Dispersant Molecules with Functional Catechol Groups for Supercapacitor Fabrication
Abstract
1. Introduction
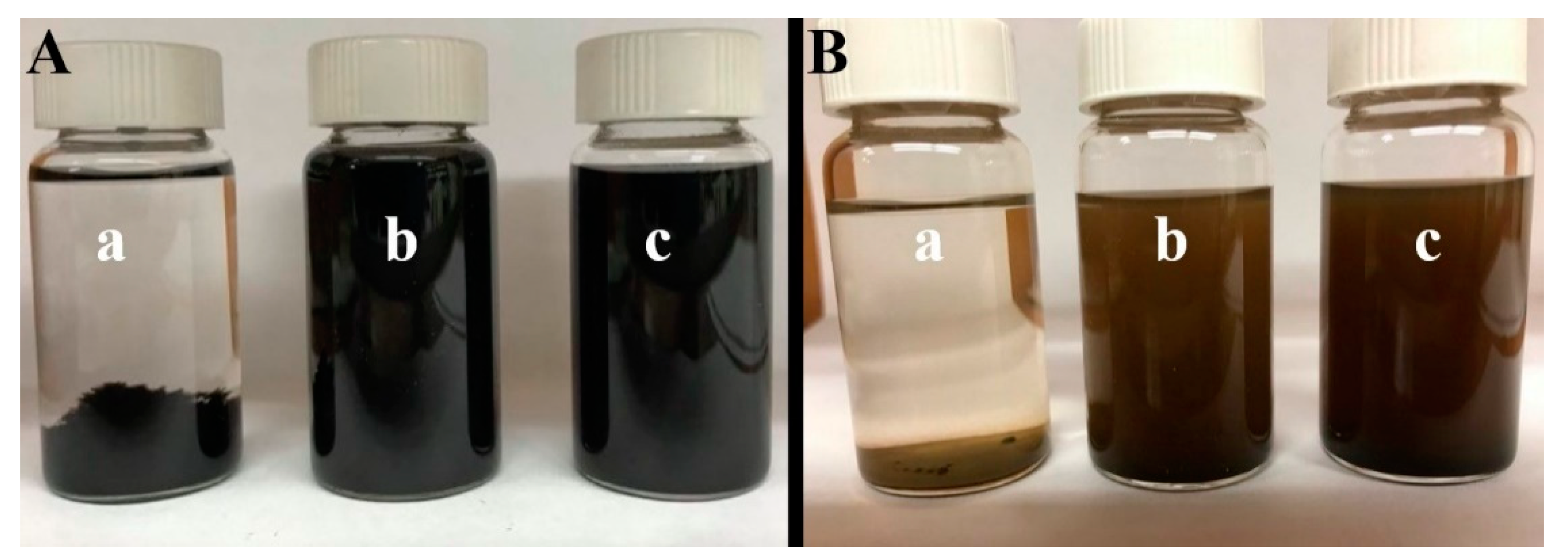
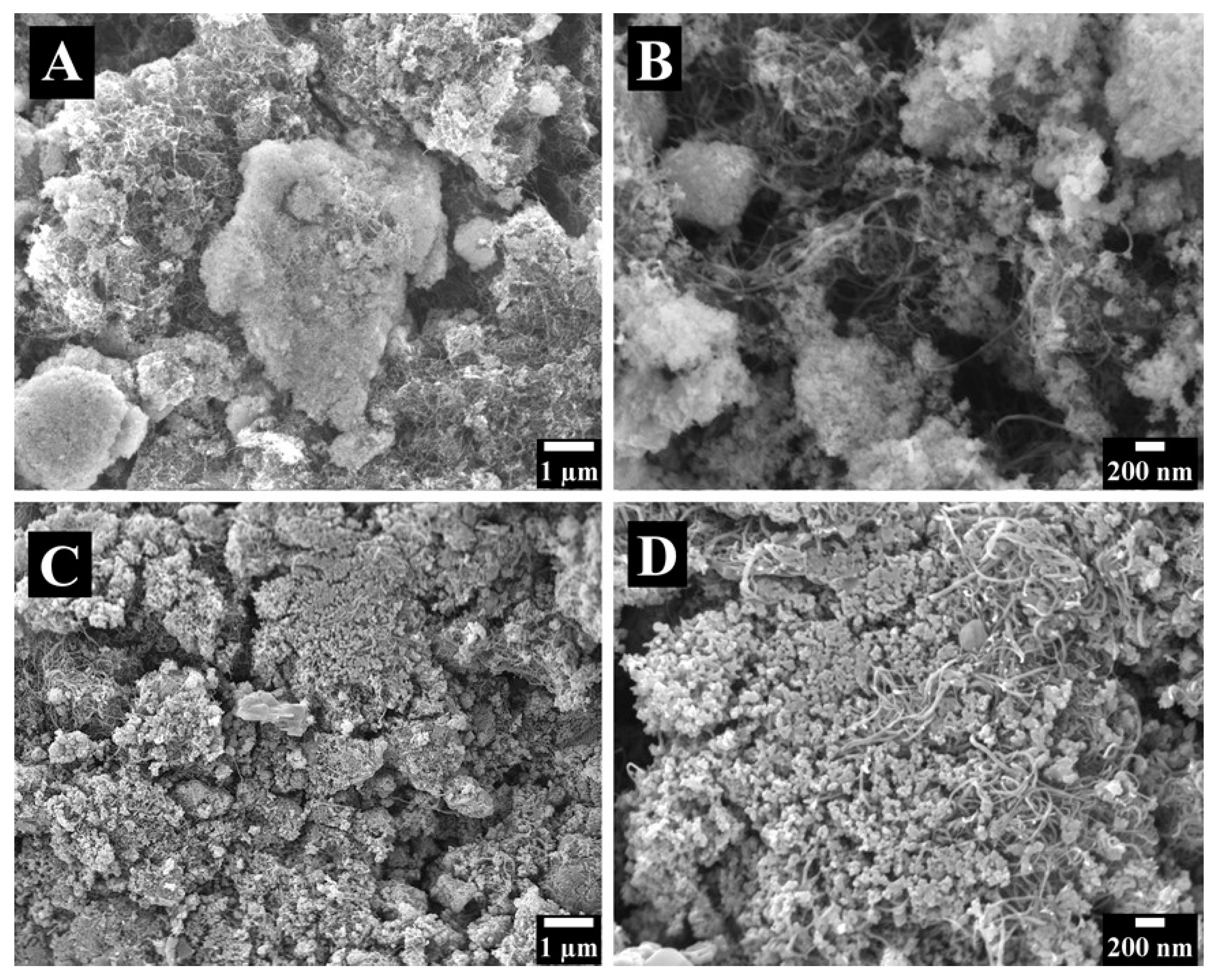
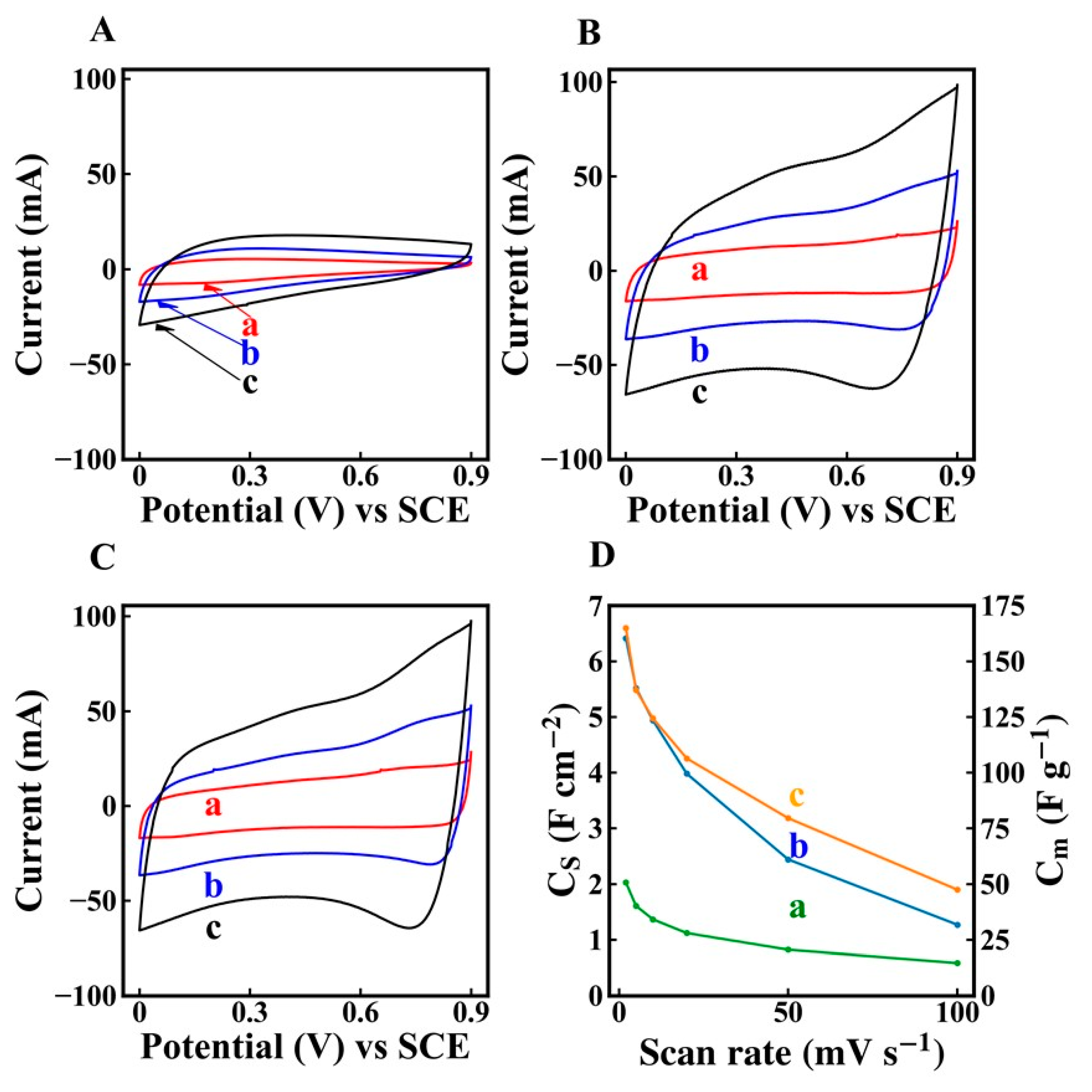
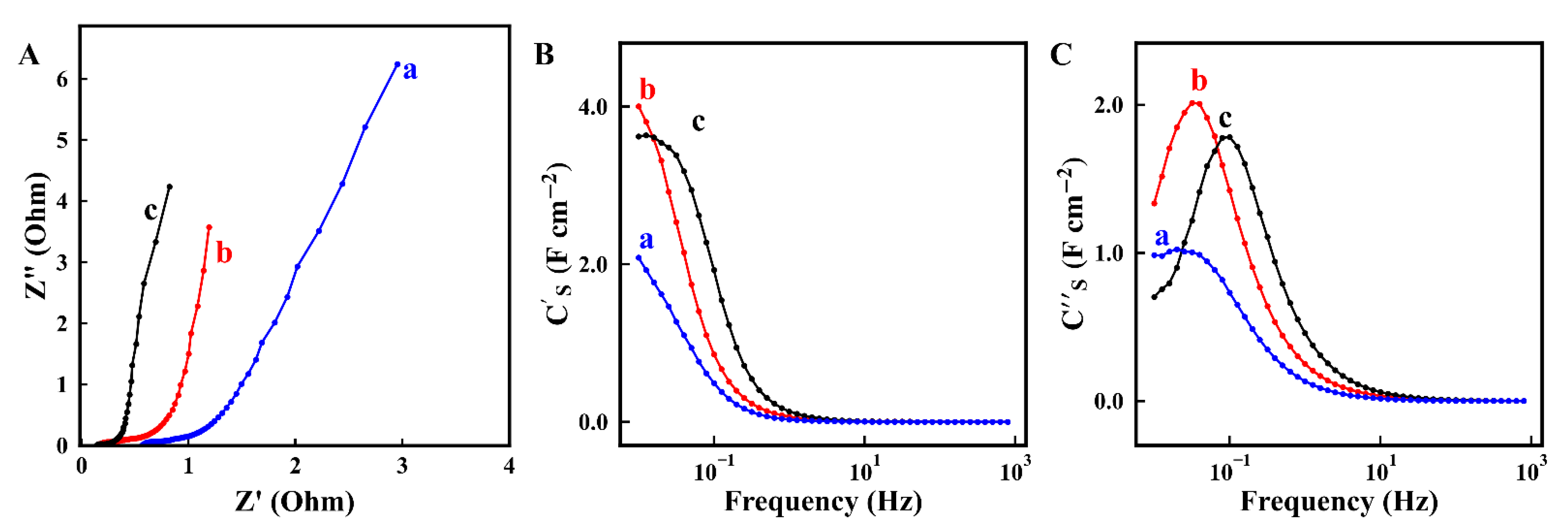
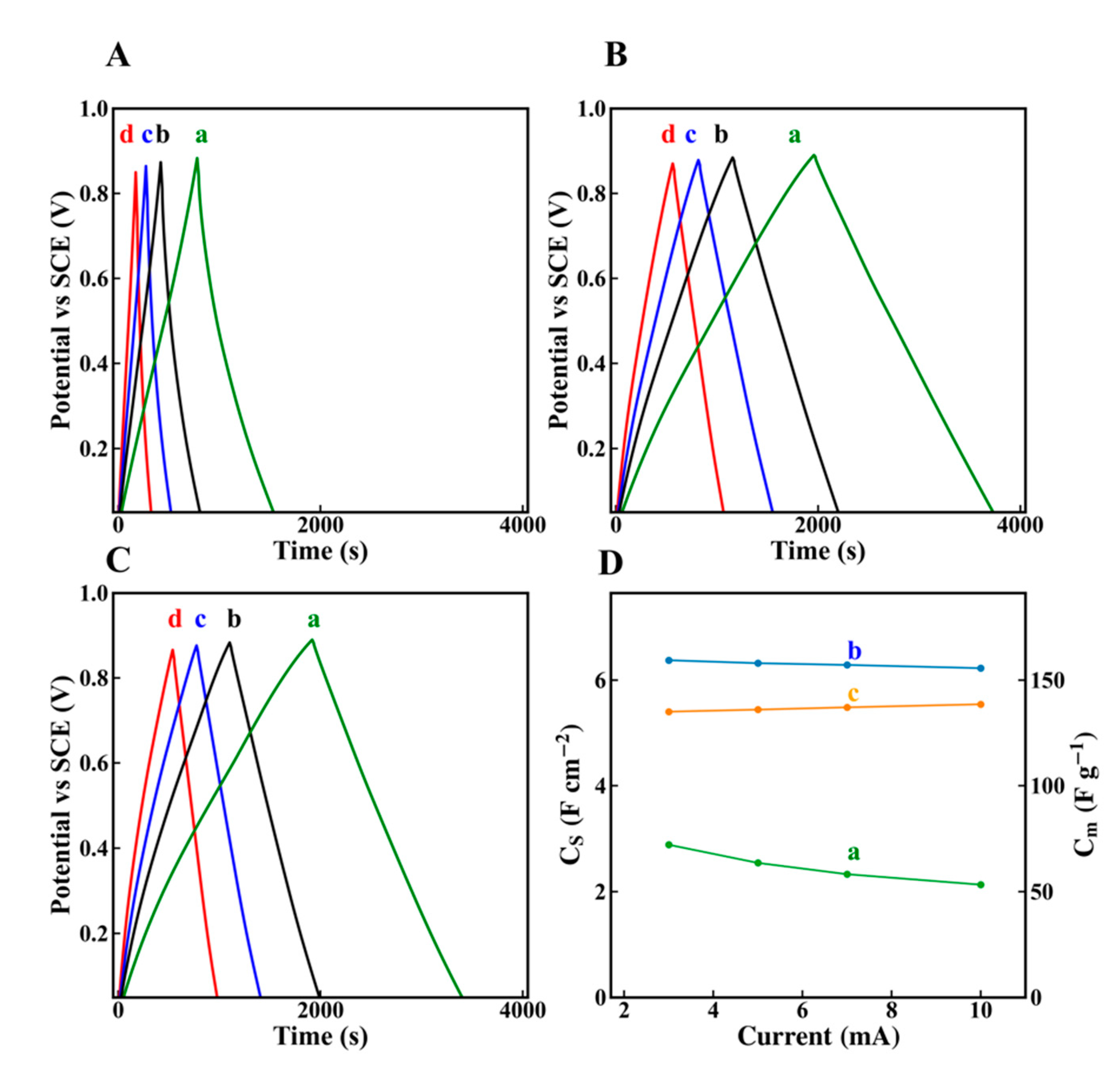
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ata, M.S.; Liu, Y.; Zhitomirsky, I. A review of new methods of surface chemical modification, dispersion and electrophoretic deposition of metal oxide particles. RSC Adv. 2014, 4, 22716–22732. [Google Scholar] [CrossRef]
- Lee, B.P.; Messersmith, P.; Israelachvili, J.; Waite, J. Mussel-Inspired Adhesives and Coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nat. Cell Biol. 2007, 448, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Petrone, L. Molecular surface chemistry in marine bioadhesion. Adv. Colloid Interface Sci. 2013, 195, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lebrette, S.; Pagnoux, C.; Abelard, P. Fabrication of titania dense layers by electrophoretic deposition in aqueous media. J. Eur. Ceram. Soc. 2006, 26, 2727–2734. [Google Scholar] [CrossRef]
- Silva, R.; Poon, R.; Milne, J.; Syed, A.; Zhitomirsky, I. New developments in liquid-liquid extraction, surface modification and agglomerate-free processing of inorganic particles. Adv. Colloid Interface Sci. 2018, 261, 15–27. [Google Scholar] [CrossRef]
- Ata, M.S.; Poon, R.; Syed, A.M.; Milne, J.; Zhitomirsky, I. New developments in non-covalent surface modification, dispersion and electrophoretic deposition of carbon nanotubes. Carbon 2018, 130, 584–598. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhitomirsky, I. Surface modification of MnO2 and carbon nanotubes using organic dyes for nanotechnology of electrochemical supercapacitors. J. Mater. Chem. A 2013, 1, 12519–12526. [Google Scholar] [CrossRef]
