The Ameliorative Effects of Fucoidan in Thioacetaide-Induced Liver Injury in Mice
Abstract
1. Introduction
2. Results
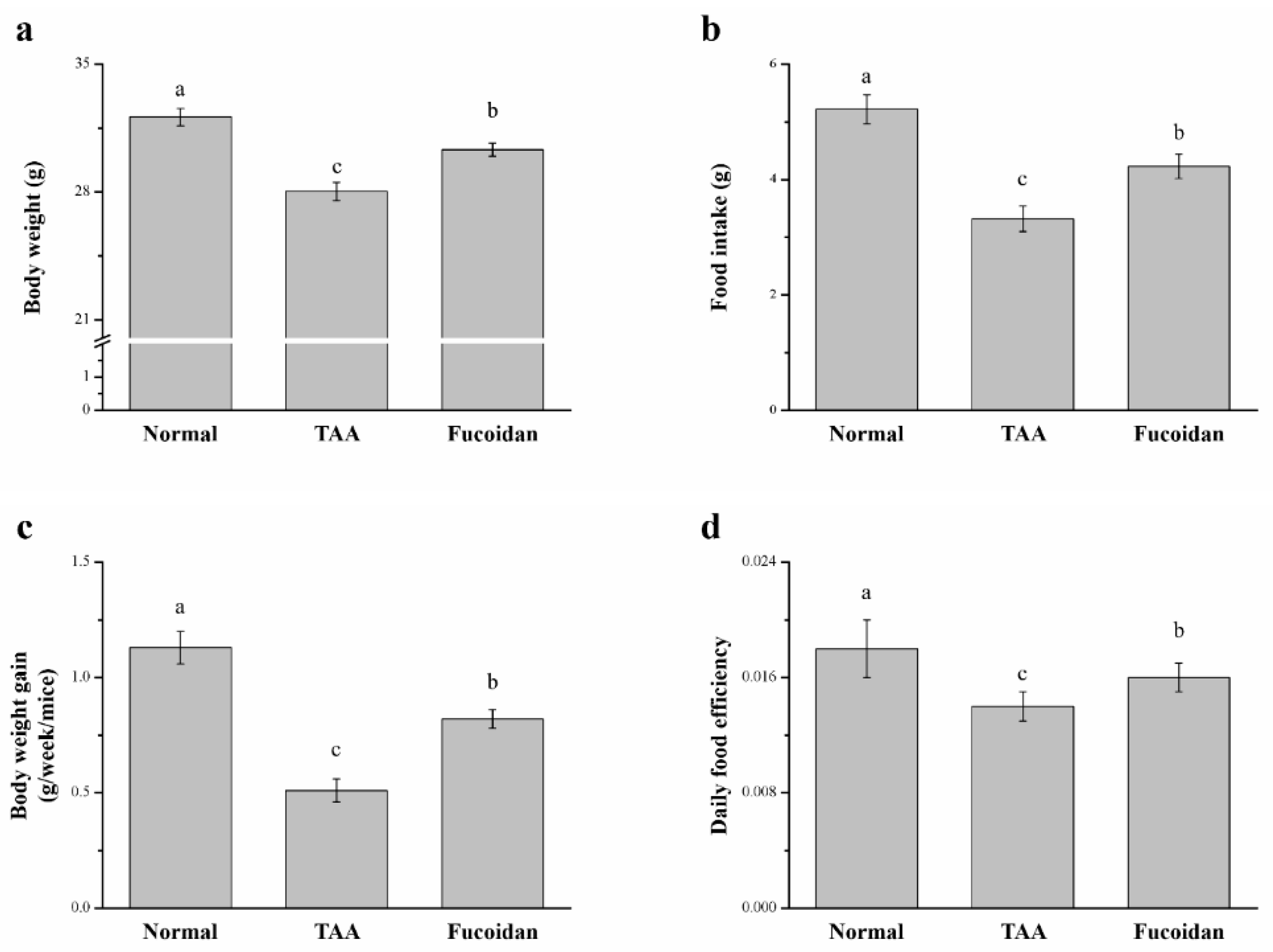
2.1. Fucoidan Affected Body Weight and Food Intake in Mice with TAA-Induced Liver Damage
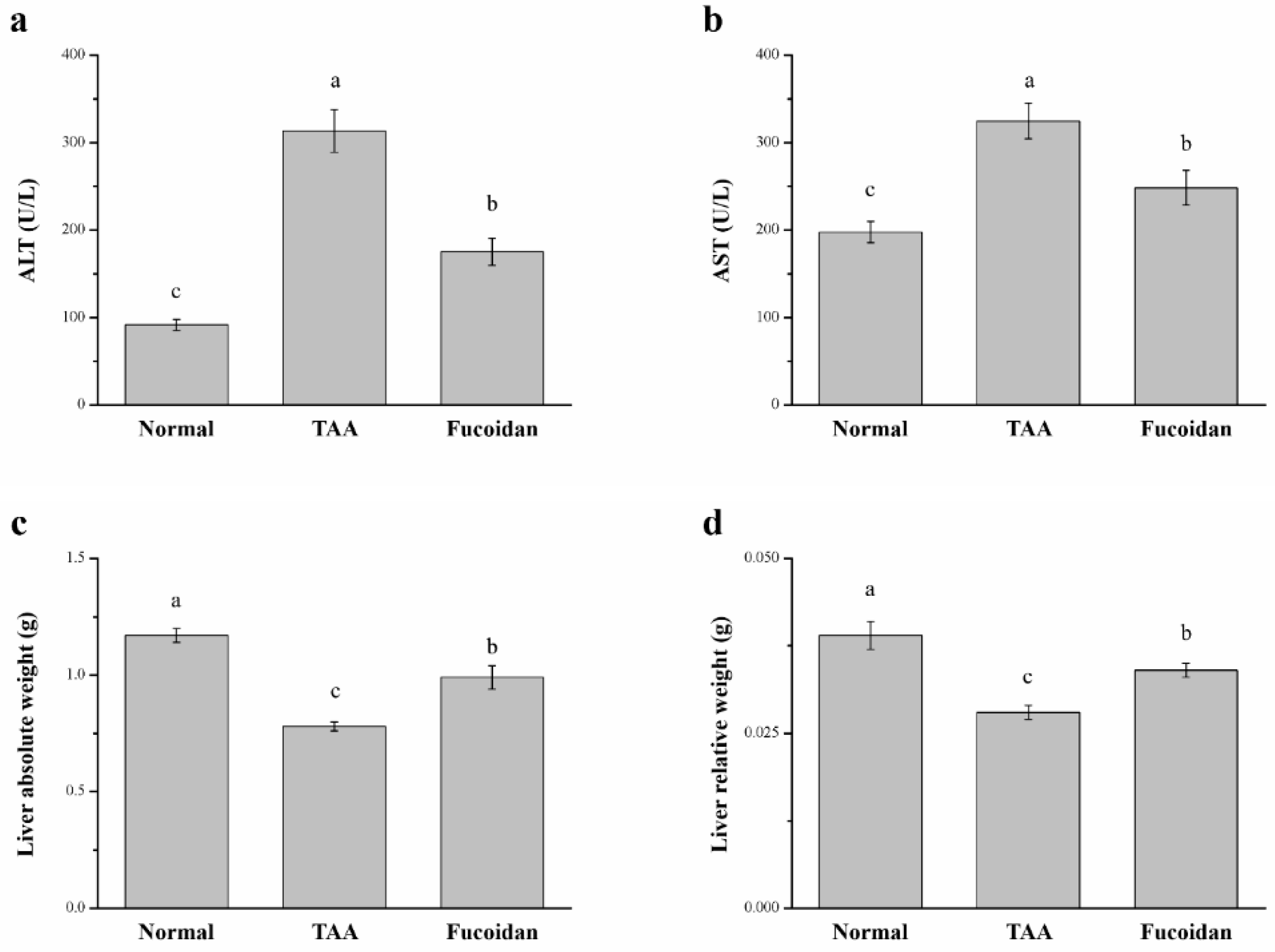
2.2. Fucoidan Affected Serum ALT, AST, and Liver Weight in Mice with TAA-Induced Liver Damage
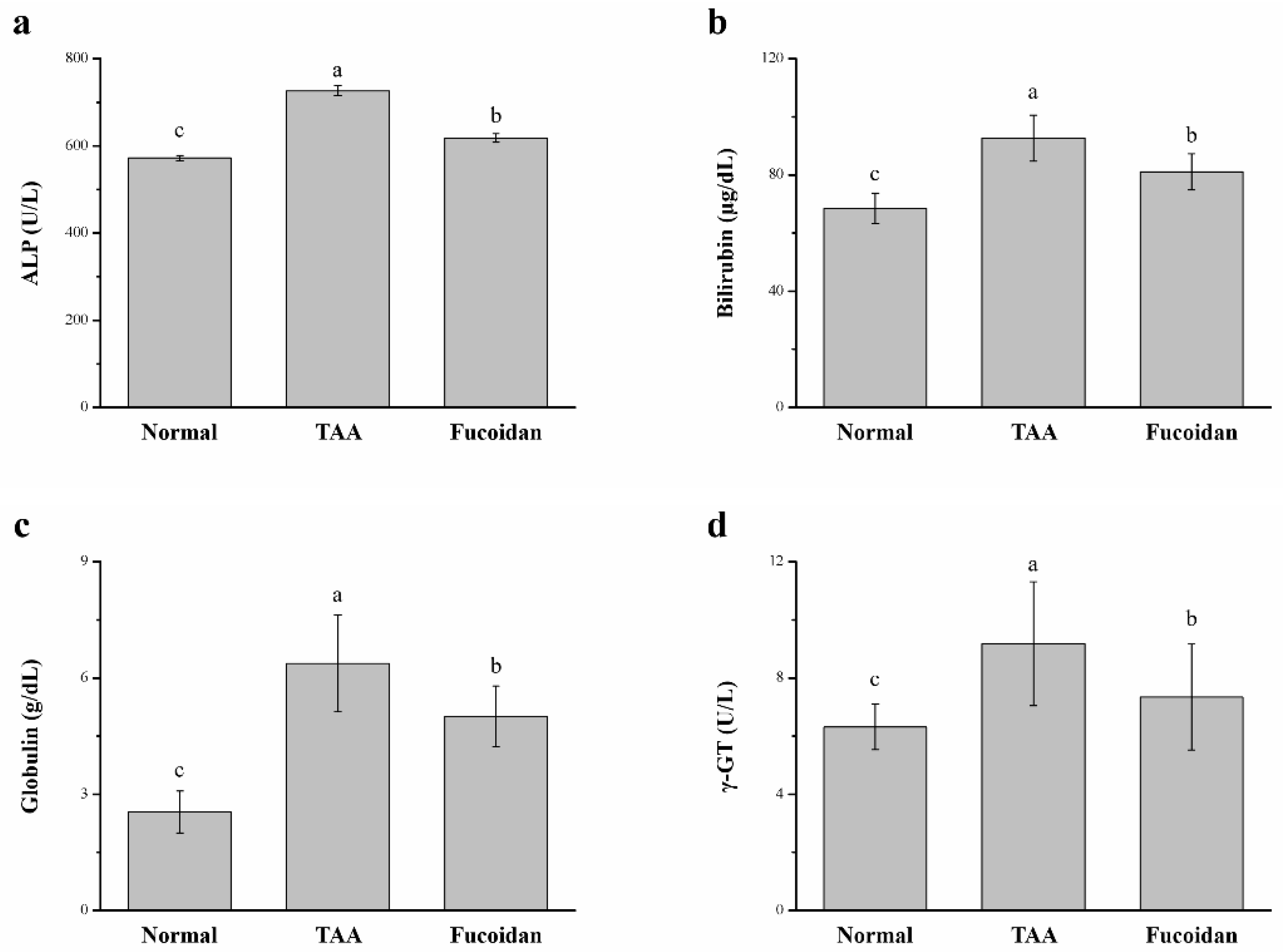
2.3. Fucoidan Affected the Serum Alkaline Phosphatase (ALP), Bilirubin, Globulin, and γ-Glutamyl Transferase (γ-GT)
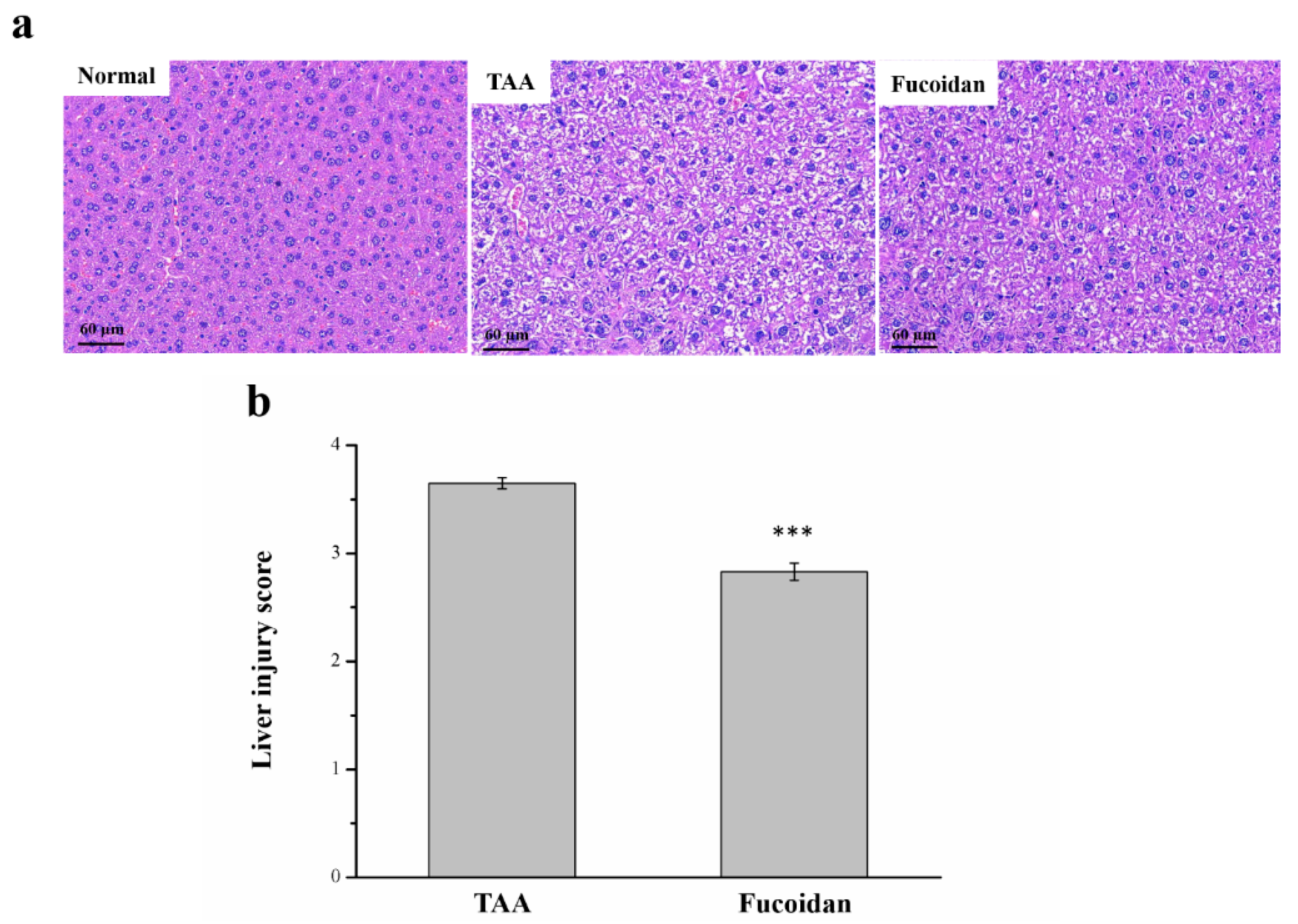
2.4. Fucoidan Affected Liver Damage
2.5. Fucoidan Affected Tumor Necrosis Factor-ɑ (TNF-ɑ), Interleukin-1β (IL-1β), Fibroblast Growth Factor-21 (FGF21), C-Reactive Protein (CRP), and Cytokine Levels in the Serum
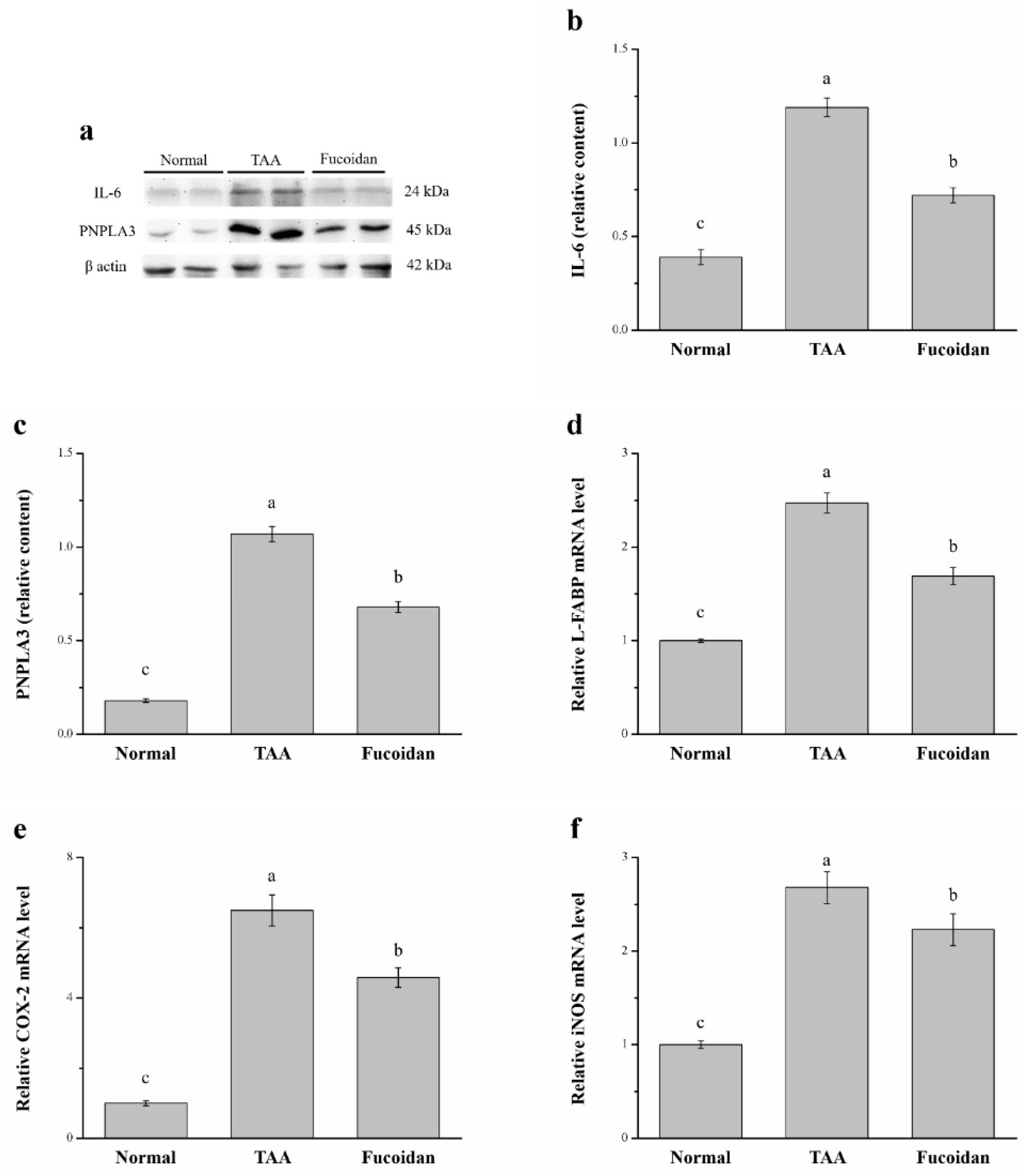
2.6. Fucoidan Affected Interleukin-6 (IL-6), Patatin-Like Phospholipid Domain Containing Protein 3 (PNPLA3), the mRNA of Liver Fatty Acid-Binding Protein (L-FABP), Cyclooxygenase-2 (COX-2), and Inducible Nitric Oxide Synthase (iNOS)
2.7. Fucoidan Affected Antioxidant Enzymes and ROS in the Liver
3. Discussion
4. Materials and Methods
4.1. Animal, Liver Injury Mouse, and Fucoidan
4.2. Body Weight and Food Intake
4.3. Liver Function Enzymes Tests
4.4. Liver Histopathological Evaluation
4.5. Determination of Serum Inflammatory Cytokines, FGF21, and CRP
4.6. Western Blotting Assay
4.7. RNA Extraction and Real-Time Quantitative PCR
4.8. Evaluation of Hepatic Catalase, GPx, SOD, and ROS
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Treyer, A.; Müsch, A. Hepatocyte polarity. Compr. Physiol. 2013, 3, 243–287. [Google Scholar] [PubMed]
- Blouin, A.; Bolender, R.P.; Weibel, E.R. Distribution of organelles and memebranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. Cell Biol. 1977, 72, 441–455. [Google Scholar] [CrossRef]
- De Mingo Pulido, Á.; de Gregorio, E.; Chandra, S.; Colell, A.; Morales, A.; Kronenberg, M.; Marí, M. Differential role of cathepsins S and B in hepatic APC-mediated NKT cell activation and cytokine secretion. Front. Immunol. 2018, 9, 391. [Google Scholar] [CrossRef] [PubMed]
- Hirsova, P.; Gores, G.J. Death receptor-mediated cell death and proinflammatory signaling in nonalcoholic steatohepatitis. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Seki, E. Toll-like receptors in liver fibrosis: Cellular crosstalk and mechanisms. Front. Physiol. 2012, 3, 138. [Google Scholar] [CrossRef]
- Fisher, C.P.; Kierzek, A.M.; Plant, N.J.; Moore, J.B. Systems biology approaches for studying the pathogenesis of non-alcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 15070. [Google Scholar] [CrossRef]
- Mermelstein, C.S.; Guma, F.C.; Mello, T.G.; Fortuna, V.A.; Guaragna, R.M.; Costa, M.L.; Borojevic, R. Induction of the lipocyte phenotype in murine hepatic stellate cells: Reorganization of the actin cytoskeleton. Cell Tissue Res. 2001, 306, 75–83. [Google Scholar] [CrossRef]
