Poly(Methyl Methacrylate) Coatings Containing Flame Retardant Additives from Suspensions in Water-2-Propanol
Abstract
1. Introduction
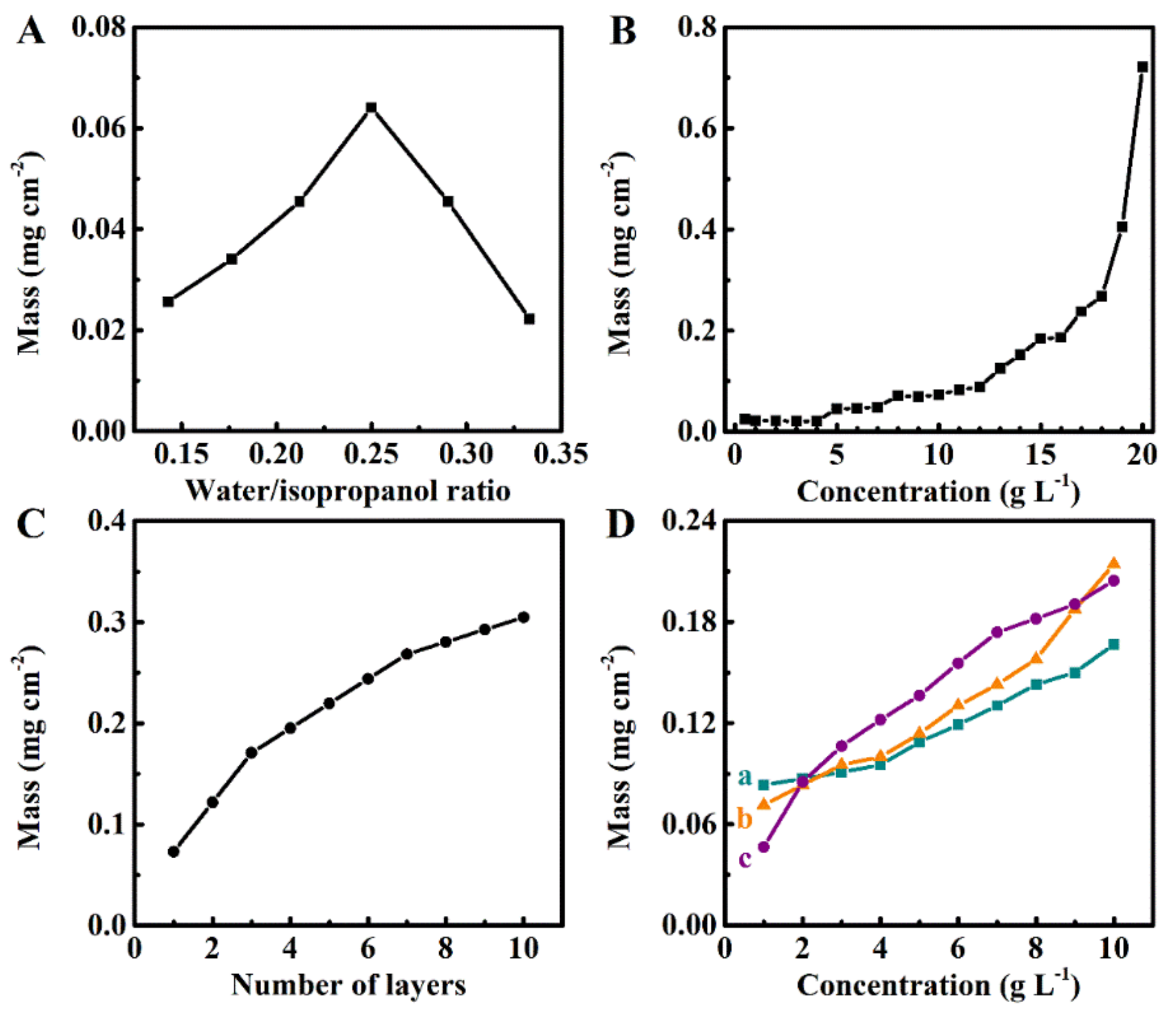
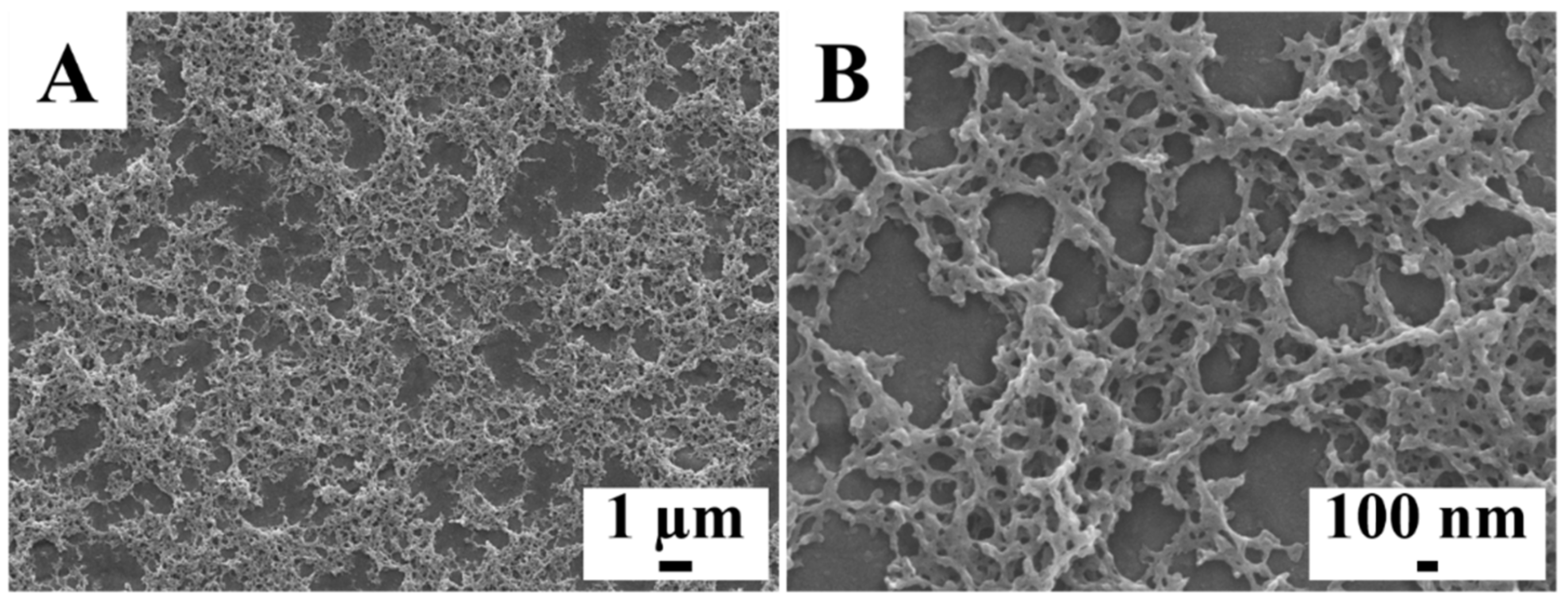
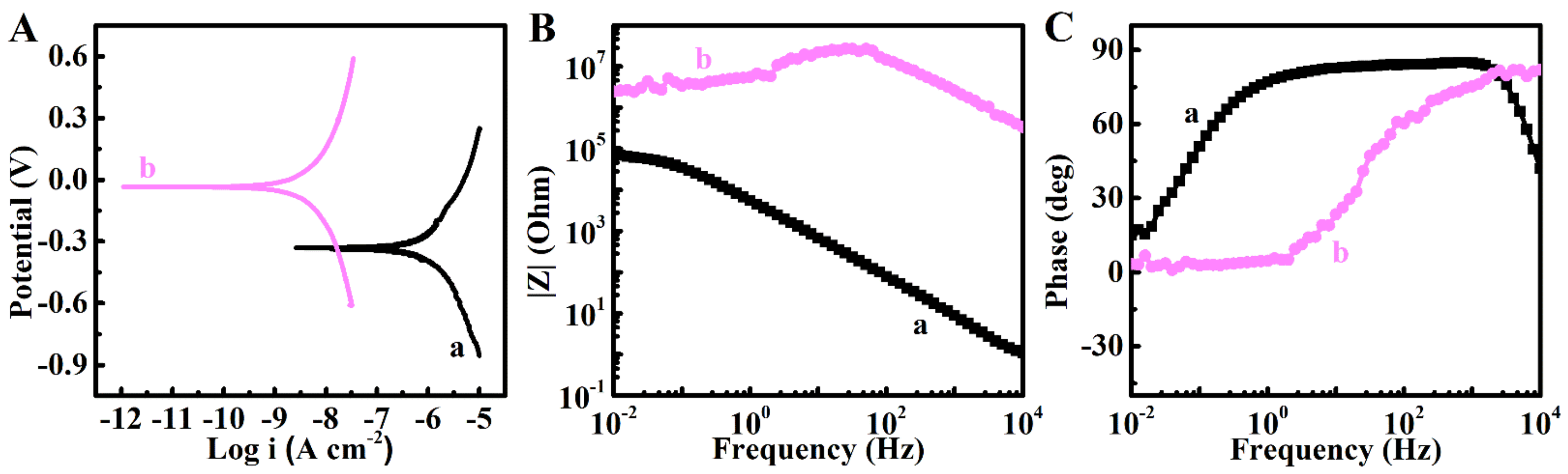
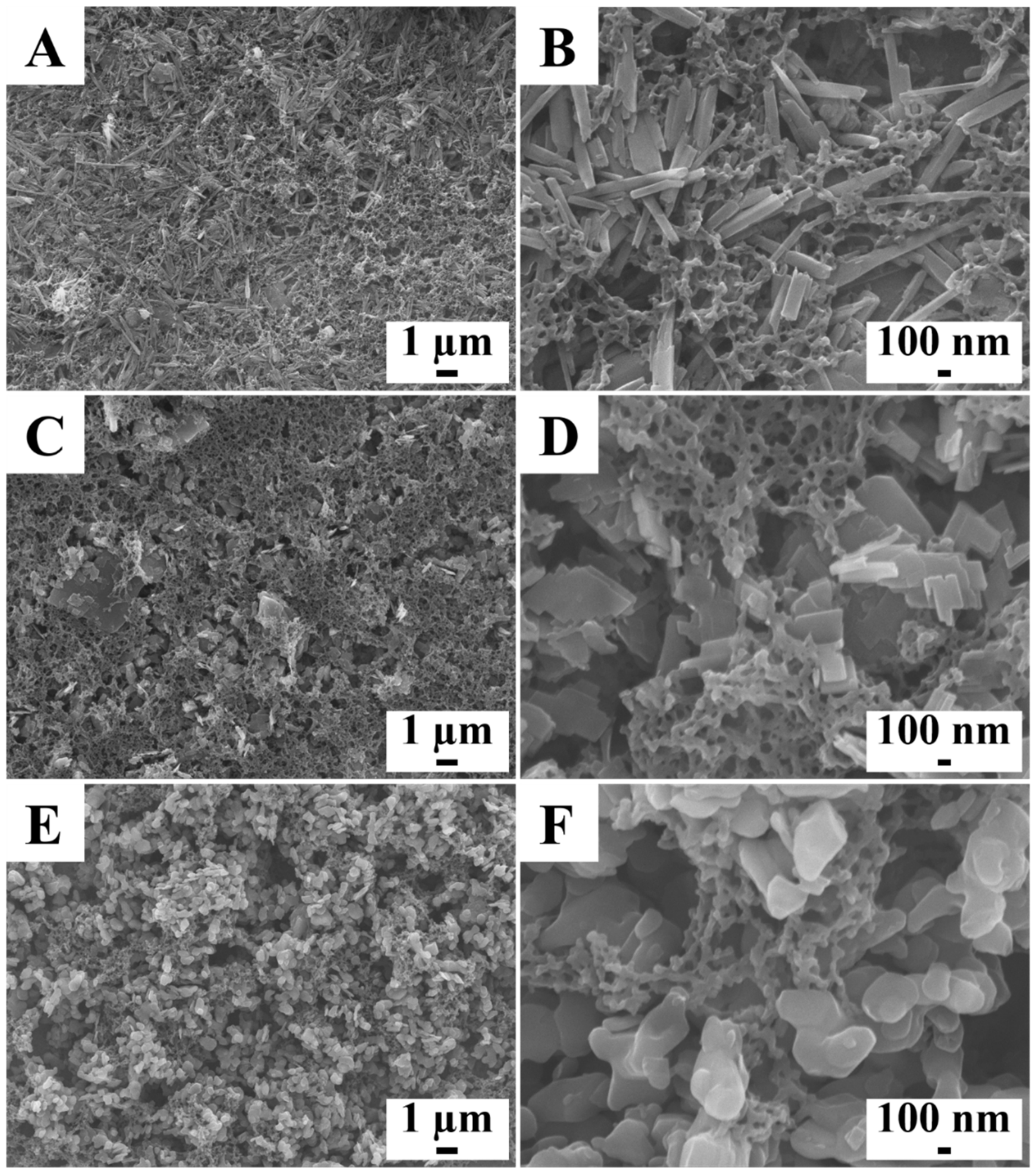
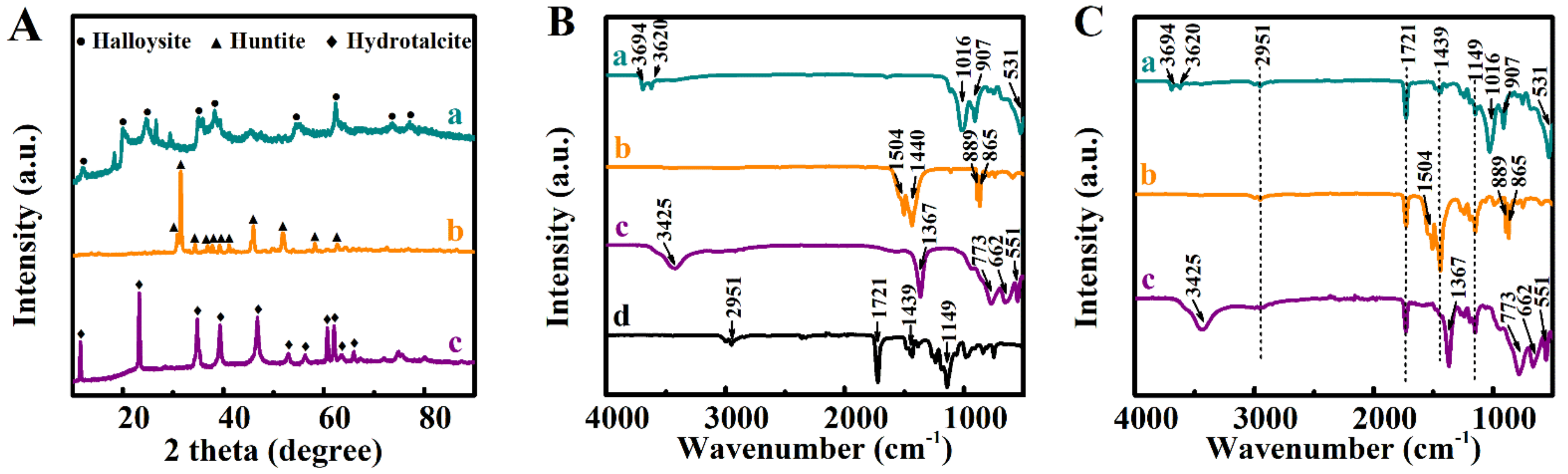
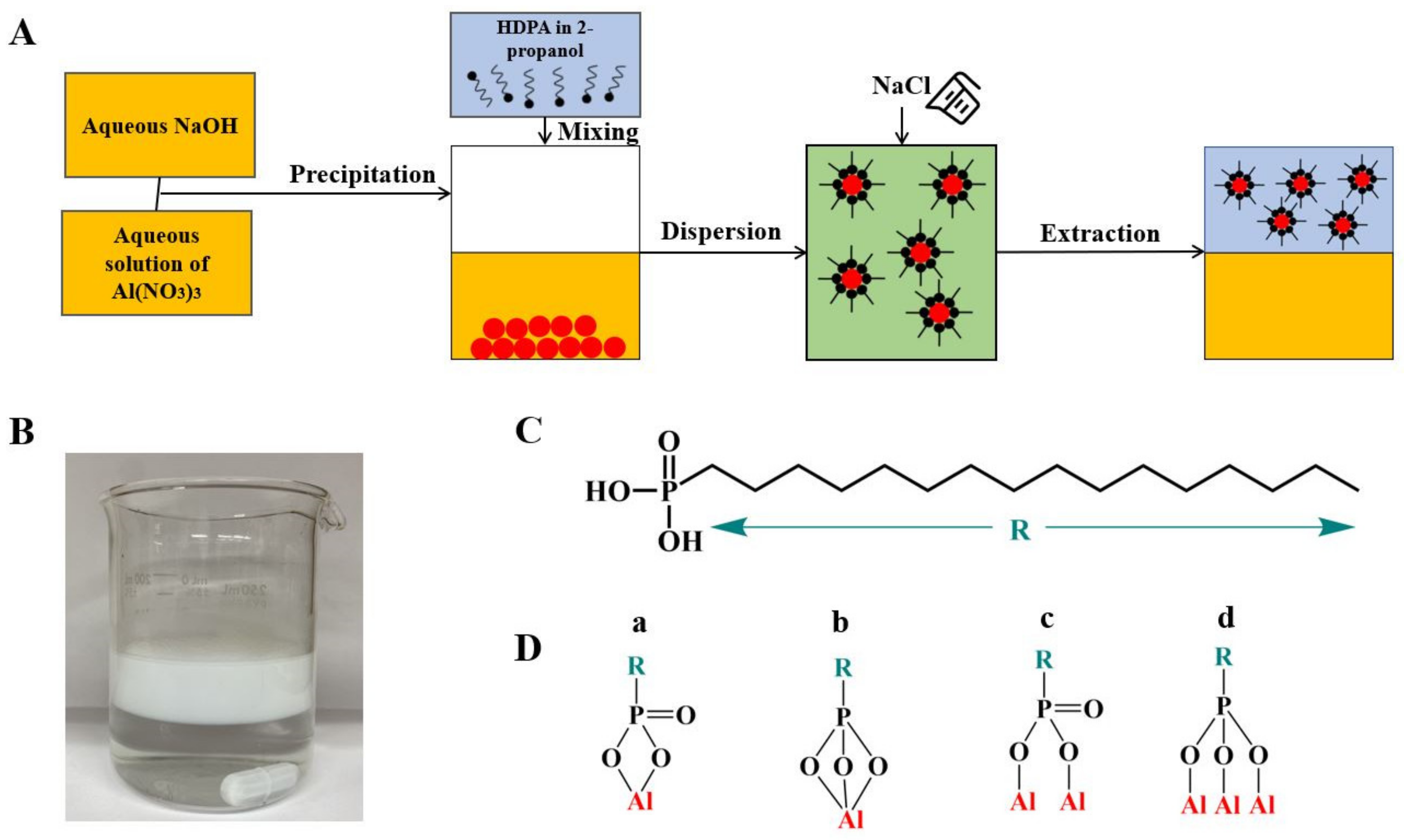
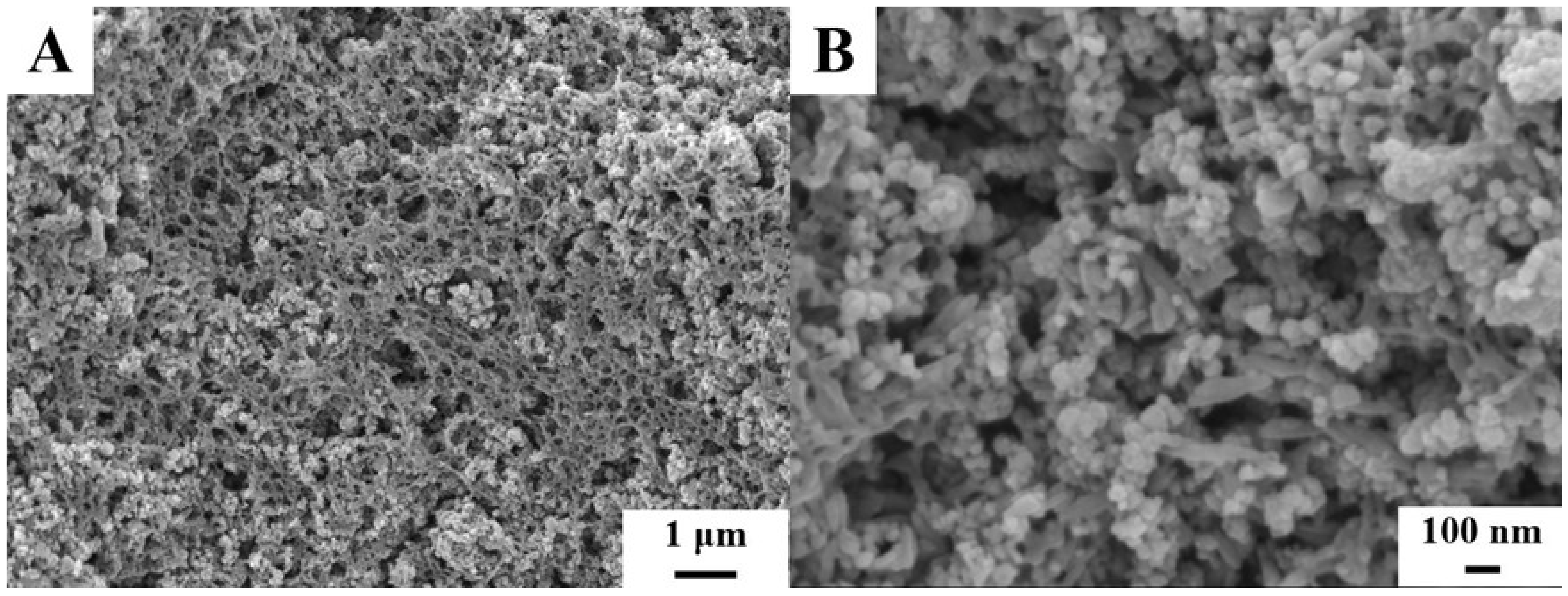
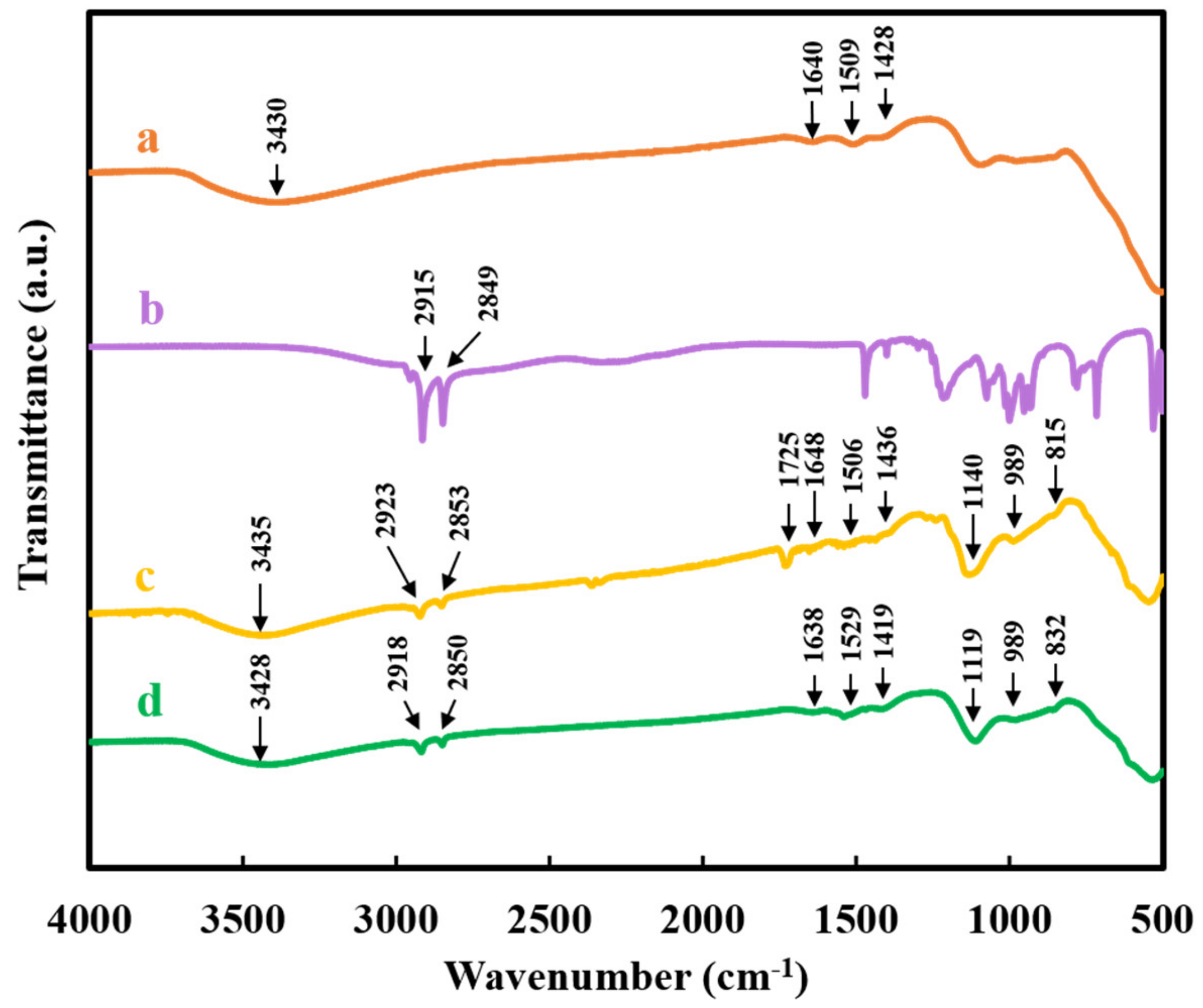
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A review of the properties and applications of poly (methyl methacrylate)(PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Jayarama Krishna, J.V.; Srivatsa Kumar, S.; Korobeinichev, O.P.; Vinu, R. Detailed kinetic analysis of slow and fast pyrolysis of poly(methyl methacrylate)-Flame retardant mixtures. Thermochim. Acta 2020, 687, 178545. [Google Scholar] [CrossRef]
- Wong, F.; Kurt-Karakus, P.; Bidleman, T.F. Fate of brominated flame retardants and organochlorine pesticides in urban soil: Volatility and degradation. Environ. Sci. Technol. 2012, 46, 2668–2674. [Google Scholar] [CrossRef]
- Xiao, H.; Shen, L.; Su, Y.; Barresi, E.; DeJong, M.; Hung, H.; Lei, Y.-D.; Wania, F.; Reiner, E.J.; Sverko, E. Atmospheric concentrations of halogenated flame retardants at two remote locations: The Canadian High Arctic and the Tibetan Plateau. Environ. Pollut. 