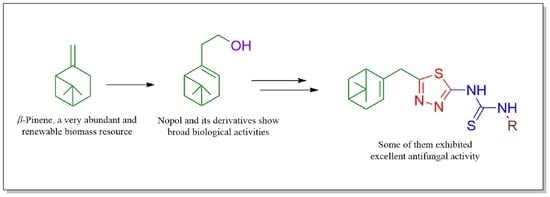
Synthesis, Antifungal Activity, and 3D-QSAR Study of Novel Nopol-Derived 1,3,4-Thiadiazole-Thiourea Compounds
Abstract
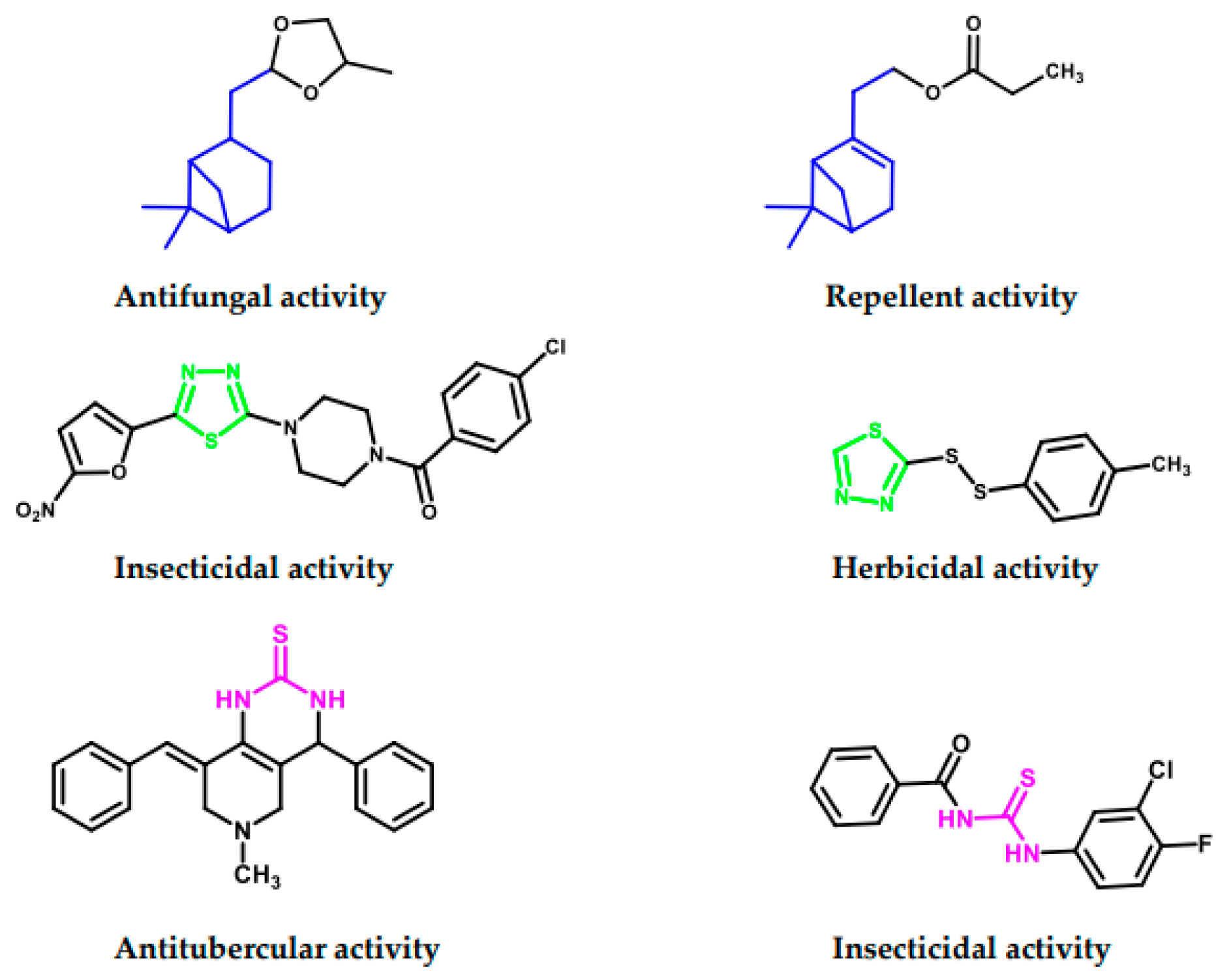
:1. Introduction
2. Results and Discussion
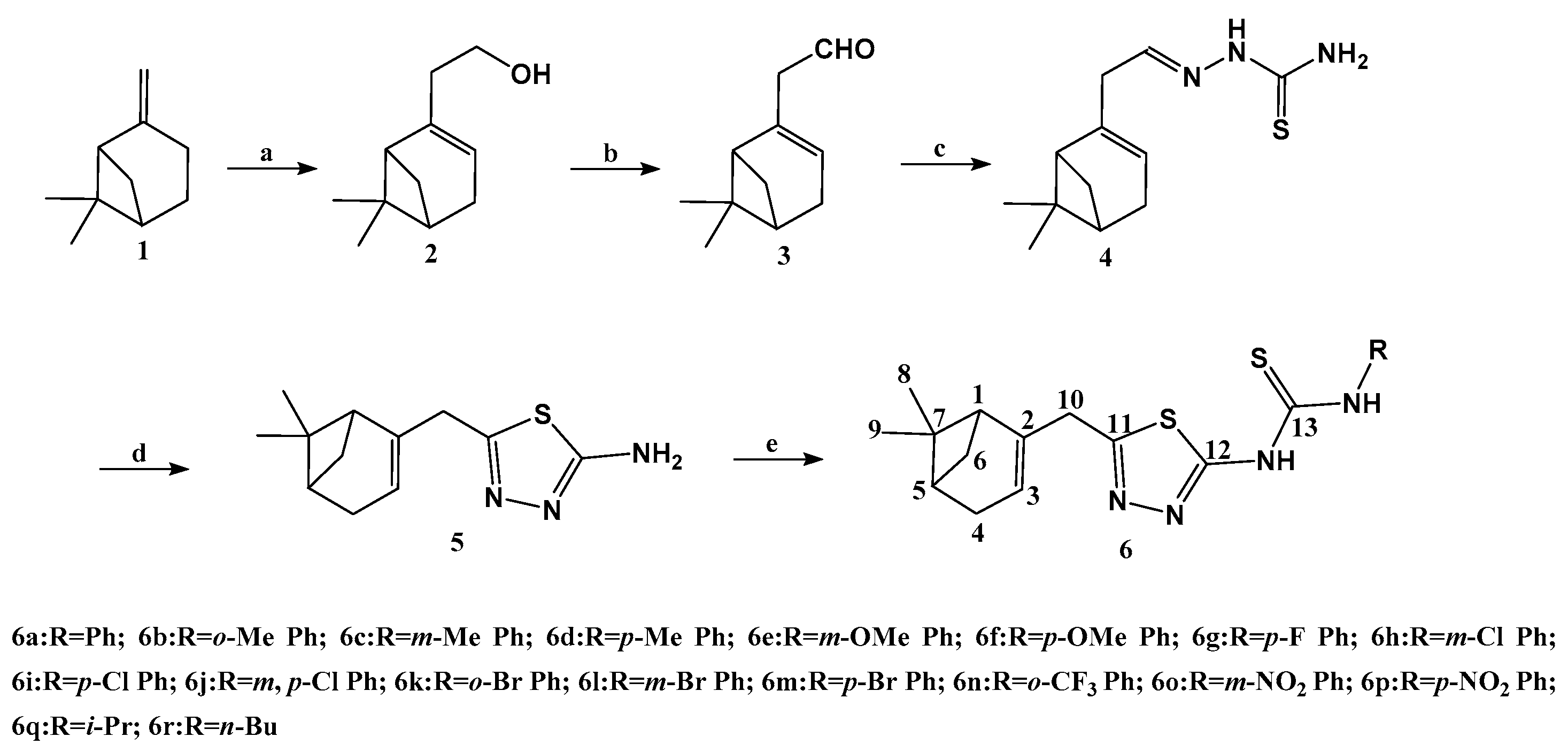
2.1. Synthesis and Characterization
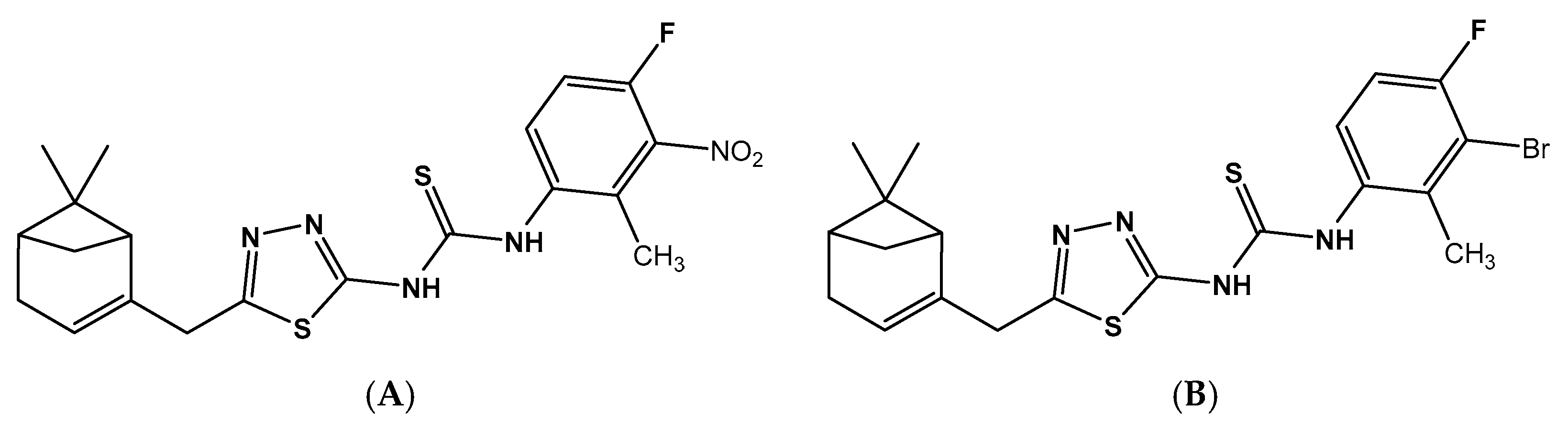
2.2. Antifungal Activity
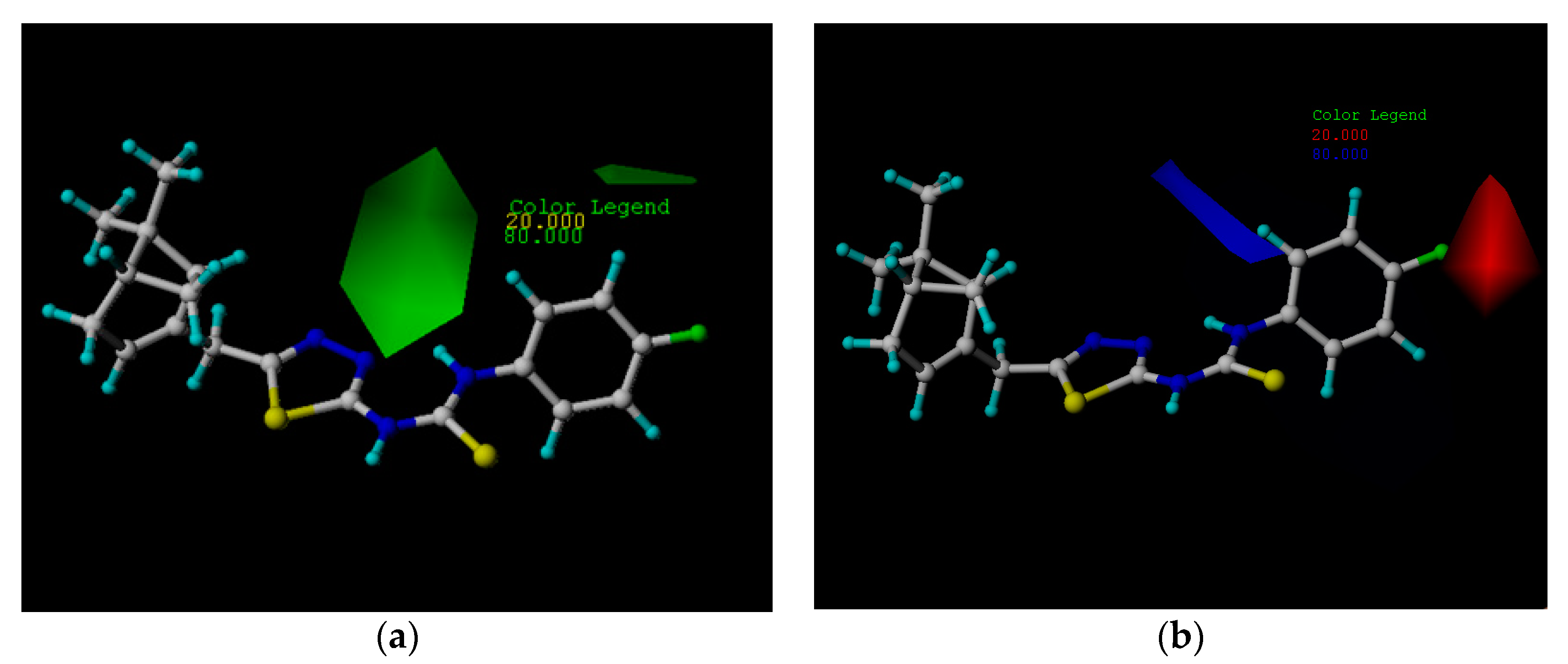
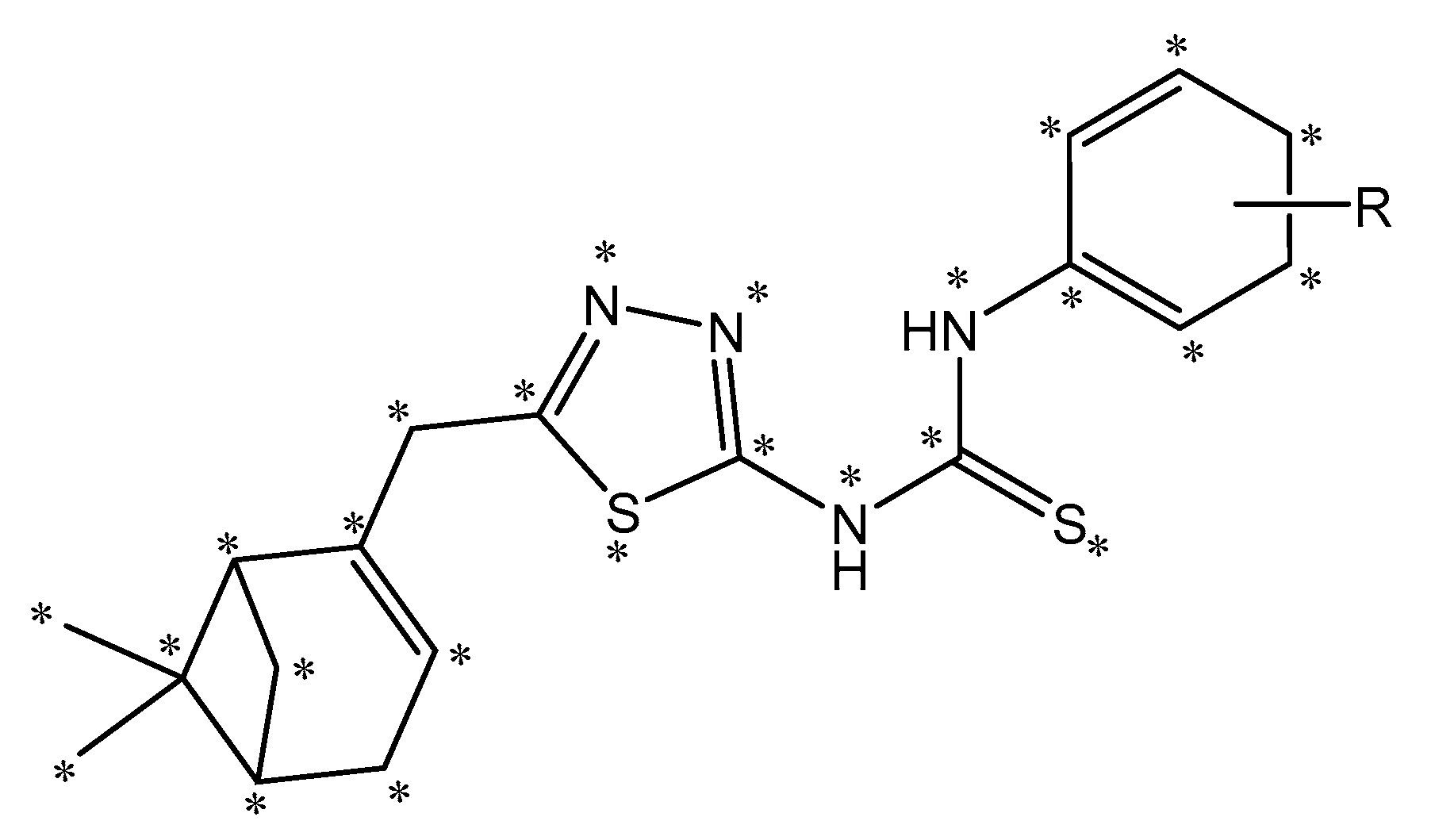
2.3. CoMFA Analysis
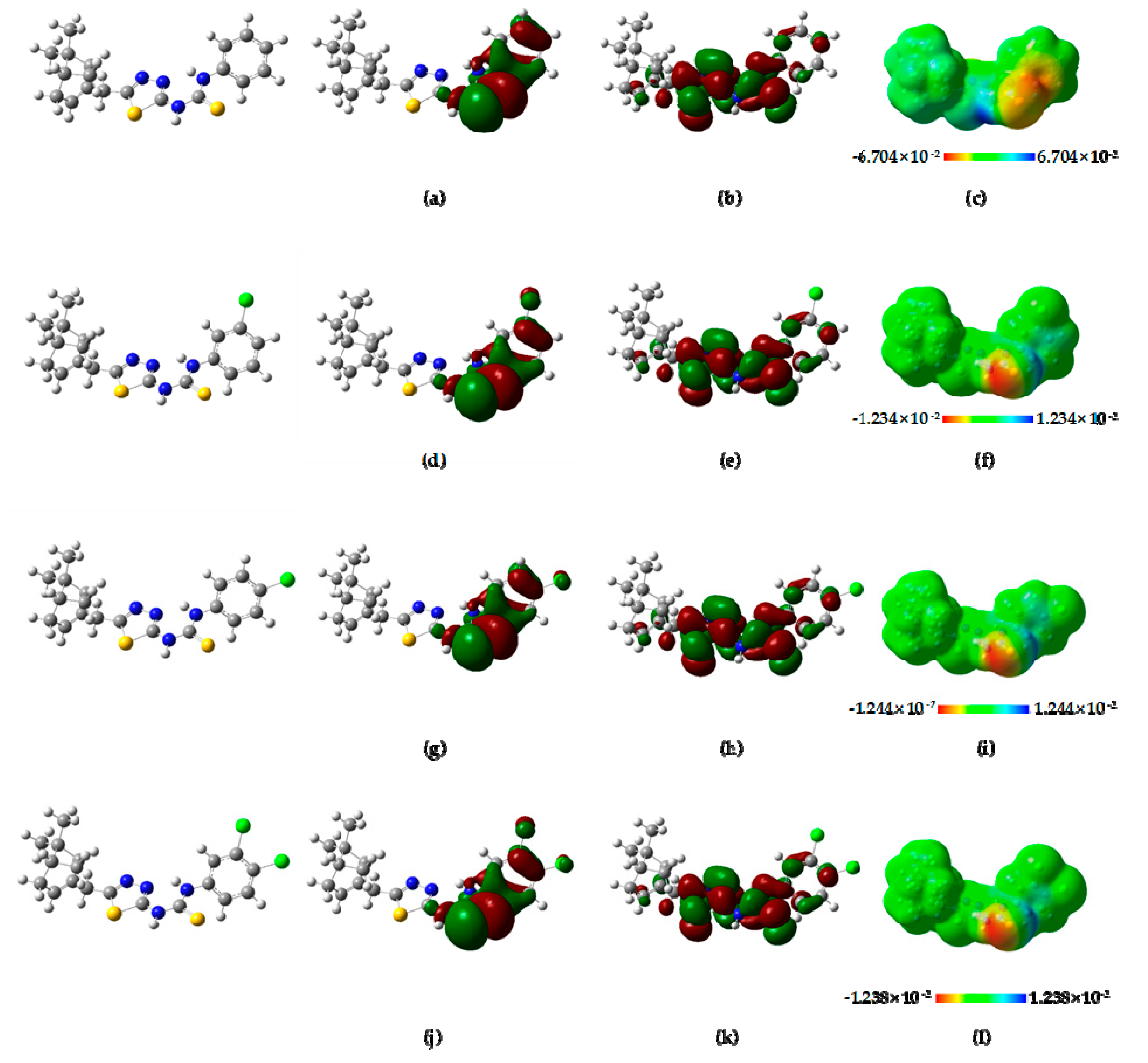
2.4. Theoretical Calculation and Analysis
3. Experimental Section
3.1. General Information
3.2. Synthesis of Nopol (2)
3.3. Synthesis of Nopol Aldehyde (3)
3.4. Synthesis of Nopol-Based Thiosemicarbazone (4)
3.5. Synthesis of 5-Nopyl-2-amino-1,3,4-thiadiazole (5)
3.6. General Procedure for Synthesis of Nopol-Based 1,3,4-Thiadiazole-thiourea Compiunds (6a–6r)
3.7. Antifungal Activity Test
3.8. 3D-QSAR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Unnikrishnan, R.P.; Endalkachew, S.D. Mesoporous iron phosphate as an active, selective and recyclable catalyst for the synthesis of nopol by Prins condensation. Chem. Commun. 2004, 7, 826–827. [Google Scholar]
- Neuenschwander, U.; Meier, E.; Hermans, I. Peculiarities of β-pinene autoxidation. ChemSusChem 2011, 4, 1613–1621. [Google Scholar] [CrossRef]
- Wang, J.; Jaenicke, S.; Chuah, G.K.; Hua, W.; Yue, Y.; Gao, Z. Acidity and porosity modulation of MWW type zeolites for nopol production by Prins condensation. Catal. Commun. 2011, 12, 1131–1135. [Google Scholar] [CrossRef]
- Xu, L.F.; Xiao, Z.Q.; Wang, P.; Fan, G.R.; Chen, J.Z.; Chen, S.X.; Wang, Z.D. The synthesis and antifungal activity of cyclic acetal hydronopic aldehyde derivatives. J. Jiangxi Norm. Univ. 2014, 38, 472–475. [Google Scholar]