- Domenicali, M.; Caraceni, P.; Giannone, F.; Baldassarre, M.; Lucchetti, G.; Quarta, C.; Bernardi, M. A novel model of CCl4-induced cirrhosis with ascites in the mouse. J. Hepatol. 2009, 51, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Kučera, O.; Červinková, Z.; Lotkova, H.; Křiváková, P.; Roušar, T.; Mužáková, V.; Rudolf, E. Protective effect of S-adenosylmethionine against galactosamine-induced injury of rat hepatocytes in primary culture. Physiol. Res. 2006, 55, 551–560. [Google Scholar] [PubMed]
- Zhan, F.; Zhao, G.; Li, X.; Yang, S.; Yang, W.; Zhou, S.; Zhang, F. Inositol-requiring enzyme 1 alpha endoribonuclease specific inhibitor STF-083010 protects the liver from thioacetamide-induced oxidative stress, inflammation and injury by triggering hepatocyte. Int. Immunopharmacol. 2019, 73, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Loh, Z.; Fitzsimmons, R.L.; Reid, R.C.; Ramnath, D.; Clouston, A.; Gupta, P.K.; Iyer, A. Inhibitors of class I histone deacetylases attenuate thioacetamide-induced liver fibrosis in mice by suppressing hepatic type 2 inflammation. Br. J. Pharmacol. 2019, 176, 3775–3790. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wei, J.; Nie, J.; Bai, F.; Zhu, X.; Zhuo, L.; Lu, Z.; Huang, Q. Inhibition of RKIP aggravates thioacetamide-induced acute liver failure in mice. Exp. Ther. Med. 2018, 16, 2992–2998. [Google Scholar] [CrossRef] [PubMed]
- Chilakapati, J.; Shankar, K.; Korrapati, M.C.; Hill, R.A.; Mahendale, H.M. Saturation toxicokinetics of thioacetamide: Role in initiation of liver injury. Drug Metab. Dispos. 2005, 33, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.M.; AlRashdi, A.S.; Abdulla, M.A.; Hassandarvish, P.; Bilgen, M. Protective activity of Panduratin A against thioacetamide-induced oxidative damage: Demonstration with in vitro experiments using WRL-68 liver cell line. BMC Complement. Altern. Med. 2013, 13, 279. [Google Scholar] [CrossRef]
- Fennema, D.; Phillips, I.R.; Shephard, E.A. Trimethylamine and trimethylamine N-oxide, a flavin-containing monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab. Dispos. 2016, 44, 1839–1850. [Google Scholar] [CrossRef]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef]
- Kannan, R.R.R.; Arumugam, R.; Anantharaman, P. Pharmaceutical potential of a fucoidan-like sulphated polysaccharide isolated from Halodule pinifolia. Int. J. Biol. Macromol. 2013, 62, 30–34. [Google Scholar] [CrossRef]
- Fitton, J.H. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef]
- Pomin, V.H. Eucanomics and galactanomics: Current status in drug discovery, mechanisms of action and role of the well-defined structures. Biochim. Biophys. Acta. Gen. Subj. 2012, 1820, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism involving the ROS-dependent JNK activation and mitochondria-mediated pathways. PLoS ONE 2011, 6, e27441. [Google Scholar] [CrossRef]