2012, 161, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Clifford, A.; Milne, J.; Mathews, R.; Zhitomirsky, I. Colloidal-electrochemical fabrication strategies for functional composites of linear polyethylenimine. J. Colloid Interface Sci. 2019, 552, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Goda, E.S.; Yoon, K.R.; El-sayed, S.H.; Hong, S.E. Halloysite nanotubes as smart flame retardant and economic reinforcing materials: A review. Thermochim. Acta 2018, 669, 173–184. [Google Scholar] [CrossRef]
- Wang, Y.; Deen, I.; Zhitomirsky, I. Electrophoretic deposition of polyacrylic acid and composite films containing nanotubes and oxide particles. J. Colloid Interface Sci. 2011, 362, 367–374. [Google Scholar] [CrossRef]
- Xuteng, X.; Xiaoyang, X.; Jihui, W.; Wenbin, H. Preparation, release and anticorrosion behavior of a multi-corrosion inhibitors-halloysite nanocomposite. Chem. Phys. Lett. 2019, 718, 69–73. [Google Scholar]
- Yildirim, S.; Celik, E. Production and characterization of the halogen-free and nanostructured flame retardant reinforced composite coatings. J. Aust. Ceram. Soc. 2020, 56, 683–695. [Google Scholar] [CrossRef]
- Savas, L.A.; Arslan, C.; Hacioglu, F.; Dogan, M. Effect of reactive and nonreactive surface modifications and compatibilizer use on mechanical and flame-retardant properties of linear low-density polyethylene filled with huntite and hydromagnesite mineral. J. Therm. Anal. Calorim. 2018, 134, 1657–1666. [Google Scholar] [CrossRef]
