Computational Study of the Electron Spectra of Vapor-Phase Indole and Four Azaindoles
Abstract
1. Introduction
2. Methods
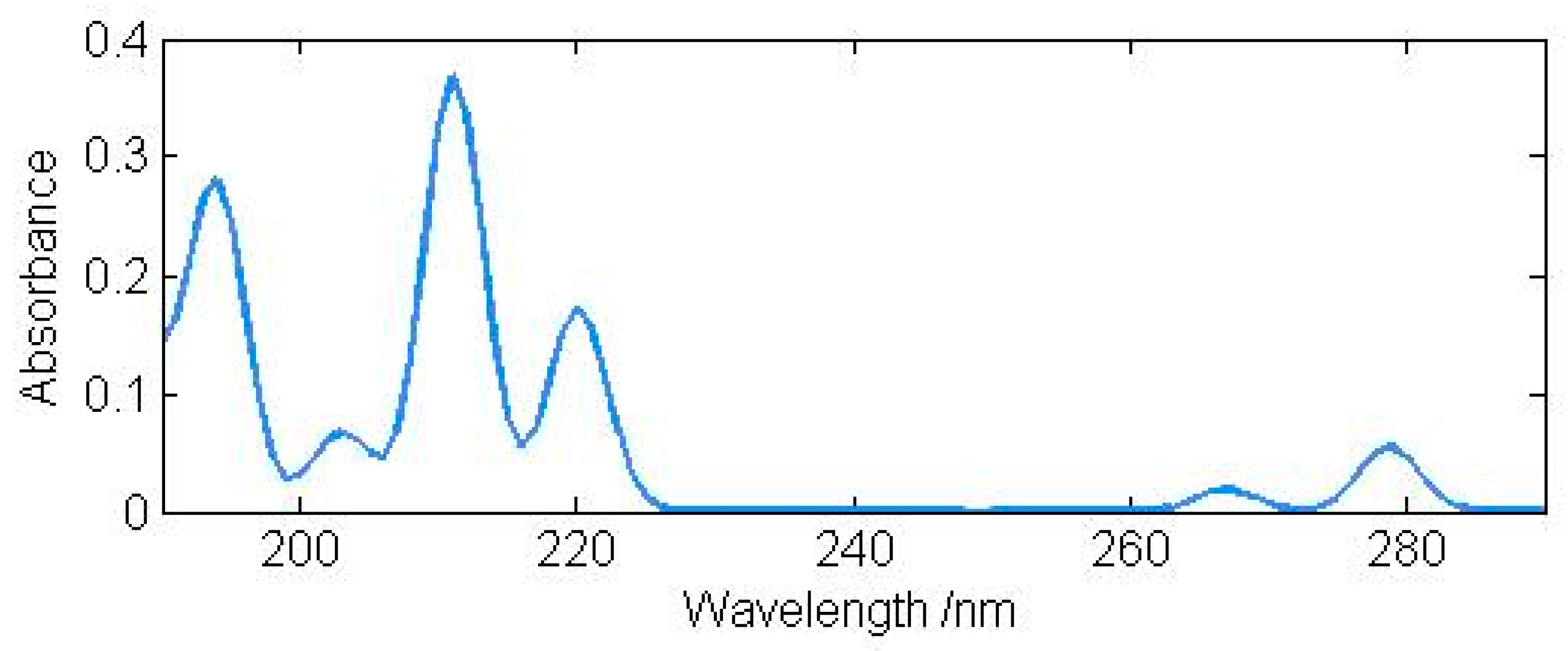
3. Results and Discussion
4. Summary
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Method | R(CO) | R(CH) | <OCH | A | B | C | AAD | OK | Dev | CK | Dev |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B3LYP/6-31G(d) | 1.2066 | 1.1105 | 122.38 | 285,068 | 38,634 | 34,023 | 1108 | 539.56 | 0.08 | 294.57 | 0.10 |
| B3LYP/6-311 + G(d,p) | 1.2019 | 1.1080 | 122.04 | 284,214 | 38,999 | 34,293 | 903 | 539.56 | 0.08 | 294.55 | 0.08 |
| B3LYP/6-311 + G(2d,p) | 1.2003 | 1.1075 | 121.89 | 283,545 | 39,135 | 34,389 | 757 | 539.56 | 0.08 | 294.55 | 0.08 |
| MP2/cc-pVTZ | 1.2102 | 1.1004 | 121.95 | 287,589 | 38,603 | 34,033 | 1962 | 539.54 | 0.06 | 294.54 | 0.07 |
| CCSD/cc-pVTZ | 1.2028 | 1.1012 | 121.94 | 287,122 | 39,022 | 34,353 | 1900 | 539.55 | 0.07 | 294.53 | 0.06 |
| CCSD(T)/cc-pVTZ | 1.2096 | 1.1030 | 121.88 | 285,816 | 38,631 | 34,031 | 1361 | 539.54 | 0.06 | 294.55 | 0.08 |
| Expt | 1.2078 | 1.1161 | 116.52 | 281,964 | 38,832 | 34,002 | (0) | 539.48 | (0) | 294.47 | (0) |
| Method | R(OO) | R(OH) | <HOO | <HOOH | A | B | C | AAD | CEBE | Dev |
|---|---|---|---|---|---|---|---|---|---|---|
| B3LYP/6-31G(d) | 1.4557 | 0.9737 | 99.68 | 118.60 | 297,104 | 26,414 | 25,366 | 4799 | 540.89 | 0.09 |
| B3LYP/6-311+G(d,p) | 1.4538 | 0.9672 | 100.50 | 120.84 | 303,402 | 26,466 | 25,364 | 2717 | 540.89 | 0.09 |
| B3LYP/6-311+G(2d,p) | 1.4516 | 0.9682 | 100.55 | 115.67 | 302,390 | 26,449 | 25,518 | 3100 | 540.90 | 0.10 |
| MP2/cc-pVTZ | 1.4498 | 0.9643 | 99.36 | 114.13 | 301,579 | 26,598 | 25,704 | 3482 | 540.92 | 0.12 |
| CCSD/cc-pVTZ | 1.4406 | 0.9610 | 100.27 | 113.09 | 305,937 | 26,836 | 25,979 | 2200 | 540.93 | 0.13 |
| CCSD(T)/cc-pVTZ | 1.4578 | 0.9640 | 99.55 | 113.93 | 302,158 | 26,301 | 25,435 | 3100 | 540.91 | 0.11 |
| Expt | 1.475 | 0.950 | 94.8 | 119.8 | 310,465 | 25,950 | 24,793 | (0) | 540.8 | (0) |
References
- van Order, R.B.; Lindwall, H.G. Indole. Chem. Rev. 1942, 30, 69–96. [Google Scholar] [CrossRef]
- Sullivan, D.; Gad, S. Indole. In Encyclopedia of Toxicology; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 1030–1031. [Google Scholar]
- Ziarani, G.M.; Moradi, R.; Ahmadi, T.; Lashgari, N. Recent Advances in the Application of Indoles in Multicomponent Reactions. RSC Adv. 2018, 8, 12069. [Google Scholar] [CrossRef]
- Chadha, N.; Silakari, O. Chapter8—Indoles: As multitarget directed ligands in medicinal chemistry. In Key Heterocycle Cores for Designing Multitargeting Molecules; Sikari, O., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 285–321. [Google Scholar]
- Sundberg, R.J. The Chemistry of Indoles. In Organic Chemistry; Blomquist, A.T., Ed.; Springer: Heidelberg, Germany, 1970; Volume 18. [Google Scholar]
- Gribble, G.W. Heterocyclic Scaffolds II: Reactions and Applications of Indoles; Springer: Heidelberg, Germany, 2010. [Google Scholar]
- Yakhontov, L.N. The Chemistry of Azaindoles [Pyrrolo[2,3]pyridines]. Russ. Chem. Rev. 1968, 37, 551–565. [Google Scholar] [CrossRef]
- Prokopov, A.A.; Yakhontov, L.N. Methods of Synthesis and the Production Technology of Therapeutic Substances. Chemistry of the Azaindoles (Review). Pharma. Chem. J. 1994, 28, 471–506. [Google Scholar] [CrossRef]
- Collier, W.B. Vibrational Frequencies for Polyatomic Molecules. I. Indole and 2,3-benzofuran Spectra and Analysis. J. Chem. Phys. 1988, 88, 7295–7306. [Google Scholar] [CrossRef]
- Walden, S.E.; Wheeler, R.A. Structure and Vibrational Analysis of Indole by Density Functional Theory and by Hartree-Fock/Density Functional Methods. J. Chem. Soc. Perkin Trans. 1996, 2, 2653–2662. [Google Scholar] [CrossRef]
- Suenram, R.D.; Lovas, F.J.; Fraser, G.T. Microwave Spectrum and 14N Quadrupole Coupling Constants of Indole. J. Mol. Spectrosc. 1988, 127, 472–480. [Google Scholar] [CrossRef]
- Caminati, W.; Bernardo, S. Microwave Spectrum and Amino Hydrogen Location in Indole. J. Mol. Struct. 1990, 223, 253–262. [Google Scholar] [CrossRef]
- Gruet, S.; Pirali, O.; Goubet, M.; Tokaryk, D.W.; Brechignac, P. High-resolution Far-infrared Spectroscopy of N-substituted Two-ring Polycyclic Aromatic Hydrocarbons: An Extended Study. J. Phys. Chem. A 2016, 120, 95–105. [Google Scholar] [CrossRef]
- Nesvadba, R.; Studecky, T.; Uhlikova, T.; Urban, S. Microwave Spectrum and Molecular Constants of Indole. J. Mol. Spectrosc. 2017, 339, 6–11. [Google Scholar] [CrossRef]
- Vavra, K.; Lukova, K.; Kania, P.; Koucky, J.; Urban, S. Rotational Spectra of Indole in the Lowest Vibrational States. J. Mol. Spectrosc. 2019, 363, 111175. [Google Scholar] [CrossRef]
