3.2.4. Specific Procedures and Characterization
2-Methyl-5-(3-phenyl-2H-azirin-2-yl)oxazole (
2a)
. Compound
2a [
27] was prepared following the general procedure GP-A from azirine
1a (463 mg, 2.5 mmol), acetonitrile (26 mL, 500 mmol) and Rh
2(oct)
4 (19.5 mg, 0.025 mmol) in DCE (250 mL) in 365 mg (73% yield, after column chromatography on silica (light petroleum/ethyl acetate, 4:1 (
v/v)) as a light brown oil.
1H-NMR (CDCl
3, 400 MHz):
δ 2.36 (s, 3H), 3.25 (s, 1H), 6.84 (s, 1H), 7.56–7.62 (m, 2H), 7.63–7.67 (m, 1H), 7.90–7.93 (m, 2H).
2-Methyl-5-(3-(4-bromophenyl)-2H-azirin-2-yl)oxazole (2b). Compound 2b was prepared following the general procedure GP-A from azirine 1b (264 mg, 1 mmol), acetonitrile (10.4 g, 200 mmol) and Rh2(oct)4 (8 mg, 0.01 mmol) in DCE (150 mL) in 135 mg (49% yield, after column chromatography on silica (light petroleum/ethyl acetate, 7:1–4:1 (v/v)) as a brown oil. 1H-NMR (CDCl3, 400 MHz): δ 2.36 (s, 3H), 3.27 (s, 1H), 6.85 (s, 1H), 7.73–7.79 (m, 4H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 13.9 (CH3), 25.3 (CH), 122.6 (C), 124.5 (CH), 128.6 (C), 131.1 (CH), 132.8 (CH), 150.7 (C), 160.9 (C), 162.1 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C12H10BrN2O+ 276.9971; found 276.9974. IR (KBr, cm−1): ν 1571, 1743, 2925.
5-(3-(Adamantan-1-yl)-2H-azirin-2-yl)-2-methyloxazole (2c). Compound 2c was prepared following the general procedure GP-A from azirine 1c (100 mg, 0.41 mmol), acetonitrile (4.3 mL, 82 mmol) and Rh2(oct)4 (3 mg, 0.0041 mmol) in DCE (100 mL) in 63 mg (61% yield, after column chromatography on silica (light petroleum/ethyl acetate, 10:1 (v/v)) as a yellow oil. 1H-NMR (CDCl3, 400 MHz): δ 1.77–1.85 (m, 6H), 1.95–1.98 (m, 6H), 2.10–2.13 (m, 3H), 2.38 (s, 3H), 2.81 (s, 1H), 6.75 (s, 1H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 13.9 (CH3), 24.0 (CH), 27.5 (CH), 35.6 (C), 36.4 (CH2), 38.2 (CH2), 123.6 (CH), 152.0 (C), 160.2 (C), 170.7 (C). HRMS (ESI) m/z: [M + Na]+ calcd. for C16H20N2NaO+ 279.1468; found 279.1458. IR (KBr, cm−1): ν 1574, 1697, 2851, 2907.
2-Ethyl-5-(3-phenyl-2H-azirin-2-yl)oxazole (2d). Compound 2d was prepared following the general procedure GP-A from azirine 1a (185 mg, 1 mmol), propionitrile (14.3 mL, 200 mmol) and Rh2(oct)4 (8 mg, 0.01 mmol) in DCE (200 mL) in 121 mg (57% yield, after column chromatography on silica (light petroleum/ethyl acetate, 11:1–7:1 (v/v)) as a light brown oil. 1H-NMR (CDCl3, 400 MHz): δ 1.26 (t, 3H, J = 7.6 Hz), 2.69 (q, 2H, J = 7.6 Hz), 3.26 (s, 1H), 6.83 (s, 1H), 7.56–7.66 (m, 3H), 7.90–7.93 (m, 2H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 11.1 (CH3), 21.7 (CH2), 25.1 (CH), 123.8 (C), 124.1 (CH), 129.3 (C), 129.9 (CH), 133.6 (CH), 151.0 (C), 162.5 (C), 165.2 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C13H12N2O+ 213.1022; found 213.1027. IR (KBr, cm−1): ν 1566, 1599, 1691, 1745, 2981.
2-Ethyl-5-(3-(4-methoxyphenyl)-2H-azirin-2-yl)oxazole (2e). Compound 2e was prepared following the general procedure GP-A from azirine 1d (215 mg, 1 mmol), propionitrile (14.3 mL, 200 mmol) and Rh2(oct)4 (8 mg, 0.01 mmol) in DCE (200 mL) in 117 mg (48% yield, after column chromatography on silica (light petroleum/ethyl acetate, 4:1 (v/v)) as a brown oil. 1H-NMR (CDCl3, 400 MHz): δ 1.26 (t, 3H, J = 7.6 Hz), 2.69 (q, 2H, J = 7.6 Hz), 3.19 (s, 1H), 3.91 (s, 3H), 6.80 (s, 1H), 7.06–7.09 (m, 2H), 7.84–7.87 (m, 2H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 11.0 (CH3), 21.7 (CH2), 24.7 (CH), 55.6 (CH3), 114.9 (CH), 116.1 (C), 123.8 (CH), 131.9 (CH), 151.3 (C), 161.1 (C), 163.8 (C), 165.02 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C14H15N2O2+ 243.1128; found 243.1131. IR (KBr, cm−1): ν 1509, 1567, 1605, 1679, 1745, 2939, 2980.
5-(3-(tert-Butyl)-2H-azirin-2-yl)-2-ethyloxazole (2f). Compound 2f was prepared following the general procedure GP-A from azirine 1e (150 mg, 0.91 mmol), propionitrile (9.7 mL, 136 mmol) and Rh2(oct)4 (7 mg, 0.0091 mmol) in DCE (150 mL) in 67 mg (38% yield, after column chromatography on silica (light petroleum/ethyl acetate, 6:1 (v/v)) as an orange oil. 1H-NMR (CDCl3, 400 MHz): δ 1.27 (t, 3H, J = 7.6 Hz), 1.34 (s, 9H), 2.70 (q, 2H, J = 7.6 Hz), 2.90 (s, 1H), 6.80 (s, 1H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 11.0 (CH3), 21.6 (CH2), 24.5 (CH), 26.0 (CH3), 33.3 (C), 123.6 (CH), 151.4 (C), 164.7 (C), 171.8 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C11H17N2O+ 193.1335; found 193.1328. IR (KBr, cm−1): ν 1568, 1700, 2971.