- Erdem, A.; Dogan, M. Production and Characterization of Green Flame Retardant Poly(lactic acid) Composites. J. Polym. Environ. 2020, 28, 2837–2850. [Google Scholar] [CrossRef]
- Zhang, T.; Luo, D.; Wojtal, P.; Zhitomirsky, I. Electrophoretic deposition of flame retardant polymer–huntite coatings. Mater. Lett. 2015, 159, 106–109. [Google Scholar] [CrossRef]
- Du, J.-Z.; Jin, L.; Zeng, H.-Y.; Feng, B.; Xu, S.; Zhou, E.-G.; Shi, X.-K.; Liu, L.; Hu, X. Facile preparation of an efficient flame retardant and its application in ethylene vinyl acetate. Appl. Sci. 2019, 168, 96–105. [Google Scholar] [CrossRef]
- Xu, S.; Li, S.-Y.; Zhang, M.; Zeng, H.-Y.; Wu, K.; Tian, X.-Y.; Chen, C.-R.; Pan, Y. Fabrication of green alginate-based and layered double hydroxides flame retardant for enhancing the fire retardancy properties of polypropylene. Carbohydr. Polym. 2020, 234, 115891. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liao, M.-C.; Zeng, H.-Y.; Zhang, Z.-Q.; Liu, X.-J.; Zhu, P.-H. Ultrafine hydrotalcite particles prepared with novel technology to improve the flame retardancy of polypropylene. Appl. Clay Sci. 2015, 108, 215–221. [Google Scholar] [CrossRef]
- Elbasuney, S. Novel multi-component flame retardant system based on nanoscopic aluminium-trihydroxide (ATH). Powder Technol. 2017, 305, 538–545. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, Y. A study of the flame-retardant properties of polypropylene/Al(OH)3/Mg(OH)2 composites. Polym. Int. 2010, 59, 539–542. [Google Scholar] [CrossRef]
