Remedia Sternutatoria over the Centuries: TRP Mediation
Abstract
1. Introduction
2. Remedia Sternutatoria: Terminology
3. Remedia Sternutatoria: Main Active Components
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Nishino, T. Physiological and pathophysiological implications of upper airway reflexes in humans. Jpn. J. Physiol. 2000, 50, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Macron, J.M.; Wallois, F.; Duron, B. Influence of vagal afferents in the sneeze reflex in cats. Neurosci. Lett. 1994, 177, 79–82. [Google Scholar] [CrossRef]
- Wallois, F.; Macron, J.M.; Duron, B. Activities of vagal receptors in the different phases of sneeze in cats. Respir. Physiol. 1995, 101, 239–255. [Google Scholar] [CrossRef]
- Moran, M.M.; McAlexander, M.A.; Bíró, T.; Szallasi, A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 601–620. [Google Scholar] [CrossRef]
- Frias, B.; Merighi, A. Capsaicin, Nociception and Pain. Molecules 2016, 21, 797. [Google Scholar] [CrossRef]
- Takaya, J.; Mio, K.; Shiraishi, T.; Kurokawa, T.; Otsuka, S.; Mori, Y.; Uesugi, M. A Potent and Site-Selective Agonist of TRPA1. J. Am. Chem. Soc. 2015, 137, 15859–15864. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Armache, J.P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015, 520, 511–517. [Google Scholar] [CrossRef]
- Srinivasan, K. Biological Activities of Red Pepper (Capsicum annuum) and Its Pungent Principle Capsaicin: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1488–1500. [Google Scholar] [CrossRef]
- Brubaker, A.P. The physiology of sneezing. J. Med. Assoc. 1919, 73, 585–587. [Google Scholar] [CrossRef]
- Songu, M.; Cingi, C. Sneeze reflex: Facts and fiction. Ther. Adv. Respir. Dis. 2009, 3, 131–141. [Google Scholar] [CrossRef]
- Askenasy, J.J. The history of sneezing. Postgrad Med. J. 1990, 66, 549–550. [Google Scholar] [CrossRef][Green Version]
- Justus Arnemann, L.A.K. Justus Arnemann’s, Ehemaligen Professors der Medizin zu Göttingen, Chirurgische Arzneimittellehre; Vandenhoeck & Ruprecht: Göttingen, Germany, 1813. [Google Scholar]
- The London Medical Dictionary Vol1 | by Bartholomew Parr. Available online: https://chestofbooks.com/health/reference/London-Medical-Dictionary/index.html (accessed on 1 February 2021).
- Dioscorides, P.; Beck, L.Y. De Materia Medica; Olms-Weidmann: Hildesheim, Germany; New York, NY, USA, 2005. [Google Scholar]
- Amida, A.D. Aetii Medici Graeci Contractae ex Veteribus Medicinae Tetrabiblos; Froben: Basel, Switzerland, 1549. [Google Scholar]
- Pearn, J. Bernard de Gordon (fl. 1270–1330): Medieval physician and teacher. J. Med. Biogr. 2013, 21, 8–11. [Google Scholar] [CrossRef]
- Leonhart, F. Opera: Dispensatorium Perfectum; Feyrabend et Huterus: Frankfurt am Main, Germany, 1567; Volume 1, pp. 399–400. [Google Scholar]
- Di Matteo, B.; Tarabella, V.; Filardo, G.; Viganò, A.; Tomba, P.; Marcacci, M. The Renaissance and the universal surgeon: Giovanni Andrea Della Croce, a master of traumatology. Int. Orthop. 2013, 37, 2523–2528. [Google Scholar] [CrossRef]
- Bencze, J. Franciscus Joel. Gyógyszerészet 1964, 386–388. [Google Scholar] [PubMed]
- Dunn, P.M. Laurent Joubert of Montpellier (1529–82) and his Erreurs Populaires. Arch. Dis. Child. Fetal Neonatal Ed. 2000, 82, F255–F256. [Google Scholar] [CrossRef] [PubMed]
- Encyclopedia.com. Available online: https://www.encyclopedia.com/religion/encyclopedias-almanacs-transcripts-and-maps/zacutus-lusitanus (accessed on 1 February 2021).
- Chalmeteus, A. Enchiridion Chirvrgicvm, Externorvm Morborvm Remedia ... Complectens. Quibus, Morbi Venerei Curandi Methodus; Andreas Wechelus: Frankfurt, Germany, 1560. [Google Scholar]
- Martin Schoock. Available online: https://en.wikipedia.org/wiki/Martin_Schoock (accessed on 1 February 2021).
- The Library. Available online: https://mineralogicalrecord.com/libdetail.asp?id=1512 (accessed on 1 February 2021).
- Justus Arnemann. Available online: https://de.wikipedia.org/wiki/Justus_Arnemann (accessed on 1 February 2021).
- Bartholomew Parr. Available online: https://en.wikipedia.org/wiki/Bartholomew_Parr#:~:text=Dr%20Bartholomew%20Parr%20FRS%20FRSE,British%20physician%20and%20medical%20author (accessed on 1 February 2021).
- Tubbs, R.S.; Gribben, W.B.; Loukas, M.; Shoja, M.M.; Tubbs, K.O.; Oakes, W.J. Franz Kaspar Hesselbach (1759–1816): Anatomist and Surgeon. World J. Surg. 2008, 32, 2527–2529. [Google Scholar] [CrossRef]
- Plantnet. Cuminum cyminum (Jansen, 1981). Available online: https://uses.plantnet-project.org/en/Cuminum_cyminum_(Jansen,_1981) (accessed on 2 February 2021).
- Sowbhagya, H.B. Chemistry, Technology, and Nutraceutical Functions of Cumin (cuminum cyminum L): An Overview. Crit. Rev. Food Sci. Nutr. 2013, 53, 1–10. [Google Scholar] [CrossRef]
- Legrand, C.; Merlini, J.M.; de Senarclens-Bezençon, C.; Michlig, S. New natural agonists of the transient receptor potential Ankyrin 1 (TRPA1) channel. Sci. Rep. 2020, 10, 11238. [Google Scholar] [CrossRef]
- Srinivasan, K. Black pepper and its pungent principle-piperine: A review of diverse physiological effects. Crit. Rev. Food Sci. Nutr. 2007, 47, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-G.; Chae, Y.; Shin, Y.; Kim, Y.-J. Chemical composition and antioxidant capacity of black pepper pericarp. Appl. Biol. Chem. 2020, 63, 35. [Google Scholar] [CrossRef]
- Gevaert, T.; Vandepitte, J.; Hutchings, G.; Vriens, J.; Nilius, B.; De Ridder, D. TRPV1 is involved in stretch-evoked contractile changes in the rat autonomous bladder model: A study with piperine, a new TRPV1 agonist. Neurourol. Urodyn. 2007, 26, 440–450; discussion 451–443. [Google Scholar] [CrossRef]
- Chen, C.Y.; Li, W.; Qu, K.P.; Chen, C.R. Piperine exerts anti-seizure effects via the TRPV1 receptor in mice. Eur. J. Pharmacol. 2013, 714, 288–294. [Google Scholar] [CrossRef]
