Construction of a Stable Lanthanide Metal-Organic Framework as a Luminescent Probe for Rapid Naked-Eye Recognition of Fe3+ and Acetone
Abstract
:1. Introduction
2. Results and Discussion
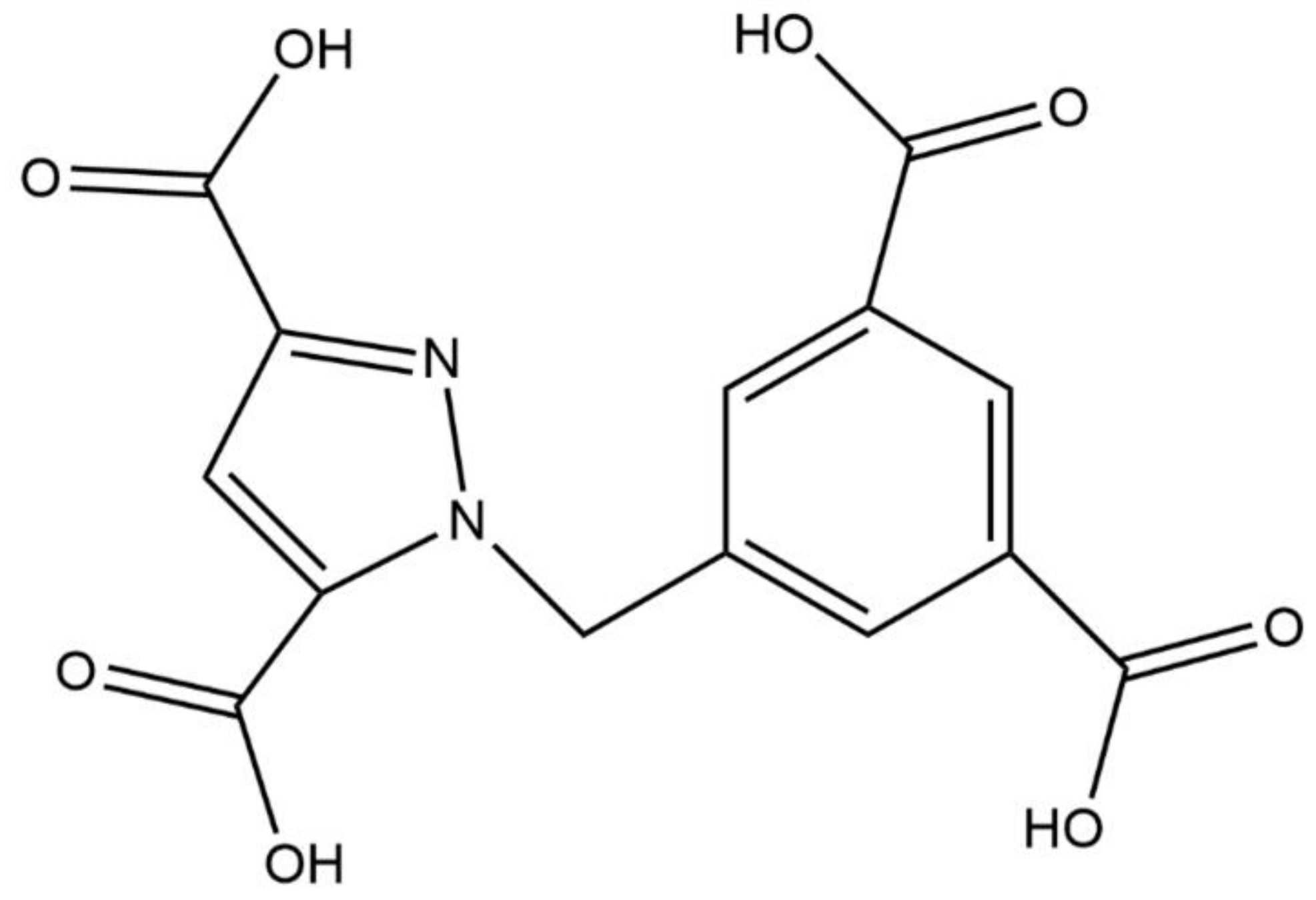
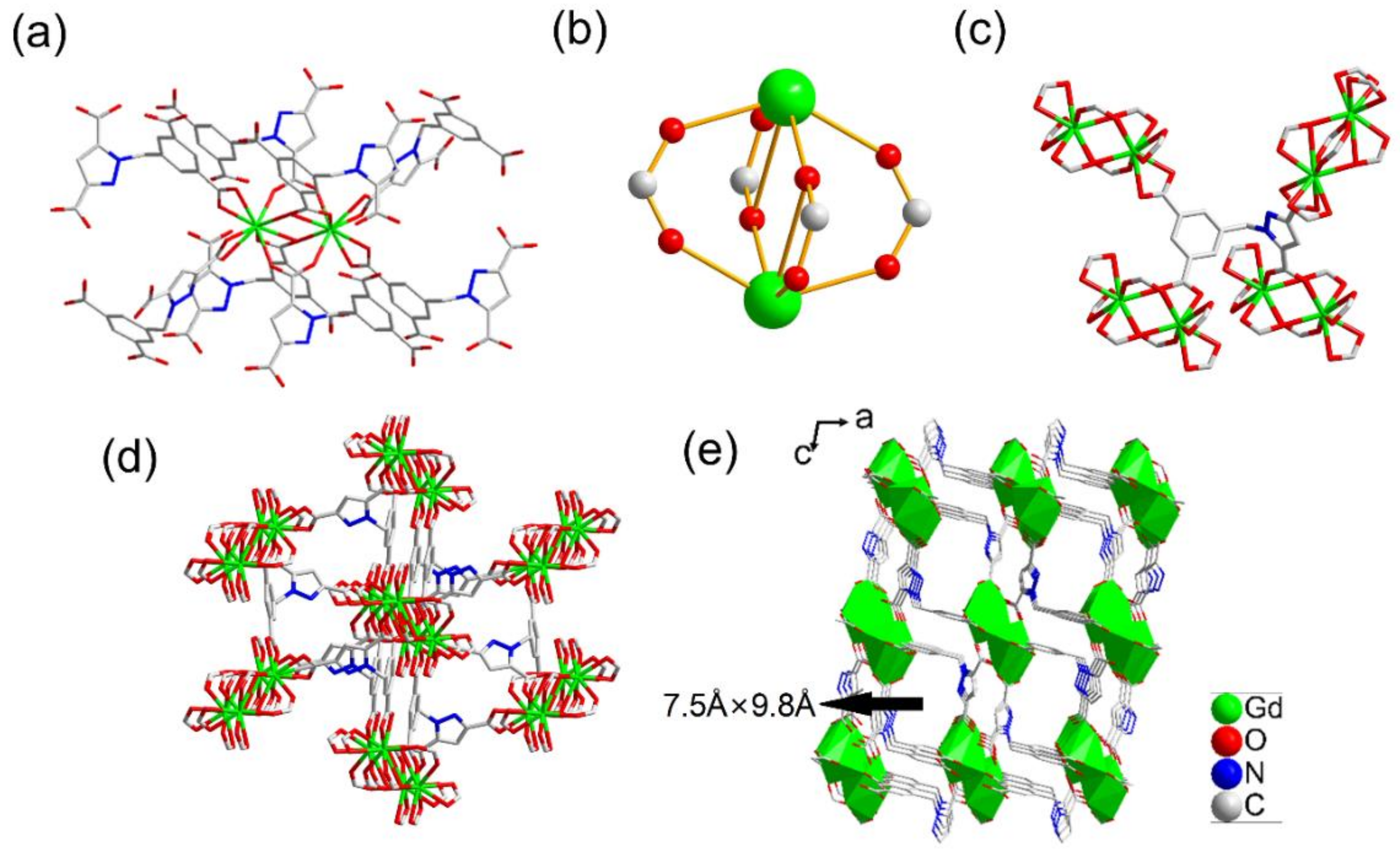
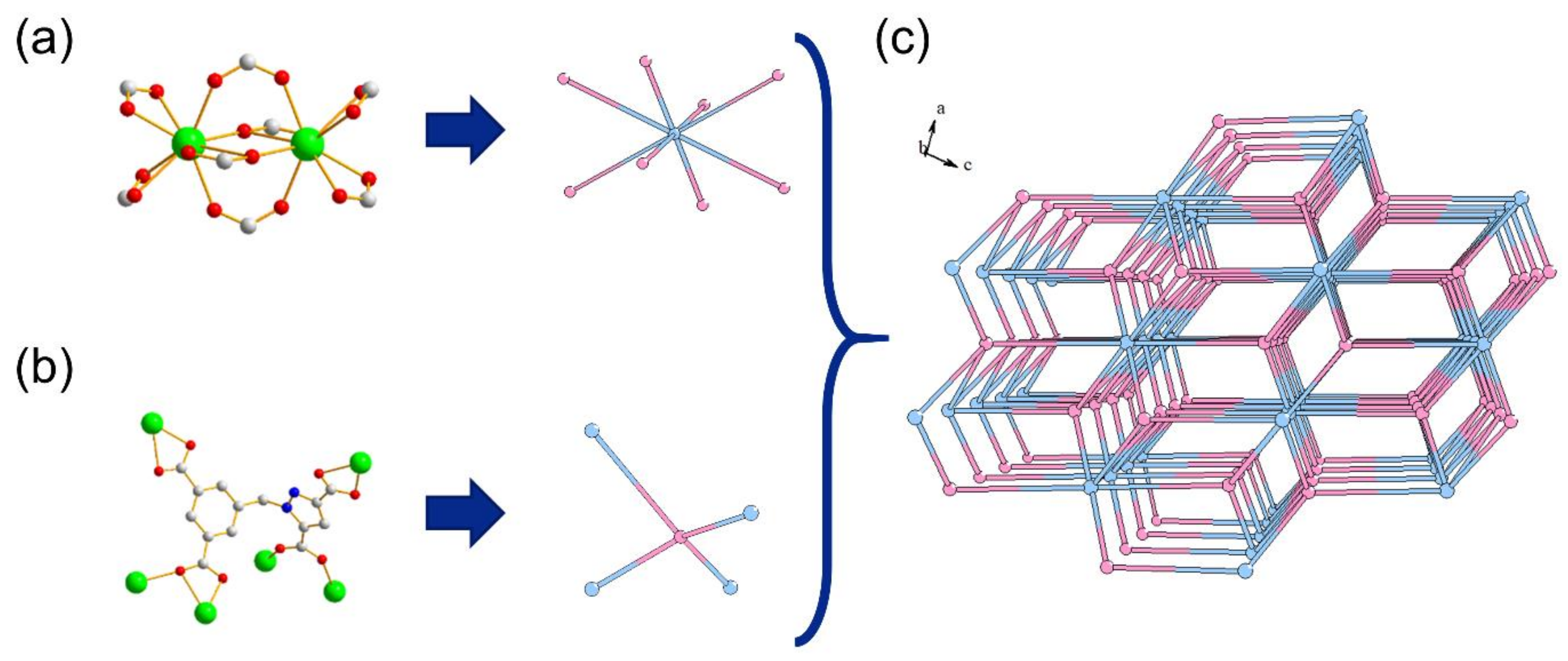
2.1. Structure Description of {[Me2NH2][LnL]·2H2O}n (Ln = Eu 1, Tb 2, Dy 3, Gd 4) (Compounds 1–4)
2.2. Framework Stability
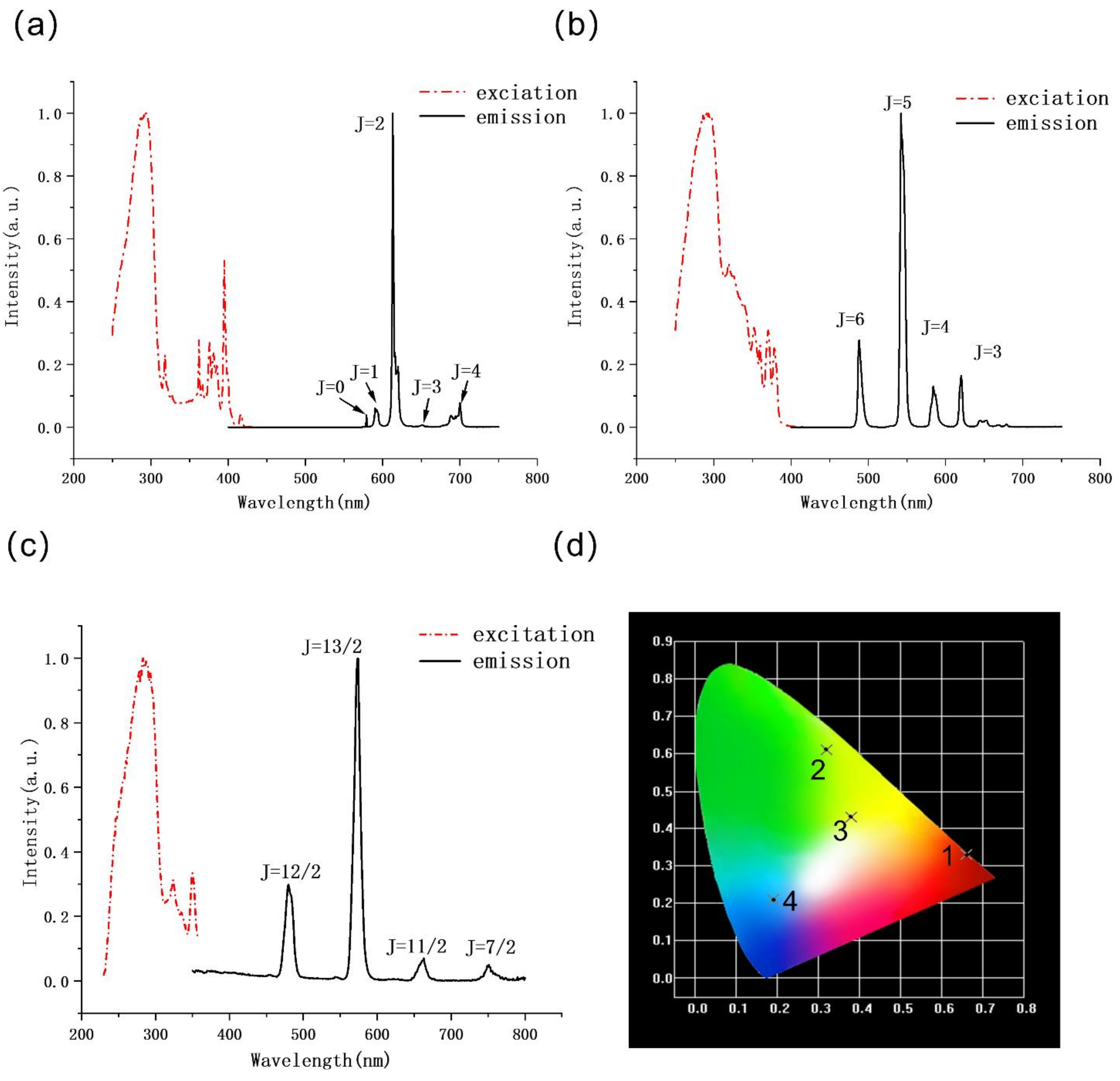
2.3. Luminescent Properties
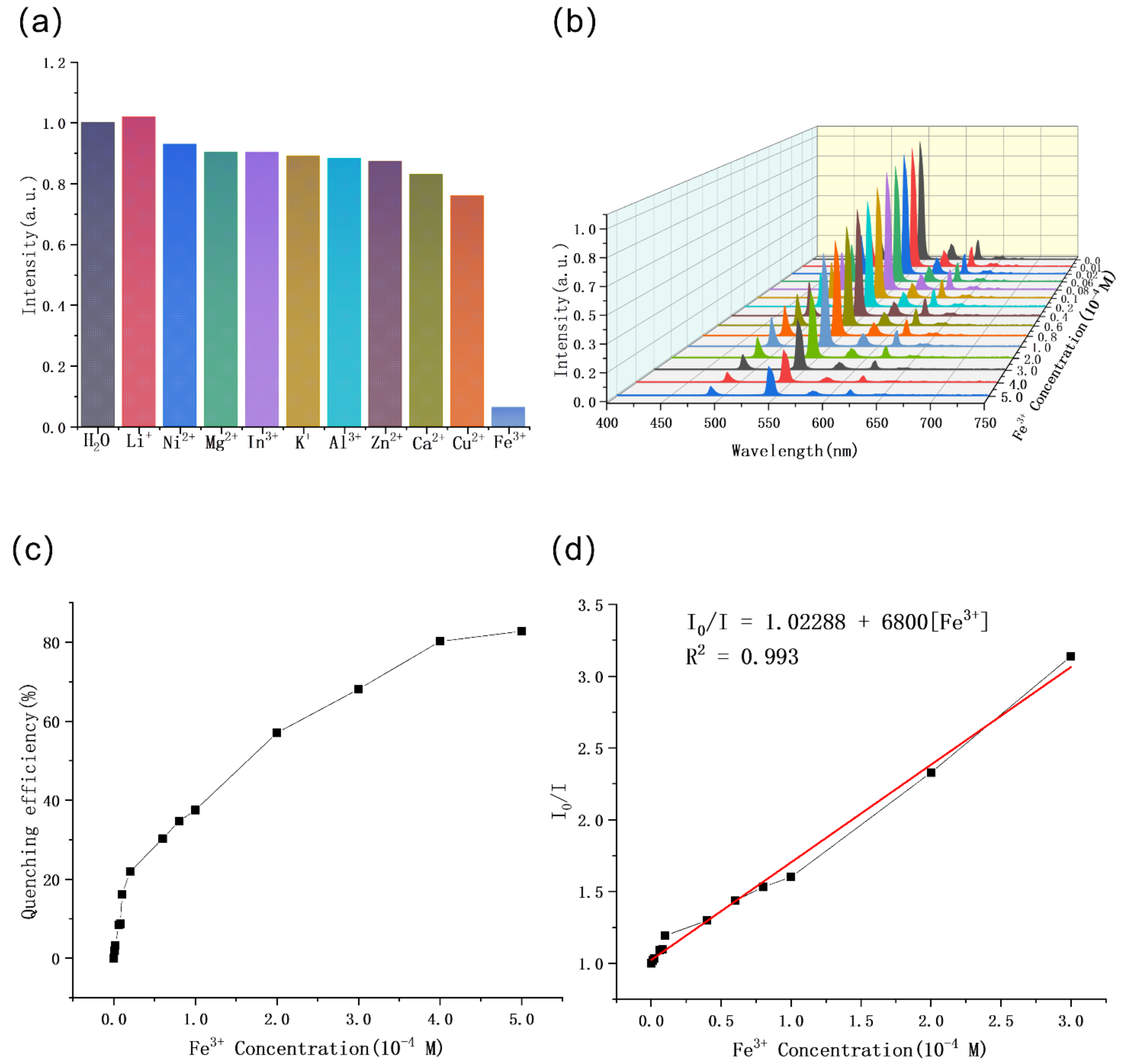
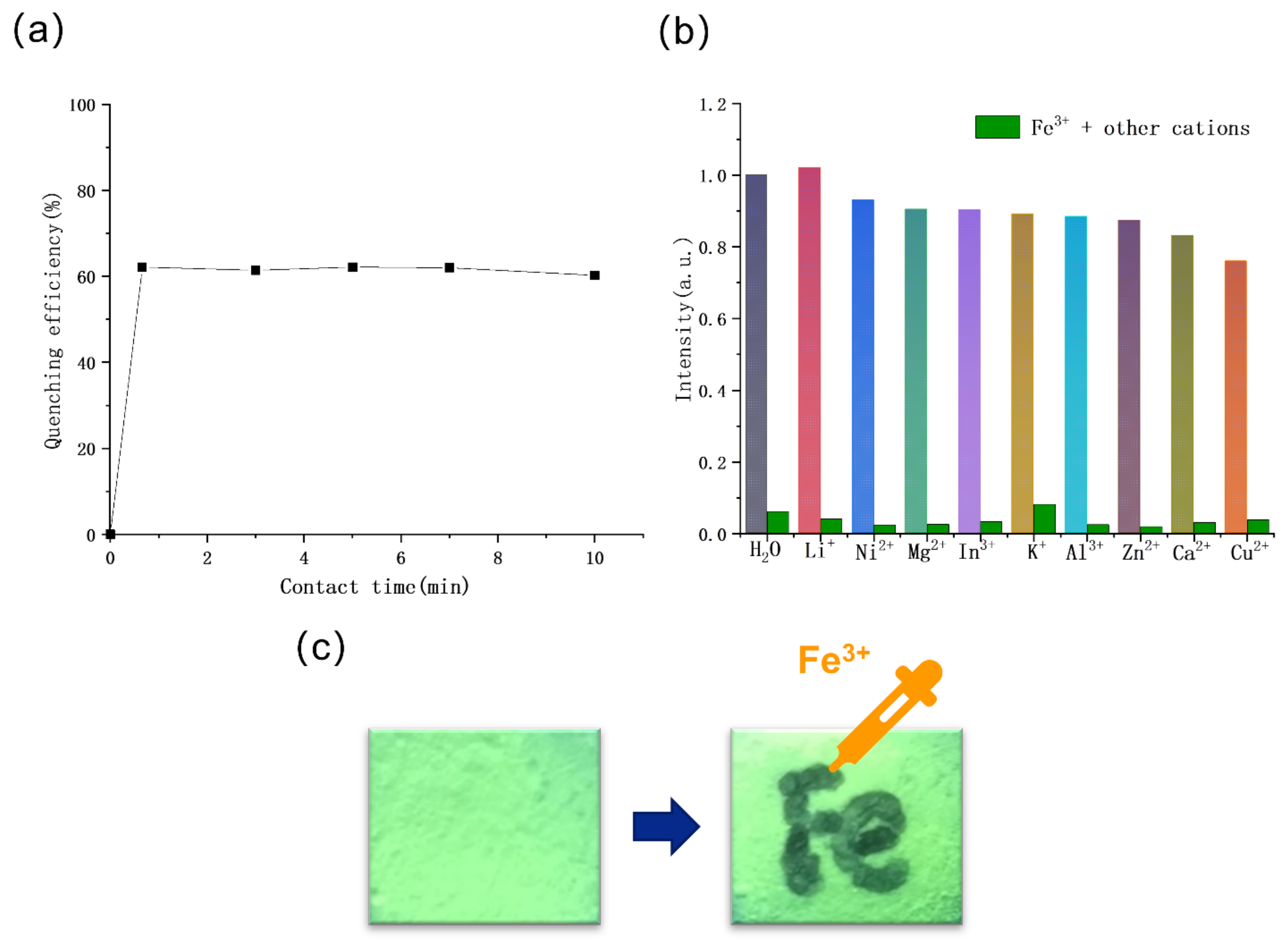
2.4. Sensing of Cations
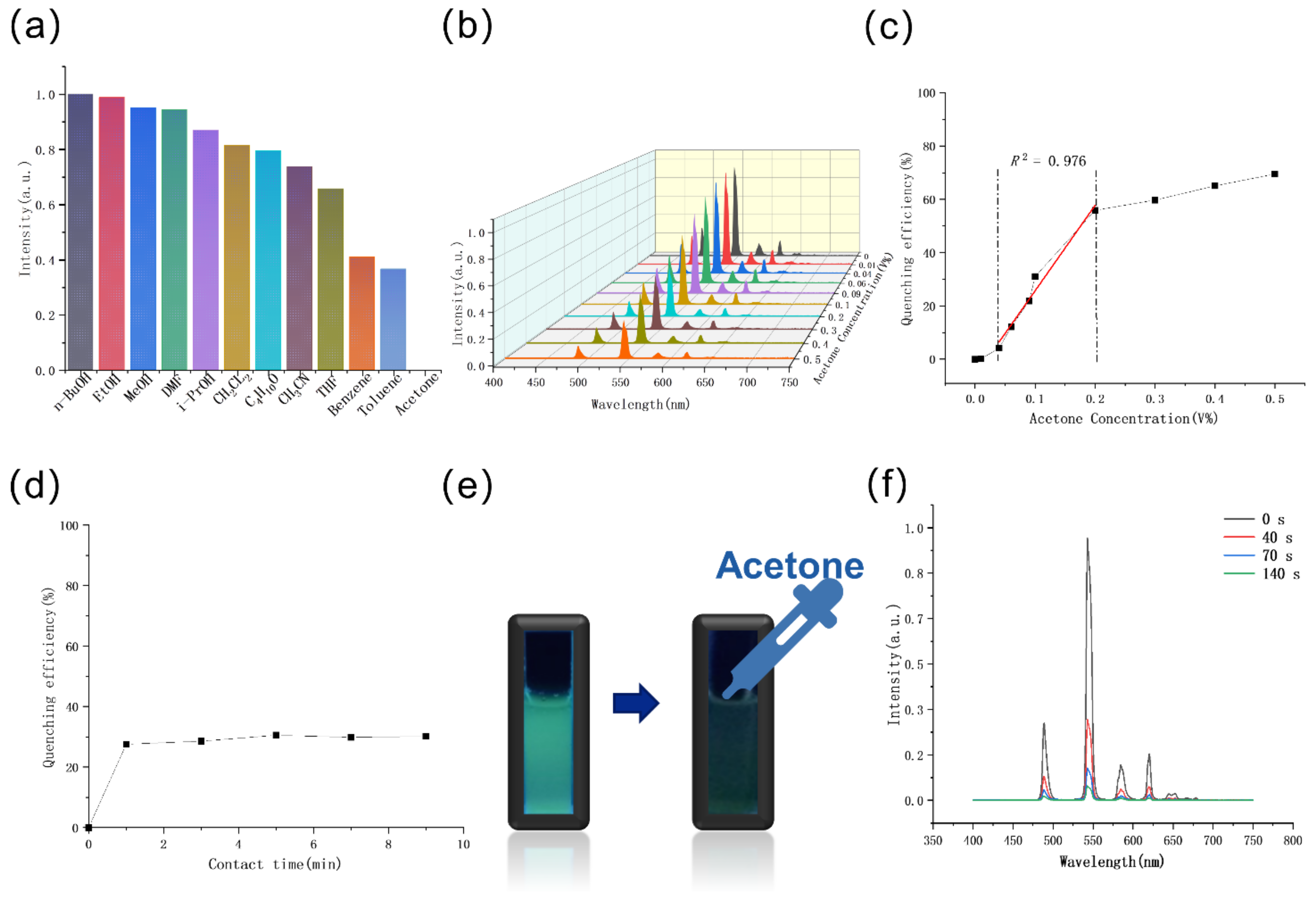
2.5. Sensing of Organic Molecules
2.6. Sensing Mechanism
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Apparatus
3.3. Synthesis of {[Me2NH2][LnL]·2H2O}n (Ln = Eu 1, Tb 2, Dy 3, Gd 4) (Compounds 1–4)
3.4. Single-Crystal X-ray Crystallography
3.5. Sensing Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yu, C.; Sun, X.; Zou, L.; Li, G.; Zhang, L.; Liu, Y. A pillar-layered Zn-LMOF with uncoordinated carboxylic acid sites: High performance for luminescence sensing Fe3+ and TNP. Inorg. Chem. 2019, 58, 4026–4032. [Google Scholar] [CrossRef]
- Li, X.; Tang, J.; Liu, H.; Gao, K.; Meng, X.; Wu, J.; Hou, H. A highly sensitive and recyclable Ln-MOF Luminescent sensor for the efficient detection of Fe(3+) and Cr(VI) anions. Chem. Asian J. 2019, 14, 3721–3727. [Google Scholar] [CrossRef]
- Yang, C.X.; Ren, H.B.; Yan, X.P. Fluorescent metal-organic framework MIL-53(Al) for highly selective and sensitive detection of Fe3+ in aqueous solution. Anal. Chem. 2013, 85, 7441–7446. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, S.; Wang, X.; Xie, C.; Zeng, D. Catalytic activation of cobalt doping sites in ZIF-71-coated ZnO nanorod arrays for enhancing gas-sensing performance to acetone. ACS Appl. Mater. Interfaces 2020, 12, 48948–48956. [Google Scholar] [CrossRef]
