Abstract
Quercetin is a flavonoid that is found in many plant materials, including commonly eaten fruits and vegetables. The compound is well known for its wide range of biological activities. In this study, 5-O-acyl derivatives of quercetin were synthesised and assessed for their antiproliferative activity against the HCT116 colon cancer and MDA-MB-231 breast cancer cell lines; and their radical scavenging activity against the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical species. Four derivatives were found to have improved the antiproliferative activity compared to quercetin whilst retaining radical scavenging activity.
1. Introduction
Quercetin (1) is a flavonoid found in a wide range of commonly eaten fruits and vegetables [1,2]. Structurally, this compound belongs to the flavonol subclass of flavonoids and consists of five hydroxy groups at the 3-, 5-, 7-, 3′- and 4′-positions (Figure 1). Quercetin displays a wide range of biological activities [3], and is particularly known for its antioxidant [3,4] and antiproliferative [5,6] properties. These activities imply that quercetin have potential for the development of health-promoting agents. However, this potential is severely hindered by its poor bioavailability [7]. Previous studies have demonstrated that by increasing the lipophilicity of flavonoids this had resulted in compounds with improved biological activity [8,9,10,11,12] as well as improved properties related to bioavailability [9,13]. It was envisaged that the strategic derivatisation of quercetin could increase its lipophilicity as well as retain, or improve, its desired bioactivity. Previous studies have identified that the hydroxy groups on 3-, 3′- and 4′- positions of quercetin significantly contribute to the compound’s radical scavenging activity [14,15]. Proposedly, the mechanism of activity occurs through two proton-coupled electron transfer steps, resulting in the structural transformation from quercetin to an ortho-qunione which further isomerises into either of the three para-quinone methide structures [16]. This mechanism suggests involvement of the three hydroxy groups already mentioned, as well as that on the 7- position. Therefore, it was rationalised the 5-O position of quercetin would be an ideal site for lipophilic derivatisation. In this report, the syntheses of seven 5-O acyl quercetin derivatives are described. The derivatives were assessed on their antiproliferative activities against two cancer cell lines as well as their radical scavenging activities against two radical species. These results were compared to quercetin to determine the effect that 5-O acylation had on these desired biological activities.
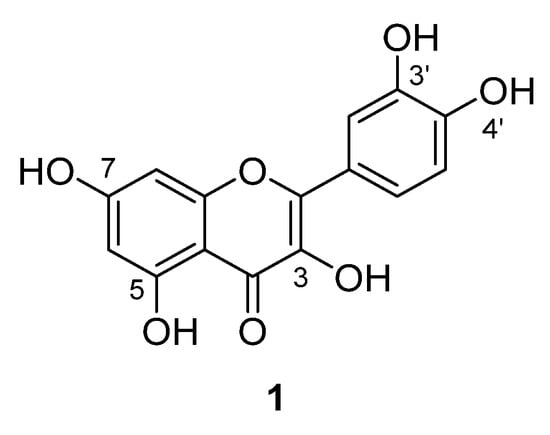
Figure 1.
Structure of quercetin, a pentahydroxy flavonol.
2. Results and Discussion
2.1. Syntheses of 5-O-Acyl Quercetin Derivatives
The syntheses of the 5-O-acyl quercetin derivatives began by protecting the catechol hydroxy groups of flavonoid 1 with a diphenyl dioxol moiety to give compound 2 (Scheme 1). Thereafter the non-hydrogen bonded hydroxy groups at the 3- and 7- positions were converted to benzyl ethers. These reactions afforded the tetra-protected quercetin 3 with an overall yield of 37%, over two steps. The experimental 1H NMR signals were in agreement with that previously reported in literature which gave confirmation of the tetra-protected product [17]. It was observed that the singlet at δ = 12.67, which has previously been identified as the signal for the 5-OH proton, was the only hydroxy proton signal observed to further confirm that this was the only available site for the subsequent reactions [18,19].
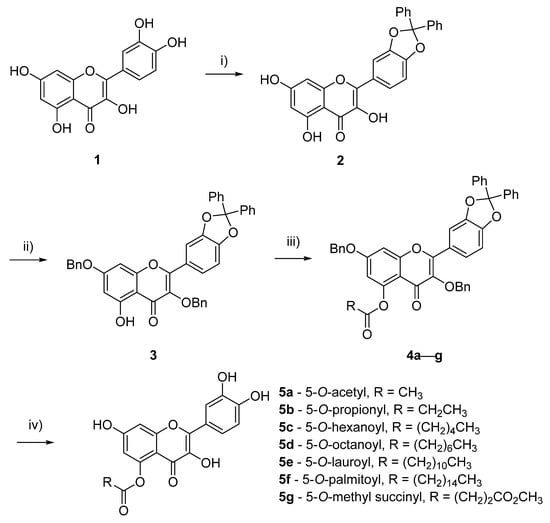
Scheme 1.