- Caminati, W.; Bernardo, S. Microwave Spectrum and Amino Hydrogen Location in 7-azaindole. J. Mol. Struct. 1990, 223, 415–424. [Google Scholar] [CrossRef]
- Hollas, J.M. Vapour-phase Ultraviolet Absorption Spectra of Indene, Indole, Coumarone and Thionaphthene. Spectrochim. Acta 1963, 19, 753–767. [Google Scholar] [CrossRef]
- Strickland, E.H.; Horwitz, J.; Billups, C. Near-ultraviolet Absorption Bands of Tryptophan. Studies using Indole and 3-methylindole as Models. Biochemistry 1970, 9, 4914–4921. [Google Scholar] [CrossRef]
- Lami, H. On the Possible Role of a Mixed Valence-Rydberg State in the Fluorescence of Indoles. J. Chem. Phys. 1977, 67, 3274–3281. [Google Scholar] [CrossRef]
- Lami, H. Presence of a Low-lying “Rydberg” Band in the Vapour Phase Absorption Spectra of Indole and 1-methyl Indole. Chem. Phys. Lett. 1977, 48, 447–450. [Google Scholar] [CrossRef]
- Ilich, P. 7-azaindole: The Low-temperature Near-UV Spectra and Electronic Structure. J. Mol. Struct. 1995, 354, 37–47. [Google Scholar] [CrossRef]
- Serrano-Andres, L.; Roos, B.J. Theoretical Study of the Absorption and Emission Spectra of Indole in the Gas Phase and in a Solvent. J. Am. Chem. Soc. 1996, 118, 185–195. [Google Scholar] [CrossRef]
- Borisevich, N.A.; Raichenok, T.F. Absorption, Fluorescence, and Fluorescence Excitation Spectra of Free Molecules of Indole and its Derivatives. J. Appl. Spectrosc. 2007, 74, 218–222. [Google Scholar] [CrossRef]
- Livingston, R.; Schalk, O.; Boguslavskiy, A.E.; Wu, G.; Bergendahl, L.T.; Stolow, A.; Paterson, M.J.; Townsend, D.J. Following the excited state relaxation dynamics of indole and 5-hydroxyindole using time-resolved photoelectron spectroscopy. Chem. Phys. 2011, 135, 194307. [Google Scholar] [CrossRef]
- Kumar, M.; Mohan, T.R.; Branton, T.A.; Trivedi, D.; Nayak, G.; Mishra, R.K.; Jana, S. Biofield Treatment: A Potential Strategy for Modification of Physical and Thermal Properties of Indole. J. Environ. Anal. Chem. 2015, 2, 152. [Google Scholar] [CrossRef]
- Borin, A.C.; Serrano-Andres, L. A Theoretical Study of the Absorption Spectra of Indole and its Analogs: Indene, Benzimidazole, and 7-azaindole. Chem. Phys. 2000, 262, 253–265. [Google Scholar] [CrossRef]
- Serrano-Andres, L.; Borin, A.C. A Theoretical Study of the Emission Spectra of Indole and its Analogs: Indene, Benzimidazole, and 7-azaindole. Chem. Phys. 2000, 262, 267–283. [Google Scholar] [CrossRef]
- Giussan, A.; Marchesell, J.; Mukamel, S.; Garavelli, M.; Nenov, A. On the Simulation of Two-dimensional Electronic Spectroscopy of Indole-containing Peptides. Photochem. Photobiol. 2017, 93, 1368–1380. [Google Scholar] [CrossRef] [PubMed]
- Fuke, K.; Yoshiuchi, H.; Kaya, K. Electronic Spectra and Tautomerism of Hydrogen-bonded Complexes of 7-azaindole in a Supersonic Jet. J. Phys. Chem. 1984, 88, 5840–5844. [Google Scholar] [CrossRef]
- Bulska, H.; Grabowska, A.; Pakula, B.; Sepiol, J.; Waluk, J. Spectroscopy of Doubly Hydrogen-bonded 7-azaindole. Reinvestigation of the Excited State Reaction. J. Lumin. 1984, 29, 65–81. [Google Scholar] [CrossRef]
- Hassan, K.H.; Hollas, J.M. Assignment of the S1-S0 Electronic Absorption Spectra of 7-azaindole and 1-azaindolizine as π*-π by Rotational Band Contour Analysis. J. Mol. Spectrosc. 1989, 138, 398–412. [Google Scholar] [CrossRef]
- Sukhodola, A.A. 7-azaindole in the Gas Phase: Absorption, Luminescence, and the Mechanism of Long-lived Luminescence. J. Appl. Spectrosc. 2018, 85, 850–855. [Google Scholar] [CrossRef]
- Serrano-Andres, L.; Merchan, M.; Borin, A.C.; Stalring, J. Theoretical Studies on the Spectroscopy of the 7-azaindole Monomer and Dimer. Int. J. Quantum Chem. 2001, 84, 181–191. [Google Scholar] [CrossRef]
- Ten, G.N.; Glukhova, O.E.; Slepchenkov, O.E.; Baranov, V.I. Theoretical Analysis of the Fluorsescence Spectra of 7-azaindole and its Tautomer. Opt. Spectrosc. 2016, 120, 359–366. [Google Scholar] [CrossRef]
- Plekan, O.; Sa’Adeh, H.; Ciavardini, A.; Callegari, C.; Cautero, G.; Dri, C.; Di Fraia, M.; Prince, K.C.; Richter, R.; Sergo, R.; et al. Experimental and Theoretical Photoemission Study of Indole and Its Derivatives in the Gas Phase. J. Phys. Chem. A 2020, 124, 4115–4127. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson; et al. Gaussian 09, Revision, A.02; Gaussian: Wallingford, CT, USA, 2009. [Google Scholar]
- ADF Program System, Release 2013; Scientific Computing & Modeling, NV: Amsterdam, 2006. For a comprehensive description of ADF, see te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931.
