[4,3 e][1,2,4]triazine sulfona-mides as carbonic anhydrase inhibitors. Bioorg. Med. Chem. 2015, 23, 3674–3680. [Google Scholar] [CrossRef] [PubMed]
- Bernat, Z.; Szymanowska, A.; Kciuk, M.; Kotwica-Mojzych, K.; Mojzych, M. Review of the Synthesis and Anticancer Properties of Pyrazolo[4,3-e][1,2,4]triazine Derivatives. Molecules 2020, 25, 3948. [Google Scholar] [CrossRef]
- Koehler, B.C.; Jäger, D.; Schulze-Bergkamen, H. Targeting cell death signaling in colorectal cancer: Current strategies and future perspectives. World J Gastroenterol. 2014, 28, 1923–1934. [Google Scholar] [CrossRef]
- Sale, M.J.; Cook, S.J. The BH3 mimetic ABT-263 synergizes with the MEK1/2 inhibitor selumetinib/AZD6244 to promote BIM-dependent tumour cell death and inhibit acquired resistance. Biochem. J. 2013, 450, 285–294. [Google Scholar] [CrossRef]
- Shima, H.; Tsurita, G.; Wada, S.; Hirohashi, Y.; Yasui, H.; Hayashi, H.; Miyakoshi, T.; Watanabe, K.; Murai, A.; Asanuma, H.; et al. Randomized phase II trial of survivin 2B peptide vaccination for patients with HLA -A24-positive pancreatic adenocarcinoma. Cancer Sci. 2019, 110, 2378–2385. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 1–19. [Google Scholar] [CrossRef]
- Tian, T.; Li, X.; Zhang, J. mTOR Signaling in cancer and mTOR inhibitors in solid tumor targeting therapy. Int. J. Mol. Sci. 2019, 20, 755. [Google Scholar] [CrossRef]
- Pópulo, H.; Lopes, J.M.; Soares, P. The mTOR Signalling Pathway in Human Cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef]
- Schellerer, V.S.; Langheinrich, M.C.; Zver, V.; Grützmann, R.; Stürzl, M.; Gefeller, O.; Naschberger, E.; Merkel, S. Soluble intercellular adhesion molecule-1 is a prognostic marker in colorectal carcinoma. Int. J. Color. Dis. 2019, 34, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Won, K.Y.; Kim, G.Y.; Lim, S.-J.; Kim, Y.W. Decreased Beclin-1 expression is correlated with the growth of the primary tumor in patients with squamous cell carcinoma and adenocarcinoma of the lung. Hum. Pathol. 2012, 43, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, L.; Zhao, S.; Guo, X.; Xu, Y.; Zheng, Z.; Lu, H.; Zheng, H. Effects of Beclin 1 overexpression on aggressive phenotypes of colon cancer cells. Oncol. Lett. 2018, 17, 2441–2450. [Google Scholar] [CrossRef]
- Sinha, A.A.; Gleason, D.F.; DeLeon, O.F.; Wilson, M.J.; Sloane, B.F. Localization of a biotinylated cathepsin B oligonucleotide probe in human prostate including invasive cells and invasive edges by in situ hybridization. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1993, 235, 233–240. [Google Scholar] [CrossRef] [PubMed]
- A Rempel, S.; Rosenblum, M.L.; Mikkelsen, T.; Yan, P.S.; Ellis, K.D.; A Golembieski, W.; Sameni, M.; Rozhin, J.; Ziegler, G.; Sloane, B.F. Cathepsin B expression and localization in glioma progression and invasion. Cancer Res. 1994, 54, 6027–6031. [Google Scholar]
- Matarrese, P.; Ascione, B.; Ciarlo, L.; Vona, R.; Leonetti, C.; Scarsella, M.; Mileo, A.M.; Catricalà, C.; Paggi, M.G.; Malorni, W. Cathepsin B inhibition interferes with metastatic potential of human melanoma: An in vitro and in vivo study. Mol. Cancer 2010, 9, 207. [Google Scholar] [CrossRef]
- Sevenich, L.; Schurigt, U.; Sachse, K.; Gajda, M.; Werner, F.; Müller, S.; Vasiljeva, O.; Schwinde, A.; Klemm, N.; Deussing, J.; et al. Synergistic antitumor effects of combined cathepsin B and cathepsin Z deficiencies on breast cancer progression and metastasis in mice. Proc. Natl. Acad. Sci. USA 2010, 107, 2497–2502. [Google Scholar] [CrossRef]