- Nawwar, M.; Poon, R.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. Fe3O4 spinel-Mn3O4 spinel supercapacitor prepared using Celestine blue as a dispersant, capping agent and charge transfer mediator. Ceram. Int. 2020, 46, 18851–18858. [Google Scholar] [CrossRef]
- Togashi, T.; Takami, S.; Kawakami, K.; Yamamoto, H.; Naka, T.; Sato, K.; Abe, K.; Adschiri, T. Continuous hydrothermal synthesis of 3,4-dihydroxyhydrocinnamic acid-modified magnetite nanoparticles with stealth-functionality against immunological response. J. Mater. Chem. 2012, 22, 9041–9045. [Google Scholar] [CrossRef]
- Sugimoto, T.; Itoh, H.; Mochida, T. Shape Control of Monodisperse Hematite Particles by Organic Additives in the Gel–Sol System. J. Colloid Interface Sci. 1998, 205, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Pagnoux, C.; Baumard, J.-F.; Nogami, M. Preparation of gold nanoparticles (GNP) aqueous suspensions by a new method involving Tiron. J. Mater. Sci. 2006, 42, 80–86. [Google Scholar] [CrossRef]
- Zhang, T.; Wojtal, P.; Rubel, O.; Zhitomirsky, I. Density functional theory and experimental studies of caffeic acid adsorption on zinc oxide and titanium dioxide nanoparticles. RSC Adv. 2015, 5, 106877–106885. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, K.; Zhitomirsky, I. Anionic dopant–dispersants for synthesis of polypyrrole coated carbon nanotubes and fabrication of supercapacitor electrodes with high active mass loading. J. Mater. Chem. A 2014, 2, 14666–14673. [Google Scholar] [CrossRef]