- Segala, M.; Chong, D.P. An Evaluation of Exchange-correlation Functionals for the Calculation of the Ionization Energies of Atoms and Molecules. J. Electron. Spectrosc. Rel. Phenom. 2009, 171, 18–23. [Google Scholar] [CrossRef]
- Chong, D.P.; van Lenthe, E.; van Gisbergen, S.; Baerends, E.J. Even-tempered Slater-type Orbitals Revisited: From Hydrogen to Krypton. J. Comput. Chem. 2004, 25, 1030–1036. [Google Scholar] [CrossRef]
- Chong, D.P. Additions and Crrections: Theoretical Study of the Electron Spectra of s-triazine Vapor. Can. J. Chem. 2010, 88, 577. [Google Scholar] [CrossRef]
- Chong, D.P. Density Functional Theory Study on the Electron Spectra of Naphthalene and Azulene. Can. J. Chem. 2010, 88, 787–796. [Google Scholar] [CrossRef]
- Chong, D.P. Density Functional Theory Study on the Electron Spectra of 1,4-benzoquinone Vapor. Mol. Phys. 2010, 108, 2459–2466. [Google Scholar] [CrossRef]
- Chong, D.P. Density Functional Theory Study on the Electron Spectra of Formamide Vapor. J. Electron. Spectrosc. Rel. Phenom. 2011, 184, 164–169. [Google Scholar] [CrossRef]
- Chong, D.P. DFT Study of the Vertical Ionization Energies of the Valence and Core Electrons of Cyclopentadiene, Pyrrole, Furan, and Thiophene. Can. J. Chem. 2011, 89, 1477–1488, Errata: The calculated core electron binding energies of pyrrole in Table 9 should read 290.75, 289.92, and 406.37 eV instead of 290.55, 289.71, and 405.57 eV, respectively, doi:10.1139/V11-121. [Google Scholar] [CrossRef]
- Takahata, Y.; Chong, D.P. DFT Calculation of Core- and Valence-Shell Electron Excitation and Ionization Energies of 2,1,3-benzothiadiazole C6H4SN2, 1,3,2,4-benzodithiadiazine, C6H4S2N2, and 1,3,5,2,4-benzotrithiadiazeoine, C6H4S3N2”. J. Electron. Spectrosc. Rel. Phenom. 2012, 185, 475–485. [Google Scholar] [CrossRef]
- Chong, D.P. Density Functional Theory Study of the Photoelectron Spectra of 5-methyltetrazole. Theoret. Comput. Chem. 2013, 12, 1250096. [Google Scholar] [CrossRef]
- Chong, D.P. Theoretical study of uric acid and its ions. J. Theoret. Comput. Sci. 2013, 1, 104. [Google Scholar] [CrossRef]
- Chong, D.P. Computational study of the electron spectra of acetamide and N-methylacetamide. Croat. Chem. Acta 2017, 90, 99–105. [Google Scholar] [CrossRef]
- Chong, D.P. Computational study of the structures and electron spectra of gas-phase nitroamines: Dimethylnitrosamine, N-nitrosopyrrolidine and 1-nitrosoaziridine. J. Electron. Spectrosc. Rel. Phenom. 2019, 232, 35–39. [Google Scholar] [CrossRef]
- Chong, D.P. Computational study of the structures and electron spectra of 12 azabenzenes. Can. J. Chem. 2019, 97, 697–703. [Google Scholar] [CrossRef]
- Nakatsuji, H.; Miyahara, T.; Fukuda, R. Symmetry-adapted-cluster/symmetry-adapted-cluster configuration interaction methodology extended to giant molecular systems: Ring molecular crystals. J. Chem. Phys. 2007, 126, 84104. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.V. An efficient, renormalizd self-energy for calculating the electron binding energies of close-shell molecules and anions. Int. J. Quantum Chem. 2005, 105, 803–808. [Google Scholar] [CrossRef]
- Cavigliasso, G.; Chong, D.P. Accurate density-functional calculation of core-electron binding energies by a total-energy difference approach. J. Chem. Phys. 1999, 111, 9485–9492. [Google Scholar] [CrossRef]
- Bellafont, N.P.; Illas, F.; Bagus, P.S. Validation of Koopmans’ theorem for density functional theory binding energies. Phys. Chem. Chem. Phys. 2015, 17, 4015–4019. [Google Scholar] [CrossRef] [PubMed]
- Bellafont, N.P.; Bagus, P.S.; Illas, F. Prediction of core level binding energies in density functional theory: Rigorous definition of initial and final state contributions and implications on the physical meaning of Kohn-Sham energies. J. Chem. Phys. 2015, 142, 214102. [Google Scholar] [CrossRef] [PubMed]
- Bellafont, N.P.; Bagus, P.S.; Sousa, C.; Illas, F. Assessing the ability of DFT methods to describe static electron correlation effects: CO core level binding energies as a representative case. J. Chem. Phys. 2017, 147, 024106. [Google Scholar] [CrossRef]
- Bellafont, N.P.; Vines, F.; Illas, F. Performance of the TPSS Functional on Predicting Core Level Binding Energies of Main Group Elements Containng Molecules: A Good Choice for Molecules Adsorbed on Metal Surfaces. J. Chem. Theory Comput. 2016, 12, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Vines, F.; Sousa, C.; Illas, F. On the prediction of core level binding energies in molecules, surfaces and solids. Phys. Chem. Chem. Phys. 2018, 20, 8403–8410. [Google Scholar] [CrossRef] [PubMed]