- Ryu, U.; Jee, S.; Rao, P.C.; Shin, J.; Ko, C.; Yoon, M.; Park, K.S.; Choi, K.M. Recent advances in process engineering and upcoming applications of metal-organic frameworks. Coord. Chem. Rev. 2021, 426, 213544. [Google Scholar] [CrossRef]
- Qin, G.; Wang, J.; Li, L.; Yuan, F.; Zha, Q.; Bai, W.; Ni, Y. Highly water-stable Cd-MOF/Tb3+ ultrathin fluorescence nanosheets for ultrasensitive and selective detection of Cefixime. Talanta 2021, 221, 121421. [Google Scholar] [CrossRef]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis and Application; Royal Society of Chemistry: London, UK, 2009. [Google Scholar] [CrossRef]
- MacGillivray, L.R.; Lukehart, C.M. Metal-Organic Framework Materials; MacGillivray, L.R., Lukehart, C.M., Eds.; John Wiley & Sons: Chichester, UK, 2014. [Google Scholar] [CrossRef]
- Zhang, J.; Biradar, A.V.; Pramanik, S.; Emge, T.J.; Asefa, T.; Li, J. A new layered metal–organic framework as a promising heterogeneous catalyst for olefin epoxidation reactions. Chem. Commun. 2012, 48, 6541–6543. [Google Scholar] [CrossRef]
- Cha, G.Y.; Chun, H.; Hong, D.Y.; Kim, J.; Cho, K.H.; Lee, U.H.; Chang, J.S.; Ryu, S.G.; Lee, H.W.; Kim, S.J.; et al. Unique design of superior metal-organic framework for removal of toxic chemicals in humid environment via direct functionalization of the metal nodes. J. Hazard. Mater. 2020, 398, 122857. [Google Scholar] [CrossRef]
- Ardila-Suárez, C.; Díaz-Lasprilla, A.M.; Díaz-Vaca, L.A.; Balbuena, P.B.; Baldovino-Medrano, V.G.; Ramírez-Caballero, G.E. Synthesis, characterization, and post-synthetic modification of a micro/mesoporous zirconium–tricarboxylate metal–organic framework: Towards the addition of acid active sites. CrystEngComm 2019, 21, 3014–3030. [Google Scholar] [CrossRef]