- Jin, L.L.; Xiao, Z.Q.; Chen, J.Z.; Fan, G.R.; Wang, P.; Wang, Z.D.; Chen, S.X. The synthesis and structural analysis for hydronopyl tri-alkyl ammonium halides. J. Jiangxi Norm. Univ. 2015, 39, 484–487. [Google Scholar]
- Wang, Z.D.; Song, J.; Chen, J.Z.; Song, Z.Q.; Shang, S.B.; Jiang, Z.K.; Han, Z.J. QSAR study of mosquito repellents from terpenoid with a six-member-ring. Bioorg. Med. Chem. Lett. 2008, 18, 2854–2859. [Google Scholar] [CrossRef]
- Baronnet, R. Terpene Derivatives. U.S. Patent 3,845,048, 14 November 1972. [Google Scholar]
- Behrouzi, F.M.; Poorrajab, F.; Ardestani, S.K.; Emami, S.; Shafiree, A.; Foroumadi, A. Synthesis and in vitro anti-leishmanial activity of 1-[5-(5-nitrofuran-2-yl)-1,3,4-thiadiazol-2-yl]- and 1-[5-(5-nitrothiophen-2-yl)-1,3,4-thiadiazol-2-yl]-4-aroylpiperazines. Bioorg. Med. Chem. 2008, 16, 4509–4515. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Wang, W.M.; Lu, W.; Niu, C.W.; Li, Y.H.; Li, Z.M.; Wang, J.G. Synthesis and biological evaluation of nonsymmetrical aromatic disulfides as novel inhibitors of acetohydroxyacid synthase. Bioorg. Med. Chem. Lett. 2013, 23, 3723–3727. [Google Scholar] [CrossRef] [PubMed]
- Banday, M.R.; Rauf, A. Synthesis and evaluation of in vitro antibacterial activity of novel 2,5-disubstituted-1,3,4-thiadiazoles from fatty acids. Chin. Chem. Lett. 2008, 19, 1427–1430. [Google Scholar] [CrossRef]
- Padmavathi, V.; Reddy, G.S.; Padmaja, A.; Kondaiah, P.; Ali-Shazia. Synthesis, antimicrobial and cytotoxic activities of 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazoles. Eur. J. Med. Chem. 2009, 44, 2106–2112. [Google Scholar] [CrossRef]