- Chong, D.P. Density-Functional Calculation of Core-electron Binding Energies of C., N., O., and F. J. Chem. Phys. 1995, 103, 1842–1845. [Google Scholar] [CrossRef]
- Pekeris, C.L. Ground State of Two-Electron Atoms. Phys. Rev. 1958, 112, 1649–1658. [Google Scholar] [CrossRef]
- Maruani, J.; Kuleff, A.I.; Chong, D.P.; Bonnelle, C. Ansatz for the Evaluation of the Relativistic Contributions to Core Ionization Energies in Complex Molecules Involving Heavy Atoms. Int. J. Quantum Chem. 2005, 104, 397–410. [Google Scholar] [CrossRef]
- Van Setten, M.J.; Costa, R.; Viñes, F.; Illas, F. Assessing GW Approaches for Predicting Core Level Binding Energies. J. Chem. Theory Comput. 2018, 14, 877–883. [Google Scholar] [CrossRef]
- Chong, D.P. Augmenting Basis Set for Time-dependent Density Functional Theory Calculation of Excitation Energies: Slater-type Orbitals for Hydrogen to Krypton. Mol. Phys. 2005, 103, 749–761, Erratum: The volume and page numbers of Ref. 20 should read 34 and 31, respectively. [Google Scholar] [CrossRef]
- Falzon, C.T.; Chong, D.P.; Wang, F. Prediction of spectroscopic constants for diatomic molecules in the ground and excited states using time-dependent density functional theory. J. Comput. Chem. 2005, 27, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Gelius, U. Molecular orbitals and line intensities in ESCA spectra. In Electron Spectroscopy; Shirley, D.A., Ed.; North-Holland Publishing Co.: Amsterdam, The Netherlands, 1972; pp. 311–334. [Google Scholar]
- Gelius, U. Recent progress in ESCA studies of gases. J. Electron. Spectrosc. Relat. Phenom. 1974, 5, 985–1057. [Google Scholar] [CrossRef]
- Nefedov, V.I.; Sergushin, N.P.; Band, I.M.; Trzhaskovskaya, M.B. Relative intensities in X-ray photoelectron spectra. J. Electron. Spectrosc. Rel. Phenom. 1973, 2, 383–403. [Google Scholar] [CrossRef]
- Chong, D.P. Adaptation of the Gelius intensity model for semiempiriacl HAM/3 molecular orbital calculations of valence photoelectron spectra excited by X.-ray radiation. Can. J. Chem. 1985, 63, 2007–2011. [Google Scholar] [CrossRef]
- Chong, D.P.; Gritsenko, O.V.; Baerends, E.J. Interpretation of the Kohn-Sham Orbital Energies as the Approximate Vertical Ionization Potentials. J. Chem. Phys. 2002, 116, 1760–1772. [Google Scholar] [CrossRef]
- Chong, D.P. Calculation of reliable non-resonant Kα X-ray emission spectra of organic molecules and other small molecules. Can. J. Chem. 2020, 98, 741–745. [Google Scholar] [CrossRef]
- Takahata, Y.; Wulfman, C.E.; Chong, D.P. Accurate calculation of N1s and C1s core electron binding energies of substituted pyridines. Correlation with basicity and with Hammett substituent constants. J. Mol. Struct. THEOCHEM 2008, 863, 33–38. [Google Scholar] [CrossRef]
- Thomas, T.D.; Børve, K.J.; Gundersen, M.; Kukk, E. Reactivity and Core-Ionization Energies in Conjugated Dienes. Carbon 1s Photoelectron Spectroscopy of 1,3-Pentadiene. J. Phys. Chem. A 2005, 109, 5085–5092. [Google Scholar] [CrossRef] [PubMed]
- Saethre, L.J.; Thomas, T.D.; Svensson, S. ChemInform Abstract: Markovnikov Addition to Alkenes. A Different View from Core-Electron Spectroscopy and Theory. Cheminform 2010, 28, 749–755. [Google Scholar] [CrossRef]
- Gusten, H.; Klasinc, L.; Ruscic, B. Photoelectron Spectroscopy of Heterocycles. Indene Analogs. Z. Naturforsch. 1976, 31a, 1051–1056. [Google Scholar] [CrossRef]
- Chrostowska, A.; Xu, S.; Mazière, A.; Boknevitz, K.; Li, B.; Abbey, E.R.; Dargelos, A.; Graciaa, A.; Liu, S.-Y. UV-Photoelectron Spectroscopy of BN Indoles: Experimental and Computational Electronic Structure Analysis. J. Am. Chem. Soc. 2014, 136, 11813–11820. [Google Scholar] [CrossRef]
| Year | Indole | A | B | C | AAD a | Dipole (ADF) b, D | α b | Δα b |
|---|---|---|---|---|---|---|---|---|
| 1988 | Suenram et al. [11] | 3877.84 | 1636.05 | 1150.90 | ||||
| 1990 | Caminati and Bernardo [12] | 3877.83 | 1636.05 | 1150.90 | 2.09 ± 0.13 | |||
| 2015 | Gruet et al. [13] | 3877.84 | 1636.05 | 1150.90 | ||||
| 2017 | Nesvadba et al. [14] | 3877.84 | 1636.05 | 1150.90 | ||||
| 2019 | Vavra et al. [15] | 3877.84 | 1636.05 | 1150.90 | (0) | |||
| 2020 | B3LYP/6-31G(d) | 3878.25 | 1631,22 | 1148.25 | 2.63 | 2.1731 (2.1798) | 103.35 | 118.93 |
| 2020 | B3LYP/6-311+G(2d,p) | 3901.39 | 1640.17 | 1154.72 | 10.50 | 2.1360 | ||