- Silva, R.M.E.; Poon, R.; Milne, J.; Syed, A.; Zhitomirsky, I. New developments in liquid-liquid extraction, surface modification and agglomerate-free processing of inorganic particles. Adv. Colloid Interface Sci. 2018, 261, 15–27. [Google Scholar] [CrossRef]
- Cowie, J.M.; Mohsin, M.A.; McEwen, I.J. Alcohol-water cosolvent systems for poly (methyl methacrylate). Polymer 1987, 28, 1569–1572. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Becer, C.R.; Guerrero-Sanchez, C.; Hoeppener, S.; Schubert, U.S. Solubility and thermoresponsiveness of PMMA in alcohol-water solvent mixtures. Aust. J. Chem. 2010, 63, 1173–1178. [Google Scholar] [CrossRef]
- Zhang, Q.; Hoogenboom, R. Polymers with upper critical solution temperature behavior in alcohol/water solvent mixtures. Prog. Polym. Sci. 2015, 48, 122–142. [Google Scholar] [CrossRef]
- Zhitomirsky, I. Cathodic electrophoretic deposition of diamond particles. Mater. Lett. 1998, 37, 72–78. [Google Scholar] [CrossRef]
- Zhitomirsky, I.; Gal-Or, L. Formation of hollow fibers by electrophoretic deposition. Mater. Lett. 1999, 38, 10–17. [Google Scholar] [CrossRef]