Syntheses of 5-O-acyl quercetin compounds: (i) Ph2CCl2, PhOPh, 175 °C, 24 h, 68%, (ii) BnBr, K2CO3, dimethylformamide (DMF), 100 °C, 24 h, 54%; (iii) Et3N, acyl chlorides, 4a 75%, 4b 94%, 4e 88%, 4f 88%; (iv) Pd(OH)2/C or Pd/C, H2, tetrahydrofuran (THF), r.t., 4 d, 5a 46%, 5b 73%, 5c 56% over 2 steps, 5d 60% over 2 steps, 5e 70%, 5f 55%, 5g 88% over two steps.
The addition of a range of acyl chlorides to compound 3, in the presence of triethylamine, afforded the 5-O-acyl-tetra-O-protected derivatives (4a–g). Compounds 4a, 4b, 4e and 4f were easily separable from the starting material and were afforded in 75–94% yields. Derivatives 4c, 4d and 4g were inseparable from 3 and were further reacted without purification. These penta-substituted quercetin products were immediately identified on thin layer chromatography (TLC) by their blue fluorescence under wavelength of 365 nm. Subsequently, the diphenyl dioxol and the benzyl groups of compounds 4a–g were simultaneously deprotected via hydrogenation catalysed by either Pd(OH)2/C or Pd/C, a reaction which required 4 days. Derivatives 5a, 5b, 5e and 5f were afforded in 46–73% yield from their corresponding analogue 4, whilst derivatives 5c, 5d and 5g were afforded in 56–88% yields over two steps from compound 3.
For all products obtained after deprotection, the four hydroxy proton signals at δ = 9.25–9.26 ppm (3-OH), δ = 11.00 (7-OH), δ = 9.49–9.53 (3′-OH) and δ = 9.25–9.28 (4′-OH) spectra were present on the 1H NMR spectra, whilst the proton signal for 5-OH was not observed. From 13C NMR spectra, it was observed that the carbon signals for C-5 of the derivatives had significantly shifted upfield, whilst the other four hydroxy bearing carbon signals remained similar, compared to that reported for quercetin [18,19]. These observations confirmed that acylation had occurred on the desired 5-O position.
Significant differences were observed in the chemical properties between 1 and derivatives 5a–g. In contrast to their starting materials, these deprotected derivatives were easily differentiated on TLC by their yellow fluorescence under wavelength of 365 nm. The Rf values of 5a and 5b, acylated with the short acyl carbon chain lengths, and 5g, with the polar methyl succinyl group, were similar to 1 (Rf = 0.57 in 1:2 petroleum ether: EtOAc) which suggested that these compounds have similar polarity to the parent flavonoid. Whilst, the Rf values of derivatives 5c, 5d, 5e and 5f, acylated with medium to long acyl carbon chains, suggested that they were less polar compared to quercetin. All seven derivatives 5a–f were readily soluble in tetrahydrofuran solvent, whereas quercetin 1 was insoluble in this solvent. This shows that acylation at the 5-O position resulted in more organic soluble molecules compared to 1 alone. It was also noted that the addition of the acyl groups at the 5-O position had greatly reduced the melting poing of the derivatives (160–189 °C) compared that reported for 1 (313–314 °C) [20].
2.2. Antiproliferative Studies against HCT116 and MDA-MB-231 Cancer Cell Lines
Previous studies have reported that quercetin 1 has antiproliferative activity against the colon HCT116 and breast MDA-MB-231 cancer cell lines and were therefore chosen to study the effect of adding the acyl groups at the 5 position [21,22,23,24]. Quercetin (1) and derivatives 5a–g were tested against these cell lines at a single concentration of 20 μM (Figure 2). The treatment of quercetin had reduced the proliferation rates of HCT116 and MDA-MB-231 cells to 1.6 (±1.1)% and 12.0 (±1.1)% of control, respectively. In comparison, it was found that derivatives 5d and 5e had similar inhibitory activity against HCT116 to flavonoid 1, with cell proliferation rates at 1.14 (±1.1)% and 0.86 (±0.7)% of control, respectively; whilst derivatives 5b, 5c, 5d and 5e had improved inhibitory activity against MDA-MB-231, with proliferation rates reduced to 9.41 (±1.0)%, 9.43 (±1.2)%, 6.59 (±1.0)% and 8.21 (±0.7)% of control, respectively.
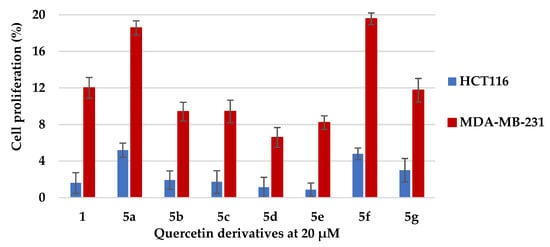
Figure 2.