2-Benzyl-5-(3-(4-bromophenyl)-2H-azirin-2-yl)oxazole (2g). Compound 2g was prepared following the general procedure GP-A from azirine 1d (200 mg, 0.76 mmol), benzyl cyanide (12.9 g, 110 mmol) and Rh2(oct)4 (6 mg, 0.0076 mmol) in DCE (200 mL) in 94 mg (35% yield, after column chromatography on silica (toluene/light petroleum/ethyl acetate, 20:1 + 0.5% triethylamine (v/v)) as a brown oil. 1H-NMR (CDCl3, 400 MHz): δ 3.30 (s, 1H), 4.06 (s, 2H), 6.89 (s, 1H), 7.26–7.35 (m, 5H), 7.75–7.81 (m, 4H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 25.3 (CH), 34.6 (CH2), 122.6 (C), 124.5 (CH), 127.1 (CH), 128.7 (CH), 128.8 (C), 131.1 (CH), 132.8 (CH), 135.3 (C), 151.3 (C), 161.9 (C), 161.9 (C), 162.5 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C18H14BrN2O+ 353.0284; found 353.0288. IR (KBr, cm−1): ν 1670, 2924.
2-Phenyl-5-(3-phenyl-2H-azirin-2-yl)oxazole (
2h). Compound
2h [
27] was prepared following the general procedure GP-A from azirine
1a (500 mg, 2.7 mmol), benzonitrile (29 mL, 284 mmol) and Rh
2(oct)
4 (2.1 mg, 0.027 mmol) in DCE (250 mL) in 302 mg (43% yield, after column chromatography on silica (light petroleum/ethyl acetate, 7:1 (
v/v)) as a brown oil.
1H-NMR (CDCl
3, 400 MHz):
δ 3.37 (s, 1H), 7.06 (s, 1H), 7.38–7.42 (m, 3H), 7.59–7.67 (m, 3H), 7.92–7.98 (m, 4H).
5-(3-(4-Fluorophenyl)-2H-azirin-2-yl)-2-phenyloxazole (2i). Compound 2i was prepared following the general procedure GP-A from azirine 1f (102 mg, 0.5 mmol), benzonitrile (10.4 mL, 100 mmol) and Rh2(oct)4 (4 mg, 0.005 mmol) in DCE (200 mL) in 78 mg (56% yield, after column chromatography on silica (light petroleum/ethyl acetate, 1:0–11:1–6:1 (v/v)) as a brown oil. 1H-NMR (CDCl3, 400 MHz): δ 3.38 (s, 1H), 7.07 (s, 1H), 7.28–7.33 (m, 2H), 7.38–7.42 (m, 3H), 7.91–7.94 (m, 2H), 7.96–8.00 (m, 2H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 25.4 (CH), 117.0 (d, CH, J = 22.4 Hz,), 119.9 (d, C, J = 3.2 Hz,), 125.8 (CH), 126.2 (CH), 127.3 (C), 128.7 (CH), 130.2 (CH), 132.3 (d, CH, J = 9.3 Hz), 136.0 (CH), 151.4 (C), 161.3 (C), 165.9 (d, C, J = 256.6 Hz). HRMS (ESI) m/z: [M + H]+ calcd. for C17H12FN2O+ 279.0928; found 279.0925. IR (KBr, cm−1): ν 1505, 1543, 1600, 1745, 3059.
5-(3-(tert-Butyl)-2H-azirin-2-yl)-2-phenyloxazole (2j). Compound 2j was prepared following the general procedure GP-A from azirine 1e (150 mg, 0.91 mmol), benzonitrile (14 mL, 137 mmol) and Rh2(oct)4 (7 mg, 0.0091 mmol) in DCE (150 mL) in 111 mg (51% yield, after column chromatography on silica (light petroleum/ethyl acetate, 9:1 (v/v)) as an orange oil. 1H-NMR (CDCl3, 400 MHz): δ 1.40 (s, 9H), 3.02 (s, 1H), 7.06 (s, 1H), 7.41–7.43 (m, 3H), 7.93–7.96 (m, 2H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 25.6 (CH), 26.1 (CH3), 33.6 (C), 125.5 (CH), 126.1 (CH), 127.4 (C), 128.7 (CH), 130.1 (CH), 152.1 (C), 160.6 (C), 171.6 (C). HRMS (ESI) m/z: [M + Na]+ calcd. for C15H16N2NaO+ 263.1155; found 263.1144. IR (KBr, cm−1): ν 1547, 1590, 2933, 2970.
5-(3-(Adamantan-1-yl)-2H-azirin-2-yl)-2-phenyloxazole (2k). Compound 2k was prepared following the general procedure GP-A from azirine 1c (150 mg, 0.62 mmol), benzonitrile (9.5 mL, 93 mmol) and Rh2(oct)4 (5 mg, 0.0062 mmol) in DCE (130 mL) in 84 mg (43% yield, after column chromatography on silica (light petroleum/ethyl acetate, 7:1 (v/v)) as a yellow oil. 1H-NMR (CDCl3, 400 MHz): δ 1.79–1.87 (m, 6H), 1.99–2.07 (m, 6H), 2.12–2.16 (m, 3H), 2.93 (s, 1H), 7.01 (s, 1H), 7.41–7.45 (m, 3H), 7.93–7.96 (m, 2H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 24.1 (CH), 27.5 (CH), 35.7 (C), 36.4 (CH2), 38.3 (CH2), 125.2 (CH), 126.1 (CH), 127.5 (C), 128.8 (CH), 130.1 (CH), 152.4 (C), 160.5 (C), 170.5 (C). HRMS (ESI) m/z: [M + Na]+ calcd. for C21H22N2NaO+ 341.1624; found 341.1621. IR (KBr, cm−1): ν 1580, 1756, 2855, 2911.
5-(3-(4-Methoxyphenyl)-2H-azirin-2-yl)-2-(p-tolyl)oxazole (2l). Compound 2l was prepared following the general procedure GP-A from azirine 1d (200 mg, 0.93 mmol), p-toluonitrile (7 g, 60 mmol) and Rh2(oct)4 (7 mg, 0.0093 mmol) in DCE (200 mL) in 58 mg (27% yield, after column chromatography on silica (light petroleum/ethyl acetate, 1:0–8:1–4:1 (v/v)) as a brown oil. 1H-NMR (CDCl3, 400 MHz): δ 2.37 (s, 3H), 3.31 (s, 1H), 3.92 (s, 3H), 7.02 (s, 1H), 7.08–7.10 (m, 2H), 7.19–7.21 (m, 2H), 7.82–7.84 (m, 2H), 7.88–7.92 (m, 2H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 21.4 (CH3), 24.9 (CH), 55.6 (CH3), 114.9 (CH), 115.9 (C), 124.7 (C), 125.4 (CH), 126.2 (CH), 129.3 (CH), 132.0 (CH), 140.4 (C), 151.7 (C), 160.9 (C), 161.2 (C), 163.9 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C19H17N2O2+ 305.1285; found 305.1296. IR (KBr, cm−1): ν 1509, 1605, 1676, 1724, 2853, 2924.