- Dong, Y.; Yin, Y.; Vu, S.; Yang, F.; Yarov-Yarovoy, V.; Tian, Y.; Zheng, J. A distinct structural mechanism underlies TRPV1 activation by piperine. Biochem. Biophys. Res. Commun. 2019, 516, 365–372. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, J. Understand spiciness: Mechanism of TRPV1 channel activation by capsaicin. Protein Cell 2017, 8, 169–177. [Google Scholar] [CrossRef]
- Khom, S.; Strommer, B.; Schöffmann, A.; Hintersteiner, J.; Baburin, I.; Erker, T.; Schwarz, T.; Schwarzer, C.; Zaugg, J.; Hamburger, M.; et al. GABAA receptor modulation by piperine and a non-TRPV1 activating derivative. Biochem. Pharmacol. 2013, 85, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Okumura, Y.; Narukawa, M.; Iwasaki, Y.; Ishikawa, A.; Matsuda, H.; Yoshikawa, M.; Watanabe, T. Activation of TRPV1 and TRPA1 by black pepper components. Biosci. Biotechnol. Biochem. 2010, 74, 1068–1072. [Google Scholar] [CrossRef]
- Watanabe, T.; Terada, Y. Food Compounds Activating Thermosensitive TRP Channels in Asian Herbal and Medicinal Foods. J. Nutr.Sci. Vitaminol. 2015, 61, S86–S88. [Google Scholar] [CrossRef]
- Jörg, J.C.G. Materialien zu einer künftigen heilmittellehre durch versuche der arzneyen an gesunden menschen gewonnen und gesammelt; C. Cnobloch: Leipzig, Germany, 1825; Volume 1. [Google Scholar]
- Müller-Schwarze, D.; Houlihan, P.W. Pheromonal activity of single castoreum constituents in beaver, Castor canadensis. J. Chem. Ecol. 1991, 17, 715–734. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Chen, X.; Cerne, R.; Syed, S.K.; Ficorilli, J.V.; Cabrera, O.; Obukhov, A.G.; Efanov, A.M. Catechol estrogens stimulate insulin secretion in pancreatic β-cells via activation of the transient receptor potential A1 (TRPA1) channel. J. Biol. Chem. 2019, 294, 2935–2946. [Google Scholar] [CrossRef]
- Lehmann, R.; Schöbel, N.; Hatt, H.; van Thriel, C. The involvement of TRP channels in sensory irritation: A mechanistic approach toward a better understanding of the biological effects of local irritants. Arch. Toxicol. 2016, 90, 1399–1413. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience 1989, 30, 515–520. [Google Scholar] [CrossRef]
- Darmani, N.A.; Henry, D.A.; Zhong, W.; Chebolu, S. Ultra-low doses of the transient receptor potential vanilloid 1 agonist, resiniferatoxin, prevents vomiting evoked by diverse emetogens in the least shrew (Cryptotis parva). Behav. Pharmacol. 2020, 31, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Stampanoni Bassi, M.; Gentile, A.; Iezzi, E.; Zagaglia, S.; Musella, A.; Simonelli, I.; Gilio, L.; Furlan, R.; Finardi, A.; Marfia, G.A.; et al. Transient Receptor Potential Vanilloid 1 Modulates Central Inflammation in Multiple Sclerosis. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lu, S.C.; Hsieh, Y.L. Establishing a Mouse Model of a Pure Small Fiber Neuropathy with the Ultrapotent Agonist of Transient Receptor Potential Vanilloid Type 1. J. Vis. Exp. JoVE 2018, 56651. [Google Scholar] [CrossRef]
- Veryser, L.; Taevernier, L.; Wynendaele, E.; Verheust, Y.; Dumoulin, A.; De Spiegeleer, B. N-alkylamide profiling of Achillea ptarmica and Achillea millefolium extracts by liquid and gas chromatography–mass spectrometry. J. Pharm. Anal. 2017, 7, 34–47. [Google Scholar] [CrossRef]
- Althaus, J.B.; Kaiser, M.; Brun, R.; Schmidt, T.J. Antiprotozoal activity of Achillea ptarmica (Asteraceae) and its main alkamide constituents. Molecules 2014, 19, 6428–6438. [Google Scholar] [CrossRef]
- Walker, J.; Ley, J.P.; Schwerzler, J.; Lieder, B.; Beltran, L.; Ziemba, P.M.; Hatt, H.; Hans, J.; Widder, S.; Krammer, G.E.; et al. Nonivamide, a capsaicin analogue, exhibits anti-inflammatory properties in peripheral blood mononuclear cells and U-937 macrophages. Mol. Nutr. Food Res. 2017, 61, 1600474. [Google Scholar] [CrossRef] [PubMed]
- Lieder, B.; Zaunschirm, M.; Holik, A.K.; Ley, J.P.; Hans, J.; Krammer, G.E.; Somoza, V. The Alkamide trans-Pellitorine Targets PPARγ via TRPV1 and TRPA1 to Reduce Lipid Accumulation in Developing 3T3-L1 Adipocytes. Front. Pharm. 2017, 8, 316. [Google Scholar] [CrossRef]
- Oláh, Z.; Rédei, D.; Pecze, L.; Vizler, C.; Jósvay, K.; Forgó, P.; Winter, Z.; Dombi, G.; Szakonyi, G.; Hohmann, J. Pellitorine, an extract of Tetradium daniellii, is an antagonist of the ion channel TRPV1. Phytomed. Int. J. Phytother. Phytopharmacol. 2017, 34, 44–49. [Google Scholar] [CrossRef]
- Lundblad, L.; Lundberg, J.M.; Anggård, A. Local and systemic capsaicin pretreatment inhibits sneezing and the increase in nasal vascular permeability induced by certain chemical irritants. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1984, 326, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Hsieh, S.-H. Analyses of tobacco alkaloids by cation-selective exhaustive injection sweeping microemulsion electrokinetic chromatography. J. Chromatogr. A 2007, 1164, 313–319. [Google Scholar] [CrossRef]
- Talavera, K.; Gees, M.; Karashima, Y.; Meseguer, V.M.; Vanoirbeek, J.A.J.; Damann, N.; Everaerts, W.; Benoit, M.; Janssens, A.; Vennekens, R.; et al. Nicotine activates the chemosensory cation channel TRPA1. Nat. Neurosci. 2009, 12, 1293–1299. [Google Scholar] [CrossRef]
- Chung, S.; Baumlin, N.; Dennis, J.S.; Moore, R.; Salathe, S.F.; Whitney, P.L.; Sabater, J.; Abraham, W.M.; Kim, M.D.; Salathe, M. Electronic Cigarette Vapor with Nicotine Causes Airway Mucociliary Dysfunction Preferentially via TRPA1 Receptors. Am. J. Respir. Crit. Care Med. 2019, 200, 1134–1145. [Google Scholar] [CrossRef]
- Kichko, T.I.; Lennerz, J.; Eberhardt, M.; Babes, R.M.; Neuhuber, W.; Kobal, G.; Reeh, P.W. Bimodal concentration-response of nicotine involves the nicotinic acetylcholine receptor, transient receptor potential vanilloid type 1, and transient receptor potential ankyrin 1 channels in mouse trachea and sensory neurons. J. Pharmacol. Exp. Ther. 2013, 347, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, W.; Zhang, Z.-S.; Yang, T.; Grant, A.; Oxford, G.; Simon, S.A. Nicotine Inhibits Voltage-Dependent Sodium Channels and Sensitizes Vanilloid Receptors. J. Neurophysiol. 2004, 91, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K. Chemical composition and antimicrobial activity of the essential oil of Ocimum basilicum L. (sweet basil) from Western Ghats of North West Karnataka, India. Anc. Sci. Life 2014, 33, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ma, Y.; Han, B. Rapid detection of the component contents in caryophylli flos by a handheld near infrared spectrometer based on digital light processing technology. J. Near Infrared Spectrosc. 2018, 26, 389–397. [Google Scholar] [CrossRef]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious Cold Ion Channel TRPA1 Is Activated by Pungent Compounds and Bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef]