- Alekseyenko, T.V.; Zhanayeva, S.Y.; Venediktova, A.A.; Zvyagintseva, T.N.; Kuz-netsova, T.A.; Besednova, N.N.; Korolenko, T.A. Antitumor and antimetastatic activity of fucoidan, a sulfated polysaccharide isolated from the Okhotsk Sea Fucus evanescens brown alga. Bull. Exp. Biol. Med. 2007, 143, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Tamauchi, H.; Iizuka, M.; Nakano, T. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida Sporophylls. Planta Med. 2006, 72, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, J.; Chang, A.K.; Liu, B.; Yang, L.; Li, Q.; Zou, X. Fucoidan extract derived from Undaria pinnatifida inhibits angiogenesis by human umbilical vein endothelial cells. Phytomedicine 2012, 19, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Chiu, Y.H.; Chan, Y.L.; Chiu, Y.H.; Wang, H.; Huang, K.C.; Wu, C.J. Prophylactic administration of fucoidan represses cancer metastasis by inhibiting vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in Lewis tumor-bearing mice. Mar. Drugs 2015, 13, 1882–1900. [Google Scholar] [CrossRef]
- Saito, A.; Yoneda, M.; Yokohama, S.; Okada, M.; Haneda, M.; Nakamura, K. Fucoidan prevents concanavalin A-induced liver injury through induction of endogenous IL-10 in mice. Hepatol. Res. 2006, 35, 190–198. [Google Scholar] [CrossRef][Green Version]
- Li, X.J.; Ye, Q.F. Fucoidan reduces inflammatory response in a rat model of hepatic ischemia–reperfusion injury. Can. J. Physiol. Pharmacol. 2015, 93, 999–1005. [Google Scholar] [CrossRef]
- Zheng, J.; Yan, Q.; Zhang, K.; Zheng, Y.; Zhao, S. Protective effects of different extracts of Eucommia ulmoides Oliv. against thioacetamide-induced hepatotoxicity in mice. Indian J. Exp. Biol. 2012, 50, 875–882. [Google Scholar] [PubMed]
- Tak, E.; Jung, D.H.; Kim, S.H.; Park, G.C.; Jun, D.Y.; Lee, J.; Jung, B.H.; Kirchner, V.A.; Hwang, S.; Song, G.W.; et al. Protective role of hypoxia-inducible factor-1alpha-dependent CD39 and CD73 in fulminant acute liver failure. Toxicol. Appl. Pharmacol. 2017, 314, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.L.; Much, C.; Gorzolla, I.C.; Schittenhelm, J.; Kloor, D.; Stahl, G.L.; Eltzschig, H.K. Extracellular adenosine production by ecto-5′-nucleotidase protects during murine hepatic ischemic. Gastroenterology 2008, 135, 1739–1750. [Google Scholar] [CrossRef]
- Keinicke, H.; Sun, G.; Mentzel, C.M.J.; Fredholm, M.; John, L.M.; Andersen, B.; Raun, K.; Kjaergaard, M. FGF21 regulates hepatic metabolic pathways to improve steatosis and inflammation. Endocr. Connect. 2020, 9, 755–768. [Google Scholar] [CrossRef]
- Kang, J.M.; Lee, W.J.; Kim, W.B.; Kim, T.Y.; Koh, J.M.; Hong, S.J.; Huh, J.; Ro, J.Y.; Chi, H.S.; Kim, M.S. Systemic inflammatory syndrome and hepatic inflammatory cell infiltration caused by an interleukin-6 producing pheochromocytoma. Endocr. J. 2005, 52, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, F.V.; Claudel, T.; Tardelli, M.; Starlinger, P.; Marra, F.; Trauner, M. PNPLA3 I148M variant impairs liver x receptor signaling and cholesterol homeostasis in human hepatic stellate cells. Hepatol. Commun. 2019, 3, 1191–1204. [Google Scholar] [CrossRef]