- Lai, W.; Guo, J.; Zheng, N.; Nie, Y.; Ye, S.; Tang, D. Selective determination of 2,4,6-trinitrophenol by using a novel carbon nanoparticles as a fluorescent probe in real sample. Anal. Bioanal. Chem. 2020, 412, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Culp, J.T.; Ohodnicki, P.R.; Cvetic, P.C.; Sanguinito, S.; Goodman, A.L.; Kwon, H.T. Alkylamine-integrated metal-organic framework-based waveguide sensors for efficient detection of carbon dioxide from humid gas streams. ACS Appl. Mater. Interfaces 2019, 11, 33489–33496. [Google Scholar] [CrossRef]
- Liu, Q.; Tan, J.Y.; Zhang, J.Y.; Zhang, N.; Deng, W. R-Substituent induced structural diversity, synergistic effect and highly selective luminescence sensing for Fe3+ detection by post-synthetically modified Cd-MOFs. CrystEngComm 2020, 22, 3871–3883. [Google Scholar] [CrossRef]
- Gogoi, C.; Reinsch, H.; Biswas, S. A pyrazine core-based luminescent Zr(iv) organic framework for specific sensing of Fe3+, picric acid and Cr2O72−. CrystEngComm 2019, 21, 6252–6260. [Google Scholar] [CrossRef]
- Zhan, Z.Z.; Liang, X.Y.; Zhang, X.L.; Jia, Y.J.; Hu, M. A water-stable europium-MOF as a multifunctional luminescent sensor for some trivalent metal ions (Fe3+, Cr3+, Al3+), PO43− ions, and nitroaromatic explosives. Dalton Trans. 2019, 48, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.S.; Jiang, X.; Kirillov, A.M.; Zhang, Y.; Hu, M.Y.; Liu, W.; Yang, L.Z.; Fang, R.; Liu, W.S. Covalent Construction of Sustainable Hybrid UiO-66-NH2@Tb-CP Material for Selective Removal of Dyes and Detection of Metal Ions. ACS Sustain. Chem. Eng. 2019, 7, 3203–3212. [Google Scholar] [CrossRef]
- Yang, L.Z.; Wang, J.; Kirillov, A.M.; Dou, W.; Xu, C.; Fang, R.; Xua, C.L.; Liu, W.S. 2D lanthanide MOFs driven by a rigid 3,5-bis(3-carboxy-phenyl)pyridine building block: Solvothermal syntheses, structural features, and photoluminescence and sensing properties. CrystEngComm 2016, 18, 6425–6436. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, X.Y.; Cheng, C.; Shao, Z.S.; Wang, H.S. Strategies to fabricate metal–organic framework (MOF)-based luminescent sensing platforms. J. Mater. Chem. C 2019, 7, 10743–10763. [Google Scholar] [CrossRef]