- Chung, G.; Im, S.T.; Kim, Y.H.; Jung, S.J.; Rhyu, M.R.; Oh, S.B. Activation of transient receptor potential ankyrin 1 by eugenol. Neuroscience 2014, 261, 153–160. [Google Scholar] [CrossRef]
- Inoue, M.; Fujita, T.; Goto, M.; Kumamoto, E. Presynaptic enhancement by eugenol of spontaneous excitatory transmission in rat spinal substantia gelatinosa neurons is mediated by transient receptor potential A1 channels. Neuroscience 2012, 210, 403–415. [Google Scholar] [CrossRef]
- Yang, B.H.; Piao, Z.G.; Kim, Y.B.; Lee, C.H.; Lee, J.K.; Park, K.; Kim, J.S.; Oh, S.B. Activation of Vanilloid Receptor 1 (VR1) by Eugenol. J. Dent. Res. 2003, 82, 781–785. [Google Scholar] [CrossRef]
- Park, C.K.; Kim, K.; Jung, S.J.; Kim, M.J.; Ahn, D.K.; Hong, S.D.; Kim, J.S.; Oh, S.B. Molecular mechanism for local anesthetic action of eugenol in the rat trigeminal system. Pain 2009, 144, 84–94. [Google Scholar] [CrossRef]
- Boscaro, V.; Boffa, L.; Binello, A.; Amisano, G.; Fornasero, S.; Cravotto, G.; Gallicchio, M. Antiproliferative, Proapoptotic, Antioxidant and Antimicrobial Effects of Sinapis nigra L. and Sinapis alba L. Extracts. Molecules 2018, 23, 3004. [Google Scholar] [CrossRef]
- Eib, S.; Schneider, D.J.; Hensel, O.; Seuß-Baum, I. Relationship between mustard pungency and allyl-isothiocyanate content: A comparison of sensory and chemical evaluations. J. Food Sci. 2020, 85, 2728–2736. [Google Scholar] [CrossRef]
- Ghawi, S.K.; Methven, L.; Niranjan, K. The potential to intensify sulforaphane formation in cooked broccoli (Brassica oleracea var. italica) using mustard seeds (Sinapis alba). Food Chem. 2013, 138, 1734–1741. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, S.Q.; Zhang, J.; Huang, G.Y.; Chen, L.Y.; Zhao, F.Y. Chemical composition, antimicrobial property and microencapsulation of Mustard (Sinapis alba) seed essential oil by complex coacervation. Food Chem. 2014, 165, 560–568. [Google Scholar] [CrossRef]
- Bautista, D.M.; Jordt, S.-E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 Mediates the Inflammatory Actions of Environmental Irritants and Proalgesic Agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef]
- Everaerts, W.; Gees, M.; Alpizar, Y.A.; Farre, R.; Leten, C.; Apetrei, A.; Dewachter, I.; van Leuven, F.; Vennekens, R.; De Ridder, D.; et al. The Capsaicin Receptor TRPV1 Is a Crucial Mediator of the Noxious Effects of Mustard Oil. Curr. Biol. 2011, 21, 316–321. [Google Scholar] [CrossRef]
- Mori, N.; Kawabata, F.; Matsumura, S.; Hosokawa, H.; Kobayashi, S.; Inoue, K.; Fushiki, T. Intragastric administration of allyl isothiocyanate increases carbohydrate oxidation via TRPV1 but not TRPA1 in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1494–R1505. [Google Scholar] [CrossRef]
- Mori, N.; Kurata, M.; Yamazaki, H.; Matsumura, S.; Hashimoto, T.; Kanazawa, K.; Nadamoto, T.; Inoue, K.; Fushiki, T. Allyl isothiocyanate increases carbohydrate oxidation through enhancing insulin secretion by TRPV1. Biosci. Biotechnol. Biochem. 2018, 82, 698–708. [Google Scholar] [CrossRef]
- Gül, S.; Demirci, B.; Başer, K.H.; Akpulat, H.A.; Aksu, P. Chemical composition and in vitro cytotoxic, genotoxic effects of essential oil from Urtica dioica L. Bull. Environ. Contam. Toxicol. 2012, 88, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef]
- Yamada, T.; Ueda, T.; Ugawa, S.; Ishida, Y.; Imayasu, M.; Koyama, S.; Shimada, S. Functional expression of transient receptor potential vanilloid 3 (TRPV3) in corneal epithelial cells: Involvement in thermosensation and wound healing. Exp. Eye Res. 2010, 90, 121–129. [Google Scholar] [CrossRef]
- Alvarenga, E.M.; Souza, L.K.M.; Araújo, T.S.L.; Nogueira, K.M.; Sousa, F.B.M.; Araújo, A.R.; Martins, C.S.; Pacífico, D.M.; de Brito, G.A.; Souza, E.P.; et al. Carvacrol reduces irinotecan-induced intestinal mucositis through inhibition of inflammation and oxidative damage via TRPA1 receptor activation. Chem. Biol. Interact. 2016, 260, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Mukaiyama, M.; Usui, T.; Nagumo, Y. Non-electrophilic TRPA1 agonists, menthol, carvacrol and clotrimazole, open epithelial tight junctions via TRPA1 activation. J. Biochem. 2020, 168, 407–415. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, Y.; Song, P.; Xie, J.; Pang, G. The investigation of allosteric regulation mechanism of analgesic effect using SD rat taste bud tissue biosensor. Biosens. Bioelectron. 2019, 126, 815–823. [Google Scholar] [CrossRef]
- Noumi, E.; Snoussi, M.; Alreshidi, M.M.; Rekha, P.D.; Saptami, K.; Caputo, L.; De Martino, L.; Souza, L.F.; Msaada, K.; Mancini, E.; et al. Chemical and Biological Evaluation of Essential Oils from Cardamom Species. Molecules 2018, 23, 2818. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.Y.; Wang, C.; Xu, N.X.; Fujita, T.; Murata, Y.; Kumamoto, E. 1,8- and 1,4-cineole enhance spontaneous excitatory transmission by activating different types of transient receptor potential channels in the rat spinal substantia gelatinosa. J. Neurochem. 2016, 136, 764–777. [Google Scholar] [CrossRef]
- Takaishi, M.; Fujita, F.; Uchida, K.; Yamamoto, S.; Sawada Shimizu, M.; Hatai Uotsu, C.; Shimizu, M.; Tominaga, M. 1,8-cineole, a TRPM8 agonist, is a novel natural antagonist of human TRPA1. Mol. Pain 2012, 8, 86. [Google Scholar] [CrossRef]