- Bansode, S.; Kamble, R. Synthesis of novel 2-(3’-aryl-sydnon-4’-ylidene)-5’-substituted-[1,3,4]-thiadiazolylamines and [1,3,4] -thiadiazol-2’-yl-3-oxo- [1,2,4]-triazoles as antimicrobial agents. Med. Chem. Res. 2012, 21, 867–873. [Google Scholar] [CrossRef]
- Hamad, N.S.; Al-Haidery, N.H.; Al-Masoudi, I.A.; Sabri, M.; Sabri, L.; Al-Masoudi, N.A. Amino Acid Derivatives, Part 4: Synthesis and anti-HIV activity of new naphthalene derivatives. Arch. Pharm. Chem. Life Sci. 2010, 343, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Sipkina, N.Y.; Polyakova, M.V.; Yakovlev, I.P. Development and Validation of a new HPLC Method for quantifcation of a novel antifungal drug based on 1,3,4-thiadiazole and its impurities. Chromatographia 2019, 82, 1633–1639. [Google Scholar] [CrossRef]
- Hamama, W.S.; Gouda, M.A.; Badr, M.H.; Zoorob, H.H. Synthesis, antioxidant, and antitumor evaluation of certain new N-substituted-2-amino-1,3,4-thiadiazoles. Med. Chem. Res. 2013, 22, 3556–3565. [Google Scholar] [CrossRef]
- Zhu, H.M.; Qin, J.H.; Ouyang, G.P. Synthesis and antitumor activities of novel 4-(5-N-substituted-1,3,4-thiadiazole- 2-thiol)benzo[4,5] furo [3,2-d] pyrimidine derivatives. Chin. J. Syn. Chem. 2012, 20, 156–160. [Google Scholar]
- Tan, X.H.; Song, X.J.; Wang, Y.G. Synthesis and biological activity of N-[5-(3-pyridyl)-1,3,4-thiadiazol-2-y1]-N’-(substituted phenyl)ureas. J. Cent. Chin. Norm. Univ. 2008, 42, 62–64. [Google Scholar]
- Wahid, A.; Basra, S.M.A.; Farooq, M. Thiourea: A molecule with immense biological significance for plants. Int. J. Agric. Biol. 2017, 19, 911–920. [Google Scholar] [CrossRef]