| 2020 | B3LYP/cc-pVTZ | 3908.14 | 1642.93 | 1156.68 | 14.32 | 2.1732 | ||
| 2020 | B3LYP/cc-pVQZ | 3909.46 | 1643.62 | 1157.14 | 15.14 | 2.1399 | ||
| 7-Azaindole | ||||||||
| 1990 | Caminati and Bernardo [12] | 3928.93 | 1702.63 | 1188.13 | (0) | 1.68 ± 0.07 | ||
| 2020 | B3LYP/6-31G(d) | 3929.63 | 1697.39 | 1185.37 | 2.90 | 1.6343 (1.7166) | 97.99 | 147.73 |
| 2020 | B3LYP/6-311+G(2d,p) | 3957.32 | 1705.05 | 1191.16 | 11.28 | 1.6435 | ||
| 2020 | B3LYP/cc-pVTZ | 3963.53 | 1708.56 | 1193.91 | 15.44 | 1.6198 | ||
| 2020 | B3LYP/cc-pVQZ | 3966.63 | 1708.97 | 1194.38 | 16.76 | 1.6293 | ||
| 4-Azaindole | ||||||||
| 2020 | B3LYP/6-31G(d) | 3914.94 | 1692.94 | 1181.87 | 4.0322 (4.1588) | 98.13 | 127.01 | |
| 5-Azaindole | ||||||||
| 2020 | B3LYP/6-31G(d) | 4014.22 | 1638.31 | 1163.47 | 4.4053 (4.5675) | 97.05 | 90.37 | |
| 6-Azaindole | ||||||||
| 2020 | B3LYP/6-31G(d) | 4012.95 | 1639.08 | 1163.75 | 3.7656 (3.9394) | 97.09 | 122.91 | |
| Ref. | Year | Molecules | Number of VIEs | AAD, eV |
|---|---|---|---|---|
| 38 | 2009 | 31 molecules | 128 | 0.26 |
| 40 | 2010 | S-triazine | 10 | 0.20 |
| 41 | 2010 | naphthalene | 19 | 0.15 |
| azulene | 9 | 0.13 | ||
| 42 | 2010 | 1,4-benzoquinone | 16 | 0.19 |
| 43 | 2011 | formamide | 9 | 0.11 |
| 44 | 2011 | cyclopentadiene | 11 | 0.26 |
| pyrrole | 10 | 0.19 | ||
| furan | 12 | 0.23 | ||
| thiophene | 11 | 0.27 | ||
| 45 | 2012 | 2,1,3-benzothiadiazole | 10 | 0.14 |
| 1,3,2,4-benzodithiadiazine | 10 | 0.09 | ||
| 1,3,5,2,4-benzodithiadiazepine | 11 | 0.15 | ||
| 46 | 2013 | 5-methyltetrazole | 9 | 0.22 |
| 47 | 2013 | uric acid | 5 | 0.14 |
| 48 | 2017 | acetamide | 9 | 0.17 |
| N-methylformamide | 8 | 0.16 | ||
| 49 | 2019 | dimethylnitrosamine | 5 | 0.13 |
| N-nitrosopyrrollidine | 6 | 0.16 | ||
| 1-nitrosoaziridine | 9 | 0.24 | ||
| 50 | 2019 | Pyridine | 12 | 0.15 |
| 1,2-diazine | 13 | 0.28 | ||
| 1,3-diazine | 12 | 0.25 | ||
| 1,4-diazine | 12 | 0.25 |
| Case | Ref | Effects Included | B | C | N | O | F | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Relativistic | Correlation | Molecular | ||||||||
| Two Electron Ions | 60 | yes | yes | no | Irel | 259.375 | 392.093 | 552.072 | 739.335 | 953.911 |
| Inr | 259.338 | 391996 | 551.865 | 738.944 | 953.234 | |||||
| Irel–Inr | 0.037 | 0.097 | 0.207 | 0.391 | 0.677 | |||||
| 59 | Crel a | 0.040 | 0.098 | 0.206 | 0.389 | 0.677 | ||||
| Typical Molecules c | 59 | yes | part | no | Crel a | 0.022 | 0.051 | 0.106 | 0.196 | 0.340 |
| Dirac-Fock Atoms | 61 | part | no | no | Crel b | 0.024 | 0.059 | 0.126 | 0.243 | 0.434 |
| Dirac-Fock Atoms | 54–58 | part | no | no | 0.06 | 0.13 | 0.25 | 0.45 | 0.75 | |
| Case | Obs | ΔPW86PW91 | ΔTPSS |
|---|---|---|---|
| CH2=C(CH3)2 | 289.83 | 289.88 | 290.66 |
| Cα in pyrrole | 289.96 | 289.92 | 289.76 |
| CH2=CHCH3 | 290.25 | 290.24 | 290.61 |
| Cm in C6H5F | 290.54 | 290.49 | 290.57 |
| Cp in C6H5F | 290.54 | 290.69 | 290.38 |
| CH2=C(CH3)2 | 290.65 | 290.76 | 290.52 |
| CH2=C(CH3)2 | 290.69 | 290.78 | 290.66 |
| CH2=CHCH3 | 290.73 | 290.79 | 290.82 |
| Cβ in pyrrole | 290.77 | 290.75 | 290.80 |
| CH2=CHCH3 | 290.81 | 290.95 | 290.17 |
| Co in C6H5F | 290.87 | 290.63 | 290.52 |
| CH4 | 290.91 | 290.95 | 290.86 |
| C2 in p-C6H4F2 | 290.99 | 290.92 | 290.89 |
| CH3COOH | 291.55 | 291.51 | 291.83 |
| CH3OCH3 | 292.34 | 292.21 | 292.17 |
| CH3OH | 292.42 | 292.54 | 292.45 |
| CH3CN | 292.45 | 292.75 | 292.70 |
| C1 in C6H5F | 292.70 | 292.75 | 292.55 |
| C1 in p-C6H4F2 | 292.95 | 292.86 | 292.74 |
| CH3CN | 292.98 | 292.84 | 292.62 |
| HCN | 293.40 | 293.56 | 293.46 |
| H2CO | 294.47 | 294.53 | 294.49 |
| CH3COOH | 295.38 | 295.06 | 294.99 |
| CO | 296.21 | 296.26 | 296.32 |
| CH2F2 | 296.40 | 296.08 | 296.20 |
| CO2 | 297.69 | 297.28 | 297.30 |
| CF4 | 301.90 | 301.14 | 301.34 |
| Cp in aniline | 289.85 | 289.85 | 290.02 |
| Co in aniline | 289.95 | 289.97 | 289.89 |
| Cm in aniline | 290.25 | 290.17 | 290.03 |
| C1 in aniline | 291.29 | 291.37 | 291.17 |
| Cp in toluene | 290.1 | 290.24 | 290.02 |
| Co in toluene | 290.2 | 290.19 | 289.89 |
| Cm in toluene | 290.4 | 290.31 | 290.03 |
| C1 in toluene | 290.9 | 290.49 | 290.17 |
| Cp in phenol | 290.2 | 290.24 | 289.94 |
| Co in phenol | 290.2 | 290.47 | 290.21 |
| Cm in phenol | 290.6 | 290.53 | 290.25 |
| C1 in phenol | 292.0 | 292.12 | 291.84 |
| Average deviation | (0) | −0.03 | −0.11 |
| Average absolute deviation | (0) | 0.14 | 0.23 |
| AD for ΔPW86PW91 + 0.08 | +0.05 | ||
| AAD for ΔPW86PW91 + 0.08 | 0.16 |