- Karashima, Y.; Damann, N.; Prenen, J.; Talavera, K.; Segal, A.; Voets, T.; Nilius, B. Bimodal Action of Menthol on the Transient Receptor Potential Channel TRPA1. J. Neurosci. 2007, 27, 9874–9884. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, S.; Wu, J.; Sun, X.; Shen, Z.; Dong, J.; Huang, J. Promotion of Mitochondrial Biogenesis via Activation of AMPK-PGC1ɑ Signaling Pathway by Ginger (Zingiber officinale Roscoe) Extract, and Its Major Active Component 6-Gingerol. J. Food Sci. 2019, 84, 2101–2111. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K. Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterol. Res. Pract. 2015, 2015, 142979. [Google Scholar] [CrossRef]
- Sugita, J.; Yoneshiro, T.; Hatano, T.; Aita, S.; Ikemoto, T.; Uchiwa, H.; Iwanaga, T.; Kameya, T.; Kawai, Y.; Saito, M. Grains of paradise (Aframomum melegueta) extract activates brown adipose tissue and increases whole-body energy expenditure in men. Br. J. Nutr. 2013, 110, 733–738. [Google Scholar] [CrossRef]
- Yoshitomi, T.; Oshima, N.; Goto, Y.; Nakamori, S.; Wakana, D.; Anjiki, N.; Sugimura, K.; Kawano, N.; Fuchino, H.; Iida, O.; et al. Construction of Prediction Models for the Transient Receptor Potential Vanilloid Subtype 1 (TRPV1)-Stimulating Activity of Ginger and Processed Ginger Based on LC-HRMS Data and PLS Regression Analyses. J. Agric. Food Chem. 2017, 65, 3581–3588. [Google Scholar] [CrossRef]
- Riera, C.E.; Menozzi-Smarrito, C.; Affolter, M.; Michlig, S.; Munari, C.; Robert, F.; Vogel, H.; Simon, S.A.; le Coutre, J. Compounds from Sichuan and Melegueta peppers activate, covalently and non-covalently, TRPA1 and TRPV1 channels. Br. J. Pharmacol. 2009, 157, 1398–1409. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Hong, C.S.; Lee, S.W.; Nam, J.H.; Kim, B.J. Effects of ginger and its pungent constituents on transient receptor potential channels. Int. J. Mol. Med. 2016, 38, 1905–1914. [Google Scholar] [CrossRef]
- Narukawa, M.; Koizumi, K.; Iwasaki, Y.; Kubota, K.; Watanabe, T. Galangal pungent component, 1′-acetoxychavicol acetate, activates TRPA1. Biosci. Biotechnol. Biochem. 2010, 74, 1694–1696. [Google Scholar] [CrossRef]
- Basri, A.M.; Taha, H.; Ahmad, N. A Review on the Pharmacological Activities and Phytochemicals of Alpinia officinarum (Galangal) Extracts Derived from Bioassay-Guided Fractionation and Isolation. Pharmacogn. Rev. 2017, 11, 43–56. [Google Scholar] [CrossRef]
- Nakamura, T.; Miyoshi, N.; Ishii, T.; Nishikawa, M.; Ikushiro, S.; Watanabe, T. Activation of transient receptor potential ankyrin 1 by quercetin and its analogs. Biosci. Biotechnol. Biochem. 2016, 80, 949–954. [Google Scholar] [CrossRef]
- Wong, K.C.; Ong, K.S.; Lim, C.L. Compositon of the essential oil of rhizomes of kaempferia galanga L. Flavour Fragr. J. 1992, 7, 263–266. [Google Scholar] [CrossRef]
- Gil, A.; De La Fuente, E.B.; Lenardis, A.E.; López Pereira, M.; Suárez, S.A.; Bandoni, A.; Van Baren, C.; Di Leo Lira, P.; Ghersa, C.M. Coriander essential oil composition from two genotypes grown in different environmental conditions. J. Agric. Food Chem. 2002, 50, 2870–2877. [Google Scholar] [CrossRef]
- Caputo, L.; Piccialli, I.; Ciccone, R.; de Caprariis, P.; Massa, A.; De Feo, V.; Pannaccione, A. Lavender and coriander essential oils and their main component linalool exert a protective effect against amyloid-β neurotoxicity. Phytother. Res. PTR 2020, 35, 486–493. [Google Scholar] [CrossRef]
- Fothergill, L.J.; Callaghan, B.; Rivera, L.R.; Lieu, T.; Poole, D.P.; Cho, H.J.; Bravo, D.M.; Furness, J.B. Effects of Food Components That Activate TRPA1 Receptors on Mucosal Ion Transport in the Mouse Intestine. Nutrients 2016, 8, 623. [Google Scholar] [CrossRef]
- Shah, S.L.; Wahid, F.; Khan, N.; Farooq, U.; Shah, A.J.; Tareen, S.; Ahmad, F.; Khan, T. Inhibitory Effects of Glycyrrhiza glabra and Its Major Constituent Glycyrrhizin on Inflammation-Associated Corneal Neovascularization. Evid. Based Complement. Altern. Med. eCAM 2018, 2018, 8438101. [Google Scholar] [CrossRef]
- Kurahara, L.H.; Hiraishi, K.; Hu, Y.; Koga, K.; Onitsuka, M.; Doi, M.; Aoyagi, K.; Takedatsu, H.; Kojima, D.; Fujihara, Y.; et al. Activation of Myofibroblast TRPA1 by Steroids and Pirfenidone Ameliorates Fibrosis in Experimental Crohn’s Disease. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 299–318. [Google Scholar] [CrossRef]
- Tu, J.; Guo, Y.; Hong, W.; Fang, Y.; Han, D.; Zhang, P.; Wang, X.; Körner, H.; Wei, W. The Regulatory Effects of Paeoniflorin and Its Derivative Paeoniflorin-6′-O-Benzene Sulfonate CP-25 on Inflammation and Immune Diseases. Front. Pharmacol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Yan, Z.; Xie, L.; Tian, Y.; Li, M.; Ni, J.; Zhang, Y.; Niu, L. Insights into the Phytochemical Composition and Bioactivities of Seeds from Wild Peony Species. Plants 2020, 9, 729. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Gao, Q.; Tao, W.; Chen, J.; Bi, J.; Ding, F.; Wang, Z. Paeoniflorin relieves LPS-induced inflammatory pain in mice by inhibiting NLRP3 inflammasome activation via transient receptor potential vanilloid 1. J. Leukoc. Biol. 2020, 108, 229–241. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, C.; Ge, Z.; Liu, Y.; Liu, Y.; Feng, W.; Li, S.; Chen, G.; Wei, T. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol. Lett. 2013, 6, 1140–1146. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Saeedi, M. Constituents of the Essential Oil of Commiphora myrrha (Nees) Engl. var. molmol. J. Essent. Oil Res. 2003, 15, 50–51. [Google Scholar] [CrossRef]
- Hu, D.; Wang, C.; Li, F.; Su, S.; Yang, N.; Yang, Y.; Zhu, C.; Shi, H.; Yu, L.; Geng, X.; et al. A Combined Water Extract of Frankincense and Myrrh Alleviates Neuropathic Pain in Mice via Modulation of TRPV1. Neural Plast. 2017, 2017, 3710821. [Google Scholar] [CrossRef]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor--a fumigant during the Black Death and a coveted fragrant wood in ancient Egypt and Babylon—A review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef]
- Xu, H.; Blair, N.T.; Clapham, D.E. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 8924–8937. [Google Scholar] [CrossRef]
