Yeast Synthetic Biology for the Production of Lycium barbarum Polysaccharides
Abstract
1. Introduction
2. LBP Biosynthetic Pathway in L. barbarum
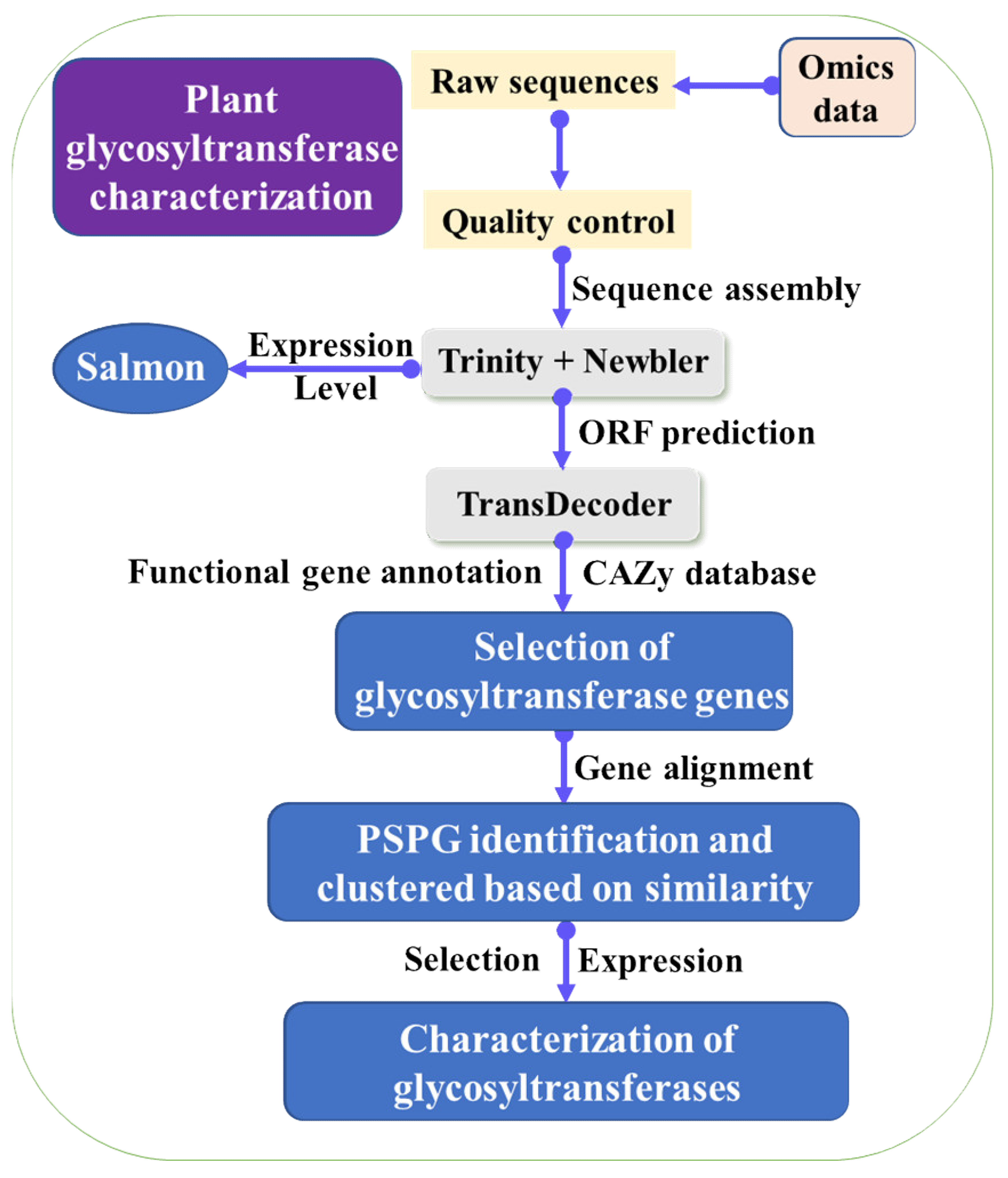
3. Mining of Key Enzymes for LBP Biosynthesis
4. S. cerevisiae is an Efficient Cell Factory for the Production of Plant Natural Products
5. Engineering Strategies for High-Level LBP Production in S. cerevisiae
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium Barbarum: A Traditional Chinese Herb and A Promising Anti-Aging Agent. Aging Dis. 2017, 8, 778–791. [Google Scholar] [CrossRef]
- Liang, B.; Jin, M.; Liu, H. Water-soluble polysaccharide from dried Lycium barbarum fruits: Isolation, structural features and antioxidant activity. Carbohydr. Polym. 2011, 83, 1947–1951. [Google Scholar] [CrossRef]
- Wetters, S.; Horn, T.; Nick, P. Goji Who? Morphological and DNA Based Authentication of a "Superfood". Front Plant Sci. 2018, 9, 1859. [Google Scholar] [CrossRef]
- Kwok, S.S.; Bu, Y.; Lo, A.C.; Chan, T.C.; So, K.F.; Lai, J.S.; Shih, K.C. A Systematic Review of Potential Therapeutic Use of Lycium Barbarum Polysaccharides in Disease. Biomed Res. Int. 2019, 2019, 4615745. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.W.; Zhang, Y.; Hayford, F.; Wang, L.; Ma, T.; Dong, Z.; Zhang, Y.; et al. Goji Berries as a Potential Natural Antioxidant Medicine: An Insight into Their Molecular Mechanisms of Action. Oxidative Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Avila, C.N.; Uecker, J.N.; Ribeiro Trindade, F.M.; Alvarado Rincon, J.A.; Penteado, J.O.; de Barros, C.C.; Janke, F.; Andreazza, R.; Schneider, J.P.; Pieniz, S. Anti-inflammatory Effect of a Goji Berry Extract (Lycium barbarum) in Rats Subjected to Inflammation by Lipopolysaccharides (LPS). Braz. Arch. Biol. Technol. 2020, 63. [Google Scholar] [CrossRef]
- Tang, W.M.; Chan, E.; Kwok, C.Y.; Lee, Y.K.; Wu, J.H.; Wan, C.W.; Chan, R.Y.; Yu, P.H.; Chan, S.W. A review of the anticancer and immunomodulatory effects of Lycium barbarum fruit. Inflammopharmacology 2012, 20, 307–314. [Google Scholar] [CrossRef]
- Xia, G.; Xin, N.; Liu, W.; Yao, H.; Hou, Y.; Qi, J. Inhibitory effect of Lycium barbarum polysaccharides on cell apoptosis and senescence is potentially mediated by the p53 signaling pathway. Mol. Med. Rep. 2014, 9, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Liu, F.; Zuo, P.; Huang, G.; Song, Z.; Wang, T.; Lu, H.; Guo, F.; Han, C.; Sun, G. Practical Application of Antidiabetic Efficacy of Lycium barbarum Polysaccharide in Patients with Type 2 Diabetes. Med. Chem. 2015, 11, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Cai, Y.; Yan, J.; Sun, M.; Corke, H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004, 76, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Skenderidis, P.; Lampakis, D.; Giavasis, I.; Leontopoulos, S.