- Rizki, I.N.; Tanaka, Y.; Okibe, N. Thiourea bioleaching for gold recycling from e-waste. Waste Manag. 2019, 84, 158–165. [Google Scholar] [CrossRef]
- Shakeel, A.; Altaf, A.A.; Qureshi, A.M.; Badshah, A. Thiourea derivatives in drug design and medicinal chemistry: A short review. J. Drug Des. Med. Chem. 2016, 2, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.Y.; Qian, X.H.; Li, Z.; Huang, Q.C.; Chen, G. Synthesis and insecticidal activity of new substituted N-aryl-N’-benzoylthiourea compounds. J. Fluorine. Chem. 2003, 121, 51–54. [Google Scholar] [CrossRef]
- Keche, A.P.; Hatnapure, G.D.; Tale, R.H.; Rodge, A.H.; Birajdar, S.S.; Kamble, V.M. A novel pyrimidine derivatives with aryl urea, thiourea and sulfonamide moieties: Synthesis, anti-inflam matory and antimicrobial evaluation. Bioorg. Med. Chem. Lett. 2012, 22, 3445–3448. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.J.; Wu, L.L.; Liu, M.; Huang, Y.D.; Wang, Y.M. Asymmetric aza-Michael additions of 4-nitrophthalimide to nitroalkenes and preliminary study of the products for herbicidal activities. Tetrahedron 2013, 69, 2613–2618. [Google Scholar] [CrossRef]
- Abbas, S.Y.; El-Sharief, M.A.M.S.; Basyouni, W.M.; Fakhr, I.M.I.; El-Gammal, E.W. Thiourea derivatives incorporating a hippuric acid moiety: Synthesis and evaluation of antibacterial and antifungal activities. Eur. J. Med. Chem. 2013, 64, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, S.M.; Kumar, R.S.; Libertsen, L.A.; Perumal, S.; Yogeeswari, P.; Sriram, D. A green expedient synthesis of pyridopyrimidine-2-thiones and their antitubercular activity. Bioorg. Med. Chem. Lett. 2011, 21, 3012–3016. [Google Scholar] [CrossRef] [PubMed]
