Determination of Toxic Pyrrolizidine Alkaloids in Traditional Chinese Herbal Medicines by UPLC-MS/MS and Accompanying Risk Assessment for Human Health
Abstract
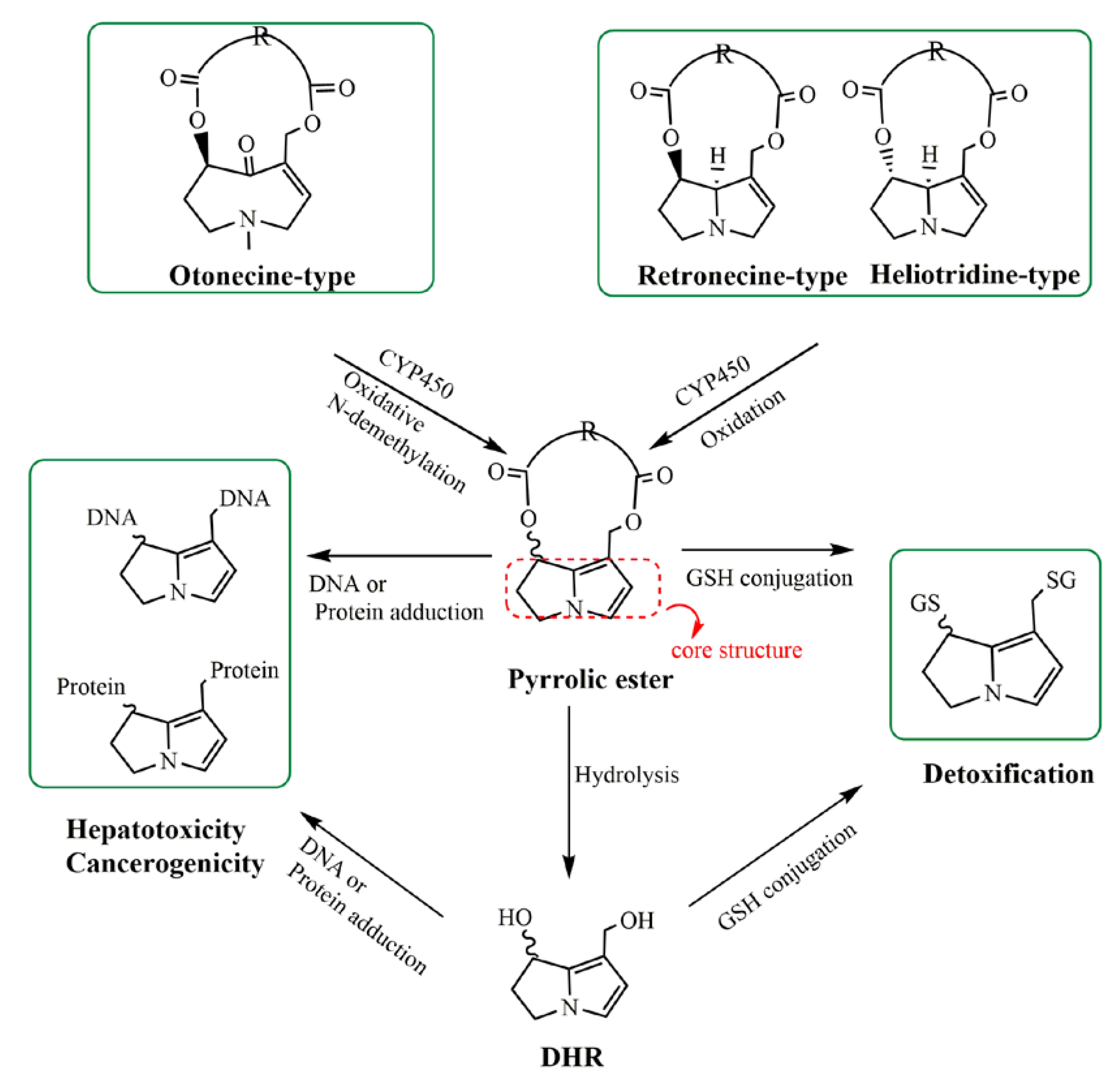
1. Introduction
2. Results
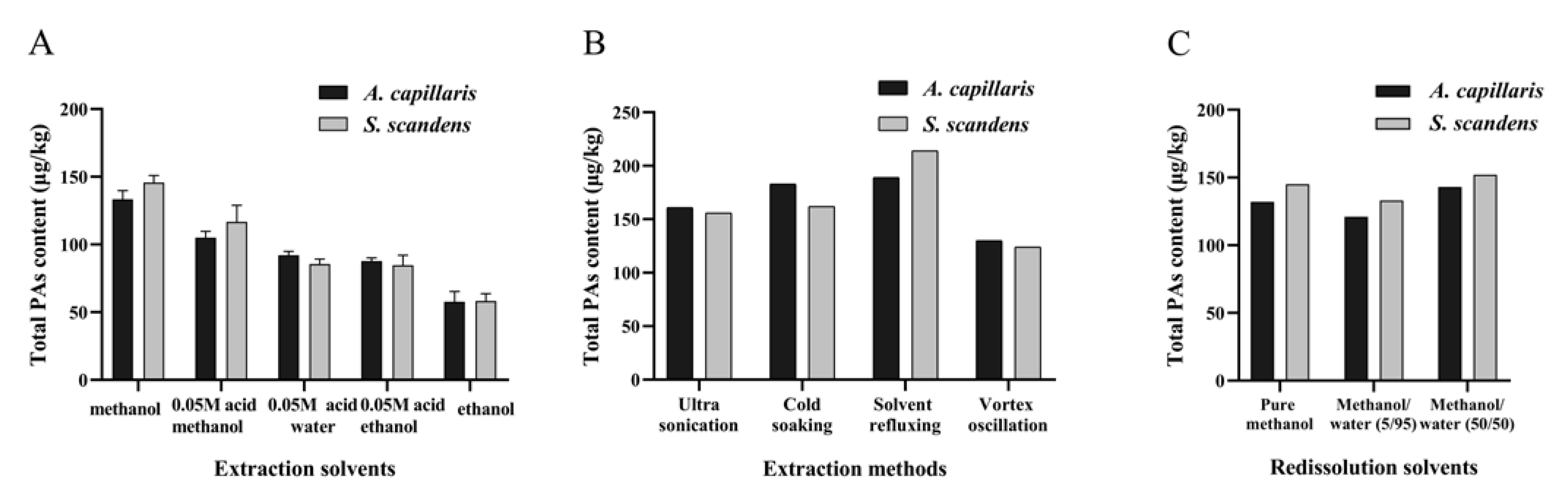
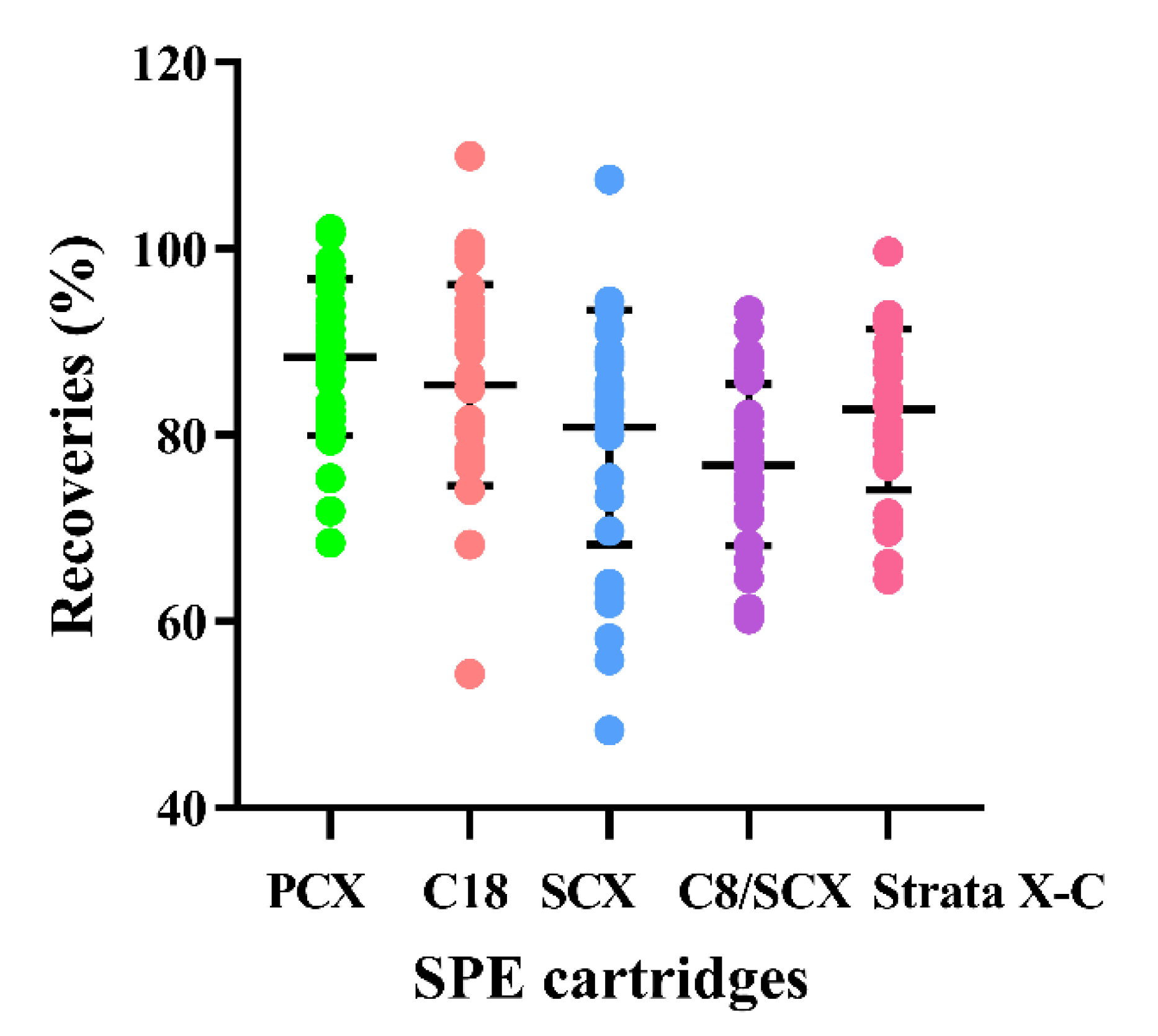
2.1. Pretreatment Method Development
2.1.1. Extraction Optimization
2.1.2. Purification Optimization
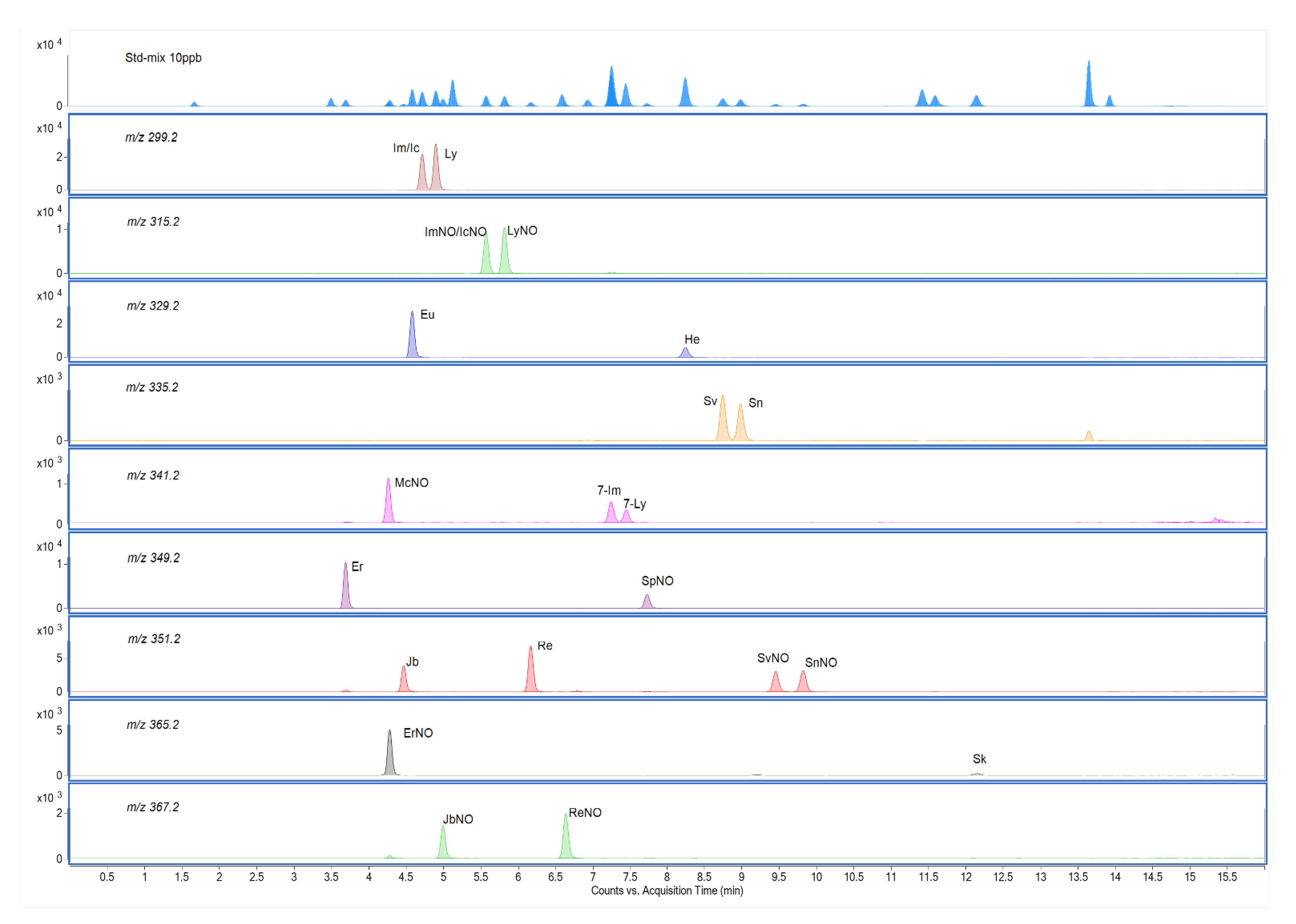
2.2. UPLC-MS/MS Method Development
2.3. Method Validation
2.3.1. Sensitivity, Linearity, Limits of Detection (LOD) and Limits of Quantification (LOQ)
2.3.2. Precision and Recovery
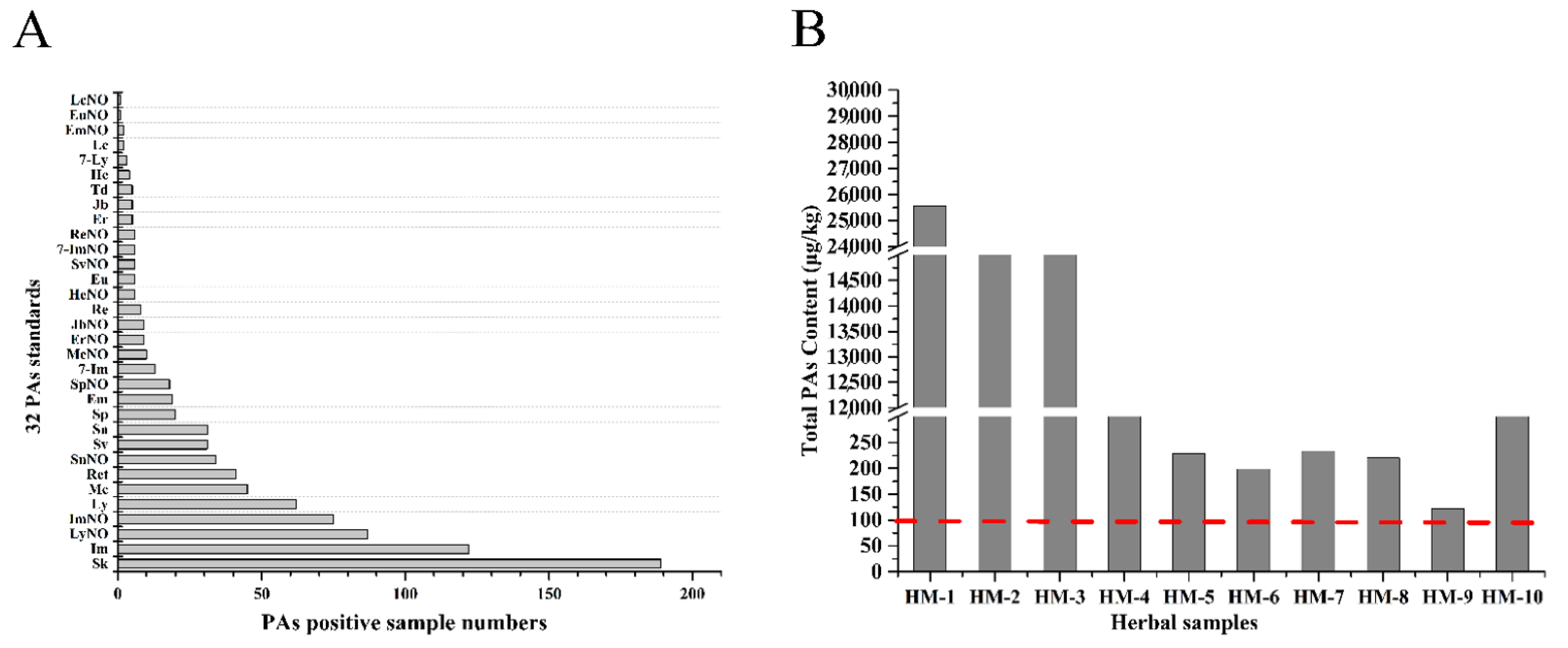
2.4. PA Concentrations in Chinese Herbal Medicines
2.5. Risk Assessment of Chinese Herbal Medicines Based on PAs Levels
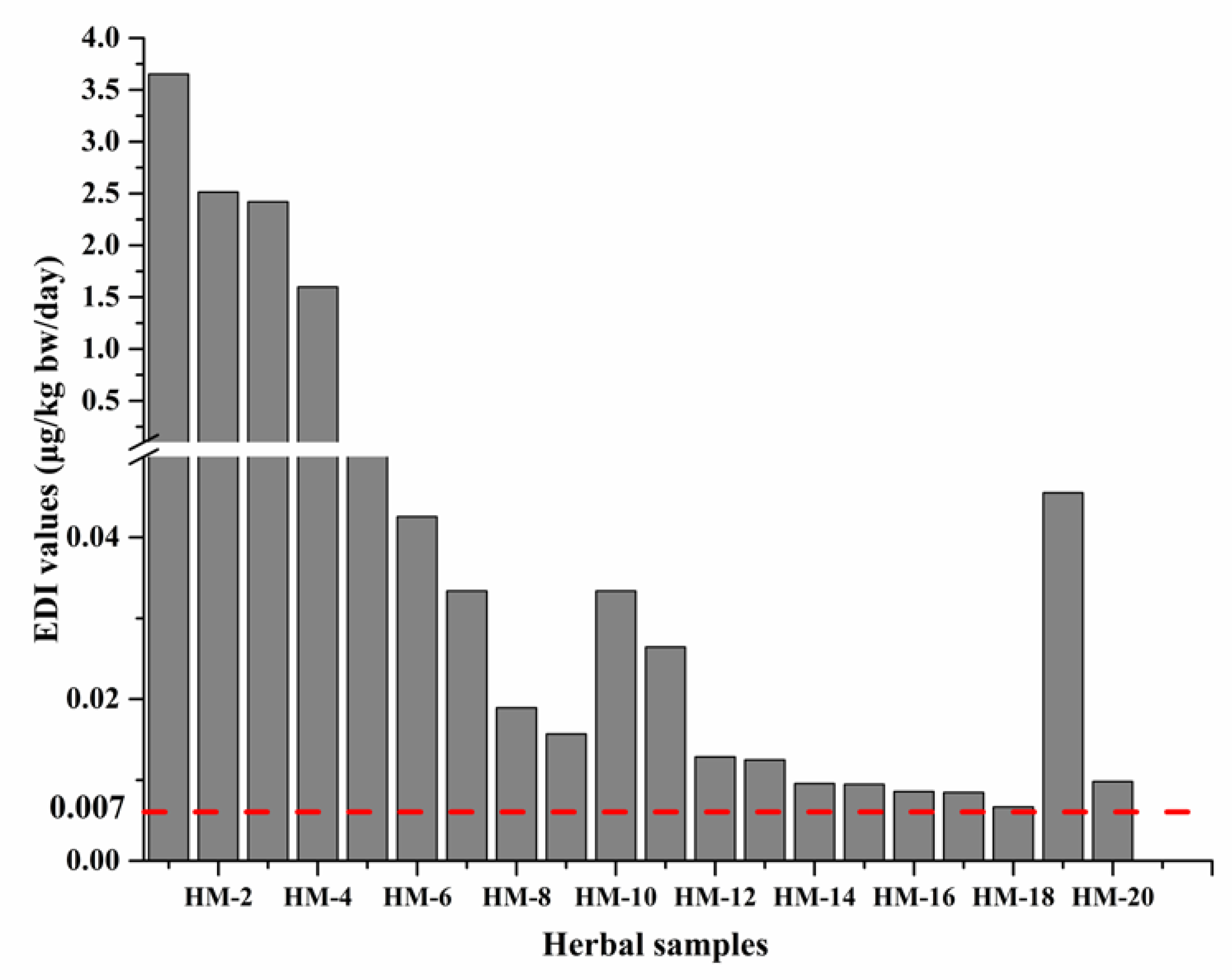
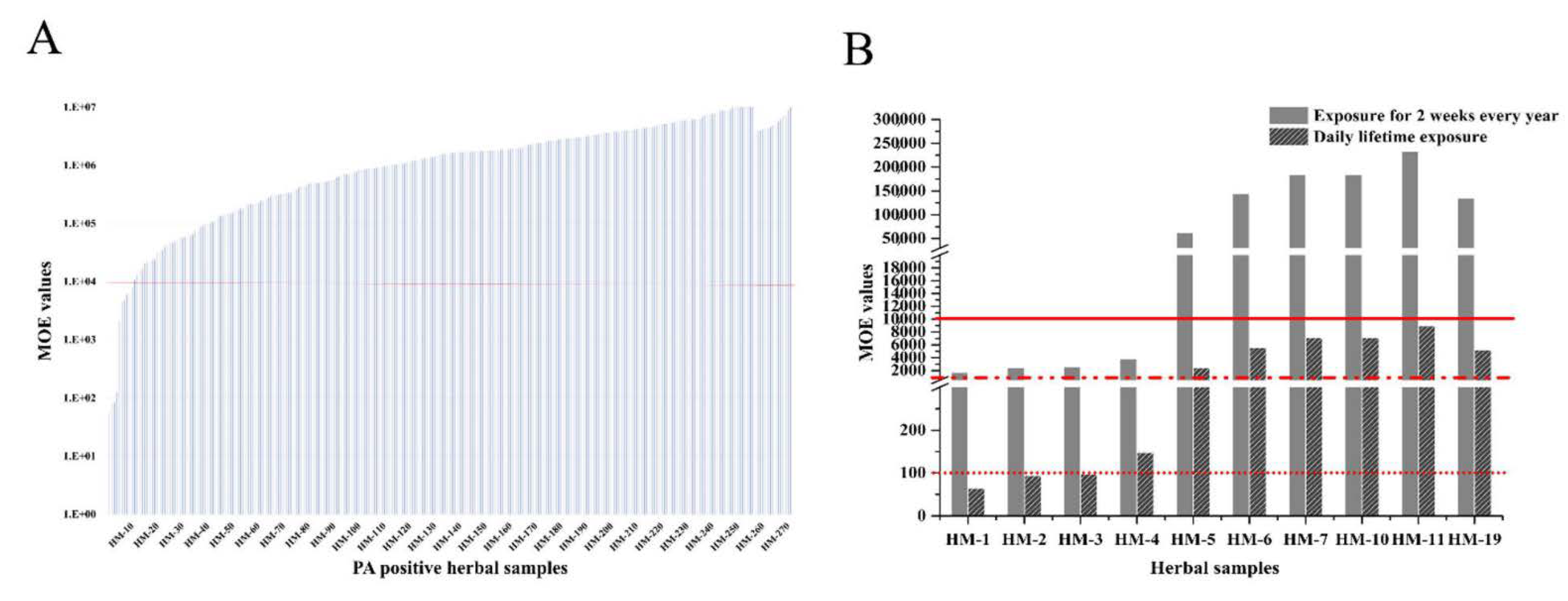
2.5.1. Acute Exposure Scenario
2.5.2. Chronic Exposure Scenario
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Collection and Preparation of Samples
4.3. PAs Extraction
4.4. LC-MS/MS Analysis
4.5. Estimated Daily Intake (EDI) of PAs Resulting from the Consumption of Herbal Medicines
4.6. Calculation of MOE
4.7. Actual Life Exposure of Chinese Herbal Medicines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mattocks, A.R. Chemistry and Toxicology of Pyrrolizidine Alkaloids; Academic Press: London, UK, 1986. [Google Scholar]
- Ma, J.; Xia, Q.; Fu, P.P.; Lin, G. Pyrrole-protein adducts—A biomarker of pyrrolizidine alkaloid-induced hepatotoxicity. J. Food Drug Anal. 2018, 26, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Roeder, E. Medicinal plants in Europe containing pyrrolizidine alkaloids. Pharmazie 1995, 50, 83–98. [Google Scholar] [PubMed]
- Stegelmeier, B.L.; Edgar, J.A.; Colegate, S.M.; Gardner, D.R.; Schoch, T.K.; Coulombe, R.A.; Molyneux, R.J. Pyrrolizidine alkaloid plants, metabolism and toxicity. J. Nat. Toxins 1999, 8, 95–116. [Google Scholar] [PubMed]