- Sevenich, L.; Werner, F.W.; Gajda, M.; Schurigt, U.; Sieber, C.; Muller, S.C.; Follo, M.Y.; Peters, C.; Reinheckel, T. Transgenic expression of human cathepsin B promotes progression and metastasis of polyoma-middle-T-induced breast cancer in mice. Oncogene 2010, 30, 54–64. [Google Scholar] [CrossRef]
- Campo, E.; Muñoz, J.; Miquel, R.; Palacín, A.; Cardesa, A.; Sloane, B.F.; Emmert-Buck, M.R. Cathepsin B expression in colorectal carcinomas correlates with tumor progression and shortened patient survival. Am. J. Pathol. 1994, 145, 301–309. [Google Scholar]
- Ruan, H.; Hao, S.; Young, P.; Zhang, H. Targeting Cathepsin B for Cancer Therapies. Horizons Cancer Res. 2015, 56, 23–40. [Google Scholar]
- Gornowicz, A.; Szymanowski, W.; Bielawska, A.; Szymanowska, A.; Czarnomysy, R.; Kałuża, Z.; Bielawski, K. Monoclonal anti-MUC1 antibody with novel octahydropyrazino[2,1-a:5,4-a’]diisoquinoline derivative as a potential multi-targeted strategy in MCF-7 breast cancer cells. Oncol. Rep. 2019, 42, 1391–1403. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, N.; Gornowicz, A.; Bielawska, A.; Surażyński, A.; Szymanowska, A.; Czarnomysy, R.; Bielawski, K. The molecular mechanism of anticancer action of novel octahydropyrazino[2,1-a:5,4-a’]diisoquinoline derivatives in human gastric cancer cells. Investig. New Drugs. 2018, 36, 970–984. [Google Scholar] [CrossRef] [PubMed]
- Lepiarczyk, M.; Kałuża, Z.; Bielawska, A.; Czarnomysy, R.; Gornowicz, A.; Bielawski, K. Cytotoxic Activity of octahydropy-razin[2,1-a:5,4-a’]diisoquinoline derivatives in human breast cancer cells. Arch. Pharm. Res. 2015, 38, 628–641. [Google Scholar] [CrossRef]
- Gornowicz, A.; Bielawska, A.; Czarnomysy, R.; Gabryel-Porowska, H.; Muszyńska, A.; Bielawski, K. The combined treatment with novel platinum(II) complex and anti-MUC1 increases apoptotic response in MDA-MB-231 breast cancer cells. Mol. Cell. Biochem. 2015, 408, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Czajkowska, A.; Gornowicz, A.; Pawłowska, N.; Czarnomysy, R.; Nazaruk, J.; Szymanowski, W.; Bielawska, A.; Bielawski, K. Anticancer Effect of a Novel Octahydropyrazino[2,1-a:5,4-a′]diisoquinoline Derivative and Its Synergistic Action with Nigella sativa in Human Gastric Cancer Cells. BioMed Res. Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gornowicz, A.; Pawłowska, N.; Czajkowska, A.; Czarnomysy, R.; Bielawska, A.; Bielawski, K.; Michalak, O.; Staszewska-Krajewska, O.; Kałuża, Z. Biological evaluation of octahydropyrazin[2,1-a:5,4-a′]diisoquinoline derivatives as potent anticancer agents. Tumor Biol. 2017, 39, 1010428317701641. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gornowicz, A.; Szymanowska, A.; Mojzych, M.; Czarnomysy, R.; Bielawski, K.; Bielawska, A. The Anticancer Action of a Novel 1,2,4-Triazine Sulfonamide Derivative in Colon Cancer Cells. Molecules 2021, 26, 2045. https://doi.org/10.3390/molecules26072045
Gornowicz A, Szymanowska A, Mojzych M, Czarnomysy R, Bielawski K, Bielawska A. The Anticancer Action of a Novel 1,2,4-Triazine Sulfonamide Derivative in Colon Cancer Cells. Molecules. 2021; 26(7):2045. https://doi.org/10.3390/molecules26072045
Chicago/Turabian StyleGornowicz, Agnieszka, Anna Szymanowska, Mariusz Mojzych, Robert Czarnomysy, Krzysztof Bielawski, and Anna Bielawska. 2021. "The Anticancer Action of a Novel 1,2,4-Triazine Sulfonamide Derivative in Colon Cancer Cells" Molecules 26, no. 7: 2045. https://doi.org/10.3390/molecules26072045
APA StyleGornowicz, A., Szymanowska, A., Mojzych, M., Czarnomysy, R., Bielawski, K., & Bielawska, A. (2021). The Anticancer Action of a Novel 1,2,4-Triazine Sulfonamide Derivative in Colon Cancer Cells. Molecules, 26(7), 2045. https://doi.org/10.3390/molecules26072045