- Huszánk, R.; Szilágyi, E.; Szoboszlai, Z.; Szikszai, Z. Investigation of chemical changes in PMMA induced by 1.6 MeV He+ irradiation by ion beam analytical methods (RBS-ERDA) and infrared spectroscopy (ATR-FTIR). Nucl. Instrum. Methods Phys. Res. B 2019, 450, 364–368. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Y.; Zhu, T.; Ruan, J.; Li, G. Effective solvent-free oxidation of cyclohexene to allylic products with oxygen by mesoporous etched halloysite nanotube supported Co2+. RSC Adv. 2018, 8, 14870–14878. [Google Scholar] [CrossRef]
- Tian, X.; Wang, J.; Zhang, H.; Cao, Z.; Zhao, M.; Guan, Y.; Zhang, Y. Establishment of transport channels with carriers for water in reverse osmosis membrane by incorporating hydrotalcite into the polyamide layer. RSC Adv. 2018, 8, 12439–12448. [Google Scholar] [CrossRef]
- Zhigang, T.; Rongqi, Z.; Zhanting, D. Separation of isopropanol from aqueous solution by salting-out extraction. J. Chem. Technol. Biotechnol. 2001, 76, 757–763. [Google Scholar] [CrossRef]
- Khayati, G.; Gholitabar, A. Liquid–liquid equilibrium of hydrophilic alcohols with three different salts of chloride: Experiment and correlation. J. Chem. Eng. Data 2016, 61, 1454–1461. [Google Scholar] [CrossRef]
- Ata, M.S.; Wojtal, P.; Zhitomirsky, I. Surface modification and electrophoretic deposition of materials using carboxyalkylphosphonic acids. Mater. Lett. 2016, 184, 320–323. [Google Scholar] [CrossRef]
- Pouran, H.M.; Banwart, S.A.; Romero-Gonzalez, M. Coating a polystyrene well-plate surface with synthetic hematite, goethite and aluminium hydroxide for cell mineral adhesion studies in a controlled environment. Appl. Geochem. 2014, 42, 60–68. [Google Scholar] [CrossRef]