5-(3-(4-Chlorophenyl)-2H-azirin-2-yl)-2-(p-tolyl)oxazole (2m). Compound 2m was prepared following the general procedure GP-A from azirine 1g (200 mg, 0.91 mmol), p-toluonitrile (10.6 g, 91 mmol) and Rh2(oct)4 (7 mg, 0.0091 mmol) in DCE (150 mL) in 95 mg (34% yield, after column chromatography on silica (light petroleum/ethyl acetate, 1:0–7:1 (v/v)) as a brown oil. 1H-NMR (CDCl3, 400 MHz): δ 2.37 (s, 3H), 3.37 (s, 1H), 7.04 (s, 1H), 7.19–7.21 (m, 2H), 7.58–7.60 (m, 2H), 7.79–7.81 (m, 2H), 7.89–7.91 (m, 2H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 21.5 (CH3), 25.5 (CH), 122.1 (C), 124.6 (C), 125.8 (CH), 126.2 (CH), 129.4 (CH), 129.9 (CH), 131.1 (CH), 140.1 (C), 140.6 (C), 150.9 (C), 161.3 (C), 161.8 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C18H14ClN2O2+ 309.0789; found 309.0792. IR (KBr, cm−1): ν 1590, 1675, 1741, 2924.
5-(3-(Adamantan-1-yl)-2H-azirin-2-yl)-2-(p-tolyl)oxazole (2n). Compound 2n was prepared following the general procedure GP-A from azirine 1c (100 mg, 0.41 mmol), p-toluonitrile (5.2 g, 41 mmol) and Rh2(oct)4 (3 mg, 0.0041 mmol) in DCE (100 mL) in 63 mg (46% yield, after column chromatography on silica (light petroleum/ethyl acetate, 1:0–10:1 (v/v)) as a yellow oil. 1H-NMR (CDCl3, 400 MHz): δ 1.79–1.87 (m, 6H), 1.98–2.07 (m, 6H), 2.12–2.15 (m, 3H), 2.39 (s, 3H), 2.92 (s, 1H), 6.99 (s, 1H), 7.23–7.25 (m, 2H), 7.82–7.84 (m, 2H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 21.5 (CH3), 24.2 (CH), 27.5 (CH), 35.7 (C), 36.4 (CH2), 38.3 (CH2), 124.8 (C), 125.1 (CH), 126.0 (CH), 129.5 (CH), 140.4 (C), 152.1 (C), 160.7 (C), 170.6 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C22H25N2O+ 333.1961; found 333.1964. IR (KBr, cm−1): ν 1590, 1758, 2856, 2903.
2-(4-Bromophenyl)-5-(3-phenyl-2H-azirin-2-yl)oxazole (2o). Compound 2o was prepared following the general procedure GP-A from azirine 1a (185 mg, 1 mmol), 4-bromobenzonitrile (25 g, 200 mmol) and Rh2(oct)4 (8 mg, 0.01 mmol) in DCE (200 mL) in 51 mg (15% yield, after column chromatographies on silica (toluene/light petroleum/ethyl acetate, 100:0:0–100:1:1–0:12:1 (v/v); hexanes/methyl acetate, 20:1 (v/v) + 0.5% NEt3) as a brown oil. The low yield of compound 2o is associated with its significant losses during chromatographic isolation due to the low solubility of the starting 4-bromobenzonitrile, which is used in a large excess in the reaction. 1H-NMR (CDCl3, 400 MHz): δ 3.36 (s, 1H), 7.05 (s, 1H), 7.52–7.55 (m, 2H), 7.59–7.63 (m, 2H), 7.65–7.69 (m, 1H), 7.77–7.81 (m, 2H), 7.94–7.971 (m, 2H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 25.2 (CH), 123.4 (C), 124.6 (C), 125.8 (CH), 126.3 (C), 127.7 (CH), 129.4 (CH), 130.0 (CH), 132.0 (CH), 133.8 (CH), 152.02 (C), 160.1 (C), 162.2 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C17H12N2O+ 339.0128; found 339.0128. IR (KBr, cm−1): ν 1599, 1675, 1728, 1741.
2-(4-Bromophenyl)-5-(3-(tert-butyl)-2H-azirin-2-yl)oxazole (2p). Compound 2p was prepared following the general procedure GP-A from azirine 1e (150 mg, 0.91 mmol), 4-bromobenzonitrile (14 g, 77 mmol) and Rh2(oct)4 (7 mg, 0.0091 mmol) in DCE (150 mL) in 89 mg (31% yield, after column chromatography on silica (light petroleum/ethyl acetate, 9:1–8:1 (v/v)) as a red oil. 1H-NMR (CDCl3, 400 MHz): δ 1.39 (s, 9H), 3.00 (s, 1H), 7.04 (s, 1H), 7.55–7.57 (m, 2H), 7.78-7.80 (m, 2H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 25.5 (CH), 26.1 (CH3), 33.6 (C), 124.6 (C), 125.5 (CH), 126.3 (C), 127.5 (CH), 132.0 (CH), 152.5 (C), 159.7 (C), 171.4 (C). HRMS (ESI) m/z: [M + Na]+ calcd. for C15H15BrN2NaO+ 341.0260; found 341.0251. IR (KBr, cm−1): ν 1573, 1754, 2971.
(E)-3-(5-(3-phenyl-2H-azirin-2-yl)oxazol-2-yl)acrylonitrile (2q). Compound 2q was prepared following the general procedure GP-A from azirine 1a (100 mg, 0.54 mmol), fumaronitrile (4.2 g, 54 mmol) and Rh2(oct)4 (4 mg, 0.0054 mmol) in DCE (150 mL) in 38 mg (30% yield, after column chromatography on silica (light petroleum/ethyl acetate, 1:0–30:1 (v/v)) as a yellow oil. 1H-NMR (CDCl3, 400 MHz): δ 3.30 (s, 1H), 6.10 (d, 1H, J = 16.5 Hz), 7.08 (d, 1H, J = 16.5 Hz), 7.13 (s, 1H), 7.59–7.63 (m, 2H), 7.66–7.70 (m, 1H), 7.90–7.93 (m, 2H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 24.9 (CH), 102.4 (CH), 116.5 (C), 122.8 (C), 127. (CH), 129.5 (CH), 130.0 (CH), 133.8 (CH), 134.0 (CH), 154.2 (C), 157.0 (C), 161.3 (C). HRMS (ESI) m/z: [M + Na]+ calcd. for C14H9N3NaO+ 258.0638; found 258.0638. IR (KBr, cm−1): ν 1516, 1746, 2217, 2854, 2924, 3062.