- Bhabak, K.P.; Bhowmick, D. Synthesis and structural characterization of some trisulfide analoges of thiouracil-based antithyroid drugs. J. Mol. Struct. 2012, 1022, 16–24. [Google Scholar] [CrossRef]
- Suhas, R.; Chandrashekar, S.; Gowda, D.C. Synthesis of uriedo and thiouriedo derivatives of peptide conjugated heterocycles—A new class of promising antimicrobials. Eur. J. Med. Chem. 2012, 48, 179–191. [Google Scholar] [CrossRef]
- Hu, Q.; Lin, G.S.; Duan, W.G.; Huang, M.; Lei, F.H. Synthesis and biological activity of novel (Z)- and (E)-verbenone oxime esters. Molecules 2017, 22, 1678. [Google Scholar] [CrossRef] [Green Version]
- Kang, G.Q.; Duan, W.G.; Lin, G.S.; Yu, Y.P.; Wang, X.Y.; Lu, S.Z. Synthesis of bioactive compounds from 3-carene (II): Synthesis, antifungal activity and 3D-QSAR study of (Z)- and (E)-3-caren-5-one oxime sulfonates. Molecules 2019, 24, 477. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.P.; Duan, W.G.; Lin, G.S.; Kang, G.Q.; Wang, X.Y.; Lei, F.H. Synthesis, biological activity and three-dimensional quantitative structure-activity relationship (3D-QSAR) study of novel 4-methyl-1,2,4-triazole-thioethers containing gem- dimethylcyclopropane ring. Chin. J. Org. Chem. 2020, 40, 1647–1657. [Google Scholar] [CrossRef]
- Chen, N.Y.; Duan, W.G.; Lin, G.S.; Liu, L.Z.; Zhang, R.; Li, D.P. Synthesis and antifungal activity of dehydroabietic acid-based 1,3,4-thiadiazole-thiazolidinone compounds. Mol. Divers. 2016, 20, 897–905. [Google Scholar] [CrossRef]
- Lin, G.S.; Bai, X.; Duan, W.G.; Cen, B.; Huang, M.; Lu, Z.S. High value-added application of sustainable natural forest product α-pinene: Synthesis of myrtenal oxime esters as potential KARI inhibitors. ACS Sustain. Chem. Eng. 2019, 7, 7862–7868. [Google Scholar] [CrossRef]