Mean cell proliferation rates (±standard error(SE) of quercetin and 5-O-quercetin derivatives against HCT116 and MDA-MB-231 cancer cell lines (cell proliferation in presence of control = 100%).
Following this single dose study to determine activity, the IC50 values of these derivatives against these cell lines were then determined (Table 1). The IC50 of the flavonoid 1 against HCT116 and MDA-MB-231 are 5.79 (±0.13) µM and 5.81 (±0.13) µM, respectively. Derivatives 5c–g displayed increased potency against both cancer cell lines, as their IC50 values had significantly decreased in comparison to flavonoid 1. From these results, the 5c, 5d, 5e and 5g displayed improved activity against both cell lines compared to quercetin, with 5e displaying the lowest IC50 values against HCT116 and MDA-MB-231 cell lines at 1.53 (±0.02) µM and 1.51 (±0.01) µM, respectively. The shorter acyl carbon chain derivatives 5a and 5b were less effective as they were observed to have reduced antiproliferative activity with higher IC50 values, whilst the longest acyl carbon chain derivative 5f displayed similar activity to quercetin against both cell lines.

Table 1.
Mean IC50 values (±SE) of quercetin and quercetin 5-O-acyl quercetin derivatives.
2.3. ABTS and DPPH Radical Scavenging Activity Studies
Quercetin (1) and derivatives 5a–g were then assessed for their radical scavenging activity, and therefore their antioxidant capacity, against the two stable radical species, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical. Trolox was used as a reference antioxidant. The Trolox concentration-activity standard curves were generated using 6 different concentrations of Trolox ranging between 40–400 µM against ABTS radical cation and 6 different concentrations of Trolox ranging between 80–800 µM against DPPH radical. The standard curves of Trolox against ABTS and DPPH displayed linearity of above 0.99 and 0.98, respectively (see supplementary material). The activities of quercetin against the ABTS radical cation were measured using 6 different concentrations ranging between 20–150 µM; and against the DPPH radical using 6 concentrations ranging between 50–500 µM. Using the Trolox standard curve equations, the percentage of the radical scavenged was converted to the Trolox equivalent (TE) concentration (Figure 3).
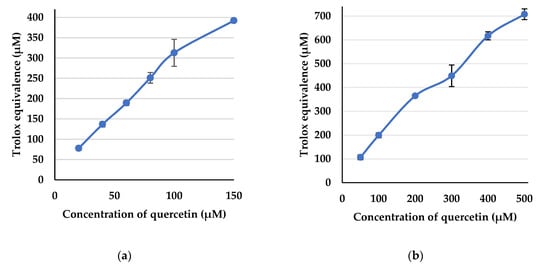
Figure 3.
Mean Trolox equivalence (TE) concentration (±SE) of quercetin at various concentrations against (a) ABTS radical cation; and (b) against DPPH radical.
The radical scavenging activity of the quercetin derivatives 5a–g was then determined (see supplementary material). These activities were compared to that of quercetin and are expressed in this report as the quercetin’s equivalence activity shown on Table 2. All derivatives, at all concentrations tested, displayed radical scavenging activity against both radicals which indicates that the acyl derivatisation at the 5-O position did not eliminate this important activity. It was generally found that the radical scavenging activities of the derivatives at each concentration against both radical species were not significantly different to that of quercetin (p > 0.05). This is with exception to 5b (20 and 40 µM) and 5c (20, 40, 60 and 150 µM) against ABTS as well as 5a (200, 400 and 500 µM) and 5c (200, 400 and 500 µM) against DPPH which were determined as significantly different (p < 0.05), and therefore displayed slightly reduced activity to that of quercetin.

Table 2.
Quercetin’s equivalence radical scavenging activity of the 5-O-acyl quercetin derivatives against ABTS radical cation and DPPH radical at various concentrations.
2.4. General Discussion
In general, the 5-O acylation of quercetin improved the antiproliferative activity and did not diminish the radical scavenging activity. From antiproliferative activity studies against the two cancer cell lines, derivatives 5c, 5d, 5e and 5g had displayed lower IC50 values, indicating improvement, whereas derivatives 5a and 5b, with shorter acyl carbon chains, had reduced effect. From radical scavenging activity studies, the activities of 5d, 5e and 5g against the radical species were not significantly different to that of quercetin, whilst 5a, 5b and 5c, at some concentrations, did have slightly reduced activity. Therefore, derivatives 5d, 5e and 5g were determined as having better, overall, activity in this study, with derivative 5e possessing the lowest IC50 values against the tested cancer cell lines and without compromise towards radical scavenging activity against the two radical species.
In comparison, it was deduced that longer acyl chain derivatives of quercetin had better overall activity compared to the shorter acyl chain derivatives, suggesting that increased lipophilic character leads to increased bioavailability and improved activity. These results are in accordance with previous studies which have shown esters of flavonoids have increased bioavailability [9,13]. Though in those cases reducing radical scavenging was seen due to derivatization of the phenols known to be critical for this activity. Derivative 5g was also effective, which suggests that the biological effects from derivatisation with polar functional groups could also be explored.