- Liu, R.; Gong, W.; Lan, H.; Gao, Y.; Liu, H.; Qu, J. Defluoridation by freshly prepared aluminum hydroxides. Chem. Eng. J. 2011, 175, 144–149. [Google Scholar] [CrossRef]
- Beran, A.; Voll, D.; Schneider, H. Dehydration and structural development of mullite precursors: An FTIR spectroscopic study. J. Eur. Ceram. Soc. 2001, 21, 2479–2485. [Google Scholar] [CrossRef]
- Tokoro, C.; Suzuki, S.; Haraguchi, D.; Izawa, S. Silicate removal in aluminum hydroxide co-precipitation process. Materials 2014, 7, 1084–1096. [Google Scholar] [CrossRef]
- Milne, J.; Silva, R.M.; Zhitomirsky, I. Surface modification and dispersion of ceramic particles using liquid-liquid extraction method for application in supercapacitor electrodes. J. Eur. Ceram. Soc. 2019, 39, 3450–3455. [Google Scholar] [CrossRef]
- Milne, J.; Zhitomirsky, I. Application of octanohydroxamic acid for liquid-liquid extraction of manganese oxides and fabrication of supercapacitor electrodes. J. Colloid Interface Sci. 2018, 515, 50–57. [Google Scholar] [CrossRef]
- Zhitomirsky, I.; Petric, A. Electrochemical deposition of yttrium oxide. J. Mater. Chem. 2000, 10, 1215–1218. [Google Scholar] [CrossRef]
- Pang, X.; Zhitomirsky, I.; Niewczas, M. Cathodic electrolytic deposition of zirconia films. Surf. Coat. Technol. 2005, 195, 138–146. [Google Scholar] [CrossRef]