- Haines, J.A. International programme on chemical safety. Lancet 1996, 348, 408–409. [Google Scholar] [CrossRef]
- Schramm, S.; Köhler, N.; Rozhon, W. Pyrrolizidine Alkaloids: Biosynthesis, Biological Activities and Occurrence in Crop Plants. Molecules 2019, 24, 498. [Google Scholar] [CrossRef] [PubMed]
- Geburek, I.; Preiss-Weigert, A.; Lahrssen-Wiederholt, M.; Schrenk, D.; These, A. In vitro metabolism of pyrrolizidine alkaloids—Metabolic degradation and GSH conjugate formation of different structure types. Food Chem. Toxicol. 2020, 135, 110868. [Google Scholar] [CrossRef] [PubMed]
- Kempf, M.; Beuerle, T.; Bühringer, M.; Denner, M.; Trost, D.; von der Ohe, K.; Bhavanam, V.B.; Schreier, P. Pyrrolizidine alkaloids in honey: Risk analysis by gas chromatography-mass spectrometry. Mol. Nutr. Food Res. 2008, 52, 1193–1200. [Google Scholar] [CrossRef]
- Moreira, R.; Pereira, D.M.; Valentão, P.; Andrade, P.B. Pyrrolizidine Alkaloids: Chemistry, Pharmacology, Toxicology and Food Safety. Int. J. Mol. Sci. 2018, 19, 1668. [Google Scholar] [CrossRef]
- Cheeke, P.R. Toxicity and metabolism of pyrrolizidine alkaloids. J. Anim. Sci. 1988, 66, 2343–2350. [Google Scholar] [CrossRef]
- Bensaude, R.J.; Monegier du Sorbier, C.; Jonville-Bera, A.P.; Autret, E.; Ouyahya, F.; Metman, E.H. Veno-occlusive disease after prolonged treatment with senecionine (Hemoluol). Gastroenterol. Clin. Biol. 1998, 22, 363–364. [Google Scholar]
- Dai, N.; Yu, Y.C.; Ren, T.H.; Wu, J.G.; Jiang, Y.; Shen, L.G.; Zhang, J. Gynura root induces hepatic veno-occlusive disease: A case report and review of the literature. World J. Gastroenterol. 2007, 13, 1628–1631. [Google Scholar] [CrossRef]
- Ka, A.S.; Michel, G.; Imbert, P.; Diakhaté, I.; Seye, M.N. Hepatic veno-occlusive disease due to dietary toxins in a five-year-old child in Senegal. Med. Trop 2006, 66, 514–515. [Google Scholar]