3. Materials and Methods
3.1. General Synthetic Procedures
General Procedure A: Acylation of phenol: To a stirred solution of phenol (1 mmol) in CH2Cl2 (25 mL) was added Et3N (3 mmol) and the mixture was stirred for 5 min. Acid chloride (2–5 mmol) was then added and the reaction mixture was stirred for 2–24 h. The reaction mixture was quenched by addition of saturated aqueous NaHCO3. This mixture was extracted with CH2Cl2 (3 × 20 mL). The combined organic extracts were dried (MgSO4) and the solvent removed in vacuo. The crude product was purified by flash chromatography to afford the desired product.
General Procedure B: Removal of diphenyl dioxol and benzyl groups: To a stirred solution of benzyl ether (1 mmol) in THF (50 mL) was added 10% Pd/C or 20% Pd(OH)2 (0.2 mmol) and the mixture was placed under an atmosphere of H2. The reaction mixture was stirred for 24 h or 4 d, as specified. This mixture was then filtered through celite, washing with THF. The solvent was removed in vacuo. The crude product was purified by flash chromatography to afford the desired product.
3.2. Specific Synthetic Procedures and Characterisation
2-(2′,2′-Diphenylbenzo[d][1′,3′]dioxol-5-yl)-3,5,7-trihydroxy-4H-chromen-4-one (2): To a stirred solution of quercetin 1 (3.0 g, 9.93 mmol) in diphenylether (60 mL) at 60 °C was added dichlorodiphenylmethane (2.85 mL, 14.89 mmol). The reaction mixture was then stirred at 175 °C for 24 h. The mixture was cooled to r.t. and petroleum ether (50 mL) was added to precipitate the crude product. The precipitate was filtered and further washed with petroleum ether (60 mL). The crude solid was dissolved in EtOAc and the solvent removed in vacuo. The resulting crude product was purified by flash chromatography (4:1 Petroleum ether:EtOAc) to give the title compound 2 (3.13 g, 68%) as a yellow solid. Rf: 0.30 (4:1 Petroleum ether:EtOAc). M.P.: 240–242 °C (literature 218–219 °C) [17]. δH (400 MHz; d6-DMSO): 6.20 (1H, d, J = 2.0 Hz, 6-H), 6.47 (1H, d, J = 2.0 Hz, 8-H), 7.22 (1H, d, J = 8.3 Hz, 7′-H), 7.43–7.49 (6H, m, Ar-H), 7.55–7.58 (4H, m, Ar-H), 7.80–7.83 (2H, m, 4′-H and 6′-H), 9.63 (1H, s, 3-OH), 10.82 (1H, s, 7-OH), 12.38 (1H, s, 5-OH). δC (100 MHz; d6-DMSO): 93.6 (C-8), 98.3 (C-6), 103.1 (C-4a), 107.8 (C-4′), 108.8 (C-7′), 117.0 (C-2′), 123.0 (C-6′), 125.2 (C-5′), 125.8 (C-2″), 128.6, 129.5 (C-3″ and C-4″), 136.4 (C-3), 139.4 (C-1″), 145.6 (C-2), 146.7 (C-3′a), 147.6 (C-7′a), 156.2 (C-8a), 160.7 (C-5), 164.1 (C-7), 176.0 (C-4). IR: νmax/cm−1; 696, 730, 747, 757, 775, 817, 827, 850, 871, 906, 951, 987, 1003, 1019, 1044, 1093, 1122, 1155, 1190, 1209, 1237, 1256, 1316, 1347, 1389, 1439, 1487, 1522, 1566, 1596, 1614, 1631, 1653, 2586, 3064, 3334. HRMS (ESI+): Found (MNa+) 489.0929, C28H18NaO7 requires 489.0945. The 1H NMR δ values are in agreement with literature [17].