- Chang, M.L.; Yang, S.S. Metabolic signature of hepatic fibrosis: From individual pathways to systems biology. Cells 2019, 8, 1423. [Google Scholar] [CrossRef] [PubMed]
- Uma, N.J.; Fakurazi, S.; Hairuszah, I. Moringa oleifera enhances liver antioxidant status via elevation of antioxidant enzymes activity and counteracts paracetamol-induced hepatotoxicity. Malays. J. Nutr. 2010, 16, 293–307. [Google Scholar] [PubMed]
- Sakai, N.; Van Sweringen, H.L.; Belizaire, R.M.; Quillin, R.C.; Schuster, R.; Blanchard, J.; Burns, J.M.; Tevar, A.D.; Edwards, M.J.; Lentsch, A.B. Interleukin-37 reduces liver inflammatory injury via effects on hepatocytes and non-parenchymal cells. J. Gastroenterol. Hepatol. 2012, 27, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. 1), 173–179. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Wei, J.; Chunhua, M.; Danhong, Y.; Jianguo, Z.; Zongqi, C.; Jian-An, B. Protective effects of Ginsenoside Rg1 against carbon tetrachloride-induced liver injury in mice through suppression of inflammation. Phytomedicine 2016, 23, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.R.; Hou, P.H.; Yang, W.C.; Wang, C.M.; Fan, P.S.; Liao, H.J.; Chen, T.P. Doxepin exacerbates renal damage, glucose intolerance, nonalcoholic fatty liver disease and urinary chromium loss in obese mice. Pharmaceuticals 2021, 14, 267. [Google Scholar] [CrossRef]
- Azam, F.; Sheikh, N.; Ali, G.; Tayyeb, A. Fagonia indica repairs hepatic damage through expression regulation of toll-like receptors in a liver injury model. J. Immunol. Res. 2018, 2018, 7967135. [Google Scholar] [CrossRef]
- Chang, G.R.; Wu, Y.Y.; Chiu, Y.S.; Chen, W.Y.; Liao, J.W.; Hsu, H.M.; Chao, T.H.; Hung, S.W.; Mao, F.C. Long-term administration of rapamycin reduces adiposity, but impairs glucose tolerance in high-fat diet-fed KK/HlJ mice. Basic Clin. Pharmacol. Toxicol. 2009, 105, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Saleem, S.; Shameem, S.; Ahmed, S.P.; Parveen, T.; Haleem, D.J. Is anorexia in thioacetamide-induced cirrhosis related to an altered brain serotonin concentration? Pol. J. Pharmacol. 2004, 56, 73–78. [Google Scholar] [PubMed]
- Hsu, H.Y.; Hwang, P.A. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin. Transl. Med. 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Hsu, W.L.; Hwang, P.A.; Chen, Y.L.; Chou, T.C. Combined administration of fucoidan ameliorates tumor and chemotherapy-induced skeletal muscle atrophy in bladder cancer-bearing mice. Oncotarget 2016, 7, 51608–51618. [Google Scholar] [CrossRef] [PubMed]
- Mahachoklertwattana, P.; Wanasuwankul, S.; Poomthavorn, P.; Choubtum, L.; Sriphrapradang, A. Short-term cyproheptadine therapy in underweight children: Effects on growth and serum insulin-like growth factor-I. J. Pediatr. Endocrinol. Metab. 2009, 22, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.R.; Chiu, Y.S.; Wu, Y.Y.; Lin, Y.C.; Hou, P.H.; Mao, F.C. Rapamycin impairs HPD-induced beneficial effects on glucose homeostasis. Br. J. Pharmacol. 2015, 172, 3793–3804. [Google Scholar] [CrossRef] [PubMed]