- Edgar, J.A.; Roeder, E.; Molyneux, R.J. Honey from plants containing pyrrolizidine alkaloids: A potential threat to health. J. Agric. Food Chem. 2002, 50, 2719–2730. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Yang, M.; Fu, P.; Ye, Y.; Lin, G. Metabolic activation of pyrrolizidine alkaloids: Insights into the structural and enzymatic basis. Chem. Res. Toxicol. 2014, 27, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Hsu, I.C.; Allen, J.R.; Chesney, C.F. Identification and toxicological effects of dehydroretronecine, a metabolite of monocrotaline. Proc. Soc. Exp. Biol. Med. 1973, 144, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J. Traditional medicine: A culture in the balance. Nature 2007, 448, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Sagi, S.; Wang, Y.H.; Zweigenbaum, J.; Wang, M.; Khan, I.A. Characterization and screening of pyrrolizidine alkaloids and N-oxides from botanicals and dietary supplements using UHPLC-high resolution mass spectrometry. Food Chem. 2015, 178, 136–148. [Google Scholar] [CrossRef]
- Bodi, D.; Ronczka, S.; Gottschalk, C.; Behr, N.; Skibba, A.; Wagner, M.; Lahrssen-Wiederholt, M.; Preiss-Weigert, A.; These, A. Determination of pyrrolizidine alkaloids in tea, herbal drugs and honey. Food Addit. Contam. A 2014, 31, 1886–1895. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Chen, H.W.; Lee, C.C.; He, B.J.; Yang, Y.C. Hepatotoxic pyrrolizidine alkaloids in Emilia sonchifolia from Taiwan. J. Food Compos. Anal. 2015, 42, 1–7. [Google Scholar] [CrossRef]
- Kaczyński, P.; Łozowicka, B. A novel approach for fast and simple determination pyrrolizidine alkaloids in herbs by ultrasound-assisted dispersive solid phase extraction method coupled to liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2020, 187, 113351. [Google Scholar] [CrossRef]