3,7-Bis(benzyloxy)-2-(2′,2′-diphenylbenzo[d][1′,3′]dioxol-5-yl)-5-hydroxy-4H-chromen-4-one (3): To a stirred solution of 2 (2.5 g, 5.36 mmol) and K2CO3 (1.63 g, 11.79 mmol) in DMF (100 mL) at r.t. was added benzyl bromide (1.40 mL, 11.79 mmol) and the reaction mixture was stirred at 110 °C for 24 h. The reaction mixture was quenched by addition of H2O (70 mL). The resulting mixture was extracted with CH2Cl2 (3 × 70 mL). The combined organic extracts were further washed with excess H2O (3 × 70 mL). The organic extract was dried (MgSO4) and the solvent removed in vacuo. The crude product was purified by flash chromatography (9:1 Petroleum ether:EtOAc) to give the title compound 3 (1.88 g, 54%) as a yellow solid. Rf: 0.48 (9:1 Petroleum ether:EtOAc). M.P.: 130–132 °C (literature 90–92 °C) [17]. δH (400 MHz; CDCl3): 5.04 (2H, s, 3-OCH2), 5.13 (2H, s, 7-OCH2Ar), 6.44 (1H, d, J = 2.5 Hz, 6-H), 6.48 (1H, d, J = 2.5 Hz, 8-H), 6.92 (1H, d, J = 8.3 Hz, 7′-H), 7.14–7.22 (3H, m, Ar-H), 7.26–7.29 (2H, m, Ar-H), 7.34–7.45 (11H, m, Ar-H), 7.50 (1H, d, J = 2.0 Hz, 4′-H), 7.57 (1H, dd, J = 8.3, 2.0 Hz, 6′-H), 7.59–7.62 (4H, m, 2″-H). δC (100 MHz; CDCl3): 70.6 (7-OCH2), 74.6 (3-OCH2), 93.2 (C-8), 98.8 (C-6), 106.3 (C-4a), 108.5 (C-7′), 109.2 (C-4′), 124.2 (C-6′), 124.4 (C-5′), 126.4 (C-2″) 128.3, 128.5, 128.9, 129.1, 129.5 (Ar-C), 135.9 (7-OCH2C(Ar)), 136.3 (3-OCH2C(Ar)), 137.4 (C-3), 139.9 (C-1″), 147.4 (C-3′a), 149.4 (C-7′a), 156.8, 156.9 (C-2 and C-8a), 162.2 (C-5), 164.6 (C-7), 178.9 (C-4). IR: νmax/cm−1; 694, 750, 776, 813, 909, 948, 987, 1017, 1042, 1092, 1155, 1182, 1202, 1237, 1257, 1308, 1331, 1382, 1448, 1488, 1596, 1654, 2874, 3032. HRMS (ESI+): Found (MNa+) 669.1872, C42H30NaO7 requires 669.1884. The 1H NMR δ values are in agreement with literature [17].
3,7-Bis(benzyloxy)-2-(2′,2′-diphenylbenzo[d][1′,3′]dioxol-5-yl)-4-oxo-4H-chromen-5-yl acetate (4a): The reaction was carried out according to general procedure A with 3 (0.23 g, 0.36 mmol), Et3N (0.15 mL, 1.08 mmol) and acetyl chloride (0.05 mL, 0.72 mmol). The crude product was purified by flash chromatography (4:1 Petroleum ether:EtOAc) to give the title compound 4a (0.19 g, 75%) as a white solid. Rf: 0.37 (4:1 Petroleum ether:EtOAc). M.P.: 167–170 °C. δH (400 MHz; CDCl3): 2.49 (3H, s, 2″-H), 5.00 (2H, s, 3-OCH2), 5.13 (2H, s, 7-OCH2), 6.69 (1H, d, J = 2.4 Hz, 6-H), 6.85 (1H, d, J = 2.4 Hz, 8-H), 6.89 (1H, d, J = 8.4 Hz, 7′-H), 7.07–7.18 (3H, m Ar-H), 7.21–7.23 (2H, m, Ar-H), 7.34–7.45 (12H, m, 4′-H and Ar-H), 7.48 (1H, dd, J = 8.4, 2.0 Hz, 6′-H), 7.57–7.63 (4H, m, 2‴-H). δC (100 MHz; CDCl3): 21.4 (C-2″), 70.9 (7-OCH2), 74.3 (3-OCH2), 99.7 (C-8), 108.3 (C-7′), 108.8 (C-6), 109.1 (C-4′), 111.7 (C-4a), 117.8 (C-2′), 124.0 (C-6′), 124.5 (C-5′), 126.4 (C-2‴), 127.7, 128.1, 128.1, 128.5, 128.6, 128.9, 129.1, 129.5 (Ar-C), 135.5 (7-OCH2C(Ar)), 136.5 (3-OCH2C(Ar)), 139.2 (C-3), 140.0 (C-1‴), 147.3 (C-3′a), 149.1 (C-7′a), 150.7 (C-8a), 155.2 (C-2), 157.9 (C-9), 162.4 (C-7), 169.9 (C-1″), 173.3 (C-4). IR: νmax/cm−1; 666, 693, 717, 733, 752, 779, 794, 823, 888, 910, 947, 981, 1019, 1044, 1078, 1151, 1184, 1207, 1234, 1260, 1293, 1335, 1366, 1398, 1443, 1496, 1596, 1625, 1763, 2923, 3032, 3062. HRMS (ESI+): Found (MNa+), 711.1966, C44H32NaO8 requires 711.1989.