- Pletincx, S.; Marcoen, K.; Trotochaud, L.; Fockaert, L.-L.; Mol, J.M.; Head, A.R.; Karslioğlu, O.; Bluhm, H.; Terryn, H.; Hauffman, T. Unravelling the chemical influence of water on the PMMA/aluminum oxide hybrid interface in situ. Sci. Rep. 2017, 7, 13341. [Google Scholar] [CrossRef]
- Nunnery, G.; Hershkovits, E.; Tannenbaum, A.; Tannenbaum, R. Adsorption of poly(methyl methacrylate) on concave Al2O3 surfaces in nanoporous membranes. Langmuir 2009, 25, 9157–9163. [Google Scholar] [CrossRef]
- Tighilt, F.-Z.; Gabouze, N.; Sam, S.; Belhousse, S.; Beldjilali, K. Morphology and specific interaction of PMMA coating with the surface of porous silicon. Surf. Sci. 2007, 601, 4217–4221. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, J.; Liu, X.; Liu, R. Polyurethane modified epoxy acrylate resins containing ε-caprolactone unit. Progress Org. Coat. 2020, 141, 105543. [Google Scholar] [CrossRef]
- Lo, T.N.; Hwang, H.S.; Lee, J.; Park, I. Synthesis of new semi-fluorinated polysilazanes and their amphiphobic coating applications. Prog. Org. Coat. 2020, 148, 105853. [Google Scholar] [CrossRef]
- Shen, Z.; Wu, Y.; Qiu, S.; Deng, H.; Hou, R.; Zhu, Y. UV-thermal dual-cured polymers with degradable and anti-bacterial function. Prog. Org. Coat. 2020, 148, 105783. [Google Scholar] [CrossRef]