- Alkiyumi, S.S.; Abdullah, M.A.; Alrashdi, A.S.; Salama, S.M.; Abdelwahab, S.I.; Hadi, A.H.A. Ipomoea aquatica extract shows protective action against thioacetamide-induced hepatotoxicity. Molecules 2012, 17, 6146–6155. [Google Scholar] [CrossRef]
- Wu, H.T.; Chuang, Y.W.; Huang, C.P.; Chang, M.H. Loss of angiotensin converting enzyme II (ACE2) accelerates the development of liver injury induced by thioacetamide. Exp. Anim. 2018, 67, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Abushouk, A.I.; Bahbah, E.I.; Bungau, S.G.; Alyousif, M.S.; Aleya, L.; Alkahtani, S. Fucoidan protects against subacute diazinon-induced oxidative damage in cardiac, hepatic, and renal tissues. Environ. Sci. Pollut. Res. 2020, 27, 11554–11564. [Google Scholar] [CrossRef]
- Schuppan, D.; Kim, Y.O. Evolving therapies for liver fibrosis. J. Clin. Investig. 2013, 123, 1887–1901. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, S.; Jiang, W.; Song, W.; Cai, L.; Wang, J. Fucoidan from sea cucumber may improve hepatic inflammatory response and insulin resistance in mice. Int. Immunopharmacol. 2016, 31, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Zhang, Y.; Wang, D.; Cheng, X.; Yang, F.; Zhang, Q.; Xue, Z.; Li, Y.; Zhang, L.; et al. Plumbagin protects liver against fulminant hepatic failure and chronic liver fibrosis via inhibiting inflammation and collagen production. Oncotarget 2016, 7, 82864–82875. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.S.; Chen, Y.C.; Chou, C.H.; Chuang, R.F.; Sheen, L.Y.; Chiu, C.H. Hepatoprotection of silymarin against thioacetamide-induced chronic liver fibrosis. J. Sci. Food. Agric. 2012, 92, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, D.K.; Jena, G.; Kumar, V. Dimethyl fumarate protects thioacetamide-induced liver damage in rats: Studies on Nrf2, NLRP3, and NF-κB. J. Biochem. Mol. Toxicol. 2020, 34, e22476. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Jung, K.H.; Lee, H.S.; Zheng, H.M.; Choi, M.J.; Lee, C.; Hong, S.S. Suppression by Fucoidan of liver fibrogenesis via the TGF-beta/Smad pathway in protecting against oxidative stress. Biosci. Biotechnol. Biochem. 2011, 75, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; Gallego, P.; Grande, L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J. Hepatol. 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Ding, W.X.; Yin, X.M. Dissection of the multiple mechanisms of TNF-α-induced apoptosis in liver injury. J. Cell. Mol. Med. 2004, 8, 445–454. [Google Scholar] [CrossRef]
- Sudo, K.; Yamada, Y.; Moriwaki, H.; Saito, K.; Seishima, M. Lack of tumor necrosis factor receptor type 1 inhibits liver fibrosis induced by carbon tetrachloride in mice. Cytokine 2005, 29, 236–244. [Google Scholar] [CrossRef]