5-(3-Phenyl-2H-azirin-2-yl)-2-vinyloxazole (2r). Compound 2r was prepared following the general procedure GP-A from azirine 1a (222 mg, 1.2 mmol), acrylonitrile (15.7 mL, 240 mmol) and Rh2(oct)4 (10 mg, 0.012 mmol) in DCE (200 mL) in 106 mg (38% yield, after column chromatography on silica (light petroleum/ethyl acetate, 5:1 (v/v)) as a yellow oil. 1H-NMR (CDCl3, 400 MHz): δ 3.30 (s, 1H), 5.53 (dd, 1H, J = 11.3, 1.1 Hz), 6.04 (dd, 1H, J = 17.7, 1.1 Hz), 6.51 (dd, 1H, J = 17.7, 11.3 Hz), 6.97 (s, 1H), 7.58–7.67 (m, 3H), 7.92–7.95 (m, 2H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 25.1 (CH), 121.4 (CH2), 123.2 (CH), 123.5 (C), 125.4 (CH), 129.4 (CH), 129.9 (CH), 133.7 (CH), 151.4 (C), 160.4 (C), 162.2 (C). HRMS (ESI) m/z: [M + Na]+ calcd. for C13H10N2NaO+ 233.0685; found 233.0677. IR (KBr, cm−1): ν 1519, 1597, 1696, 1745, 3061.
2-(Chloromethyl)-5-(3-phenyl-2H-azirin-2-yl)oxazole (2s). Compound 2s was prepared following the general procedure GP-A from azirine 1a (200 mg, 1.1 mmol), chloroacetonitrile (13.8 mL, 220 mmol) and Rh2(oct)4 (8 mg, 0.01 mmol) in DCE (150 mL) in 67 mg (26% yield, after column chromatography on silica (light petroleum/ethyl acetate, 5:1 (v/v)) as a yellow oil. 1H-NMR (CDCl3, 400 MHz): δ 3.29 (s, 1H), 4.53 (d, 2H, J = 0.8 Hz), 6.93 (s, 1H), 7.58–7.63 (m, 2H), 7.65–7.69 (m, 1H), 7.91–7.94 (m, 2H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 24.9 (CH), 35.8 (CH2), 123.3 (C), 124.8 (CH), 129.4 (CH), 130.0 (CH), 133.8 (CH), 153.3 (C), 158.2 (C), 161.8 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C12H10ClN2O+ 213.0476; found 233.0476. IR (KBr, cm−1): ν 1526, 1598, 1689, 2854, 2926, 3035, 3143.
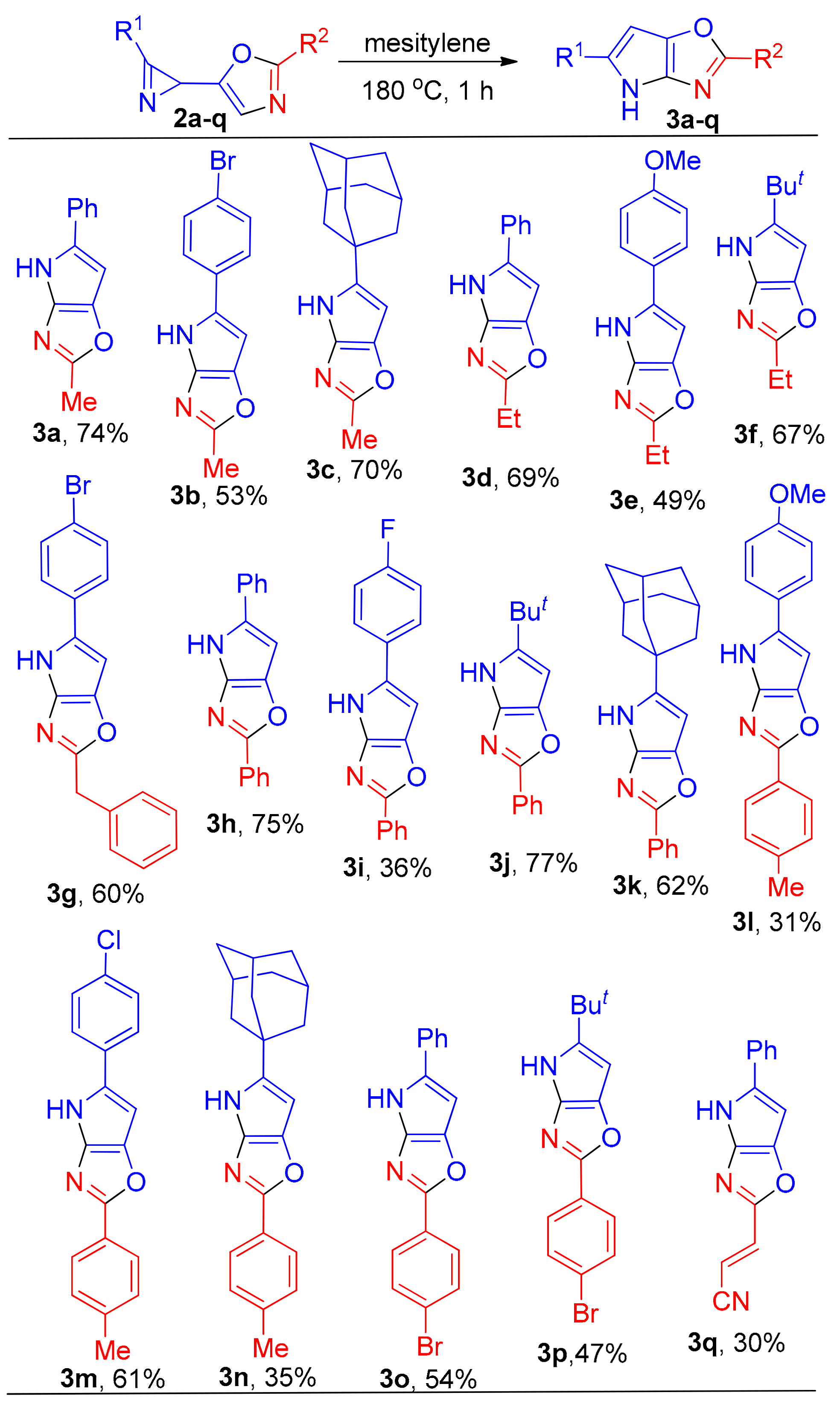
2-Methyl-5-phenyl-4H-pyrrolo[2,3-d]oxazole (3a). Compound 3a was prepared following the general procedure GP-B from azirine 2a (100 mg, 0.5 mmol) in mesitylene (1.0 mL) in 74 mg (74% yield, after column chromatography on silica (chloroform/methanol, 0:1–100:1 (v/v)) as a light brown solid: mp 194–195 °C (chloroform). 1H-NMR (DMSO-d6, 400 MHz): δ 2.52 (s, 3H), 6.60 (d, 1H, J = 1.7 Hz), 7.15–7.19 (m, 1H), 7.33–7.37 (m, 2H), 7.64–7.67 (m, 2H), 11.60 (s, 1H). 13C{1H}-NMR (DMSO-d6, 100 MHz): δ 14.8 (CH3), 87.7 (CH), 123.4 (CH), 125.9 (CH), 128.7 (CH), 131.1 (C), 133.4 (C), 139.0 (C), 140.9 (C), 162.6 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C12H11N2O+ 199.0866; found 199.0873. IR (KBr, cm−1): ν 1509, 1556, 1606, 3167, 3205.