- Alpizar, Y.A.; Gees, M.; Sanchez, A.; Apetrei, A.; Voets, T.; Nilius, B.; Talavera, K. Bimodal effects of cinnamaldehyde and camphor on mouse TRPA1. Pflüg. Arch. Eur. J. Physiol. 2013, 465, 853–864. [Google Scholar] [CrossRef]
- Antsyshkina, A.M. The Genus Asarum L.: A Phytochemical and Ethnopharmacological Review. Syst. Rev. Pharm. 2020, 11, 472–502. [Google Scholar] [CrossRef]
- Afroz, A.; Howlett, N.; Shukla, A.; Ahmad, F.; Batista, E.; Bedard, K.; Payne, S.; Morton, B.; Mansfield, J.H.; Glendinning, J.I. Gustatory receptor neurons in Manduca sexta contain a TrpA1-dependent signaling pathway that integrates taste and temperature. Chem. Senses 2013, 38, 605–617. [Google Scholar] [CrossRef]
- Moon, H.; Kim, M.J.; Son, H.J.; Kweon, H.J.; Kim, J.T.; Kim, Y.; Shim, J.; Suh, B.C.; Rhyu, M.R. Five hTRPA1 Agonists Found in Indigenous Korean Mint, Agastache rugosa. PLoS ONE 2015, 10, e0127060. [Google Scholar] [CrossRef]
- Fico, G.; Bader, A.; Flamini, G.; Cioni, P.L.; Morelli, I. Essential Oil of Nigella damascena L. (Ranunculaceae) Seeds. J. Essent. Oil Res. 2003, 15, 57–58. [Google Scholar] [CrossRef]
- Rchid, H.; Nmila, R.; Bessière, J.M.; Sauvaire, Y.; Chokaïri, M. Volatile Components of Nigella damascena L. and Nigella sativa L. Seeds. J. Essent. Oil Res. 2004, 16, 585–587. [Google Scholar] [CrossRef]
- Moretti, A.; D’Antuono, L.F.; Elementi, S. Essential Oils of Nigella sativa L. and Nigella damascena L. Seed. J. Essent. Oil Res. 2004, 16, 182–183. [Google Scholar] [CrossRef]
- Aikkal, R. Phytochemical analysis, carminative, enzyme inhibitor, and anticancer activities of beta-elemene. Retrieved March 2016. [Google Scholar] [CrossRef]
- Andriana, Y.; Xuan, T.D.; Quy, T.N.; Tran, H.D.; Le, Q.T. Biological Activities and Chemical Constituents of Essential Oils from Piper cubeba Bojer and Piper nigrum L. Molecules 2019, 24, 1876. [Google Scholar] [CrossRef]
- Alminderej, F.; Bakari, S.; Almundarij, T.I.; Snoussi, M.; Aouadi, K.; Kadri, A. Antioxidant Activities of a New Chemotype of Piper cubeba L. Fruit Essential Oil (Methyleugenol/Eugenol): In Silico Molecular Docking and ADMET Studies. Plants 2020, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.G.; de Souza, J.M.; Wakabayashi, K.A.; Laurentiz Rda, S.; Vinhólis, A.H.; Rezende, K.C.; Simaro, G.V.; Bastos, J.K.; Rodrigues, V.; Esperandim, V.R.; et al. In vitro efficacy of the essential oil of Piper cubeba L. (Piperaceae) against Schistosoma mansoni. Parasitol. Res. 2012, 110, 1747–1754. [Google Scholar] [CrossRef]
- Bos, R.; Woerdenbag, H.; Kayser, O.; Quax, W.; Ruslan, K.; Elfami. Essential Oil Constituents of Piper cubeba L. fils. from Indonesia. J. Essent. Oil Res. 2007, 19, 14–17. [Google Scholar] [CrossRef]
- Vriens, J.; Watanabe, H.; Janssens, A.; Droogmans, G.; Voets, T.; Nilius, B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl. Acad. Sci. USA 2004, 101, 396–401. [Google Scholar] [CrossRef]
- Kwan, K.Y.; Allchorne, A.J.; Vollrath, M.A.; Christensen, A.P.; Zhang, D.S.; Woolf, C.J.; Corey, D.P. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 2006, 50, 277–289. [Google Scholar] [CrossRef]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Bessac, B.F.; Jordt, S.E. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology 2008, 23, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.P.; Corey, D.P. TRP channels in mechanosensation: Direct or indirect activation? Nat. Rev. Neurosci. 2007, 8, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Siemens, J. TRP ion channels in thermosensation, thermoregulation and metabolism. Temperature 2015, 2, 178–187. [Google Scholar] [CrossRef]
- Nishida, M.; Kurose, H. Roles of TRP channels in the development of cardiac hypertrophy. Naunyn-Schmiedeberg’s Arch. pharmacol. 2008, 378, 395–406. [Google Scholar] [CrossRef]
- Liu, H.; Dilger, J.P.; Lin, J. The Role of Transient Receptor Potential Melastatin 7 (TRPM7) in Cell Viability: A Potential Target to Suppress Breast Cancer Cell Cycle. Cancers 2020, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dilger, J.P.; Lin, J. Lidocaine Suppresses Viability and Migration of Human Breast Cancer Cells: TRPM7 as a Target for Some Breast Cancer Cell Lines. Cancers 2021, 13, 234. [Google Scholar] [CrossRef]
- Ohba, T.; Watanabe, H.; Murakami, M.; Takahashi, Y.; Iino, K.; Kuromitsu, S.; Mori, Y.; Ono, K.; Iijima, T.; Ito, H. Upregulation of TRPC1 in the development of cardiac hypertrophy. J. Mol. Cell. Cardiol. 2007, 42, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Nassini, R.; Materazzi, S.; Benemei, S.; Geppetti, P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev. Physiol. Biochem. Pharmacol. 2014, 167, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Shin, S.H.; Auh, C.K.; Chun, J. Human skeletal dysplasia caused by a constitutive activated transient receptor potential vanilloid 4 (TRPV4) cation channel mutation. Exp. Mol. Med. 2012, 44, 707–722. [Google Scholar] [CrossRef]
- Bevan, S.; Andersson, D.A. TRP channel antagonists for pain--opportunities beyond TRPV1. Curr. Opin. Investig. Drugs 2009, 10, 655–663. [Google Scholar]
- Gouin, O.; L’Herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhé, V.; Plée-Gautier, E.; Carré, J.L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef]
- Fernandes, E.; Fernandes, M.; Keeble, J. The functions of TRPA1 and TRPV1: Moving away from sensory nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Mihara, S.; Shibamoto, T. The role of flavor and fragrance chemicals in TRPA1 (transient receptor potential cation channel, member A1) activity associated with allergies. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol. 2015, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Staruschenko, A.; Jeske, N.A.; Akopian, A.N. Contribution of TRPV1-TRPA1 Interaction to the Single Channel Properties of the TRPA1 Channel. J. Biol. Chem. 2010, 285, 15167–15177. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient Receptor Potential (TRP) Channels. Sub-cell. Biochem. 2018, 87, 141–165. [Google Scholar] [CrossRef]