- Kim, S.; Park, C.B. Mussel-inspired transformation of CaCO3 to bone minerals. Biomaterials 2010, 31, 6628–6634. [Google Scholar] [CrossRef]
- Yu, M.; Deming, T.J. Synthetic Polypeptide Mimics of Marine Adhesives. Macromolecules 1998, 31, 4739–4745. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef] [PubMed]
- Dalsin, J.L.; Hu, B.-H.; Lee, B.P.; Messersmith, P.B. Mussel Adhesive Protein Mimetic Polymers for the Preparation of Nonfouling Surfaces. J. Am. Chem. Soc. 2003, 125, 4253–4258. [Google Scholar] [CrossRef]
- Winkler, T.E.; Ben-Yoav, H.; Chocron, S.E.; Kim, E.; Kelly, D.L.; Payne, G.F.; Ghodssi, R. Electrochemical Study of the Catechol-Modified Chitosan System for Clozapine Treatment Monitoring. Langmuir 2014, 30, 14686–14693. [Google Scholar] [CrossRef]
- Clifford, A.; Pang, X.; Zhitomirsky, I. Biomimetically modified chitosan for electrophoretic deposition of composites. Colloids Surf. A Physicochem. Eng. Asp. 2018, 544, 28–34. [Google Scholar] [CrossRef]
- Wang, G.-L.; Xu, J.-J.; Chen, H.-Y. Dopamine sensitized nanoporous TiO2 film on electrodes: Photoelectrochemical sensing of NADH under visible irradiation. Biosens. Bioelectron. 2009, 24, 2494–2498. [Google Scholar] [CrossRef]
- Varaganti, S.; Ramakrishna, G. Dynamics of Interfacial Charge Transfer Emission in Small Molecule Sensitized TiO2 Nanoparticles: Is It Localized or Delocalized? J. Phys. Chem. C 2010, 114, 13917–13925. [Google Scholar] [CrossRef]