- Jaros, S.W.; Sokolnicki, J.; Wołoszyn, A.; Haukka, M.; Kirillov, A.M.; Smoleński, P. A novel 2D coordination network built from hexacopper(i)-iodide clusters and cagelike aminophosphine blocks for reversible “turn-on” sensing of aniline. J. Mater. Chem. C 2018, 6, 1670–1678. [Google Scholar] [CrossRef]
- Li, Y.; Qin, C.; Li, Q.; Wang, P.; Miao, X.; Jin, H.; Ao, W.; Cao, L. Supramolecular organic frameworks with controllable shape and aggregation-induced emission for tunable luminescent materials through aqueous host–guest complexation. Adv. Opt. Mater. 2020, 8, 1902154. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A.; Dhakshinamoorthy, A.; Garcia, H. Mixed-metal MOFs: Unique opportunities in metal-organic framework (MOF) functionality and design. Angew. Chem. Int. Ed. Engl. 2019, 58, 15188–15205. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, F.; Liu, C.; Chen, L.; Gai, Y.; Pang, J.; Su, K.; Wan, X.; Hong, M. Self-assembly syntheses, structural characterization, and luminescent properties of lanthanide coordination polymers constructed by three triazole-carboxylate ligands. Cryst. Growth Des. 2016, 16, 2266–2276. [Google Scholar] [CrossRef]
- Gao, X.; Sun, G.; Ge, F.; Zheng, H. Three anionic indium-organic frameworks for highly efficient and selective dye adsorption, lanthanide adsorption, and luminescence regulation. Inorg. Chem. 2019, 58, 8396–8407. [Google Scholar] [CrossRef]
- Peng, C.; Song, X.; Yin, J.; Zhang, G.; Fei, H. Intrinsic white-light-emitting metal-organic frameworks with structurally deformable secondary building units. Angew. Chem. Int. Ed. Engl. 2019, 58, 7818–7822. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Zeng, Y.; Liang, Z.; Guo, W.; Zhi, C.; Wu, Y.; Zhong, R.; Qu, C.; Zou, R. Tuning expanded pores in metal-organic frameworks for selective capture and catalytic conversion of carbon dioxide. ChemSusChem 2018, 11, 3751–3757. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, J.; Sun, C.; Liu, H.; Wang, W.; Smith, E.; Jiang, L.; Chen, X.; Snape, C. Design and development of 3D hierarchical ultra-microporous CO2-sieving carbon architectures for potential flow-through CO2 capture at typical practical flue gas temperatures. J. Mater. Chem. A 2020, 8, 17025–17035. [Google Scholar] [CrossRef]