- Aloum, L.; Alefishat, E.; Adem, A.; Petroianu, G. Ionone Is More than a Violet’s Fragrance: A Review. Molecules 2020, 25, 5822. [Google Scholar] [CrossRef] [PubMed]
- Van Gerven, L.; Alpizar, Y.A.; Wouters, M.M.; Hox, V.; Hauben, E.; Jorissen, M.; Boeckxstaens, G.; Talavera, K.; Hellings, P.W. Capsaicin treatment reduces nasal hyperreactivity and transient receptor potential cation channel subfamily V, receptor 1 (TRPV1) overexpression in patients with idiopathic rhinitis. J. Allergy Clin. Immunol. 2014, 133, 1332–1339. [Google Scholar] [CrossRef]
- Caterina, M.J.; Park, U. Chapter 4 TRPV1: A Polymodal Sensor in the Nociceptor Terminal. In Current Topics in Membranes; Academic Press: Cambridge, MA, USA, 2006; Volume 57, pp. 113–150. [Google Scholar]
- Bang, S.; Hwang, S.W. Polymodal Ligand Sensitivity of TRPA1 and Its Modes of Interactions. J. Gen. Physiol. 2009, 133, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, M.; Fiorio Pla, A.; Prevarskaya, N.; Gkika, D. Human transient receptor potential (TRP) channel expression profiling in carcinogenesis. Int. J. Dev. Biol. 2015, 59, 399–406. [Google Scholar] [CrossRef]
- Benítez-Angeles, M.; Morales-Lázaro, S.L.; Juárez-González, E.; Rosenbaum, T. TRPV1: Structure, Endogenous Agonists, and Mechanisms. Int. J. Mol. Sci. 2020, 21, 3421. [Google Scholar] [CrossRef] [PubMed]
- Chernov-Rogan, T.; Gianti, E.; Liu, C.; Villemure, E.; Cridland, A.P.; Hu, X.; Ballini, E.; Lange, W.; Deisemann, H.; Li, T.; et al. TRPA1 modulation by piperidine carboxamides suggests an evolutionarily conserved binding site and gating mechanism. Proc. Natl. Acad. Sci. USA 2019, 116, 26008–26019. [Google Scholar] [CrossRef] [PubMed]
- Meents, J.E.; Ciotu, C.I.; Fischer, M.J.M. TRPA1: A molecular view. J. Neurophysiol. 2019, 121, 427–443. [Google Scholar] [CrossRef]
- Hinman, A.; Chuang, H.-h.; Bautista, D.M.; Julius, D. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. USA 2006, 103, 19564–19568. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, L.J.; Dubin, A.E.; Evans, M.J.; Marr, F.; Schultz, P.G.; Cravatt, B.F.; Patapoutian, A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007, 445, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiao, X.; Cheng, W.; Yang, W.; Yu, P.; Song, Z.; Yarov-Yarovoy, V.; Zheng, J. Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat. Chem. Biol. 2015, 11, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Elokely, K.; Velisetty, P.; Delemotte, L.; Palovcak, E.; Klein, M.L.; Rohacs, T.; Carnevale, V. Understanding TRPV1 activation by ligands: Insights from the binding modes of capsaicin and resiniferatoxin. Proc. Natl. Acad. Sci. USA 2016, 113, E137–E145. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, D.; Caprioglio, D.; Appendino, G.; Minassi, A.; Schiano-Moriello, A.; Di Marzo, V.; De Petrocellis, L. Discovery of non-electrophilic capsaicinoid-type TRPA1 ligands. Bioorg. Med. Chem. Lett. 2015, 25, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
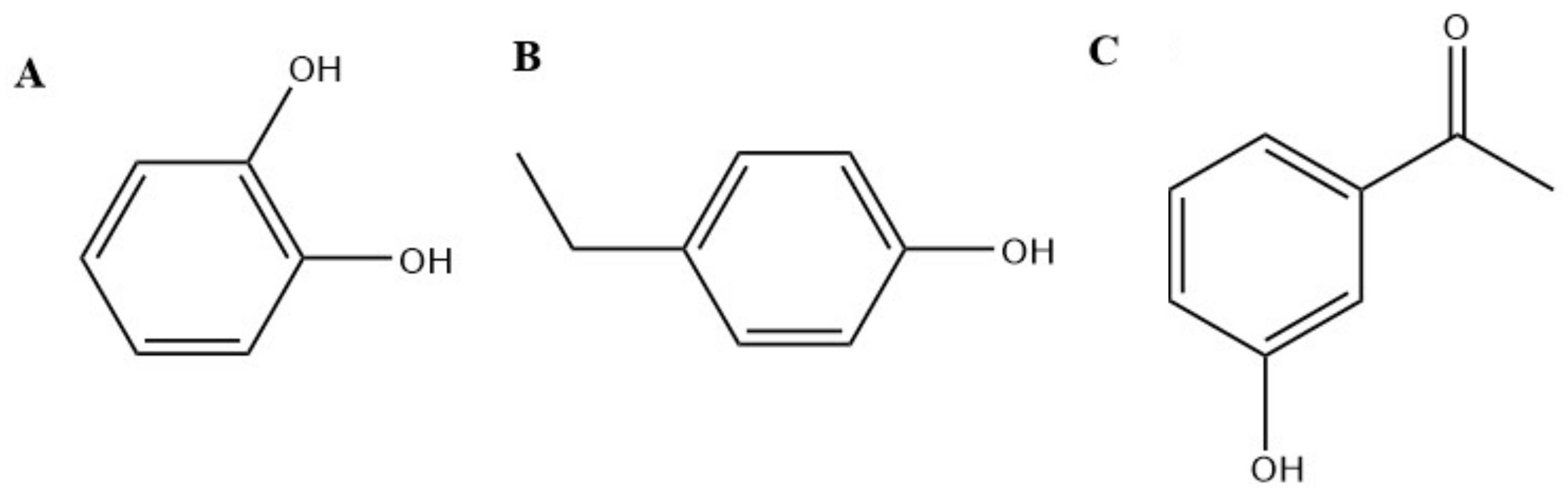
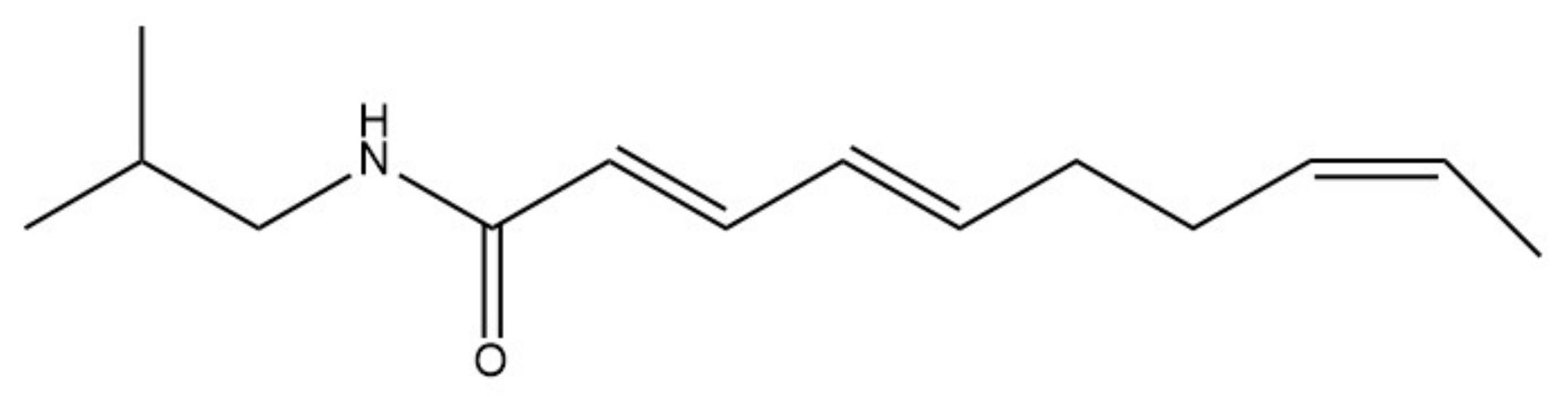
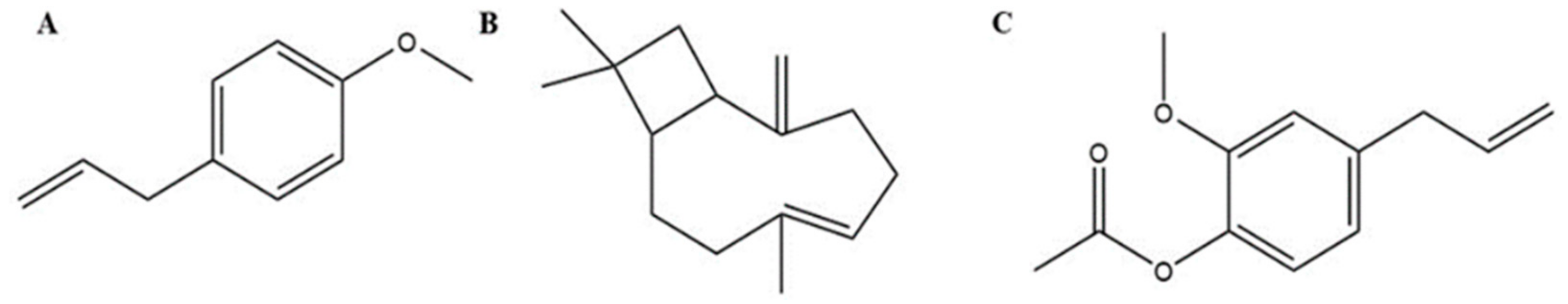
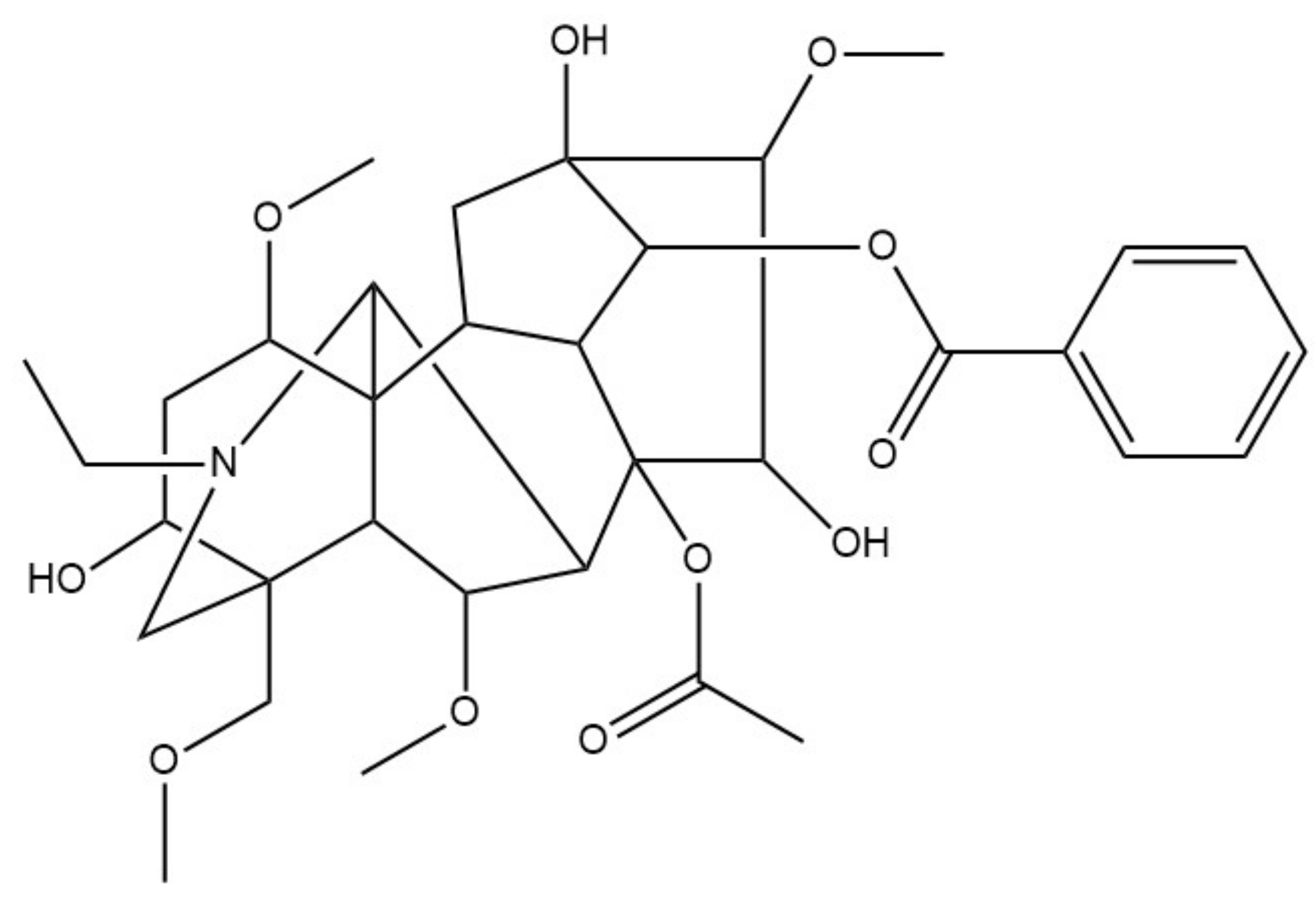


| Name as Reported over History | Current Name | Physicians | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pedanius (~40–90) | Aëtius (≈†574) | De Gordon (1270–1330) | De Montagna (1400–1460) | Fuchs (1501–1566) | Dalla Croce (1509–1575) | Joel (1510–1579) | Joubert (1529–1582) | Zacuth (1575–1642) | Chalmeteus (~ 1560:) | Schoock (1614–1669) | Woyt (1671–1709) | Andrioli (1672–1713) | Arnemann (1763–1806) | Par (1750–1810) | Hesselbach (1788–1856) | ||
| Cumini | Cuminum cyminum | X | X | ||||||||||||||
| Piperis | Piper nigrum | X | X | X | X | X | X | X | X | X | X | ||||||
| Herba castorii/castoreum | Castoreum | X | X | X | X | X | X | X | X | X | |||||||
| Euphorbio | Euphorbia resinifera | X | X | X | X | X | X | X | X | ||||||||
| Ptarmice | Achillea ptarmica | X | X | X | |||||||||||||
| Nicotianae | Tobacco leaves | X | X | X | X | X | |||||||||||
| Ocimum basilicum | Ocimum basilicum | X | |||||||||||||||
| Cariophlli | Caryophylli flos | X | |||||||||||||||
| Sinapis | X | X | |||||||||||||||
| Urtica | Urtica dioica | X | |||||||||||||||
| Ranunculo | X | ||||||||||||||||
| Cardamom | Elettaria cardamomum | X | |||||||||||||||
| Aframomum-Grana paradise | Aframomum melegueta | ||||||||||||||||
| Ginger | Zingiber officinale | X | X | ||||||||||||||
| Galangal | X | X | |||||||||||||||
| Coriander | Coriandrum sativum | X | |||||||||||||||
| Salt liquorice-Salzlakritz | Glycyrrhiza glabra | X | |||||||||||||||
| Paeoniae | X | ||||||||||||||||
| Myrrhae | Commiphora myrrha | X | |||||||||||||||
| Kampfer | Camphor | X | |||||||||||||||
| Asarum | X | X | |||||||||||||||
| Nigella damascena | Nigella damascena | X | |||||||||||||||
| Cubebarum | Piper Cubeba | X | |||||||||||||||
| Cyclamini | X | X | |||||||||||||||
| Elleboro albo | Veratrum album | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| Daphnoides-Spurge Laurel | Daphne laureola | X | |||||||||||||||
| Pyrethri | X | X | X | X | |||||||||||||
| Mercurius dulcis | Hydrargyrum chloratum dulce | X | |||||||||||||||
| Chinapuder | China powder | X | |||||||||||||||
| Nitro | Saltpeter | X | |||||||||||||||
| Aloe | X | X | |||||||||||||||
| Herba lanaria | Saponaria officinalis | X | X | X | |||||||||||||
| Staphydis agriae | Staphysagria macrosperma | X | X | ||||||||||||||
- Pedanius Dioscorides (c. 40–90 AD) was a Greek physician, pharmacologist, botanist, and author of a multivolume encyclopedia of herbal medicine [14].
- Aëtius of Amida (≈† 574) was a Greek physician at the court of Byzantine Emperor Justinian [15].
- Gordon, Bernard de (1270–1330) was a French physician teacher; his books, specifically Lilium medicinae, formed an important part of the medical curriculum of medieval Europe [16].
- De Montagna, Bartholomaeus (ca. 1400–ca. 1460) was an Italian physician and professor in Padua and Bologna.