- Sultan, M.; Ben-Ari, Z.; Masoud, R.; Pappo, O.; Harats, D.; Kamari, Y.; Safran, M. Interleukin-1α and Interleukin-1β play a central role in the pathogenesis of fulminant hepatic failure in mice. PLoS ONE 2017, 12, e0184084. [Google Scholar] [CrossRef]
- Kamari, Y.; Shaish, A.; Vax, E.; Shemesh, S.; Kandel-Kfir, M.; Arbel, Y.; Harats, D. Lack of interleukin-1α or interleukin-1β inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J. Hepatol. 2011, 55, 1086–1094. [Google Scholar] [CrossRef]
- Markova, M.; Pivovarova, O.; Hornemann, S.; Sucher, S.; Frahnow, T.; Wegner, K.; Pfeiffer, A.F. Isocaloric diets high in animal or plant protein reduce liver fat and inflammation in individuals with type 2 diabetes. Gastroenterology 2017, 152, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Wang, Y.; Li, H.; Jia, W.; Man, K.; Lo, C.M.; Xu, A. Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1α-mediated antioxidant capacity in mice. Hepatology 2014, 60, 977–989. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Pang, X.; Zhang, X.; Wang, S.; Wu, D. Rosiglitazone attenuates angiotensin II-induced c-reactive protein expression in hepatocytes via inhibiting AT1/ROS/MAPK signal pathway. Int. Immunopharmacol. 2016, 31, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Liu, T.C.; Yin, M.C. Beneficial effects of histidine and carnosine on ethanol-induced chronic liver injury. Food Chem. Toxicol. 2008, 46, 1503–1509. [Google Scholar] [CrossRef]
- Devière, J.; Content, J.; Denys, C.; Vandenbussche, P.; Schandené, L.; Wybran, J.; Dupont, E. High interleukin-6 serum levels and increased production by leucocytes in alcoholic liver cirrhosis. Correlation with IgA serum levels and lymphokines production. Clin. Exp. Immunol. 1989, 77, 221. [Google Scholar] [PubMed]
- Oyanagi, Y.; Takahashi, T.; Matsui, S.; Takahashi, S.; Boku, S.; Takahashi, K.; Asakura, H. Ehanced expression of interleukin-6 in chronic hepatitis C. Liver 1999, 19, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, F.V.; Claudel, T.; Tardelli, M.; Caligiuri, A.; Stulnig, T.M.; Marra, F.; Trauner, M. The PNPLA3 I148M variant modulates the fibrogenic phenotype of human hepatic stellate cells. Hepatology 2017, 65, 1875–1890. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Alisi, A.; Galmozzi, E.; Bartuli, A.; Del Menico, B.; Alterio, A.; Nobili, V. I148M patatin-like phospholipase domain-containing 3 gene variant and severity of pediatric nonalcoholic fatty liver disease. Hepatology 2010, 52, 1274–1280. [Google Scholar] [CrossRef]
- Bruschi, F.V.; Tardelli, M.; Einwallner, E.; Claudel, T.; Trauner, M. PNPLA3 I148M up-regulates hedgehog and yap signaling in human hepatic stellate cells. Int. J. Mol. Sci. 2020, 21, 8711. [Google Scholar] [CrossRef] [PubMed]
- Cakir, O.O.; Toker, A.; Ataseven, H.; Demir, A.; Polat, H. The importance of liver-fatty acid binding protein in diagnosis of liver damage in patients with acute hepatitis. J. Clin. Diagn. Res. 2017, 11, OC17–OC21. [Google Scholar]