- Aryanejad, S.; Motlagh, N.V. Investigation of the carbon dioxide adsorption behavior and the heterogeneous catalytic efficiency of a novel Ni-MOF with nitrogen-rich channels. RSC Adv. 2020, 10, 29772–29779. [Google Scholar] [CrossRef]
- Singh, G.; Lee, J.; Karakoti, A.; Bahadur, R.; Yi, J.; Zhao, D.; AlBahily, K.; Vinu, A. Emerging trends in porous materials for CO2 capture and conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jana, D.; Zhao, Y. Metal-organic framework derived nanozymes in biomedicine. Acc. Chem. Res. 2020, 53, 1389–1400. [Google Scholar] [CrossRef]
- Ma, D.D.; Zhu, Q.L. MOF-based atomically dispersed metal catalysts: Recent progress towards novel atomic configurations and electrocatalytic applications. Coord. Chem. Rev. 2020, 422, 213483. [Google Scholar] [CrossRef]
- Chen, L.J.; Zhao, X.; Liu, Y.Y.; Yan, X.P. Macrophage membrane coated persistent luminescence nanoparticle@MOF-derived mesoporous carbon core-shell nanocomposites for autofluorescence-free imaging-guided chemotherapy. J. Mater. Chem. B 2020, 8, 8071–8083. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, H.; Xu, C.; Tong, Z.; Zhang, S.; Bai, Y.; Chen, Y.; Xu, Q.; Zhou, L.; Ding, H.; et al. A nanomedicine fabricated from gold nanoparticles-decorated metal-organic framework for cascade chemo/chemodynamic cancer therapy. Adv. Sci. 2020, 7, 2001060. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.M.; Li, C.J.; Wu, X.; Zhang, J.H.; Wang, Z.; Wang, H.P.; Fan, Y.N.; Pan, M.; Su, C.Y. Homometallic Ln(iii)-complexes from an ILCT ligand with sensitized vis-NIR emission, excitation-dependent PL color tuning and white-light emission. J. Mater. Chem. C 2018, 6, 3254–3259. [Google Scholar] [CrossRef]