2-(3′,4′-Dihydroxyphenyl)-3,7-dihydroxy-4-oxo-4H-chromen-5-yl acetate (5a): The reaction was carried out according to general procedure B with 4a (0.17 g, 0.24 mmol) and 20% Pd(OH)2/C (34 mg, 0.05 mmol). The reaction was stirred for 4 d. The crude product was purified by flash chromatography (1:1 Petroleum ether: EtOAc) to give the title compound 5a (38 mg, 46%) as a yellow solid. Rf: 0.48 (1:2 Petroleum ether:EtOAc). M.P.: 165–168 °C. δH (400 MHz; d6-DMSO): 3.32 (3H, s, 2″-H), 6.55 (1H, d, J = 2.1 Hz, 6-H), 6.83 (1H, d, J = 2.1 Hz, 8-H), 6.88 (1H, d, J = 8.4 Hz, 5′-H), 7.51 (1H, dd, J = 8.4, 1.9 Hz, 6′-H), 7.66 (1H, d, J = 1.9 Hz, 2′-H), 9.04 (1H, s, 3-OH), 9.25 (1H, s, 4′-OH), 9.50 (1H, s, 3′-OH), 11.02 (1H, s, 7-OH). δC (100 MHz; d6-DMSO): 20.9 (C-2″), 100.1 (C-8), 107.6 (C-4a), 108.1 (C-6), 114.8 (C-2′), 115.6 (C-5′), 119.5 (C-6′), 122.0 (C-1′), 137.2 (C-3), 144.1 (C-2), 145.0 (C-3′), 147.3 (C-4′), 149.8 (C-5), 156.9 (C-8a), 161.7 (C-7), 168.8 (C-1″), 170.6 (C-4). IR: νmax/cm−1; 678, 395, 737, 785, 809, 845, 864, 910, 937, 997, 1027, 1043, 1079, 1124, 1160, 1190, 1207, 1239, 1272, 1327, 1368, 1418, 1454, 1516, 1548, 1592, 1621, 1643, 1733, 2922, 3301. HRMS (ESI+): Found (MNa+) 367.0423, C17H12NaO8 requires 367.0424.
3,7-Bis(benzyloxy)-2-(2′,2′-diphenylbenzo[d][1′,3′]dioxol-5-yl)-4-oxo-4H-chromen-5-yl propionate (4b): The reaction was carried out according to general procedure A with 3 (0.22 g, 0.34 mmol), Et3N (0.14 mL, 1.01 mmol) and propionyl chloride (0.06 mL, 0.67 mmol). The crude product was purified by flash chromatography (4:1 Petroleum ether:EtOAc) to give the title compound 4b (0.22 g, 94%) as white solid. Rf: 0.58 (4:1 Petroleum ether:EtOAc). M.P.: 125–128 °C. δH (400 MHz; CDCl3): 1.34 (3H, t, J = 7.5 Hz, 3″-H), 2.83 (2H, q, J = 7.5 Hz, ″-H), 4.99 (2H, s, 3-OCH2), 5.13 (2H, s, 7-OCH2), 6.68 (1H, d, J = 2.4 Hz, 6-H), 6.84 (1H, d, J = 2.4 Hz, 8-H), 6.89 (1H, d, J = 8.4 Hz, 7′-H), 7.07–7.17 (3H, m Ar-H), 7.21–7.23 (2H, m, Ar-H), 7.36–7.44 (12H, m, 4′-H, Ar-H), 7.48 (1H, dd, J = 8.4, 1.7 Hz, 6′-H), 7.56–7.61 (4H, m, 2‴-H). δC (100 MHz; CDCl3): 9.0 (C-3″), 27.8 (C-2″), 70.8 (7-OCH2), 74.3 (3-OCH2), 99.6 (C-8), 108.3 (C-7′), 108.8 (C-6), 109.1 (C-4′), 111.9 (C-4a), 117.8 (C-2′), 123.9 (C-6′), 124.6 (C-5′), 126.4 (C-2‴), 127.7, 128.1, 128.1, 128.5, 128.6, 128.9, 129.2, 129.5 (Ar-C), 135.6 (7-OCH2C(Ar)), 136.5 (3-OCH2C(Ar)), 139.2 (C-3), 140.0 (C-1‴), 147.3 (C-3′a), 149.0 (C-7′a), 150.9 (C-8a), 155.1 (C-2), 157.9 (C-9), 162.4 (C-7), 173.2 (C-1″), 173.2 (C-4). IR: νmax/cm−1; 667, 693, 728, 746, 779, 810, 839, 885, 914, 949, 981, 1019, 1042, 1085, 1127, 1155, 1187, 1212, 1233, 1259, 1295, 1333, 1360, 1380, 1400, 1442, 1496, 1566, 1596, 1627, 1763, 1956, 2941, 3032, 3063. HRMS (ESI+): Found (MNa+) 725.2133, C45H34NaO8 requires 725.2146.