- Wang, B.L.; Shi, Y.X.; Zhang, S.J.; Ma, Y.; Wang, H.X.; Zhang, L.Y.; Wei, W.; Liu, X.H.; Li, Y.H.; Li, Z.M.; et al. Syntheses, biological activities and SAR studies of novel carboxamide compounds containing piperazine and arylsulfonyl moieties. Eur. J. Med. Chem. 2016, 117, 167–178. [Google Scholar] [CrossRef]
- Zhu, X.P.; Lin, G.S.; Duan, W.G.; Li, Q.M.; Li, F.Y.; Lu, S.Z. Synthesis and antiproliferative evaluation of novel longifolene-derived tetralone derivatives bearing 1,2,4-triazole moiety. Molecules 2020, 25, 986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorai, P.; Dussault, P.H.; Hu, C.H. Synthesis of spiro-bisperoxyketals. Org. Lett. 2008, 10, 2401–2404. [Google Scholar] [CrossRef]
- Lin, G.S.; Duan, W.G.; Li, Z.S.; Bai, X.; Hu, Q.; Lei, F.H. Synthesis and properties of myrtenal-based thiadiazole-amide compounds. Fine. Chem. 2017, 34, 588–595. [Google Scholar]
- Javid, M.T.; Rahim, F.; Taha, M.; Rehman, H.U.; Nawaz, M.; Wadood, A.; Imran, S.; Uddin, I.; Mosaddik, A.; Khan, K.M. Synthesis, in vitro alpha-glucosidase inhibitory potential and molecular docking study of thiadiazole analogs. Bioorg. Chem. 2018, 78, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Shi, Y.X.; Ma, Y.; Zhang, C.Y.; Dong, W.L.; Pan, L.; Wang, B.L.; Li, B.J.; Li, Z.M. Synthesis, antifungal activities and 3D-QSAR study of N-(5-substituted-1,3,4-thiadiazol-2-yl)cyclopropane- carboxamides. Eur. J. Med. Chem. 2009, 44, 2782–2786. [Google Scholar] [CrossRef]


| Compounds | Inhibition Rate (%) against the Tested Fungi | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| F. oxysporum f. sp. cucumerinum | C. arachidicola | P. piricola | A. solani | G. zeae | R. solani | B. myadis | C. orbicalare | Average (I) | |