- Rajh, T.; Chen, L.X.; Lukas, K.; Liu, T.; Thurnauer, M.C.; Tiede, D.M. Surface Restructuring of Nanoparticles: An Efficient Route for Ligand−Metal Oxide Crosstalk. J. Phys. Chem. B 2002, 106, 10543–10552. [Google Scholar] [CrossRef]
- Nagesha, D.K.; Plouffe, B.D.; Phan, M.; Lewis, L.H.; Sridhar, S.; Murthy, S.K. Functionalization-induced improvement in magnetic properties of Fe3O4 nanoparticles for biomedical applications. J. Appl. Phys. 2009, 105, 317. [Google Scholar] [CrossRef]
- Huang, W.; Jiang, P.; Wei, C.; Zhuang, D.; Shi, J. Low-temperature one-step synthesis of covalently chelated ZnO/dopamine hybrid nanoparticles and their optical properties. J. Mater. Res. 2008, 23, 1946–1952. [Google Scholar] [CrossRef]
- Bloemen, M.; Debruyne, D.; Demeyer, P.-J.; Clays, K.; Gils, A.; Geukens, N.; Bartic, C.; Verbiest, T. Catechols as ligands for CdSe–ZnS quantum dots. RSC Adv. 2014, 4, 10208. [Google Scholar] [CrossRef]
- Tallman, D.E.; Vang, C.; Wallace, G.G.; Bierwagen, G.P. Direct Electrodeposition of Polypyrrole on Aluminum and Aluminum Alloy by Electron Transfer Mediation. J. Electrochem. Soc. 2002, 149, C173–C179. [Google Scholar] [CrossRef]
- Chen, S.; Zhitomirsky, I. Influence of dopants and carbon nanotubes on polypyrrole electropolymerization and capacitive behavior. Mater. Lett. 2013, 98, 67–70. [Google Scholar] [CrossRef]
- Shi, C.; Zhitomirsky, I. Electrodeposition of composite polypyrrole–carbon nanotube films. Surf. Eng. 2011, 27, 655–661. [Google Scholar] [CrossRef]
- Fan, X.; Lin, L.; Dalsin, J.L.; Messersmith, P.B. Biomimetic Anchor for Surface-Initiated Polymerization from Metal Substrates. J. Am. Chem. Soc. 2005, 127, 15843–15847. [Google Scholar] [CrossRef] [PubMed]