2-(3′,4′-Dihydroxyphenyl)-3,7-dihydroxy-4-oxo-4H-chromen-5-yl propionate (5b): The reaction was carried out according to general procedure B with 4b (0.21 g, 0.3 mmol) and 20% Pd(OH)2/C (42 mg, 0.06 mmol). The reaction was stirred for 4 d. The crude product was purified by flash chromatography (1:1 Petroleum ether:EtOAc) to give the title compound 5b (78 mg, 73%) as a yellow solid. Rf: 0.46 (1:2 Petroleum ether:EtOAc). M.P.: 180–183 °C. δH (400 MHz; d6-DMSO): 1.76 (3H, t, J = 7.5 Hz, 3″-H), 2.70 (2H, q, J = 7.5 Hz, 2″-H), 6.55 (1H, d, J = 2.0 Hz, 6-H), 6.82 (1H, d, J = 2.0 Hz, 8-H), 6.88 (1H, d, J = 8.7 Hz, 5′-H), 7.51 (1H, dd, J = 8.7, 2.0 Hz, 6′-H), 7.66 (1H, d, J = 2.0 Hz, 2′-H), 9.00 (1H, s, 3-OH), 9.25 (1H, s, 4′-OH), 9.49 (1H, s, 3′-OH), 11.01 (1H, s, 7-OH). δC (100 MHz; d6-DMSO): 8.6 (C-3″), 26.9 (C-2″), 100.1 (C-8), 107.7 (C-4a), 108.2 (C-6), 114.9 (C-2′), 115.6 (C-5′), 119.5 (C-6′), 122.0 (C-1′), 137.2 (C-3), 144.1 (C-2), 145.0 (C-3′), 147.3 (C-4′), 149.9 (C-5), 156.9 (C-8a), 161.7 (C-7), 170.7 (C-1″), 172.1 (C-4). IR: νmax/cm−1; 694, 726, 788, 814, 841, 874, 900, 927, 997, 1091, 1154, 1189, 1267, 1311, 1364, 1412, 1448, 1508, 1547, 1593, 1735, 2944, 3323. HRMS (ESI+): Found (MNa+) 381.0577, C18H14NaO8 requires 381.0581.
2-(3′,4′-Dihydroxyphenyl)-3,7-dihydroxy-4-oxo-4H-chromen-5-yl hexanoate (5c): The reaction was carried out firstly according to general procedure A with 3 (0.2 g, 0.31 mmol), Et3N (0.13 mL, 0.93 mmol) and hexanoyl chloride (0.09 mL, 0.62 mmol) to give a crude ester which was taken to the next step without further purification. Then, according to general procedure B using the above produced ester (0.24 g, 0.32 mmol) and 20% Pd(OH)2/C (45 mg, 0.06 mmol). The reaction mixture was stirred for 4 d. The crude product was purified by flash chromatography (1:1 Petroleum ether:EtOAc) to give the title compound 5c (72 mg, 56% over 2 steps) as a yellow solid. Rf: 0.63 (1:2 Petroleum ether:EtOAc). M.P.: 160–163 °C. δH (400 MHz; d6-DMSO): 0.91 (3H, t, J = 7.1 Hz, 6″-H), 1.32–1.39 (4H, m, 4″-H and 5″-H), 1.68 (2H, p, J = 7.5 Hz, 3″-H), 2.66 (2H, t, J = 7.5 Hz, 2″-H), 6.53 (1H, d, J = 2.1 Hz, 6-H), 6.82 (1H, d, J = 2.1 Hz, 8-H), 6.88 (1H, d, J = 8.5 Hz, 5′-H), 7.51 (1H, dd, J = 8.5, 2.2 Hz, 6′-H), 7.66 (1H, d, J = 2.2 Hz, 2′-H), 8.98 (1H, s, 3-OH), 9.25 (1H, s, 4′-OH), 9.50 (1H, s, 3′-OH), 11.00 (1H, s, 7-OH). δC (100 MHz; d6-DMSO): 13.9 (C-6″), 21.9 (C-5″), 23.6 (C-3″), 30.7 (C-4″), 33.5 (C-2″), 100.1 (C-8), 107.7 (C-4a), 108.2 (C-6), 114.8 (C-2′), 115.6 (C-5′), 119.5 (C-6′), 122.0 (C-1′), 137.2 (C-3), 144.0 (C-2), 145.0 (C-3′), 147.3 (C-4′), 149.9 (C-5), 156.9 (C-8a), 161.7 (C-7), 170.7 (C-1″), 171.3 (C-4). IR: νmax/cm−1; 662, 692, 727, 747, 791, 822, 845, 883, 928, 991, 1105, 1140, 1187, 1214, 1249, 1277, 1312, 1379, 1415, 1446, 1509, 1518, 1536, 1584, 1624, 1722, 2854, 2924, 2956, 3319. HRMS (ESI+): Found (MNa+) 423.1034, C21H20NaO8 requires 423.1050.