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Chemical Properties, Fatty-Acid Composition, and Antioxidant Activity of Goji Berry (Lycium barbarum L. and Lycium chinense Mill.) Fruits. Antioxidants 2019, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.-Q.; Zheng, Z.-Y.; Xu, X.; Hu, Z.-H. Variation in fruit sugar composition of Lycium barbarum L. and Lycium chinense Mill. of different regions and varieties. Biochem. Syst. Ecol. 2010, 38, 275–284. [Google Scholar] [CrossRef]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium barbarum polysaccharides: Extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef]
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, Structural Characterization, and Biological Functions of Lycium Barbarum Polysaccharides: A Review. Biomol. 2019, 9, 389. [Google Scholar] [CrossRef]
- Zhang, Q.; Lv, X.; Wu, T.; Ma, Q.; Teng, A.; Zhang, Y.; Zhang, M. Composition of Lycium barbarum polysaccharides and their apoptosis-inducing effect on human hepatoma SMMC-7721 cells. Food Nutr. Res. 2015, 59, 28696. [Google Scholar] [CrossRef]
- Wang, W.-F.; Yang, J.-L.; Shi, Y.-P. Quality evaluation of six bioactive constituents in goji berry based on capillary electrophoresis field amplified sample stacking. Electrophoresis 2018, 39, 2117–2124. [Google Scholar] [CrossRef]
- Zhou, Y.; Lai, Y.; Chen, Z.; Qu, H.; Ma, S.; Wang, Y.; Jiang, Y. Evolution of physiological characteristics and nutritional quality in fresh goji berry (Lycium barbarum) stored under different temperatures. J. Food Process. Preserv. 2020, 44, e14835. [Google Scholar] [CrossRef]
- Song, H.H.; Bi, J.F.; Chen, Q.Q.; Zhou, M.; Wu, X.Y.; Song, J.X. Structural and health functionality of dried goji berries as affected by coupled dewaxing pre-treatment and hybrid drying methods. Int. J. Food Prop. 2018, 21, 2527–2538. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. Aims Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Schmid, J.; Sieber, V. Enzymatic transformations involved in the biosynthesis of microbial exo-polysaccharides based on the assembly of repeat units. Chembiochem 2015, 16, 1141–1147. [Google Scholar] [CrossRef]
- Wei, Y.; Gossing, M.; Bergenholm, D.; Siewers, V.; Nielsen, J. Increasing cocoa butter-like lipid production of Saccharomyces cerevisiae by expression of selected cocoa genes. Amb Express 2017, 7, 34. [Google Scholar] [CrossRef]
- Wei, Y.; Siewers, V.; Nielsen, J. Cocoa butter-like lipid production ability of non-oleaginous and oleaginous yeasts under nitrogen-limited culture conditions. Appl. Microbiol. Biotechnol. 2017, 101, 3577–3585. [Google Scholar] [CrossRef]
- Bergenholm, D.; Gossing, M.; Wei, Y.; Siewers, V.; Nielsen, J. Modulation of saturation and chain length of fatty acids in Saccharomyces cerevisiae for production of cocoa butter-like lipids. Biotechnol. Bioeng. 2018, 115, 932–942. [Google Scholar] [CrossRef]