- Guo, H.; Wu, N.; Xue, R.; Liu, H.; Wang, M.; Yao, W.; Wang, X.; Yang, W. An Eu(III)-functionalized Sr-based metal-organic framework for fluorometric determination of Cr(III) and Cr(VI) ions. Mikrochim. Acta 2020, 187, 374. [Google Scholar] [CrossRef] [PubMed]
- Harbuzaru, B.V.; Corma, A.; Rey, F.; Atienzar, P.; Jordá, J.L.; García, H.; Ananias, D.; Carlos, L.D.; Rocha, J. Metal–organic nanoporous structures with anisotropic photoluminescence and magnetic properties and their use as sensors. Angew. Chem. Int. Ed. 2008, 47, 1080–1083. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Min, H.; Shi, W.; Cheng, P. Multicenter metal-organic framework-based ratiometric fluorescent sensors. Adv. Mater. 2020, 32, e1805871. [Google Scholar] [CrossRef] [PubMed]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Wang, L.N.; Zhang, Y.H.; Jiang, S.; Liu, Z.Z. Three coordination polymers based on 3-(3′,5′-dicarboxylphenoxy)phthalic acid and auxiliary N-donor ligands: Syntheses, structures, and highly selective sensing for nitro explosives and Fe3+ ions. CrystEngComm 2019, 21, 4557–4567. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, C.; Cen, P.; Jin, X.; Liang, C.; Yang, J.; Liu, X. Stable Ln-MOFs as multi-responsive photoluminescence sensors for the sensitive sensing of Fe3+, Cr2O72−, and nitrofuran. CrystEngComm 2020, 22, 1695–1704. [Google Scholar] [CrossRef]
- Hao, Z.; Yang, G.; Song, X.; Zhu, M.; Meng, X.; Zhao, S.; Song, S.; Zhang, H. A europium(iii) based metal–organic framework: Bifunctional properties related to sensing and electronic conductivity. J. Mater. Chem. A 2014, 2, 237–244. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Desai, A.V.; Ghosh, S.K. A fluorescent metal–organic framework for highly selective detection of nitro explosives in the aqueous phase. Chem. Commun. 2014, 50, 8915–8918. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Joarder, B.; Chaudhari, A.K.; Mukherjee, S.; Ghosh, S.K. Highly Selective Detection of Nitro Explosives by a Luminescent Metal–Organic Framework. Angew. Chem. Int. Ed. 2013, 52, 2881–2885. [Google Scholar] [CrossRef]