- Luo, Z.; Li, X.; Wang, L.; Chang, C.; Fu, Q. Development of UPLC-Q-TOF-MS Coupled with Cation-exchange Solid-phase Extraction Method for the Determination of Ten Pyrrolizidine Alkaloids in Herbal Medicines. Anal. Sci. 2019, 35, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- CONTAM. Scientific Opinion on Pyrrolizidine alkaloids in food and feed. EFSA J. 2011, 9, 2406. [Google Scholar] [CrossRef]
- Chan, P. NTP technical report on the toxicity studies of Riddelliine (CAS No. 23246-96-0) Administered by Gavage to F344 Rats and B6C3F1 Mice. Toxic. Rep. Ser. 1993, 27, 1–d9. [Google Scholar] [PubMed]
- Kaltner, F.; Rychlik, M.; Gareis, M.; Gottschalk, C. Occurrence and Risk Assessment of Pyrrolizidine Alkaloids in Spices and Culinary Herbs from Various Geographical Origins. Toxins 2020, 12, 155. [Google Scholar] [CrossRef]
- Picron, J.F.; Herman, M.; Van Hoeck, E.; Goscinny, S. Analytical strategies for the determination of pyrrolizidine alkaloids in plant based food and examination of the transfer rate during the infusion process. Food Chem. 2018, 266, 514–523. [Google Scholar] [CrossRef]
- Merz, K.H.; Schrenk, D. Interim relative potency factors for the toxicological risk assessment of pyrrolizidine alkaloids in food and herbal medicines. Toxicol. Lett. 2016, 263, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl Kraupp, B. Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA J. 2017, 15, e04908. [Google Scholar]
- WHO. Pyrrolizidine alkaloids health and safety guide. In Proceedings of World Health Organization for the International Programme on Chemical Safety; WHO: Geneva, Switzerland, 1989. [Google Scholar]
- Suparmi, S.; Mulder, P.P.J.; Rietjens, I. Detection of pyrrolizidine alkaloids in jamu available on the Indonesian market and accompanying safety assessment for human consumption. Food Chem. Toxicol. 2020, 138, 111230. [Google Scholar] [CrossRef]