- Wei, Y.; Bergenholm, D.; Gossing, M.; Siewers, V.; Nielsen, J. Expression of cocoa genes in Saccharomyces cerevisiae improves cocoa butter production. Microb. Cell Factories 2018, 17, 11. [Google Scholar] [CrossRef]
- Wei, Y.; Ji, B.; Siewers, V.; Xu, D.; Halkier, B.A.; Nielsen, J. Identification of genes involved in shea butter biosynthesis from Vitellaria paradoxa fruits through transcriptomics and functional heterologous expression. Appl. Microbiol. Biotechnol. 2019, 103, 3727–3736. [Google Scholar] [CrossRef]
- Yan, X.; Fan, Y.; Wei, W.; Wang, P.; Liu, Q.; Wei, Y.; Zhang, L.; Zhao, G.; Yue, J.; Zhou, Z. Production of bioactive ginsenoside compound K in metabolically engineered yeast. Cell Res. 2014, 24, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wei, Y.; Fan, Y.; Liu, Q.; Wei, W.; Yang, C.; Zhang, L.; Zhao, G.; Yue, J.; Yan, X.; et al. Production of bioactive ginsenosides Rh2 and Rg3 by metabolically engineered yeasts. Metab. Eng. 2015, 29, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wang, P.; Wei, Y.; Liu, Q.; Yang, C.; Zhao, G.; Yue, J.; Yan, X.; Zhou, Z. Characterization of Panax ginseng UDP-glycosyltransferases catalyzing protopanaxatriol and biosyntheses of bioactive ginsenosides F1 and Rh1 in metabolically engineered yeasts. Mol. Plant. 2015, 8, 1412–1424. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, C.; Wei, W.; Wei, Y.; Liu, Q.; Zhao, G.; Yue, J.; Yan, X.; Wang, P.; Zhou, Z. The unprecedented diversity of UGT94-family UDP-glycosyltransferases in Panax plants and their contribution to ginsenoside biosynthesis. Sci. Rep. 2020, 10, 15394. [Google Scholar] [CrossRef] [PubMed]
- Orlean, P. Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics 2012, 192, 775–818. [Google Scholar] [CrossRef] [PubMed]
- Bzducha-Wróbel, A.; Błażejak, S.; Kieliszek, M.; Pobiega, K.; Falana, K.; Janowicz, M. Modification of the cell wall structure of Saccharomyces cerevisiae strains during cultivation on waste potato juice water and glycerol towards biosynthesis of functional polysaccharides. J. Biotechnol. 2018, 281, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, Y.B.; Jiang, Y.; Prasad, K.N.; Yang, J.; Qu, H.; Wang, Y.; Jia, Y.; Mo, H.; Yang, B. Structure identification of a polysaccharide purified from Lycium barbarium fruit. Int. J. Biol. Macromol. 2016, 82, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, Y.; Zhu, R.; Yu, J.; Lu, W.; Pan, C.; Yao, W.; Gao, X. Structure characterization, chemical and enzymatic degradation, and chain conformation of an acidic polysaccharide from Lycium barbarum L. Carbohydr. Polym. 2016, 147, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Mestrom, L.; Przypis, M.; Kowalczykiewicz, D.; Pollender, A.; Kumpf, A.; Marsden, S.R.; Bento, I.; Jarzębski, A.B.; Szymańska, K.; Chruściel, A.; et al. Leloir Glycosyltransferases in Applied Biocatalysis: A Multidisciplinary Approach. Int. J. Mol. Sci. 2019, 20, 5263. [Google Scholar] [CrossRef]