- Wu, Z.F.; Fu, Z.H.; Velasco, E.; Xing, K.; Wang, H.; Zou, G.D.; Huang, X.Y.; Li, J. A robust and multifunctional calcium coordination polymer as a selective fluorescent sensor for acetone and iron (+3) and as a tunable proton conductor. J. Mater. Chem. C 2020, 8, 16784–16789. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Jiang, F.; Wu, M.; Pang, J.; Wan, X.; Hong, M. A water-stable 3D Eu-MOF based on a metallacyclodimeric secondary building unit for sensitive fluorescent detection of acetone molecules. CrystEngComm 2019, 21, 321–328. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, P.; Liu, J.; Chen, X.; Guo, X.; Jin, H.; Chai, J.; Wang, L.; Fan, Y. Multi-responsive luminescent sensor based on three dimensional lanthanide metal–organic framework. New J. Chem. 2018, 42, 19485–19493. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Meng, Y.; Shi, X.; Sun, J.; Zhao, L.; Chen, D.; Hao, H.; Li, D.; Dou, J.; et al. Di-functional luminescent sensors based on Y3+ doped Eu3+ and Tb3+ coordination polymers: Fast response and visible detection of Cr3+, Fe3+ ions in aqueous solutions and acetone. RSC Adv. 2020, 10, 32232–32240. [Google Scholar] [CrossRef]
- Gao, L.L.; Zhao, Q.N.; Li, M.M.; Fan, L.M.; Niu, X.Y.; Wang, X.Q.; Hu, T.P. Magnetic properties and luminescence sensing of five coordination polymers based on a rigid terphenyl-tetracarboxylic acid. CrystEngComm 2017, 19, 6651–6659. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, L.; Cui, Y.; Chen, B.; Zapata, F.; Qian, G. Molecular sensing with lanthanide luminescence in a 3D porous metal-organic framework. J. Alloys Compd. 2009, 484, 601–604. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXL-97, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Empirical formula | C16H14EuN3O8 | C16H14TbN3O8 | C16H14DyN3O8 | C16H14GdN3O8 |