2-Methyl-5-(4-bromophenyl)-4H-pyrrolo[2,3-d]oxazole (3b). Compound 3b was prepared following the general procedure GP-B from azirine 2b (98 mg, 0.35 mmol) in mesitylene (1.5 mL) in 52 mg (53% yield, after evaporation of solvent and washing with cold ether) as a brown solid: mp 274–276 °C (mesitylene). 1H-NMR (DMSO-d6, 400 MHz): δ 2.52 (s, 3H), 6.66 (d, J = 1.6 Hz, 1H), 7.52–7.55 (m, 2H), 7.60–7.63 (m, 2H), 11.68 (s, 1H). 13C{1H}-NMR (DMSO-d6, 100 MHz): δ 14.8 (CH3), 88.3 (CH), 118.4 (C), 125.3 (CH), 129.8 (C), 131.6 (CH), 132.7 (C), 139.4 (C), 140.9 (C), 162.0 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C12H10BrN2O+ 276.9971; found 276.9965. IR (KBr, cm−1): ν 1558, 3131, 3214.
5-(Adamantan-1-yl)-2-methyl-4H-pyrrolo[2,3-d]oxazole (3c). Compound 3c was prepared following the general procedure GP-B from azirine 2c (56 mg, 0.22 mmol) in mesitylene (1.0 mL) in 35 mg (70% yield, after column chromatography on silica (light petroleum/ethyl acetate, 7:1 (v/v)) as a light brown solid: mp 204–206 °C (light petroleum/ethyl acetate). 1H-NMR (CDCl3, 400 MHz): δ 1.73–1.86 (m, 6H), 1.94–1.95 (m, 6H), 2.07–2.09 (m, 3H), 2.55 (s, 3H), 5.83 (d, 1H, J = 1.8 Hz), 8.96 (s, 1H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 15.0 (CH3), 27.5 (CH), 28.5 (CH), 34.2 (C), 36.7 (CH2), 43.0 (CH2), 85.6 (CH), 136.1 (C), 140.7 (C), 143.0 (C), 160.5 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C16H21N2O+ 257.1648; found 257.1647. IR (KBr, cm−1): ν 1552, 1663, 1714, 2847, 2905, 3211.
2-Ethyl-5-phenyl-4H-pyrrolo[2,3-d]oxazole (3d). Compound 3d was prepared following the general procedure GP-B from azirine 2d (75 mg, 0.35 mmol) in mesitylene (1.0 mL) in 52 mg (69% yield, after column chromatography on silica (toluene/chloroform, 100:1 (v/v)) as a light brown solid: mp 155–157 °C (toluene). 1H-NMR (CDCl3, 400 MHz): δ 1.43 (t, 3H, J = 7.6 Hz), 2.97 (q, 2H, J = 7.6 Hz), 6.45 (d, 1H, J = 1.6 Hz), 7.21-7.26 (s, 1H), 7.37–7.40 (m, 2H), 7.52–7.55 (m, 2H), 9.26 (s, 1H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 11.8 (CH3), 23.1 (CH2), 88.4 (CH), 124.0 (CH), 126.5 (CH), 128.9 (CH), 132.2 (C), 133.6 (C), 138.7 (C), 141.9 (C), 166.74 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C13H13N2O+ 213.1022; found 213.1015. IR (KBr, cm−1): ν 1508, 1551, 1604, 3204.
2-Ethyl-5-(4-methoxyphenyl)-4H-pyrrolo[2,3-d]oxazole (3e). Compound 3e was prepared following the general procedure GP-B from azirine 2e (82 mg, 0.34 mmol) in mesitylene (1.5 mL) in 40 mg (49% yield, after column chromatography on silica (light petroleum/ethyl acetate, 5:1 + 5% chloroform (v/v)) as a brown solid: mp 214–216 °C (light petroleum/ethyl acetate). 1H-NMR (DMSO-d6, 400 MHz): δ 1.40 (t, 3H, J = 7.6 Hz), 2.92 (q, 2H, J = 7.6 Hz), 3.84 (s, 3H), 6.30 (d, 1H, J = 1.6 Hz), 6.92–6.95 (m, 2H), 7.44–7.47 (m, 2H), 9.03 (s, 1H). 13C{1H}-NMR (DMSO-d6, 100 MHz): δ 11.9 (CH3), 22.6 (CH2), 55.6 (CH3), 87.1 (CH), 114.7 (CH), 125.4 (CH), 126.7 (C), 131.8 (C), 138.7 (C), 141.3 (C), 158.2 (C), 165.8 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C14H15N2O2+ 243.1128; found 243.1122. IR (KBr, cm−1): ν 1517, 1551, 1613, 2959, 3233.
5-(tert-Butyl)-2-ethyl-4H-pyrrolo[2,3-d]oxazole (3f). Compound 3f was prepared following the general procedure GP-B from azirine 2f (59 mg, 0.31 mmol) in mesitylene (1.5 mL) in 39 mg (67% yield, after column chromatography on silica (light petroleum/ethyl acetate, 7:1 (v/v)) as a light brown solid: mp 151–154 °C (light petroleum/ethyl acetate). 1H-NMR (CDCl3, 400 MHz): δ 1.35 (s, 9H), 1.37 (t, 3H, J = 7.5 Hz), 2.87 (q, 2H, J = 7.5 Hz,), 5.86 (d, 1H, J = 1.7 Hz), 8.82 (s, 1H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 11.8 (CH3), 22.8 (CH2), 30.6 (CH3), 32.4 (C), 86.0 (CH), 136.4 (C), 140.4 (C), 142.4 (C), 165.3 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C11H17N2O+ 193.1335; found 193.1333. IR (KBr, cm−1): ν 1525, 1550, 1584, 2867, 2963, 3236.