- Ma, Y.; Reddy, V.R.; Devi, M.J.; Song, L.; Cao, B. De novo characterization of the Goji berry (Lycium barbarium L.) fruit transcriptome and analysis of candidate genes involved in sugar metabolism under different CO2 concentrations. Tree Physiol. 2019, 39, 1032–1045. [Google Scholar]
- Chen, J.H.; Zhang, D.Z.; Zhang, C.; Xu, M.L.; Yin, W.L. Physiological characterization, transcriptomic profiling, and microsatellite marker mining of Lycium ruthenicum. J. Zhejiang Univ. Sci. B 2017, 18, 1002–1021. [Google Scholar] [CrossRef]
- Chen, J.W.; Zhang, S.L.; Zhang, L.C. Sugar transport, metabolism, accumulation and their regulation in fruits. J. Plant Physiol. Mol. Biol. 2004, 30, 1–10. [Google Scholar]
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef]
- Stein, O.; Granot, D. An Overview of Sucrose Synthases in Plants. Front Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef]
- Deng, S.; Yao, C.; Zhang, X.; Jia, Z.; Shan, C.; Luo, X.; Lin, L. Involvement of UDP-glucose pyrophosphorylase from Verticillium dahliae in cell morphogenesis, stress responses, and host infection. Fungal Biol. 2020, 124, 648–660. [Google Scholar] [CrossRef]
- Zan, X.Y.; Wu, X.H.; Cui, F.J.; Zhu, H.A.; Sun, W.J.; Jiang, L.H.; Tao, T.L.; Zhao, X. UDP-glucose pyrophosphorylase gene affects mycelia growth and polysaccharide synthesis of Grifola frondosa. Int. J. Biol. Macromol. 2020, 161, 1161–1170. [Google Scholar] [CrossRef]
- Wang, G.; Du, X.; Ji, J.; Guan, C.; Li, Z.; Josine, T.L. De novo characterization of the Lycium chinense Mill. leaf transcriptome and analysis of candidate genes involved in carotenoid biosynthesis. Gene 2015, 555, 458–463. [Google Scholar] [CrossRef]
- Chen, C.; Xu, M.; Wang, C.; Qiao, G.; Wang, W.; Tan, Z.; Wu, T.; Zhang, Z. Characterization of the Lycium barbarum fruit transcriptome and development of EST-SSR markers. PLoS ONE 2017, 12, e0187738. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wei, Y.; Ji, B.; Nielsen, J. Advances in Metabolic Engineering of Saccharomyces cerevisiae for Cocoa Butter Equivalent Production. Front. Bioeng. Biotechnol. 2020, 8, 1194. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.Y.; Seki, H.; Fujisawa, Y.; Shimoda, Y.; Hiraga, S.; Nomura, Y.; Saito, K.; Ishimoto, M.; Muranaka, T. A cellulose synthase-derived enzyme catalyses 3-O-glucuronosylation in saponin biosynthesis. Nat. Commun. 2020, 11, 5664. [Google Scholar] [CrossRef] [PubMed]