- EFSA. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA J. 2012, 10, 2579. [Google Scholar]
- Tang, J.; Hattori, M. Pyrrolizidine alkaloids-containing Chinese medicines in the Chinese pharmacopoeia and related safety concerns. Yao Xue Xue Bao Acta Pharm. Sin. 2011, 46, 762–772. [Google Scholar]
- Wu, Q.Z.; Zhao, D.X.; Xiang, J.; Zhang, M.; Zhang, C.F.; Xu, X.H. Antitussive, expectorant, and anti-inflammatory activities of four caffeoylquinic acids isolated from Tussilago farfara. Pharm. Biol. 2016, 54, 1117–1124. [Google Scholar] [CrossRef]
- Lin, G.; Wang, J.Y.; Li, N.; Li, M.; Gao, H.; Ji, Y.; Zhang, F.; Wang, H.; Zhou, Y.; Ye, Y.; et al. Hepatic sinusoidal obstruction syndrome associated with consumption of Gynura segetum. J. Hepatol. 2011, 54, 666–673. [Google Scholar] [CrossRef]
- Ruan, J.; Gao, H.; Li, N.; Xue, J.; Chen, J.; Ke, C.; Ye, Y.; Fu, P.P.; Zheng, J.; Wang, J.; et al. Blood Pyrrole-Protein Adducts--A Biomarker of Pyrrolizidine Alkaloid-Induced Liver Injury in Humans. J. Environ. Sci. Health C 2015, 33, 404–421. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mulder, P.P.J.; Louisse, J.; Peijnenburg, A.; Wesseling, S.; Rietjens, I. Risk assessment for pyrrolizidine alkaloids detected in (herbal) teas and plant food supplements. Regul. Toxicol. Pharmacol. 2017, 86, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Louisse, J.; Rijkers, D.; Stoopen, G.; Holleboom, W.J.; Delagrange, M.; Molthof, E.; Mulder, P.P.J.; Hoogenboom, R.; Audebert, M.; Peijnenburg, A. Determination of genotoxic potencies of pyrrolizidine alkaloids in HepaRG cells using the γH2AX assay. Food Chem. Toxicol. 2019, 131, 110532. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mulder, P.P.J.; Peijnenburg, A.; Rietjens, I. Risk assessment of intake of pyrrolizidine alkaloids from herbal teas and medicines following realistic exposure scenarios. Food Chem. Toxicol. 