- Özenirler, S.; Degertekin, C.K.; Erkan, G.; Elbeğ, Ş.; Tuncer, C.; Kandilc, U.; Akyol, G. Serum liver fatty acid binding protein shows good correlation with liver histology in NASH. Hepatogastroenterology 2013, 60, 1095–1100. [Google Scholar] [PubMed]
- Giannitrapani, L.; Ingrao, S.; Soresi, M.; Florena, A.M.; Spada, E.L.; Sandonato, L.; Montalto, G. Cyclooxygenase-2 expression in chronic liver diseases and hepatocellular carcinoma: An immunohistochemical study. Ann. N. Y. Acad. Sci. 2009, 1155, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, M.; Ramadori, P.; Blaschke, M.; Sultan, S.; Khan, S.; Malik, I.A.; Schultze, F.C. Immunodetection of cyclooxygenase-2 (COX-2) is restricted to tissue macrophages in normal rat liver and to recruited mononuclear phagocytes in liver injury and cholangiocarcinoma. Histochem. Cell Biol. 2012, 137, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Raso, G.M.; Meli, R.; Di Carlo, G.; Pacilio, M.; Di Carlo, R. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A. 1. Life Sci. 2001, 68, 921–931. [Google Scholar] [CrossRef]
- Diesen, D.L.; Kuo, P.C. Nitric oxide and redox regulation in the liver: Part II. Redox biology in pathologic hepatocytes and implications for intervention. J. Surg. Res. 2011, 167, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Cressman, D.E.; Greenbaum, L.E.; DeAngelis, R.A.; Ciliberto, G.; Furth, E.E.; Poli, V.; Taub, R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 1996, 274, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Meth. Enzymol. 1990, 186, 1–85. [Google Scholar]
- Espinoza, S.E.; Guo, H.; Fedarko, N.; DeZern, A.; Fried, L.P.; Xue, Q.L.; Walston, J.D. Glutathione peroxidase enzyme activity in aging. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, Y.; Hu, X.; Liu, Z.; Chen, J.; Lu, Y.; Liao, X. The hepatoprotective role of reduced glutathione and its underlying mechanism in oxaliplatin-induced acute liver injury. Oncol. Lett. 2018, 15, 2266–2272. [Google Scholar] [CrossRef]
- Jones, D.P.; Eklöw, L.; Thor, H.; Orrenius, S. Metabolism of hydrogen peroxide in isolated hepatocytes: Relative contributions of catalase and glutathione peroxidase in decomposition of endogenously generated H2O2. Arch. Biochem. Biophys. 1981, 210, 505–516. [Google Scholar] [CrossRef]
- Verkerk, A.; Jongkind, J.F. Vascular cells under peroxide induced oxidative stress: A balance study on in vitro peroxide handling by vascular endothelial and smooth muscle cells. Arch. Biochem. Biophys. 1992, 17, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Goth, L.; Meszaros, I.; Nemeth, H. Serum catalase enzyme activity in liver diseases. Acta. Biol. Hung. 1987, 38, 287–290. [Google Scholar]