| Case | Obs | ΔPW86PW91 | ΔTPSS [56] | ΔB3LYP [56] | QsGW [62] |
|---|---|---|---|---|---|
| N in pyridine | 404.88 | 404.76 | 404.43 | ||
| NH3 | 405.56 | 405.77 | 405.52 | 405.32 | 407.84 |
| N in pyrrole | 406.15 | 406.37 | 405.96 | ||
| HCONH2 | 406.38 | 406.55 | |||
| HCN | 406.78 | 406.94 | 406.67 | 406.66 | 408.78 |
| NNO | 408.71 | 408.59 | |||
| N2 | 409.98 | 410.02 | |||
| NNO | 412.59 | 412.47 | |||
| AAD(4) for N1s | (0) | 0.18 | 0.20 | ||
| AAD(8) for N1s | (0) | 0.14 | |||
| HCONH2 | 537.77 | 537.78 | |||
| CH3OCH3 | 538.74 | 538.71 | 538.04 | ||
| CH3OH | 539.48 | 539.15 | 538.59 | ||
| H2CO | 539.48 | 539.48 | 539.03 | ||
| H2O | 539.90 | 540.01 | 539.45 | 539.39 | 542.13 |
| CO2 | 541.28 | 541.36 | 540.96 | ||
| CO | 542.55 | 542.72 | 542.21 | 542.10 | |
| AAD(6) for O1s | (0) | 0.12 | 0.52 | ||
| AAD(7) for O1s | (0) | 0.10 | |||
| HF | 694.23 | 694.28 | 693.53 | ||
| CH2F2 | 693.65 | 693.71 | 692.89 | ||
| CF4 | 695.56 | 695.38 | 694.58 | ||
| F2 | 696.69 | 696.52 | 695.89 | ||
| AAD(4) for F1s | (0) | 0.04 | 0.81 |
| State | Experiment | Theory | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1963 a | 1970 b | 1977 c | 1995 d | 1996 e | 2007 f | 2011 g | 2015 h | CASPT2 | CASPT2 | CCR(3) | RASPT2 | DFT k | |
| 1996 e | 2000 i | 2011 g | 2017 j | 2020 k | |||||||||
| 2 1A’ | 4.37 | 4.32 | 4.35 m | 4.37 (0.045) | 4.37 | 4.37 | 4.32 | 4.43 (0.050) | 4.43 (0.050) | 4.76 (0.038) | 4.31 (0.38) | 4.45 (0.0543) | |
| 3 1A’ | 4.77 | 4.67 s | 4.77 (0.123) | 4.63 | 4.79 | 4.73 (0.081) | 4.73 (0.081) | 5.12 (0.1018) | 4.64 (0.23) | 4.64 (0.0187) | |||
| 1 1A” | 4.86 | 4.784.87 | 4.90 | 4.85 (0.001) | 5.02 (0.0022) | 5.84 (0.14) | 5.34 (0.0015) | ||||||
| 4 1A’ | 5.27 | 5.21 (0.004) | 5.84 (0.458) | 5.96 (0.11) | 5.62 (0.1761) | ||||||||
| 2 1A” | 5.71 | 5.33 (0.003) | 6.01 (0.36) | 5.75 (0.0011) | |||||||||
| 3 1A” | 5.36 (0.001) | 6.17 (0.54) | 5.76 (0.0010) | ||||||||||
| 5 1A’ | 5.55 | 5.90 | 6.02 | 5.65 (0.002) | 6.16 (0.003) | 6.42 (0.10) | 5.87 (0.3682) | ||||||
| 4 1A” | 5.37 (0.002) | 6.55 (0.11) | 6.08 (0.0018) | ||||||||||
| 6 1A’ | 6.04 h | 6.02 (~0.6) | 5.84 (0.458) | 6.44 (0.257) | 7.40 (0.88) | 6.08 (0.0571) | |||||||
| 5 1A” | 5.81 (0.001) | 7.39 (0.17) | 6.17 (0.0019) | ||||||||||
| 7 1A’ | 6.34 h | 6.35 | 5.94 (0.012) | 6.71 (0.138) | 6.38 (0.2593) | ||||||||
| State | Experiment | Theory | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| INDO/SI | CASPT2 | CASPT2 | TDDFT | This Work i | ||||||
| 1984 a | 1984 b | 1989 c | 1995 d | 2018 e | 1995 d | 2000 f | 2001 g | 2016 h | 2020 | |
| 2 1A’ | 4.29 | 4.15 | 4.29 | 4.28 | 4.29 | 4.28 (0.17) | 4.22 (0.043) | 4.22 (0.043) | 4.56 | 4.20 (0.0456) |
| 1 1A” | 5.27 (0.008) | 5.27 (0.008) | 4.84 | 4.60 (0.0018) | ||||||
| 3 1A’ | 4.49 | 4.49 | 4.55 (0.09) | 4.49 (0.072) | 4.49 (0.072) | 4.62 (0.0571) | ||||
| 2 1A” | 5.64 (0.0002) | |||||||||
| 4 1A’ | 5.76 | 5.77 (0.065) | 5.77 (0.065) | 5.69 (0.3733) | ||||||
| 3 1A” | 5.82 (0.0034) | |||||||||
| 5 1A’ | 5.99 | 5.93 (0.148) | 5.93 (0.148) | 6.02 (0.0518) | ||||||
| 4 1A” | 6.10 (0.0000) | |||||||||
| 5 1A” | 6.25 (0.0029) | |||||||||
| 6 1A’ | 6.26 (0.377) | 6.26 (0.377) | 6.36 (0.0718) | |||||||
| 6 1A” | 6.47 (0.0018) | |||||||||
| 7 1A’ | 6.46 (0.079) | 6.46 (0.079) | 6.51 (0.0763) | |||||||
| 7 1A” | 6.52 (0.0085) | |||||||||
| 8 1A” | 6.52 (0.0008) | |||||||||
| 8 1A’ | 6.71 (0.265) | 6.71 (0.265) | 6.64 (0.2339) | |||||||
| State | n = 4 | n = 5 | n = 6 |
|---|---|---|---|
| 2 1A’ | 4.23 (0.0383) | 4.46 (0.0479) | 4.40 (0.0521) |
| 3 1A’ | 4.62 (0.0646) | 4.85 (0.0079) | 4.82 (0.0211) |
| 4 1A’ | 5.66 (0.2827) | 5.67 (0.1402) | 5.80 (0.1531) |
| 5 1A’ | 5.93 (0.0382) | 5.86 (0.1474) | 5.86 (0.2207) |
| 6 1A’ | 5.98 (0.1296) | 5.94 (0.1817) | 5.97 (0.0103) |
| 7 1A’ | 6.43 (0.1104) | 6.44 (0.2553) | 6.21 (0.1286) |
| 8 1A’ | 6.47 (0.0378 | 6.49 (0.0751) | 6.47 (0.3539) |
| 1 1A” | 4.26 (0.0016) | 4.33 (0.0004) | 4.33 (0.0012) |
| 2 1A” | 5.27 (0.0005) | 5.18 (0.0025) | 5.29 (0.0006) |
| 3 1A” | 5.62 (0.0017) | 5.49 (0.0017) | 5.62 (0.00010 |
| 4 1A” | 5.93 (0.0008) | 6.03 (0.0001) | 6.06 (0.0013) |
| 5 1A” | 6.12 (0.0020) | 6.16 (0.0007) | 6.09 (0.0015) |
| 6 1A” | 6.16 (0.0012) | 6.32 (0.0077) | 6.31 (0.0001) |
| 7 1A” | 6.43 (0.0001 | 6.35 (0.0018) | 6.41 (0.0034) |
| MO | 2020 | 2020 | 1976 | 2014 | |||||
|---|---|---|---|---|---|---|---|---|---|
| This Work | Plekan et al. a | Gusten b | Chrostowska c | ||||||
| HAM/3 d | mKT e | DFT f | Ave. | Obs | P3+ | Ave. | Obs | Obs | |