2019, 130, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Lehotay, S.J.; Geis-Asteggiante, L. Variability of matrix effects in liquid and gas chromatography-mass spectrometry analysis of pesticide residues after QuEChERS sample preparation of different food crops. J. Chromatogr. A 2012, 1270, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Frenich, A.G.; Romero-González, R.; Gómez-Pérez, M.L.; Vidal, J.L. Multi-mycotoxin analysis in eggs using a QuEChERS-based extraction procedure and ultra-high-pressure liquid chromatography coupled to triple quadrupole mass spectrometry. J. Chromatogr. A 2011, 1218, 4349–4356. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fan, Y.; He, W.; Hu, D.; Wu, A.; Wu, W. Development and Application of a QuEChERS-Based Liquid Chromatography Tandem Mass Spectrometry Method to Quantitate Multi-Component Alternaria Toxins in Jujube. Toxins 2018, 10, 382. [Google Scholar] [CrossRef]
- COT. COT statement on pyrrolizidine alkaloids in food. 2008, p. 6. Available online: https://cot.food.gov.uk/sites/default/files/cot/cotstatementpa200806.pdf (accessed on 1 March 2021).
- BfR. Updated risk assessment on levels of 1,2-unsaturated pyrrolizidine alkaloids (PAs) in foods. Available online: https://www.bfr.bund.de/cm/349/updated-risk-assessment-on-levels-of-1-2-unsaturated-pyrrolizidine-alkaloids-pas-in-foods.pdf (accessed on 1 March 2021).
- HMPC. Public Statement on Contamination of Herbal Medicinal products/Traditional Herbal Medicinal Products with Pyrrolizidine Alkaloids Transitional Recommendations for Risk Management and Quality Control. Available online: https://www.ema.europa.eu/en/documents/public-statement/public-statement-contamination-herbal-medicinal-products/traditional-herbal-medicinal-products-pyrrolizidine-alkaloids_en.pdf (accessed on 1 March 2021).