2-Benzyl-5-(4-bromophenyl)-4H-pyrrolo[2,3-d]oxazole (3g). Compound 3g was prepared following the general procedure GP-B from azirine 2g (94 mg, 0.27 mmol) in mesitylene (1.5 mL) in 56 mg (60% yield, after column chromatography on silica (light petroleum/ethyl acetate, 4:1 + 5% chloroform (v/v)) as a brown solid: mp 227–230 °C (light petroleum/ethyl acetate). 1H-NMR (DMSO-d6, 400 MHz): δ 4.22 (s, 2H), 6.66 (d, 1H, J = 1.7 Hz), 7.26–7.29 (m, 1H), 7.32–7.37 (m, 4H), 7.53–7.56 (m, 2H), 7.60–7.63 (m, 2H), 11.72 (s, 1H). 13C{1H}-NMR (DMSO-d6, 100 MHz): δ 34.9 (CH2), 88.4 (CH), 118.6 (C), 125.4 (CH), 126.8 (CH), 128.6 (CH), 128.7 (CH), 130.3 (C), 131.6 (CH), 132.5 (C), 136.1 (C), 139.3 (C), 141.2 (C), 163.5 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C18H14BrN2O+ 353.0284; found 353.0284. IR (KBr, cm−1): ν 1506, 3210.
2,5-Diphenyl-4H-pyrrolo[2,3-d]oxazole (3h). Compound 3h was prepared following the general procedure GP-B from azirine 2h (85 mg, 0.33 mmol) in mesitylene (1.0 mL) in 64 mg (75% yield, after column chromatography on silica (toluene/chloroform, 100:1 (v/v)) as a light brown solid: mp 246–247 °C (toluene). 1H-NMR (DMSO-d6, 400 MHz): δ 6.75 (d, 1H, J = 1.6 Hz), 7.21–7.25 (m, 1H), 7.38–7.42 (m, 2H), 7.48–7.50 (m, 1H), 7.51–7.56 (m, 2H), 7.72–7.74 (m, 2H), 8.01–8.04 (m, 2H), 11.86 (s, 1H). 13C{1H}-NMR (DMSO-d6, 100 MHz): δ 87.9 (CH), 123.8 (CH), 125.3 (CH), 126.4 (CH), 128.1 (C), 128.8 (CH), 129.1 (CH), 129.8 (CH), 133.0 (C), 133.4 (C), 140.3 (C), 141.7 (C), 160.8 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C17H13N2O+ 261.1022; found 261.1015. IR (KBr, cm−1): ν 1467, 1600, 3256.
5-(4-Fluorophenyl)-2-phenyl-4H-pyrrolo[2,3-d]oxazole (3i). Compound 3i was prepared following the general procedure GP-B from azirine 2i (120 mg, 0.43 mmol) in mesitylene (1.5 mL) in 43 mg (36% yield, after evaporation of solvent and washing with acetonitrile) as a brown solid: mp 248–251 °C (mesitylene). 1H-NMR (DMSO-d6, 400 MHz): δ 6.72 (d, 1H, J = 1.6 Hz), 7.23–7.28 (m, 2H), 7.46–7.50 (m, 1H), 7.51–7.56 (m, 2H), 7.74–7.77 (m, 2H), 8.00–8.03 (m, 2H), 11.86 (s, 1H). 13C{1H}-NMR (DMSO-d6, 100 MHz): δ 88.5 (CH), 116.2 (d, CH, J = 21.6 Hz), 125.8 (CH), 126.2 (d, CH, J = 7.8 Hz), 128.5 (C), 129.6 (CH), 130.2 (d, C, J = 3.2 Hz), 130.3 (CH), 132.9 (C), 140.7 (C), 142.1 (C), 161.2 (C), 161.4 (d, C, J = 243.7 Hz). HRMS (ESI) m/z: [M + H]+ calcd. for C17H12FN2O+ 279.0928; found 279.0932. IR (KBr, cm−1): ν 1513, 1563, 3252.
5-(tert-Butyl)-2-phenyl-4H-pyrrolo[2,3-d]oxazole (3j). Compound 3j was prepared following the general procedure GP-B from azirine 2j (87 mg, 0.36 mmol) in mesitylene (1.5 mL) in 67 mg (77% yield, after column chromatography on silica (light petroleum/ethyl acetate, 9:1 (v/v)) as a light brown solid: mp 119–121 °C (light petroleum/ethyl acetate). 1H-NMR (CDCl3, 400 MHz): δ 1.35 (s, 9H), 5.96 (d, 1H, J = 1.7 Hz), 7.35–7.39 (m, 1H), 7.41–7.46 (m, 2H), 8.03–8.05 (m, 2H), 8.19 (s, 1H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 30.5 (CH3), 32.6 (C), 86.5 (CH), 125.7 (CH), 128.7 (CH), 129.0 (C), 129.2 (CH), 138.0 (C), 141.2 (C), 144.2 (C), 160.8 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C15H17N2O+ 241.1335; found 241.1337. IR (KBr, cm−1): ν 1541, 1571, 1605, 2963, 3134, 3215.
5-(Adamantan-1-yl)-2-phenyl-4H-pyrrolo[2,3-d]oxazole (3k). Compound 3k was prepared following the general procedure GP-B from azirine 2k (50 mg, 0.15 mmol) in mesitylene (1.0 mL) in 31 mg (62% yield, after column chromatography on silica (light petroleum/ethyl acetate, 10:1 (v/v)) as a light grey solid: mp 205–207 °C (light petroleum/ethyl acetate). 1H-NMR (CDCl3, 400 MHz): δ 1.69–1.79 (m, 6H), 1.92–1.93 (m, 6H), 2.04–2.06 (m, 3H), 5.93 (d, 1H, J = 1.7 Hz), 7.35–7.39 (m, 1H), 7.41–7.45 (m, 2H), 8.03–8.06 (m, 2H), 8.34 (s, 1H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 28.5 (CH), 34.4 (C), 36.6 (CH2), 42.8 (CH2), 85.9 (CH), 125.7 (CH), 128.7 (CH), 129.0 (C), 129.2 (CH), 137.7 (C), 141.3 (C), 144.9 (C), 160.7 (C). HRMS (ESI) m/z: [M + Na]+ calcd. for C21H22N2NaO+ 341.1624; found 341.1627. IR (KBr, cm−1): ν 1569, 2848, 2900, 3264.