- Kim, K.J.; Yoon, K.Y.; Lee, B.Y. Fucoidan regulate blood glucose homeostasis in C57BL/KSJ m+/+db and C57BL/KSJ db/db mice. Fitoterapia 2012, 83, 1105–1109. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Li, S.; Dai, W.; Feng, J.; Wu, L.; Liu, T.; Chen, K.; Xia, Y.; Lu, J.; et al. The natural product fucoidan ameliorates hepatic ischemia-reperfusion injury in mice. Biomed. Pharmacother. 2017, 94, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.Z.; Wang, Y.T.; Zhuo, Y.L.; Zhu, K.J.; Wang, X.Z.; Liu, A.J. Fucoidan inhibits LPS-induced acute lung injury in mice through regulating GSK-3 beta-Nrf2 signaling pathway. Arch. Pharm. Res. 2020, 43, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Smathers, R.L.; Fritz, K.S.; Galligan, J.J.; Shearn, C.T.; Reigan, P.; Marks, M.J.; Petersen, D.R. Characterization of 4-HNE modified L-FABP reveals alterations in structural and functional dynamics. PLoS ONE 2012, 7, 38459. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Hsieh, H.L.; Shih, R.H.; Chi, P.L.; Cheng, S.E.; Yang, C.M. Up-regulation of COX-2/PGE2 by endothelin-1 via MAPK-dependent NF-kappaB pathway in mouse brain microvascular endothelial cells. Cell Commun. Signal. 2013, 11, 8. [Google Scholar] [CrossRef]
- Mungrue, I.N.; Gros, R.; You, X.; Pirani, A.; Azad, A.; Csont, T.; Schulz, R.; Butany, J.; Stewart, D.J.; Husain, M. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J. Clin. Investig. 2002, 109, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Tirkey, N.; Pilkhwal, S.; Kuhad, A.; Chopra, K. Hesperidin, a citrus bioflavonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidney. BMC Pharmacol. 2005, 5, 2. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Cao, K.; Dong, Z.; Feng, Z.; Liu, J. Combination of β-glucan and morus alba l. leaf extract promotes metabolic benefits in mice fed a high-fat diet. Nutrients 2017, 9, 1110. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-Y.; Yang, W.-C.; Lin, C.-F.; Wang, C.-M.; Liu, H.-Y.; Lin, C.-S.; Lin, J.-W.; Lin, W.-L.; Lin, T.-C.; Fan, P.-S.; et al. The Ameliorative Effects of Fucoidan in Thioacetaide-Induced Liver Injury in Mice. Molecules 2021, 26, 1937. https://doi.org/10.3390/molecules26071937
Tsai M-Y, Yang W-C, Lin C-F, Wang C-M, Liu H-Y, Lin C-S, Lin J-W, Lin W-L, Lin T-C, Fan P-S, et al. The Ameliorative Effects of Fucoidan in Thioacetaide-Induced Liver Injury in Mice. Molecules. 2021; 26(7):1937. https://doi.org/10.3390/molecules26071937
Chicago/Turabian StyleTsai, Ming-Yang, Wei-Cheng Yang, Chuen-Fu Lin, Chao-Min Wang, Hsien-Yueh Liu, Chen-Si Lin, Jen-Wei Lin, Wei-Li Lin, Tzu-Chun Lin, Pei-Shan Fan, and et al. 2021. "The Ameliorative Effects of Fucoidan in Thioacetaide-Induced Liver Injury in Mice" Molecules 26, no. 7: 1937. https://doi.org/10.3390/molecules26071937
APA StyleTsai, M.-Y., Yang, W.-C., Lin, C.-F., Wang, C.-M., Liu, H.-Y., Lin, C.-S., Lin, J.-W., Lin, W.-L., Lin, T.-C., Fan, P.-S., Hung, K.-H., Lu, Y.-W., & Chang, G.-R. (2021). The Ameliorative Effects of Fucoidan in Thioacetaide-Induced Liver Injury in Mice. Molecules, 26(7), 1937. https://doi.org/10.3390/molecules26071937