- EFSA. Opinion of the Scientific Committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA J. 2005, 282, 1–31. [Google Scholar]
- Doull, J.; Rozman, K.K. Using Haber’s law to define the margin of exposure. Toxicology 2000, 149, 1–2. [Google Scholar] [CrossRef]
| PAs | P. frutescens | Average | R. rosea | Average | S. oblata | Average | C. sinensis | Average | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbr. | 50 μg/kg | 100 μg/kg | 200 μg/kg | 50 μg/kg | 100 g/kg | 200 μg/kg | 50 μg/kg | 100 μg/kg | 200 μg/kg | 50 μg/kg | 100 μg/kg | 200 μg/kg | ||||
| Ret | 52.96 | 50.23 | 44.33 | 49.17 | 34.05 | 31.48 | 28.58 | 31.37 | 33.80 | 32.45 | 28.92 | 31.73 | 28.29 | 25.93 | 22.58 | 25.60 |
| Mc | 87.51 | 81.26 | 76.29 | 81.69 | 93.52 | 83.99 | 81.09 | 86.20 | 80.36 | 77.07 | 73.45 | 76.96 | 91.93 | 87.35 | 83.57 | 87.62 |
| Er | 92.22 | 82.64 | 79.19 | 84.68 | 92.24 | 83.75 | 80.78 | 85.59 | 82.58 | 77.49 | 75.82 | 78.63 | 95.34 | 89.42 | 86.64 | 90.47 |
| McNO | 93.11 | 87.48 | 84.56 | 88.39 | 99.22 | 92.92 | 93.90 | 95.35 | 91.41 | 88.53 | 85.60 | 88.51 | 95.78 | 92.95 | 90.91 | 93.21 |
| ErNO | 93.30 | 88.68 | 81.93 | 87.97 | 99.11 | 94.69 | 91.74 | 95.18 | 92.86 | 88.15 | 84.76 | 88.59 | 97.35 | 93.44 | 91.38 | 94.06 |
| Jb | 95.29 | 89.09 | 84.03 | 89.47 | 95.34 | 87.42 | 83.72 | 88.83 | 87.94 | 82.76 | 80.34 | 83.68 | 96.02 | 90.20 | 87.06 | 91.09 |
| Eu | 116.50 | 109.30 | 102.67 | 109.49 | 106.92 | 98.97 | 98.63 | 101.51 | 123.26 | 114.61 | 111.40 | 116.42 | 115.77 | 108.56 | 104.45 | 109.59 |
| Im | 104.19 | 96.34 | 91.69 | 97.41 | 100.51 | 91.80 | 87.10 | 93.14 | 101.71 | 95.22 | 94.75 | 97.23 | 101.15 | 94.47 | 93.20 | 96.27 |
| Ly | 112.08 | 99.29 | 91.32 | 100.90 | 118.46 | 102.36 | 97.05 | 105.96 | 119.29 | 108.72 | 107.15 | 111.72 | 117.58 | 105.60 | 104.14 | 109.11 |
| JbNO | 92.05 | 86.68 | 83.15 | 87.29 | 95.84 | 91.72 | 93.77 | 93.77 | 90.24 | 87.77 | 86.67 | 88.23 | 99.58 | 93.67 | 93.10 | 95.45 |
| EuNO | 113.80 | 106.74 | 102.79 | 107.77 | 112.71 | 103.62 | 106.20 | 107.51 | 111.69 | 108.06 | 106.05 | 108.60 | 111.70 | 103.73 | 105.91 | 107.11 |
| ImNO | 136.31 | 125.89 | 120.85 | 127.68 | 127.33 | 115.07 | 119.21 | 120.54 | 133.24 | 122.01 | 123.50 | 126.25 | 129.96 | 116.92 | 120.13 | 122.34 |
| LyNO | 114.13 | 106.84 | 103.62 | 108.20 | 111.30 | 105.04 | 108.91 | 108.41 | 112.67 | 106.88 | 106.47 | 108.67 | 114.17 | 106.61 | 107.32 | 109.37 |
| Re | 88.24 | 85.83 | 81.80 | 85.29 | 98.16 | 92.80 | 87.90 | 92.95 | 96.13 | 94.37 | 91.86 | 94.12 | 99.43 | 96.15 | 93.41 | 96.33 |
| ReNO | 97.58 | 92.65 | 88.85 | 93.03 | 101.48 | 96.09 | 98.72 | 98.76 | 101.12 | 93.88 | 94.61 | 96.53 | 103.04 | 97.81 | 97.75 | 99.53 |
| Td | 98.47 | 93.47 | 87.79 | 93.25 | 99.88 | 92.35 | 92.91 | 95.05 | 96.87 | 93.51 | 92.58 | 94.32 | 98.56 | 94.97 | 94.82 | 96.12 |
| Sp | 100.76 | 94.30 | 88.73 | 94.60 | 101.32 | 91.52 | 87.85 | 93.57 | 102.39 | 93.53 | 94.67 | 96.86 | 103.53 | 95.24 | 93.72 | 97.50 |
| 7-Im | 108.18 | 101.53 | 94.91 | 101.54 | 107.08 | 98.40 | 96.02 | 100.50 | 107.56 | 104.56 | 100.25 | 104.12 | 103.72 | 100.51 | 96.29 | 100.17 |
| He | 109.93 | 102.09 | 97.39 | 103.14 | 107.59 | 98.34 | 96.99 | 100.97 | 108.75 | 103.93 | 100.47 | 104.39 | 106.52 | 101.40 | 98.85 | 102.26 |
| 7-Ly | 111.21 | 100.62 | 96.59 | 102.81 | 107.41 | 96.70 | 96.79 | 100.30 | 111.66 | 101.03 | 98.92 | 103.87 | 107.92 | 99.05 | 97.60 | 101.52 |
| SpNO | 99.31 | 95.89 | 91.65 | 95.62 | 100.17 | 94.36 | 98.88 | 97.80 | 100.08 | 95.67 | 95.34 | 97.03 | 102.86 | 97.71 | 99.56 | 100.04 |
| 7ImNO | 80.81 | 77.37 | 75.52 | 77.90 | 89.81 | 86.90 | 88.52 | 88.41 | 83.31 | 81.43 | 79.87 | 81.54 | 88.80 | 87.43 | 85.12 | 87.12 |
| HeNO | 105.60 | 97.82 | 95.78 | 99.74 | 106.34 | 96.47 | 102.04 | 101.62 | 104.15 | 99.02 | 99.01 | 100.73 | 104.00 | 98.86 | 98.52 | 100.46 |
| Sv | 100.08 | 93.29 | 87.62 | 93.66 | 99.35 | 89.16 | 85.48 | 91.33 | 99.25 | 93.91 | 92.12 | 95.09 | 99.89 | 91.94 | 91.49 | 94.44 |
| Sn | 101.29 | 96.03 | 90.98 | 96.10 | 101.42 | 92.35 | 87.74 | 93.84 | 100.07 | 94.97 | 93.77 | 96.27 | 100.19 | 95.83 | 95.42 | 97.15 |
| SvNO | 104.14 | 97.65 | 94.73 | 98.84 | 105.24 | 99.60 | 102.36 | 102.40 | 104.85 | 99.38 | 96.48 | 100.24 | 107.33 | 99.20 | 99.17 | 101.90 |
| SnNO | 103.66 | 96.53 | 94.35 | 98.18 | 105.18 | 99.56 | 101.74 | 102.16 | 103.00 | 95.50 | 95.41 | 97.97 | 101.84 | 99.27 | 99.26 | 100.12 |