- De Souza, F.; Spinelli, A. Caffeic acid as a green corrosion inhibitor for mild steel. Corros. Sci. 2009, 51, 642–649. [Google Scholar] [CrossRef]
- Black, K.C.L.; Liu, Z.; Messersmith, P.B. Catechol Redox Induced Formation of Metal Core−Polymer Shell Nanoparticles. Chem. Mater. 2011, 23, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, G.; Fernández-García, M. Direct preparation of PNIPAM coating gold nanoparticles by catechol redox and surface adhesion chemistry. RSC Adv. 2014, 4, 11740–11749. [Google Scholar] [CrossRef]
- Stallings, M.D.; Morrison, M.M.; Sawyer, D.T. Redox chemistry of metal-catechol complexes in aprotic media. 1. Electrochemistry of substituted catechols and their oxidation products. Inorg. Chem. 1981, 20, 2655–2660. [Google Scholar] [CrossRef]
- Touzé, E.; Gohier, F.; Daffos, B.; Taberna, P.-L.; Cougnon, C. Improvement of electrochemical performances of catechol-based supercapacitor electrodes by tuning the redox potential via different-sized O-protected catechol diazonium salts. Electrochim. Acta 2018, 265, 121–130. [Google Scholar] [CrossRef]
- Fireman-Shoresh, S.; Turyan, I.; Mandler, D.; Avnir, D.; Marx, S. Chiral Electrochemical Recognition by Very Thin Molecularly Imprinted Sol−Gel Films. Langmuir 2005, 21, 7842–7847. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Huang, J.; Li, T.; Chen, Z.; Liu, X.; Liu, S. Rapid electrochemical detection of DNA damage and repair with epigallocatechin gallate, chlorogenic acid and ascorbic acid. Electrochem. Commun. 2008, 10, 1198–1200. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Niu, N.; Chen, Z.; Li, S.; Liu, S.-X.; Li, J. Fluorescent Poly (vinyl alcohol) Films Containing Chlorogenic Acid Carbon Nanodots for Food Monitoring. ACS Appl. Nano Mater. 2020, 3, 7611–7620. [Google Scholar] [CrossRef]