5-(4-Methoxyphenyl)-2-(p-tolyl)-4H-pyrrolo[2,3-d]oxazole (3l). Compound 3l was prepared following the general procedure GP-B from azirine 2l (58 mg, 0.19 mmol) in mesitylene (1.0 mL) in 18 mg (31% yield, after column chromatography on silica (light petroleum/ethyl acetate, 1:0–4:1 (v/v)) as a light brown solid: mp 217–219 °C (light petroleum/ethyl acetate). 1H-NMR (DMSO-d6, 400 MHz): δ 2.37 (s, 3H), 3.78 (s, 3H), 6.58 (d, 1H, J = 1.6 Hz), 6.96–6.98 (m, 2H), 7.32–7.34 (m, 2H), 7.63–6.65 (m, 2H), 7.87–7.89 (m, 2H), 11.65 (s, 1H). 13C{1H}-NMR (DMSO-d6, 400 MHz): δ 21.5 (CH3), 55.6 (CH3), 87.3 (CH), 114.8 (CH), 125.6 (CH), 125.7 (CH), 126.0 (C), 126.3 (C), 130.2 (CH), 133.7 (C), 139.9 (C), 140.1 (C), 142.0 (C), 158.5 (C), 161.0 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C19H17N2O2+ 305.1285; found 305.1296. IR (KBr, cm−1): ν 1604, 1661, 3252.
5-(4-Chlorophenyl)-2-(p-tolyl)-4H-pyrrolo[2,3-d]oxazole (3m). Compound 3m was prepared following the general procedure GP-B from azirine 2m (76 mg, 0.25 mmol) in mesitylene (1.5 mL) in 47 mg (61% yield, after evaporation of solvent and washing with cold ether) as a light brown solid: mp 237–239 °C (mesitylene). 1H-NMR (DMSO-d6, 400 MHz): δ 2.38 (s, 3H), 6.78 (d, 1H, J = 1.5 Hz), 7.34–7.36 (m, 2H), 7.44–7.46 (m, 2H), 7.72–7.74 (m, 2H), 7.90–7.92 (m, 2H), 11.89 (s, 1H). 13C{1H}-NMR (DMSO-d6, 400 MHz): δ 21.5 (CH3), 89.0 (CH), 125.7 (CH), 125.8 (C), 125.9 (CH), 129.3 (CH), 130.2 (CH), 131.0 (C), 132.2 (C), 132.5 (C), 140.3 (C), 141.0 (C), 141.9 (C), 161.9 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C18H14ClN2O+ 309.0789; found 309.0777. IR (KBr, cm−1): ν 1551, 3247.
5-(Adamantan-1-yl)-2-(p-tolyl)-4H-pyrrolo[2,3-d]oxazole (3n). Compound 3n was prepared following the general procedure GP-B from azirine 2n (60 mg, 0.18 mmol) in mesitylene (1.0 mL) in 21 mg (35% yield, after column chromatography on silica (light petroleum/ethyl acetate, 7:1 (v/v)) as a light brown solid: mp 198–201 °C (light petroleum/ethyl acetate). 1H-NMR (CDCl3, 400 MHz): δ 1.70–1.79 (m, 6H), 1.92–1.93 (m, 6H), 2.06 (s, 3H), 2.39 (s, 3H), 5.92 (s, 1H), 7.23–7.25 (m, 2H), 7.92–7.94 (m, 2H), 8.31 (s, 1H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 21.4 (CH), 28.5 (CH), 34.4 (C), 36.7 (CH2), 42.9 (CH2), 85.8 (CH), 125.7 (CH), 126.3 (C), 129.4 (CH), 137.6 (C), 139.3 (C), 141.0 (C), 144.6 (C), 161.0 (C). HRMS (ESI) m/z: [M + Na]+ calcd. for C22H24N2NaO+ 355.1781; found 355.1781. IR (KBr, cm−1): ν 1551, 1570, 2847, 2908, 3261.
2-(4-Bromophenyl)-5-phenyl-4H-pyrrolo[2,3-d]oxazole (3o). Compound 3o was prepared following the general procedure GP-B from azirine 2o (48 mg, 0.14 mmol) in mesitylene (0.5 mL) in 26 mg (54% yield, after evaporation of solvent and washing with cold ether) as a light brown solid: mp 244–245 °C (mesitylene). 1H-NMR (DMSO-d6, 400 MHz): δ 6.74 (d, 1H, J = 1.5 Hz), 7.21–7.24 (m, 1H), 7.37–7.41 (m, 2H), 7.70–7.73 (m, 4H), 7.92–7.94 (m, 2H), 11.89 (s, 1H). 13C{1H}-NMR (DMSO-d6, 100 MHz): δ 87.9 (CH), 123.0 (C), 123.8 (CH), 126.5 (CH), 127.1 (CH), 127.2 (C), 128.8 (CH), 132.2 (CH), 132.9 (C), 133.8 (C), 140.3 (C), 141.9 (C), 159.8 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C17H12N2O+ 339.0128; found 339.0131. IR (KBr, cm−1): ν 1597, 3262.
2-(4-Bromophenyl)-5-(tert-butyl)-4H-pyrrolo[2,3-d]oxazole (3p). Compound 3p was prepared following the general procedure GP-B from azirine 2p (91 mg, 0.29 mmol) in mesitylene (1.5 mL) in 44 mg (47% yield, after column chromatography on silica (light petroleum/ethyl acetate, 10:1 (v/v)) as a light brown solid: mp 181–183 °C (light petroleum/ethyl acetate). 1H-NMR (CDCl3, 400 MHz): δ 1.35 (s, 9H), 5.95 (d, 1H, J = 1.7 Hz), 7.54–7.56 (m, 2H), 7.87-7.89 (m, 2H), 8.07 (s, 1H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 30.5 (CH3), 32.6 (C), 86.5 (CH), 123.3 (C), 127.0 (CH), 127.9 (C), 131.9 (CH), 138.0 (C), 141.4 (C), 144.7 (C), 159.7 (C). HRMS (ESI) m/z: [M + H]+ calcd. for C15H16BrN2O+ 319.0441; found 319.0434. IR (KBr, cm−1): ν 1534, 1566, 2955, 3225.