| Em | 107.55 | 101.47 | 99.18 | 102.73 | 105.32 | 99.30 | 98.47 | 101.03 | 106.05 | 102.22 | 100.38 | 102.88 | 105.90 | 100.02 | 98.84 | 101.59 |
| EmNO | 108.87 | 107.19 | 113.70 | 109.92 | 111.86 | 113.25 | 129.58 | 118.23 | 112.14 | 111.47 | 116.28 | 113.29 | 113.34 | 113.89 | 125.30 | 117.51 |
| Sk | 105.56 | 100.38 | 95.60 | 100.51 | 105.89 | 100.33 | 104.05 | 103.42 | 105.62 | 101.63 | 99.82 | 102.36 | 106.31 | 101.57 | 101.08 | 102.99 |
| Lc | 92.85 | 88.45 | 90.21 | 90.50 | 94.69 | 89.25 | 93.62 | 92.52 | 89.74 | 87.28 | 91.01 | 89.34 | 97.76 | 93.19 | 95.74 | 95.56 |
| LcNO | 90.74 | 85.54 | 81.06 | 85.78 | 94.34 | 88.08 | 90.09 | 90.84 | 85.59 | 81.88 | 81.63 | 83.03 | 96.76 | 90.92 | 92.21 | 93.30 |
| Ret | 52.96 | 50.23 | 44.33 | 49.17 | 34.05 | 31.48 | 28.58 | 31.37 | 33.80 | 32.45 | 28.92 | 31.73 | 28.29 | 25.93 | 22.58 | 25.60 |
| Mc | 87.51 | 81.26 | 76.29 | 81.69 | 93.52 | 83.99 | 81.09 | 86.20 | 80.36 | 77.07 | 73.45 | 76.96 | 91.93 | 87.35 | 83.57 | 87.62 |
| Sample ID | Herbal Samples | Numbers of PAs Detected and Top Three | Total PAs Content (μg/kg) | Recommended Maximum Daily Use (g) | PAs Maximum Daily Consumption (g) | EDI (μg/kg bw/ Day) | MOE Values (Life Long Time) | MOE Values (Shorter Exposure) |
|---|---|---|---|---|---|---|---|---|
| HM-1 | Arnebia euchroma | 7 (LyNO, ImNO, EmNO) | 25567.4 | 10 | 255.6 | 3.652 | 64.8 | 1687.0 |
| HM-2 | Tussilago farfara | 10 (Sk, SnNO, Sn) | 17600.2 | 10 | 176.0 | 2.514 | 94.2 | 2450.7 |
| HM-3 | Eupatorium fortunei | 5 (Im, Ly, LyNO) | 16943.6 | 10 | 169.4 | 2.420 | 97.9 | 2545.7 |
| HM-4 | Eupatorium lindleyanum | 5 (LyNO, Im, Ly) | 1863.6 | 60 | 111.8 | 1.597 | 148.3 | 3857.4 |
| HM-5 | Senecio scandens | 12 (Sk, SnNO, SpNO) | 229.0 | 30 | 6.8 | 0.098 | 2414.5 | 62,777.8 |
| HM-6 | Laggera pterodonta | 9 (Im, ImNO, JbNO) | 198.5 | 15 | 2.9 | 0.042 | 5569.5 | 144,808.1 |
| HM-7 | Artemisia scoparia | 6 (Im, Ly, LyNO) | 233.6 | 10 | 3.5 | 0.033 | 7100.9 | 184,625.2 |
| HM-8 | Cassia angustifolia | 9 (Mc, McNO, Td) | 220.6 | 6 | 1.3 | 0.018 | 12,528.8 | 325,751.0 |
| HM-9 | Euphorbia hirta | 6 (Im, ImNO, LyNO) | 121.9 | 9 | 1.0 | 0.015 | 15,121.6 | 393,163.7 |
| HM-10 | Gynura japonica | 6 (SnNO, SpNO, Sk) | 531.0 | 6 | 3.1 | 0.045 | 5206.7 | 135,375.7 |
| HM-11 | Scutellaria barbata | 5 (LyNO, Ly, ImNO) | 61.7 | 30 | 1.8 | 0.026 | 8958.3 | 232,917.5 |
| HM-12 | Euphorbia humifusa | 6 (LyNO, Ly, ImNO) | 45.0 | 20 | 0.9 | 0.012 | 18,421.0 | 478,947.3 |
| HM-13 | Picria fel-terrae | 6 (LyNO, Ly, Im) | 58.2 | 15 | 0.8 | 0.012 | 18,983.8 | 493,580.5 |
| HM-14 | Cirsium japonicum | 7 (Im, Sk, LyNO) | 44.6 | 15 | 0.6 | 0.009 | 24,798.2 | 644,753.3 |
| HM-15 | Abrus cantoniensis | 6 (LyNO, Ly, ImNO) | 22.1 | 30 | 0.6 | 0.009 | 25,067.9 | 651,767.9 |
| HM-16 | Equisetum hyemale | 6 (LyNO, Ly, ImNO) | 66.6 | 9 | 0.5 | 0.008 | 27,677.6 | 719,619.6 |
| HM-17 | Apocynum venetum | 5(LyNO, Ly, ImNO) | 49.3 | 12 | 0.5 | 0.008 | 28,071.0 | 729,847.7 |
| HM-18 | Siphonostegia chinensis | 13 (Im, ImNO, LyNO) | 51.8 | 9 | 0.4 | 0.006 | 35,530.7 | 923,798.5 |
| HM-19 | Achillea alpina | 4 (Sk, Im, Ly) | 51.9 | 45 | 2.3 | 0.033 | 7103.4 | 184,688.5 |
| HM-20 | Phryma leptostachya | 6 (LyNO, Ly, ImNO) | 45.7 | 15 | 0.6 | 0.009 | 24,179.6 | 628,671.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, M.; Chen, L.; Qiao, Y.; Ma, S.; Sun, D.; Si, J.; Liao, Y. Determination of Toxic Pyrrolizidine Alkaloids in Traditional Chinese Herbal Medicines by UPLC-MS/MS and Accompanying Risk Assessment for Human Health. Molecules 2021, 26, 1648. https://doi.org/10.3390/molecules26061648
Wang J, Zhang M, Chen L, Qiao Y, Ma S, Sun D, Si J, Liao Y. Determination of Toxic Pyrrolizidine Alkaloids in Traditional Chinese Herbal Medicines by UPLC-MS/MS and Accompanying Risk Assessment for Human Health. Molecules. 2021; 26(6):1648. https://doi.org/10.3390/molecules26061648
Chicago/Turabian StyleWang, Junchi, Meng Zhang, Lihua Chen, Yue Qiao, Siqi Ma, Dian Sun, Jianyong Si, and Yonghong Liao. 2021. "Determination of Toxic Pyrrolizidine Alkaloids in Traditional Chinese Herbal Medicines by UPLC-MS/MS and Accompanying Risk Assessment for Human Health" Molecules 26, no. 6: 1648. https://doi.org/10.3390/molecules26061648
APA StyleWang, J., Zhang, M., Chen, L., Qiao, Y., Ma, S., Sun, D., Si, J., & Liao, Y. (2021). Determination of Toxic Pyrrolizidine Alkaloids in Traditional Chinese Herbal Medicines by UPLC-MS/MS and Accompanying Risk Assessment for Human Health. Molecules, 26(6), 1648. https://doi.org/10.3390/molecules26061648