- Salimi, A.; Hallaj, R. Adsorption and Reactivity of Chlorogenic Acid at a Hydrophobic Carbon Ceramic Composite Electrode: Application for the Amperometric Detection of Hydrazine. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2004, 16, 1964–1971. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Vancamp, J.; Demeulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Cheng, M.-C.; Lu, Y.-C.; Wu, J.; Ma, Y.-H. Gallate-induced nanoparticle uptake by tumor cells: Structure-activity relationships. Colloids Surf. B Biointerfaces 2019, 179, 28–36. [Google Scholar] [CrossRef]
- Jovanović, I.N.; Miličević, A.; Jadreško, D.; Hranjec, M. Electrochemical oxidation of synthetic amino-substituted benzamides with potential antioxidant activity. J. Electroanal. Chem. 2020, 870, 114244. [Google Scholar] [CrossRef]
- Vangari, M.; Pryor, T.; Jiang, L. Supercapacitors: Review of Materials and Fabrication Methods. J. Energy Eng. 2013, 139, 72–79. [Google Scholar] [CrossRef]
- Mao, N.; Wang, H.; Sui, Y.; Cui, Y.; Pokrzywinski, J.; Shi, J.; Liu, W.; Chen, S.; Wang, X.; Mitlin, D. Extremely high-rate aqueous supercapacitor fabricated using doped carbon nanoflakes with large surface area and mesopores at near-commercial mass loading. Nano Res. 2017, 10, 1767–1783. [Google Scholar] [CrossRef]