- Deen, I.; Pang, X.; Zhitomirsky, I. Electrophoretic deposition of composite chitosan–halloysite nanotube–hydroxyapatite films. Colloids Surf. A Physicochem. Eng. Aspects 2012, 410, 38–44. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, X.; Veldhuis, S.; Zhitomirsky, I. Sodium deoxycholate as a versatile dispersing and coating-forming agent: A new facet of electrophoretic deposition technology. Colloids Surfaces A Physicochem. Eng. Aspects 2020, 588, 124382. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, Z.; Sakib, S.; Mathews, R.; Zhitomirsky, I. Poly(Methyl Methacrylate) Coatings Containing Flame Retardant Additives from Suspensions in Water-2-Propanol. Molecules 2021, 26, 1974. https://doi.org/10.3390/molecules26071974
Li X, Wang Z, Sakib S, Mathews R, Zhitomirsky I. Poly(Methyl Methacrylate) Coatings Containing Flame Retardant Additives from Suspensions in Water-2-Propanol. Molecules. 2021; 26(7):1974. https://doi.org/10.3390/molecules26071974
Chicago/Turabian StyleLi, Xuelin, Zhengzheng Wang, Sadman Sakib, Ritch Mathews, and Igor Zhitomirsky. 2021. "Poly(Methyl Methacrylate) Coatings Containing Flame Retardant Additives from Suspensions in Water-2-Propanol" Molecules 26, no. 7: 1974. https://doi.org/10.3390/molecules26071974
APA StyleLi, X., Wang, Z., Sakib, S., Mathews, R., & Zhitomirsky, I. (2021). Poly(Methyl Methacrylate) Coatings Containing Flame Retardant Additives from Suspensions in Water-2-Propanol. Molecules, 26(7), 1974. https://doi.org/10.3390/molecules26071974