| Formula weight | 528.27 | 535.23 | 538.80 | 533.55 |
| Crystal system | monoclinic | monoclinic | monoclinic | monoclinic |
| Space group | P21/c | P21/c | P21/c | P21/c |
| a/Å | 9.1791(2) | 9.2477(2) | 9.1815(5) | 9.2303(5) |
| b/Å | 10.1636(3) | 10.1192(2) | 10.1647(4) | 10.1530(4) |
| c/Å | 21.7390(5) | 21.7150(5) | 21.7438(9) | 21.8214(9) |
| α/° | 90 | 90 | 90 | 90 |
| β/° | 99.829(2) | 100.542(2) | 99.809(4) | 100.353(4) |
| γ/° | 90 | 90 | 90 | 90 |
| Volume/Å3 | 1998.32(9) | 1997.78(8) | 1999.62(16) | 2011.70(16) |
| Z | 4 | 4 | 4 | 4 |
| F (000) | 924 | 909 | 936 | 1076 |
| Goodness-of-fit on F2 | 1.082 | 1.021 | 1.07 | 1.03 |
| R1 (I > 2σ (I)) | 0.0311 | 0.0282 | 0.0387 | 0.0259 |
| wR2 (reflections) | 0.0848 | 0.0587 | 0.0701 | 0.1056 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yu, M.; Chen, L.; Li, Z.; Li, S.; Jiang, F.; Hong, M. Construction of a Stable Lanthanide Metal-Organic Framework as a Luminescent Probe for Rapid Naked-Eye Recognition of Fe3+ and Acetone. Molecules 2021, 26, 1695. https://doi.org/10.3390/molecules26061695
Wang J, Yu M, Chen L, Li Z, Li S, Jiang F, Hong M. Construction of a Stable Lanthanide Metal-Organic Framework as a Luminescent Probe for Rapid Naked-Eye Recognition of Fe3+ and Acetone. Molecules. 2021; 26(6):1695. https://doi.org/10.3390/molecules26061695
Chicago/Turabian StyleWang, Jiayishuo, Muxin Yu, Lian Chen, Zhijia Li, Shengchang Li, Feilong Jiang, and Maochun Hong. 2021. "Construction of a Stable Lanthanide Metal-Organic Framework as a Luminescent Probe for Rapid Naked-Eye Recognition of Fe3+ and Acetone" Molecules 26, no. 6: 1695. https://doi.org/10.3390/molecules26061695
APA StyleWang, J., Yu, M., Chen, L., Li, Z., Li, S., Jiang, F., & Hong, M. (2021). Construction of a Stable Lanthanide Metal-Organic Framework as a Luminescent Probe for Rapid Naked-Eye Recognition of Fe3+ and Acetone. Molecules, 26(6), 1695. https://doi.org/10.3390/molecules26061695