- Pant, B.; Pant, H.R.; Park, M. Fe1−xS Modified TiO2 NPs Embedded Carbon Nanofiber Composite via Electrospinning: A Potential Electrode Material for Supercapacitors. Molecules 2020, 25, 1075. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, X.; Ma, Y.; Song, H.; Pi, C.; Zheng, Y.; Gao, B.; Fu, J.; Chu, P.K. In-Situ Synthesis of Heterostructured Carbon-Coated Co/MnO Nanowire Arrays for High-Performance Anodes in Asymmetric Supercapacitors. Molecules 2020, 25, 3218. [Google Scholar] [CrossRef] [PubMed]
- Malkova, A.N.; Sipyagina, N.A.; Gozhikova, I.O.; Dobrovolsky, Y.A.; Konev, D.V.; Baranchikov, A.E.; Ivanova, O.S.; Ukshe, A.E.; Lermontov, S.A. Electrochemical Properties of Carbon Aerogel Electrodes: Dependence on Synthesis Temperature. Molecules 2019, 24, 3847. [Google Scholar] [CrossRef]
- Li, J.; Zhitomirsky, I. Cathodic electrophoretic deposition of manganese dioxide films. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 248–253. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, K.; Zhitomirsky, I. Polypyrrole coated carbon nanotubes for supercapacitor devices with enhanced electrochemical performance. J. Power Sources 2014, 268, 233–239. [Google Scholar] [CrossRef]
- Biswas, N.; Kapoor, S.; Mahal, H.S.; Mukherjee, T. Adsorption of CGA on colloidal silver particles: DFT and SERS study. Chem. Phys. Lett. 2007, 444, 338–345. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, K.; Zhitomirsky, I. Asymmetric supercapacitor, based on composite MnO2-graphene and N-doped activated carbon coated carbon nanotube electrodes. Electrochim. Acta 2017, 233, 142–150. [Google Scholar] [CrossRef]
- Sikkema, R.; Baker, K.; Zhitomirsky, I. Electrophoretic deposition of polymers and proteins for biomedical applications. Adv. Colloid Interface Sci. 2020, 284, 102272. [Google Scholar] [CrossRef]