- Della Croce, Giovanni Andrea (1509–1575) was an Italian surgeon who authored “Universal Surgery Complete with All the Relevant Parts for the Optimum Surgeon”, which was a keystone for traumatology [18].
- Joel, Franciscus primus (Joó Ferenc; 1510–1579) was a Hungarian-born pharmacist and physician, professor, and then, the rector of the University in Greifswald, Duchy of Pomerania [19].
- Joubert, Laurent (1529–1582) was the Chancellor of the Faculty of Medicine at the University of Montpellier [20].
- Zacuth, Abraham (1575–1642) was famous for his precise descriptions of diseases, such as the plague and blackwater fever [21].
- Chalmeteus, Antonius (Antoine Chaumette) was a French surgeon who authored “Enchiridion chirurgicum, externorum morborum remedia complectens” [22].
- Schoock, Martin (1614–1669) was a Dutch historian and professor at the University of Deventer and the University of Frankfurt (Oder) [23].
- Woyt, Johann Jacob (1671–1709) was a German physician and professor of medicine at the University of Königsberg (Prussian city that is now Kaliningrad, Russia) [24].
- Andrioli, Michele Angelo (1672–1713) was an Italian physician who worked in Klagenfurt, Austria. He was a prolific author who assisted Barbato, Girolamo in the discovery of blood serum.
- Arnemann, Justus (1763–1806) was a German surgeon and professor of medicine at the University of Göttingen [25].
- Parr, Bartholomew (1750–1810) was a British surgeon and Fellow of the Royal Society of Edinburgh and London [26].
- Hesselbach, Adam Kaspar (1788–1856) was a German professor of surgery in Würzburg and Bamberg. He was the son of Franz Kaspar Hesselbach, a famous surgeon known for his contribution to hernia surgeries [27].
| Sneeze-Inducing Remedies | Main Component | TRP Channel Agonist |
|---|---|---|
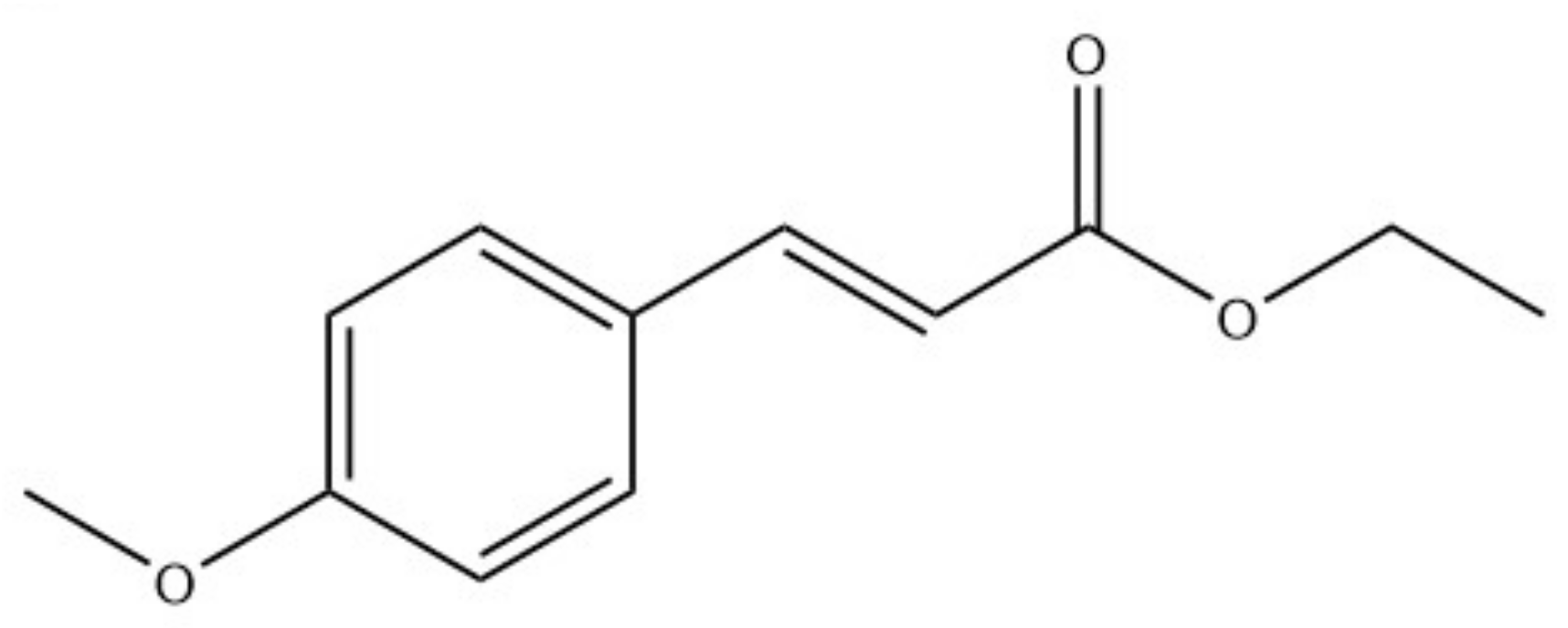