- Jozwiak, A.; Sonawane, P.D.; Panda, S.; Garagounis, C.; Papadopoulou, K.K.; Abebie, B.; Massalha, H.; Almekias-Siegl, E.; Scherf, T.; Aharoni, A. Plant terpenoid metabolism co-opts a component of the cell wall biosynthesis machinery. Nat. Chem. Biol. 2020, 16, 740–748. [Google Scholar] [CrossRef]
- Nimpiboon, P.; Tumhom, S.; Nakapong, S.; Pongsawasdi, P. Amylomaltase from Thermus filiformis: Expression in Saccharomyces cerevisiae and its use in starch modification. J. Appl. Microbiol. 2020, 129, 1287–1296. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, L.; Zhang, M.; Wei, Y.; Lin, W. Recent Advances on Feasible Strategies for Monoterpenoid Production in Saccharomyces cerevisiae. Front. Bioeng. Biotechnol. 2020, 8, 1372. [Google Scholar] [CrossRef]
- Guan, R.; Wang, M.; Guan, Z.; Jin, C.-Y.; Lin, W.; Ji, X.; Wei, Y. Metabolic engineering for glycyrrhetinic acid production in Saccharomyces cerevisiae. Front. Bioeng. Biotechnol. 2020, 8, 1318. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Huang, M.; Chen, Y.; Siewers, V.; Nielsen, J. Global rewiring of cellular metabolism renders Saccharomyces cerevisiae Crabtree negative. Nat. Commun. 2018, 9, 3059. [Google Scholar] [CrossRef]
- Mans, R.; van Rossum, H.M.; Wijsman, M.; Backx, A.; Kuijpers, N.G.; van den Broek, M.; Daran-Lapujade, P.; Pronk, J.T.; van Maris, A.J.; Daran, J.M. CRISPR/Cas9: A molecular Swiss army knife for simultaneous introduction of multiple genetic modifications in Saccharomyces cerevisiae. Fems Yeast Res. 2015, 15. [Google Scholar] [CrossRef]
- Jigami, Y.; Odani, T. Mannosylphosphate transfer to yeast mannan. Biochim. Biophys. Acta 1999, 1426, 335–345. [Google Scholar] [CrossRef]
- Zhou, X.; He, J.; Wang, L.; Wang, Y.; Du, G.; Kang, Z. Metabolic Engineering of Saccharomyces cerevisiae to Improve Glucan Biosynthesis. J. Microbiol. Biotechnol. 2019, 29, 758–764. [Google Scholar] [CrossRef]
- Ye, S.; Kim, J.W.; Kim, S.R. Metabolic Engineering for Improved Fermentation of L-Arabinose. J. Microbiol. Biotechnol. 2019, 29, 339–346. [Google Scholar] [CrossRef]
- Oka, T.; Jigami, Y. Reconstruction of de novo pathway for synthesis of UDP-glucuronic acid and UDP-xylose from intrinsic UDP-glucose in Saccharomyces cerevisiae. Febs J. 2006, 273, 2645–2657. [Google Scholar] [CrossRef]
- Bahia, F.M.; de Almeida, G.C.; de Andrade, L.P.; Campos, C.G.; Queiroz, L.R.; da Silva, R.L.V.; Abdelnur, P.V.; Corrêa, J.R.; Bettiga, M.; Parachin, N.S. Rhamnolipids production from sucrose by engineered Saccharomyces cerevisiae. Sci. Rep. 2018, 8, 2905. [Google Scholar] [CrossRef] [PubMed]
- van den Brink, J.; Akeroyd, M.; van der Hoeven, R.; Pronk, J.T.; de Winde, J.H.; Daran-Lapujade, P. Energetic limits to metabolic flexibility: Responses of Saccharomyces cerevisiae to glucose-galactose transitions. Microbiology 2009, 155 Pt 4, 1340–1350. [Google Scholar] [CrossRef]