- Su, Y.; Zhitomirsky, I. Hybrid MnO2/carbon nanotube-VN/carbon nanotube supercapacitors. J. Power Sources 2014, 267, 235–242. [Google Scholar] [CrossRef]
- Rorabeck, K.; Zhitomirsky, I. Application of Octanohydroxamic Acid for Salting out Liquid–Liquid Extraction of Materials for Energy Storage in Supercapacitors. Molecules 2021, 26, 296. [Google Scholar] [CrossRef]
- Chen, R.; Yu, M.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. The Development of Pseudocapacitor Electrodes and Devices with High Active Mass Loading. Adv. Energy Mater. 2020, 10, 1903848. [Google Scholar] [CrossRef]
- Shi, K.; Zhitomirsky, I. Fabrication of Polypyrrole-Coated Carbon Nanotubes Using Oxidant–Surfactant Nanocrystals for Supercapacitor Electrodes with High Mass Loading and Enhanced Performance. ACS Appl. Mater. Interfaces 2013, 5, 13161–13170. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rorabeck, K.; Zhitomirsky, I. Dispersant Molecules with Functional Catechol Groups for Supercapacitor Fabrication. Molecules 2021, 26, 1709. https://doi.org/10.3390/molecules26061709
Rorabeck K, Zhitomirsky I. Dispersant Molecules with Functional Catechol Groups for Supercapacitor Fabrication. Molecules. 2021; 26(6):1709. https://doi.org/10.3390/molecules26061709
Chicago/Turabian StyleRorabeck, Kaelan, and Igor Zhitomirsky. 2021. "Dispersant Molecules with Functional Catechol Groups for Supercapacitor Fabrication" Molecules 26, no. 6: 1709. https://doi.org/10.3390/molecules26061709
APA StyleRorabeck, K., & Zhitomirsky, I. (2021). Dispersant Molecules with Functional Catechol Groups for Supercapacitor Fabrication. Molecules, 26(6), 1709. https://doi.org/10.3390/molecules26061709