| 6a (R = Ph) | 34.1 | 64.3 | 64.3 | 60.6 | 54.8 | 19.8 | 37.7 | 35.5 | 46.4 |
| 6b (R = o-Me Ph) | 37.8 | 67.1 | 75.2 | 72.3 | 47.9 | 19.8 | 29.1 | 35.5 | 48.1 |
| 6c (R = m-Me Ph) | 28.5 | 52.9 | 86.1 | 61.3 | 56.6 | 17.3 | 33.4 | 25.7 | 45.2 |
| 6d (R = p-Me Ph) | 30.4 | 50.0 | 66.5 | 33.1 | 47.9 | 53.9 | 24.9 | 29.6 | 42.0 |
| 6e (R = m-OMe Ph) | 28.5 | 64.3 | 55.7 | 65.0 | 58.3 | 14.9 | 24.9 | 27.6 | 42.4 |
| 6f (R = p-OMe Ph) | 21.1 | 50.0 | 68.7 | 50.9 | 39.3 | 17.3 | 22.8 | 25.7 | 37.0 |
| 6g (R = p-F Ph) | 21.1 | 70.0 | 64.3 | 77.3 | 56.6 | 17.3 | 27.0 | 27.6 | 45.2 |
| 6h (R = m-Cl Ph) | 30.5 | 80.6 | 55.5 | 69.7 | 56.8 | 19.3 | 36.5 | 33.6 | 47.8 |
| 6i (R = p-Cl Ph) | 29.8 | 69.3 | 80.2 | 61.3 | 58.1 | 22.3 | 38.4 | 28.7 | 48.5 |
| 6j (R = m, p-Cl Ph) | 36.1 | 66.3 | 66.3 | 70.3 | 65.4 | 21.8 | 39.7 | 49.3 | 51.9 |
| 6k (R = o-Br Ph) | 32.2 | 64.3 | 75.2 | 69.0 | 47.9 | 14.9 | 35.5 | 33.5 | 46.6 |
| 6l (R = m-Br Ph) | 26.7 | 52.9 | 51.3 | 71.0 | 30.7 | 19.8 | 35.5 | 29.6 | 39.7 |
| 6m (R = p-Br Ph) | 34.1 | 67.1 | 64.3 | 61.3 | 27.2 | 17.3 | 29.1 | 31.6 | 41.5 |
| 6n (R = o-CF3 Ph) | 34.1 | 61.4 | 57.8 | 72.8 | 79.0 | 16.1 | 31.3 | 37.5 | 48.8 |
| 6o (R = m-NO2 Ph) | 26.7 | 72.9 | 53.5 | 70.9 | 56.6 | 17.3 | 29.1 | 25.7 | 44.1 |
| 6p (R = p-NO2 Ph) | 35.9 | 72.9 | 36.1 | 63.8 | 51.4 | 17.3 | 35.5 | 37.5 | 43.8 |
| 6q (R = i-Pr) | 34.1 | 70.0 | 86.1 | 60.6 | 58.3 | 17.3 | 22.8 | 29.6 | 47.4 |
| 6r (R = n-Bu) | 28.5 | 61.4 | 75.2 | 63.8 | 51.4 | 14.9 | 24.9 | 27.6 | 43.5 |
| Average (II) | 30.6 | 64.3 | 65.7 | 64.2 | 52.5 | 19.9 | 31.0 | 31.7 | - |
| Chlorothalonil | 100 | 73.3 | 75.0 | 73.9 | 73.1 | 96.1 | 90.4 | 91.3 | - |