- Lafraya, Á.; Sanz-Aparicio, J.; Polaina, J.; Marín-Navarro, J. Fructo-oligosaccharide synthesis by mutant versions of Saccharomyces cerevisiae invertase. Appl. Env. Microbiol. 2011, 77, 6148. [Google Scholar] [CrossRef] [PubMed]
- Franken, J.; Brandt, B.A.; Tai, S.L.; Bauer, F.F. Biosynthesis of Levan, a Bacterial Extracellular Polysaccharide, in the Yeast Saccharomyces cerevisiae. PLoS ONE 2013, 8, e77499. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Bae, J.H.; Sung, B.H.; Kim, M.J.; Kim, C.H.; Oh, B.R.; Sohn, J.H. Efficient production of levan using a recombinant yeast Saccharomyces cerevisiae hypersecreting a bacterial levansucrase. J. Ind. Microbiol. Biotechnol. 2019, 46, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104 Pt B, 1415–1421. [Google Scholar] [CrossRef]
- Ambardar, S.; Gupta, R.; Trakroo, D.; Lal, R.; Vakhlu, J. High Throughput Sequencing: An Overview of Sequencing Chemistry. Indian J. Microbiol. 2016, 56, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Y.; Tian, J.; Zhu, Y.; Fan, J. Changes in sucrose metabolism in maize varieties with different cadmium sensitivities under cadmium stress. PLoS ONE 2020, 15, e0243835. [Google Scholar] [CrossRef]
- Scossa, F.; Benina, M.; Alseekh, S.; Zhang, Y.; Fernie, A.R. The Integration of Metabolomics and Next-Generation Sequencing Data to Elucidate the Pathways of Natural Product Metabolism in Medicinal Plants. Planta Med. 2018, 84, 855–873. [Google Scholar] [CrossRef]
- Han, R.; Rai, A.; Nakamura, M.; Suzuki, H.; Takahashi, H.; Yamazaki, M.; Saito, K. De Novo Deep Transcriptome Analysis of Medicinal Plants for Gene Discovery in Biosynthesis of Plant Natural Products. Methods Enzym. 2016, 576, 19–45. [Google Scholar]
- Voigt, C.A. Synthetic biology 2020–2030: Six commercially-available products that are changing our world. Nat. Commun. 2020, 11, 6379. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Wang, L.; Wang, M.; Du, R.; Qin, S.; Jin, C.-Y.; Wei, Y. Yeast Synthetic Biology for the Production of Lycium barbarum Polysaccharides. Molecules 2021, 26, 1641. https://doi.org/10.3390/molecules26061641
Peng J, Wang L, Wang M, Du R, Qin S, Jin C-Y, Wei Y. Yeast Synthetic Biology for the Production of Lycium barbarum Polysaccharides. Molecules. 2021; 26(6):1641. https://doi.org/10.3390/molecules26061641
Chicago/Turabian StylePeng, Jinjin, Luan Wang, Mengge Wang, Rui Du, Shangshang Qin, Cheng-Yun Jin, and Yongjun Wei. 2021. "Yeast Synthetic Biology for the Production of Lycium barbarum Polysaccharides" Molecules 26, no. 6: 1641. https://doi.org/10.3390/molecules26061641
APA StylePeng, J., Wang, L., Wang, M., Du, R., Qin, S., Jin, C.-Y., & Wei, Y. (2021). Yeast Synthetic Biology for the Production of Lycium barbarum Polysaccharides. Molecules, 26(6), 1641. https://doi.org/10.3390/molecules26061641