(E)-3-(5-phenyl-4H-pyrrolo[2,3-d]oxazol-2-yl)acrylonitrile (3q). Compound 3q was prepared following the general procedure GP-B from azirine 2q (84 mg, 0.36 mmol) in mesitylene (1.0 mL) in 25 mg (30% yield, after evaporation of solvent and washing with cold ether) as a yellow solid: mp 251–253 °C (mesitylene). 1H-NMR (DMSO-d6, 400 MHz): δ 6.46 (d, 1H, J = 16.3 Hz), 6.78 (d, 1H, J = 1.5 Hz), 7.27–7.31 (m, 1H), 7.41–7.45 (m, 2H), 7.55 (d, 1H, J = 16.3 Hz), 7.76–7.78 (m, 2H), 12.12 (s, 1H). 13C{1H}-NMR (DMSO-d6, 100 MHz): δ 88.2 (CH), 99.3 (CH), 118.7 (C), 124.8 (CH), 127.8 (CH), 129.4 (CH), 132.7 (C), 135.1 (CH), 138.0 (C), 141.8 (C), 143.7 (C), 158.2 (C). HRMS (ESI) m/z: [M + Na]+ calcd. for C14H9N3NaO+ 258.0638; found 258.0643. IR (KBr, cm−1): ν 1555, 1603, 1628, 1744, 2218, 2924, 3212.
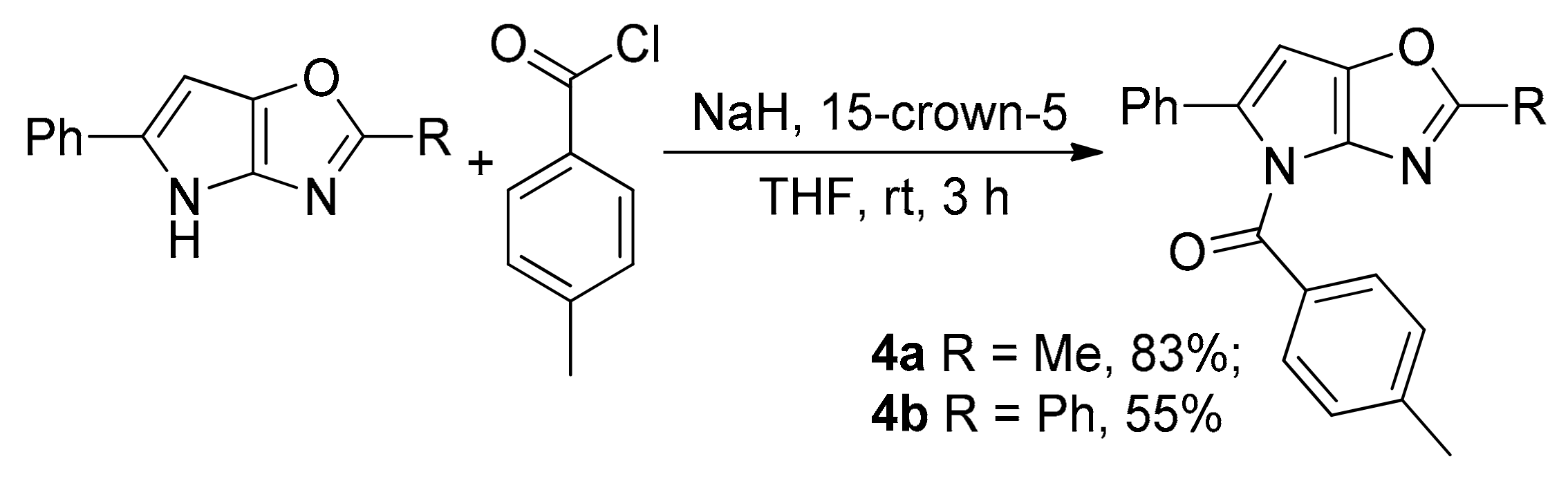
(2-Methyl-5-phenyl-4H-pyrrolo[2,3-d]oxazol-4-yl)(p-tolyl)methanone (4a). Compound 4a was prepared following the general procedure GP-C from 4H-pyrrolo[2,3-d]oxazole 3a (100 mg, 0.51 mmol), NaH (30 mg, 0.76 mmol), 15-crown-5 (112 mg, 0.51 mmol), 4-methylbenzoyl chloride (154 mg, 1.0 mmol) in tetrahydrofuran (5.0 mL) in 132 mg (83% yield, after column chromatography on silica (light petroleum/ethyl acetate, 10:1 (v/v)) as a yellow solid: mp 121–124 °C (light petroleum/ethyl acetate). 1H-NMR (CDCl3, 400 MHz): δ 2.44 (s, 3H), 2.52 (s, 3H), 6.43 (s, 1H), 7.21–7.25 (m, 2H), 7.27–7.234 (m, 5H), 7.85–7.87 (m, 2H). 13C{1H}-NMR (CDCl3, 100 MHz): δ 15.1 (CH3), 21.8 (CH3), 98.2 (CH), 127.2 (CH), 127.8 (CH), 128.3 (CH), 129.1 (CH), 130.0 (C), 131.2 (CH), 133.3 (C), 136.5 (C), 140.9 (C), 141.0 (C), 144.7 (C), 162.4 (C), 166.7 (C). HRMS (ESI) m/z: [M + Na]+ calcd. for C20H16N2NaO+ 339.1104; found 339.1104. IR (KBr, cm−1): ν 1611, 1705, 2919.
(2,5-Diphenyl-4H-pyrrolo[2,3-d]oxazol-4-yl)(p-tolyl)methanone (4b). Compound 4b was prepared following the general procedure GP-C from 4H-pyrrolo[2,3-d]oxazole 3h (58 mg, 0.22 mmol), NaH (14 mg, 0.33 mmol), 15-crown-5 (49 mg, 0.55 mmol), 4-methylbenzoyl chloride (68 mg, 0.44 mmol) in tetrahydrofuran (3.0 mL) in 46 mg (55% yield, after column chromatography on silica (light petroleum/ethyl acetate, 20:1 (v/v)) as a yellow solid: mp 156–158 °C (light petroleum/ethyl acetate). 1H-NMR (CDCl3, 400 MHz): δ 2.47 (s, 3H), 6.53 (s, 1H), 7.25–7.34 (m, 5H), 7.36–7.43 (m, 5H), 7.90–7.92 (m, 2H), 7.97–8.00 (m, 2H). 13C{1H}-NMR (CDCl3, 400 MHz): δ 21.8 (CH3), 98.1 (CH), 126.2 (CH), 127.4 (CH), 127.8 (CH), 128.1 (C), 128.3 (CH), 128.7 (CH), 129.0 (CH), 129.95 (C), 129.99 (CH), 131.4 (CH), 133.2 (C), 137.8 (C), 141.4 (C), 142.3 (C), 144.7 (C), 162.2 (C), 166.8 (C). HRMS (ESI) m/z: [M + Na]+ calcd. for C25H18N2NaO+ 401.1260; found 401.1262. IR (KBr, cm−1): ν 1609, 1699, 2854, 2924.