| Cumini | Cuminaldehyde | TRPA1 |
| Piperis | Piperine | TRPV1 and TRPA1 |
| Castoreum | Acetophenone | TRPA1 |
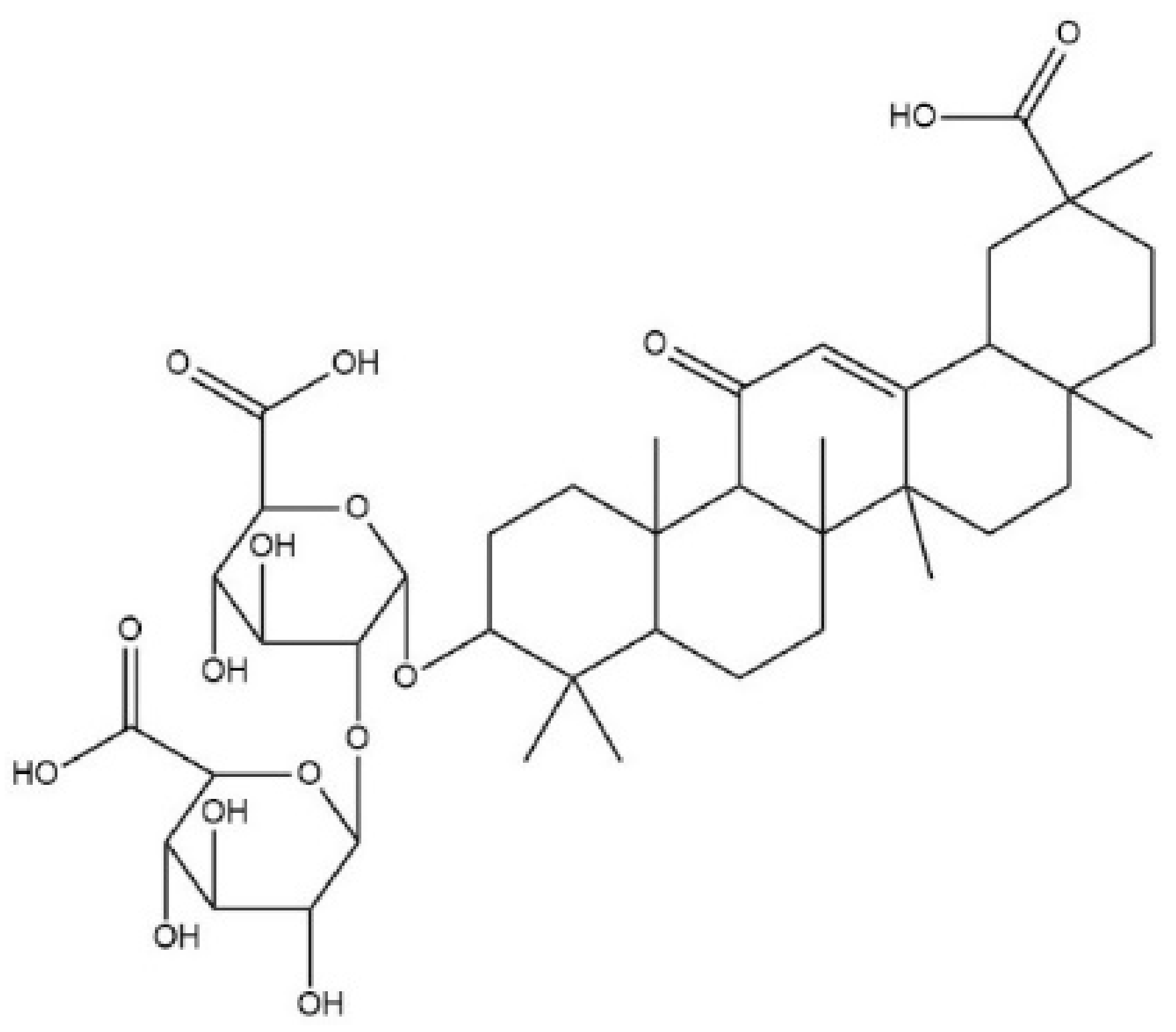
| Euphorbio | Resiniferatoxin | TRPV1 |
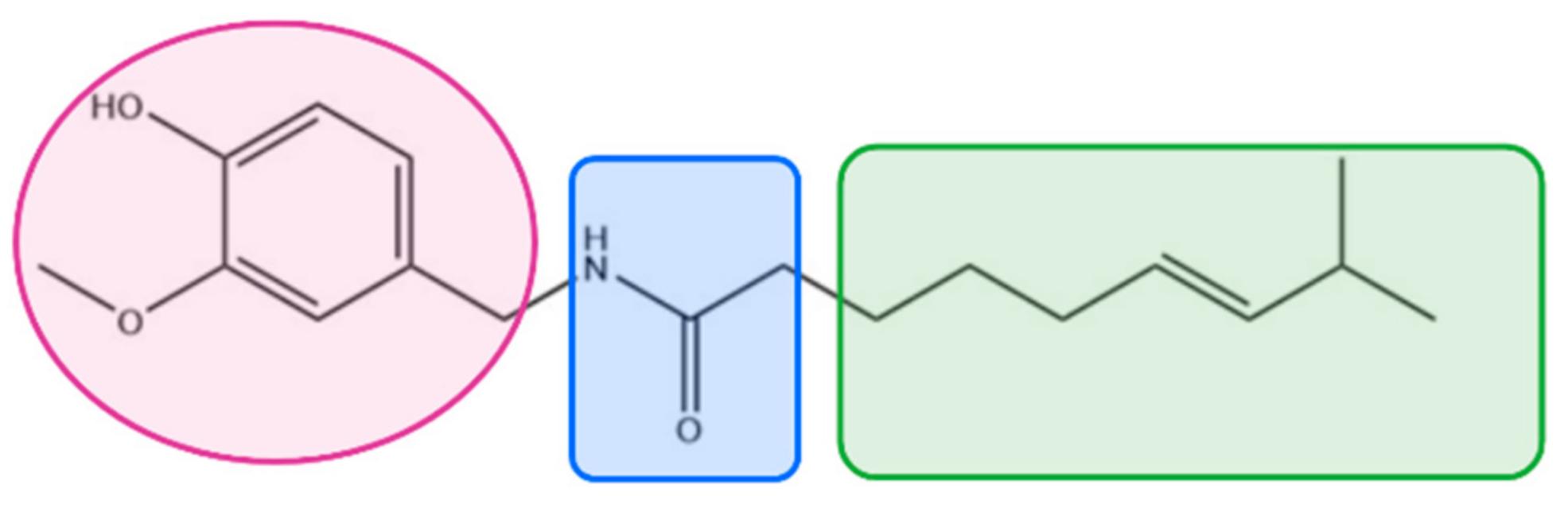
| Ptarmice | trans-Pellitorine * | TRPV1 and TRPA1 (indirect) |
| Nicotianae | Nicotine ** | TRPA1 |
| Ocimum basilicum/Cariophlli/ Cubebarum | Eugenol | TRPA1 and TRPV1 |
| Sinapis | Allyl isothiocyanate ** | TRPA1 and TRPV1 |
| Urtica dioica | Carvacol | TRPA1 |
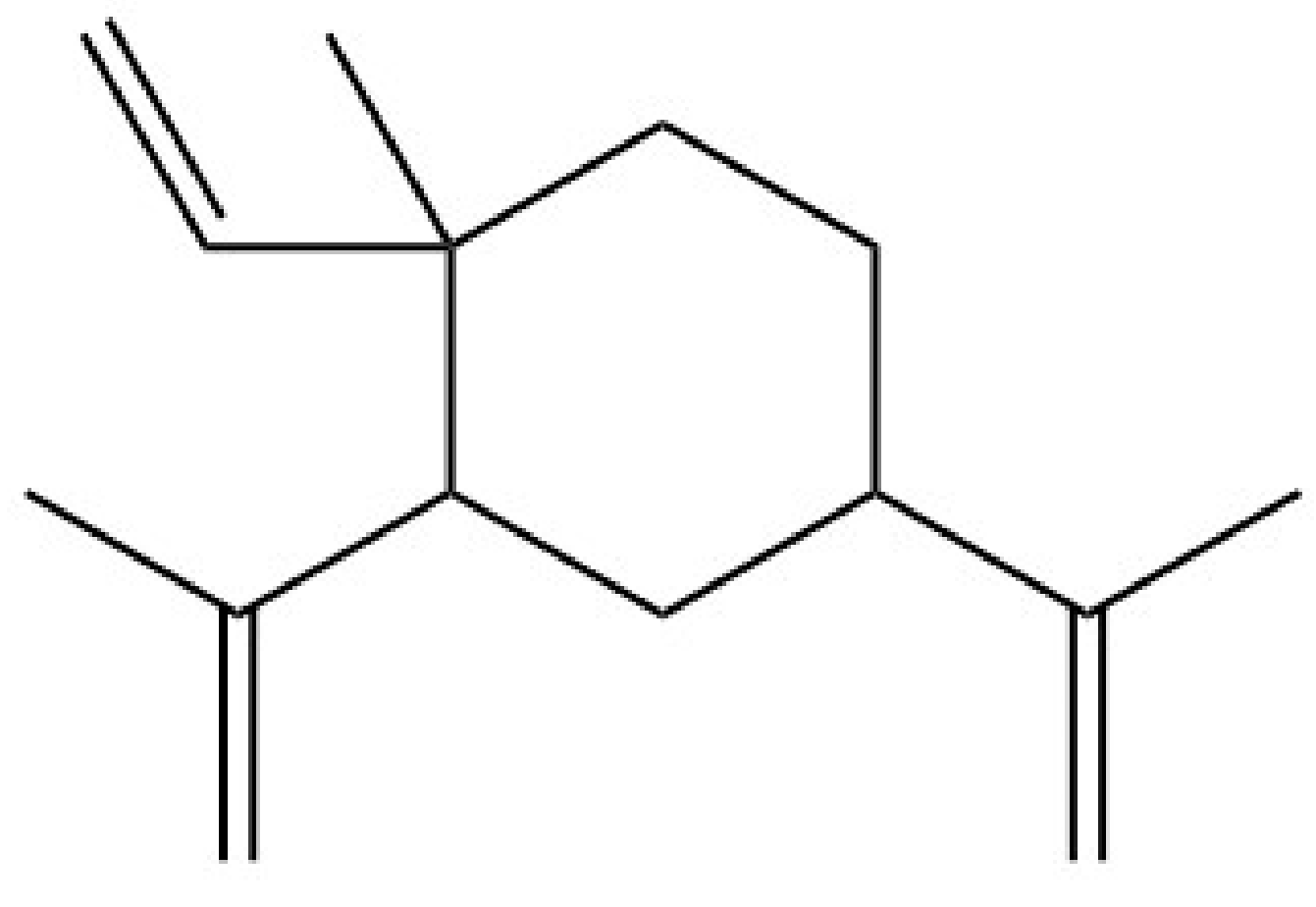
| Cardamom | 1,8-cineole ** | TRPA1 |
| Aframomum-Grana paradise/ Ginger | 6-paradol | TRPV1 and TRPA1 |
| 6-gingerol | TRPV1 and TRPA1 | |
| 6-shogaol | TRPV1 and TRPA1 | |
| Galangal | 1′-acetoxychavicol acetate | TRPA1 |
| Galangan | TRPA1 | |
| Coriander | Linalool | TRPA1 |
| Salt liquorice-Salzlakritz | Glycyrrhizin → glycyrrhetininc acid | TRPA1 |
| Camphor/Cubebarum | Camphor ** | TRPA1 and TRPV1 |
| Asarum | Aristolochic acid | TRPA1 |
| Asarum/Cubebarum | Methyl eugenol | TRPA1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloum, L.; Alefishat, E.; Shaya, J.; Petroianu, G.A. Remedia Sternutatoria over the Centuries: TRP Mediation. Molecules 2021, 26, 1627. https://doi.org/10.3390/molecules26061627
Aloum L, Alefishat E, Shaya J, Petroianu GA. Remedia Sternutatoria over the Centuries: TRP Mediation. Molecules. 2021; 26(6):1627. https://doi.org/10.3390/molecules26061627
Chicago/Turabian StyleAloum, Lujain, Eman Alefishat, Janah Shaya, and Georg A. Petroianu. 2021. "Remedia Sternutatoria over the Centuries: TRP Mediation" Molecules 26, no. 6: 1627. https://doi.org/10.3390/molecules26061627
APA StyleAloum, L., Alefishat, E., Shaya, J., & Petroianu, G. A. (2021). Remedia Sternutatoria over the Centuries: TRP Mediation. Molecules, 26(6), 1627. https://doi.org/10.3390/molecules26061627