| Compounds | MW | ED | ED[a] | Residue |
|---|---|---|---|---|
| 6a | 370.13 | −2.381 | −2.368 | −0.013 |
| 6b | 384.14 | −2.168 | −2.197 | 0.029 |
| 6c | 384.14 | −2.385 | −2.401 | 0.016 |
| 6d | 384.14 | −2.890 | −2.907 | 0.017 |
| 6e | 400.14 | −2.333 | −2.317 | −0.016 |
| 6f | 400.14 | −2.587 | −2.578 | −0.009 |
| 6g | 388.12 | −2.057 | −2.079 | 0.022 |
| 6h | 404.09 | −2.245 | −2.246 | 0.001 |
| 6i | 404.09 | −2.407 | −2.400 | −0.007 |
| 6j | 438.05 | −2.267 | −2.282 | 0.015 |
| 6k | 448.04 | −2.304 | −2.264 | −0.040 |
| 6l | 448.04 | −2.262 | −2.260 | −0.002 |
| 6m | 448.04 | −2.452 | −2.443 | −0.009 |
| 6n | 438.12 | −2.214 | −2.225 | 0.011 |
| 6o | 415.11 | −2.231 | −2.218 | −0.013 |
| 6p * | 415.11 | −2.372 | −2.360 | −0.012 |
| Contribution (%) | ||||||
|---|---|---|---|---|---|---|
| q2 | r2 | S | F | Steric | Electrostatic | |
| CoMFA | 0.753 | 0.992 | 0.021 | 294.200 | 52.9 | 47.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Duan, W.-G.; Lin, G.-S.; Fan, Z.-T.; Wang, X. Synthesis, Antifungal Activity, and 3D-QSAR Study of Novel Nopol-Derived 1,3,4-Thiadiazole-Thiourea Compounds. Molecules 2021, 26, 1708. https://doi.org/10.3390/molecules26061708
Chen M, Duan W-G, Lin G-S, Fan Z-T, Wang X. Synthesis, Antifungal Activity, and 3D-QSAR Study of Novel Nopol-Derived 1,3,4-Thiadiazole-Thiourea Compounds. Molecules. 2021; 26(6):1708. https://doi.org/10.3390/molecules26061708
Chicago/Turabian StyleChen, Ming, Wen-Gui Duan, Gui-Shan Lin, Zhong-Tian Fan, and Xiu Wang. 2021. "Synthesis, Antifungal Activity, and 3D-QSAR Study of Novel Nopol-Derived 1,3,4-Thiadiazole-Thiourea Compounds" Molecules 26, no. 6: 1708. https://doi.org/10.3390/molecules26061708