| 5π | 8.29 (0.0517) | 9,23 | 7.78 | 7.90 | 7.91 | 7.91 | 7.9 | ||
| 4π | 8.85 (0.0467) | 9.65 | 8.24 | 8.32 | 8.25 | 8.37 | 8.5 | ||
| 3π | 9.99 (0.0418) | 10.96 | 9.81 | 9.82 | 9.88 | 9.78 | 9.9 | ||
| 26σ | 11.93 (0.0394) | 12.06 | 11.35 | 10.97 | 11.68 | 11.03 | 11.05 | ||
| 2π | 11.12 (0.0450) | 12.30 | 11.38 | 11.55 | 11.29 | 11.52 | 11.45 | ||
| 25σ | 12.40 (0.0485) | 12.58 | 11.97 | 12.20 | 12.27 | 12.26 | 12.25 | ||
| 24σ | 13.26 (0.0572) | 13.52 | 12.93 | 13.02 | 13.29 | 12.72 | 13.0 | ||
| 23σ | 13.55 (0.0402) | 14.00 | 13.51 | (13.7) | 13.88 | 13.16 | |||
| 1π | 13.24 (0.0535) | 14.49 | 13.75 | 13.80 | 13.66 | 13.77 | |||
| 22σ | 13.97 (0.0463) | 14.46 | 14.02 | (14.1) | 14.37 | (14.04) | |||
| 21σ | 14.44 (0.0617) | 14.68 | 14.08 | 14.25 | 14.52 | 14.25 | |||
| 20σ | 15.06 (0.0710) | 15.54 | 15.12 | 15.30 | 15.58 | 15.05 | |||
| 19σ | 15.55 (0.1286) | 15.86 | 15.45 | 15.80 | 15.88 | 15.33 | |||
| 18σ | 16.61 (0.1039) | 17.31 | 17.04 | 17.00 | 17.48 | 17.03 | |||
| 17σ | 17.50 (0.2076) | 18.38 | 18.26 | (18.2) | |||||
| 16σ | 18.48 (0.2547) | 18.73 | 18.68 | 18.52 | |||||
| 15σ | 18.74 (0.1813) | 19.45 | 19.42 | 19.25 | |||||
| 14σ | 21.84 (0.4390) | 21.83 | 22.06 | ||||||
| 13σ | 22.51 (0.4648) | 22.29 | 22.58 | ||||||
| 12σ | 23.95 (0.5046) | 23.50 | 23.91 | ||||||
| 11σ | 25.96 (0.5796) | 24.94 | 25.48 | ||||||
| 10σ | 30.18 (0.6925) | 28.62 | 29.43 | ||||||
| C8 | 289.72 | 289.72 | 289.85 | 289.89 | 289.49 | 289.61 | |||
| C7 | 289.82 | 289.77 | 289.54 | ||||||
| C3 | 289.67 | 289.78 | 289.55 | ||||||
| C9 | 289.83 | 289.79 | 289.57 | ||||||
| C6 | 289.97 | 290.00 | 289.76 | ||||||
| C4 | 289.92 | 290.02 | 289.77 | ||||||
| C5 | 290.76 | (290.76) g | 290.78 | 290.86 | 290.61 | 290.64 | |||
| C2 | 290.78 | 290.79 | 290.66 | ||||||
| N1 | 406.00 | 405.82 | 405.45 | ||||||
| MO | 4-Azaindole | 5-Azaindole | 6-Azaindole | 7-Azaindole |
|---|---|---|---|---|
| 5π | 8.29 (0.0552) | 8.16 (0.0499) | 8.25 (0.0572) | 8.22 (0.0530) |
| 4π | 8.61 (0.0566) | 9.09 (0.0667) | 8.81 (0.0492) | 8.67 (0.0552) |
| 3π | 10.44 (0.0429) | 10.19 (0.0408) | 10.33 (0.0511) | 10.58 (0.0511) |
| 2π | 12.18 (0.0655) | 12.34 (0.0636) | 12.30 (0.0631) | 12.04 (0.0601) |
| 1π | 14.15 (0.0528) | 14.18 (0.0530) | 14.20 (0.0533) | 14.27 (0.0549) |
| 26σ | 9.18 (0.2224) | 9.16 (0.2354) | 9.22 (0.2353) | 9.57 (0.2135) |
| 25σ | 12.44 (0.0493) | 11.92 (0.0446) | 12.04 (0.0546) | 12.52 (0.0443) |
| 24σ | 13.06 (0.0508) | 13.24 (0.0554) | 12.79 (0.0453) | ~12.9 b (0.089) |
| 23σ | 13.41 (0.0802) | 13.98(0.0693) | 14.08 (0.0546) | 13.22 (0.0751) |
| 22σ | 14.03 (0.0637) | 14.29 (0.0501) | 14.13 (0.0444) | 14.06 (0.0575) |
| 21σ | 14.73 (0.0502) | ~14.7 b (0.0504) | 14.73 (0.0593) | 14.73 (0.0542) |
| 20σ | 15.63 (0.0683) | 15.21 (0.0840) | 15.39 (0.0813) | 15.41 (0.0640) |
| 19σ | 15.96 (0.1277) | 16.03 (0.1192) | 15.90 (0.1275) | 15.90 (0.1173) |
| 18σ | 17.31 (0.1197) | 17.57 (0.1003) | 17.60 (0.1058) | 17.41 (0.1203) |
| 17σ | 18.66 (0.2008) | 18.82 (0.212) | 18.79 (0.2240) | 18.82 (0.2020) |
| 16σ | 19.22 (0.2565) | 19.21 (0.2483) | 19.18 (0.2619) | 19.18 (0.2450) |
| 15σ | 20.11 (0.2364) | 20.17 (0.2310) | 20.11 (0.2213) | 20.08 (0.2544) |
| 14σ | 22.60 (0.4581) | 22.56 (0.4403) | ~22.8 b (0.4536) | 22.65 (0.4506) |
| 13σ | 23.77 (0.4929) | ~23.4 b (0.5260) | 23.30 (0.4915) | 23.37 (0.4881) |
| 12σ | 24.57 (0.5415) | 24.04 (0.5327) | 24.69 (0.5583) | 24.85 (0.5656) |
| 11σ | 28.12 (0.6945) | 28.20 (0.7018) | 28.19 (0.6963) | 27.98 (0.6628) |
| 10σ | 29.75 (0.7095) | ~29.9 b (0.6993) | 29.86 (0.7016) | 29.84 (0.7255) |
| C3 (289.77) c | C3 290.18 | C4 290.28 | C5 290.05 | |
| C6 290.07 | C9 290.34 | C3 290.34 | C3 290.06 | |
| C7 290.57 | C7 (290.37) c | C9 290.70 | C9 290.34 | |
| C5 290.61 | C6 290.59 | C5 290.71 | C4 290.35 | |
| C9 290.80 | C4 290.71 | C7 291.07 | C6 290.67 | |
| C8 (291.01) c | C2 291.17 | C2 291.34 | C2 291.05 | |
| C2 291.05 | C6 (291.37) c | C8 (291.23) c | C8 (291.54) c | |
| N4 404.06 | N5 404.01 | N6 404.21 | N7 404.36 | |
| N1 406.30 | N1 406.39 | N1 406.57 | N1 406.13 |
| Indole | 7-Azaindole | ||
|---|---|---|---|
| Core Hole @ N1 | N1 | N7 | |
| 5π | 398.22 (0.0061) | 397.91 (0.0040) | 396.14 (0.0019) |
| 4π | 397.76 (0.0040) | 397.46 (0.0082) | 395.69 (0.0028) |
| 3π | 396.19 (0.0037) | 395.55 (0.0027) | 393.78 (0.0076) |
| 2π | 394.62 (0.0064) | 394.09 (0.0082) | 392.32 (0.0084) |
| 1π | 392.25 (0.0019) | 391.86 (0.0111) | 390.09 (0.0042) |
| 26σ | 394.65 (0.0005) | 396.56 (0.0007) | 394.79 (0.0252) |
| 25σ | 394.03 (0.0009) | 393.61 (0.0004) | 391.84 (0.0033) |
| 24σ | 393.07 (0.0033) | 393.23 (0.0023) | 391.46 (0.0038) |
| 23σ | 302.49 (0.0004) | 392.91 (0.0027) | 391.14 (0.0026) |
| 22σ | 391.98 (0.0143) | 392.07 (0.0024) | 390.30 (0.0020) |
| 21σ | 391.92 (0.0079) | 391.40 (0.0071) | 389.63 (0.0033) |
| 20σ | 390.88 (0.0006) | 390.72 (0.0036) | 388.95 (0.0043) |
| 19σ | 390.55 (0.0084) | 390.23 (0.0058) | 388.46 (0.0010) |
| 18σ | 388.96 (0.0068) | 388.72 (0.0076) | 386.95 (0.0030) |
| 17σ | 387.74 (0.0051) | 387.31 (0.0095) | 385.54 (0.0010) |
| 16σ | 387.32 (0.0072) | 386.95 (0.0027) | 385.18 (0.0066) |
| 15σ | 386.58 (0.0065) | 386.05 (0.0057) | 384.28 (0.0002) |
| 14σ | 383.94 (0.0022) | 383.48 (0.0038) | 381.71 (0.0029) |
| 13σ | 383.42 (0.0024) | 382.78 (0.0011) | 380.99 (0.0002) |
| 12σ | 382.09 (0.0016) | 381.28 (0.0006) | 379.51 (0.0001) |
| 11σ | 380.52 (0.0003) | 378.15 (0.0003) | 376.38 (0.0017) |
| 10σ | 376.57 (0.0005) | 376.29 (0.0005) | 374.52 (0.0004) |
| 4-Azaindole | 5-Azaindole | 6-Azaindole | ||||
|---|---|---|---|---|---|---|
| N1 | N4 | N1 | N5 | N1 | N6 | |
| 5π | 398.01 (0.0039) | 395.77 (0.0038) | 398.23 (0.0047) | 395.85 (0.0002) | 398.32 (0.0088) | 395.96 (0.0018) |
| 4π | 397.69 (0.0092) | 395.45 (0.0018) | 397.30 (0.0076) | 394.92 (0.0099) | 397.76 (0.0008) | 395.40 (0.0052) |
| 3π | 395.86 (0.0004) | 393.62 (0.0261) | 396.20 (0.0027) | 393.82 (0.0002) | 396.24 (0.0056) | 393.88 (0.0032) |
| 2π | 394.12 (0.0002) | 391.88 (0.0053) | 394.05 (0.0063) | 391.67 (0.0130) | 394.27 (0.0061) | 391.91 (0.0128) |
| 1π | 392.15 (0.0120) | 389.91 (0.0022) | 392.21 (0.0132) | 389.83 (0.0010) | 392.37 (0.0131) | 390.01 (0.0013) |
| 26σ | 397.12 (0.0005) | 394.88 (0.0055) | 397.23 (0.0001) | 394.85 (0.0276) | 397.35 (0.0003) | 394.99 (0.0273) |
| 25σ | 393.86 (0.0089) | 391.62 (0.0110) | 394.47 (0.0003) | 392.09 (0.0046) | 394.53 (0.0014) | 392.17 (0.0044) |
| 24σ | 393,24 (0.0025) | 391.00 (0.0038) | 393.15 (0.0035) | 390.77 (0.0010) | 393.76 (0.0005) | 391.42 (0.0025) |
| 23σ | 392.89 (0.0021) | 390.65 (0.0011) | 392.41 (0.0013) | 390.03 (0.0030) | 392.49(0.0015) | 390.13 (0.0032) |
| 22σ | 392.27 (0.0020) | 390.03 (0.0020) | 392.10 (0.0035) | 389.72 (0.0024) | 392.44(0.0015) | 390.08 (0.0005) |
| 21σ | 391.57 (0.0070) | 389.33 (0.0035) | 391.69 (0.0073) | 389.31 (0.0027) | 391.84 (0.0091) | 389.48 (0.0032) |
| 20σ | 390.67 (0.0002) | 388.43 (0.0037) | 391.18 (0.0071) | 388.80 (0.0044) | 391.18 (0.0018) | 388.82 (0.0058) |
| 19σ | 390.34 (0.0104) | 388.10 (0.0029) | 390.36 (0.0064) | 387.98 (0.0028) | 390.67 (0.0085) | 388.31 (0.0007) |
| 18σ | 388.99 (0.0073) | 386.75 (0.0004) | 388.82 (0.0067) | 386.44 (0.0022) | 388.97 (0.0071) | 386.61 (0.0043) |
| 17σ | 387.64 (0.0085) | 385.40 (0.0017) | 387.57 (0.0084) | 385.19 (0.0006) | 387.78 (0.0074) | 385.42 (0.0006) |
| 16σ | 387.08 (0.0034) | 384.84 (0.0053) | 387.18 (0.0044) | 384.80 (0.0036) | 387.39 (0.0054) | 385.03 (0.0004) |
| 15σ | 386.19 (0.0058) | 383.95 (0.0019) | 386.22 (0.0072) | 383.84 (0.0022) | 386.46 (0.0057) | 384.10 (0.0044) |
| 14σ | 383.70 (0.0034) | 381.46 (0.0011) | 383.83 (0.0020) | 381.45 (0.0025) | 383.77 (0.0009) | 381.41 (0.0021) |
| 13σ | 382.53 (0.0018) | 380.29 (0.0010) | 382.99 (0.0019) | 380.61 (0.0004) | 383.27 (0.0039) | 380.91 (0.0006) |
| 12σ | 381.73 (0.0009) | 379.49 (0.0002) | 382.35 (0.0013) | 379.97 (0.0002) | 381.88 (0.0009) | 379.52 (0.0001) |
| 11σ | 378.18 (0.0000) | 375.94 (0.0019) | 378.19 (0.0000) | 375.81 (0.0019) | 378.38 (0.0000) | 376.02 (0.0019) |
| 10σ | 376.55 (0.0005) | 374.31 (0.0001) | 376.49 (0.0005) | 374.11 (0.0000) | 376.71 (0.0005) | 374.35 (0.0000) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chong, D.P. Computational Study of the Electron Spectra of Vapor-Phase Indole and Four Azaindoles. Molecules 2021, 26, 1947. https://doi.org/10.3390/molecules26071947
Chong DP. Computational Study of the Electron Spectra of Vapor-Phase Indole and Four Azaindoles. Molecules. 2021; 26(7):1947. https://doi.org/10.3390/molecules26071947
Chicago/Turabian StyleChong, Delano P. 2021. "Computational Study of the Electron Spectra of Vapor-Phase Indole and Four Azaindoles" Molecules 26, no. 7: 1947. https://doi.org/10.3390/molecules26071947
APA StyleChong, D. P. (2021). Computational Study of the Electron Spectra of Vapor-Phase Indole and Four Azaindoles. Molecules, 26(7), 1947. https://doi.org/10.3390/molecules26071947