2-(3′,4′-Dihydroxyphenyl)-3,7-dihydroxy-4-oxo-4H-chromen-5-yl octanoate (5d): The reaction was carried out firstly according to general procedure A with 3 (0.2 g, 0.31 mmol), Et3N (0.13 mL, 0.93 mmol) and octanoyl chloride (0.19 mL, 0.62 mmol) The product was taken into the next step without purification. Then, according to general procedure B using the above produced ester (0.24 g, 0.31 mmol) and 20% Pd(OH)2/C (43 mg, 0.06 mmol). The reaction was stirred for 4 d. The crude product was purified by flash chromatography (2:1 Petroleum ether:EtOAc) to give the title compound 5d (80 mg, 60% over 2 steps) as a yellow solid. Rf: 0.28 (1:1 Petroleum ether:EtOAc). M.P.: 178–181 °C. δH (400 MHz; d6-DMSO): 0.88 (3H, t, J = 6.9 Hz, 8″-H), 1.21–1.34 (6H, broad m, 5″-H, 6″-H and 7″-H), 1.33–1.38 (2H, m, 4″-H), 1.67 (2H, p, J = 7.6 Hz, 3″-H), 2.66 (2H, t, J = 7.6 Hz, 2″-H), 6.53 (1H, d, J = 2.5 Hz, 6-H), 6.82 (1H, d, J = 2.5 Hz, 8-H), 6.88 (1H, d, J = 8.5 Hz, 5′-H), 7.51 (1H, dd, J = 8.5, 2.2 Hz, 6′-H), 7.66 (1H, d, J = 2.2 Hz, 2′-H), 8.98 (1H, s, 3-OH), 9.26 (1H, s, 4′-OH), 9.51 (1H, s, 3′-OH), 11.01 (1H, s, 7-OH). δC (100 MHz; d6-DMSO): 14.0 (C-8″), 22.1 (C-7″), 24.0 (C-3″), 28.5, 28.5 (C-4″ and C-5″), 31.2 (C-6″), 33.5 (C-2″), 100.1 (C-8), 107.8 (C-4a), 108.2 (C-6), 114.9 (C-2′), 115.6 (C-5′), 119.5 (C-6′), 122.0 (C-1′), 137.2 (C-3), 144.1 (C-2), 145.0 (C-3′), 147.3 (C-4′), 149.9 (C-5), 156.9 (C-8a), 161.7 (C-7), 170.7 (C-1″), 171.4 (C-4). IR: νmax/cm−1; 662, 696, 718, 727, 747, 789, 823, 843, 882, 925, 990, 1006, 1114, 1139, 1158, 1185, 1214, 1277, 1315, 1356, 1383, 1400, 1417, 1446, 1509, 1541, 1583, 1624, 1634, 1724, 2854, 2920, 2952, 3335. HRMS (ESI+): Found (MNa+) 451.1368, C23H24NaO8 requires 451.1363.
3,7-Bis(benzyloxy)-2-(2′,2′-diphenylbenzo[d][1′,3′]dioxol-5-yl)-4-oxo-4H-chromen-5-yl dodecanoate (4e): The reaction was carried out according to general procedure A with 3 (0.2 g, 0.31 mmol), Et3N (0.13 mL, 0.93 mmol) and lauroyl chloride (0.14 mL, 0.62 mmol). The crude product was purified by flash chromatography (9:1 Petroleum ether:EtOAc) to give the title compound 4e (0.23 g, 88%) as white solid. Rf: 0.23 (9:1 Petroleum ether:EtOAc). M.P.: 75–76 °C. δH (400 MHz; CDCl3): 0.88 (3H, t, J = 6.9 Hz, 12″-H), 1.22–1.41 (14H, broad m, 5″-H, 6″-H, 7″-H, 8″-H, 9″-H, 10″-H and 11″-H), 1.43–1.50 (2H, m, 4″-H), 1.83 (2H, p, J = 7.6 Hz, 3″-H), 2.79 (2H, t, J = 7.6 Hz, 2″-H), 4.99 (2H, s, 3-OCH2), 5.13 (2H, s, 7-OCH2), 6.67 (1H, d, J = 2.4 Hz, 6-H), 6.84 (1H, d, J = 2.4 Hz, 8-H), 6.88 (1H, d, J = 8.5 Hz, 7′-H), 7.07–7.18 (3H, m Ar-H), 7.21–7.23 (2H, m, Ar-H), 7.34–7.44 (12H, m, 4′-H and Ar-H), 7.48 (1H, dd, J = 8.5, 1.8 Hz, 6′-H), 7.56–7.61 (4H, m, 2‴-H). δC (100 MHz; CDCl3): 14.3 (C-12″), 22.8 (C-11″), 24.7 (C-3″), 29.4, 29.5, 29.5, 29.7, 29.7, 29.8, 29.8, 31.1 (C-4″, C-5″, C-6″, C-7″, C-8″ and C-9″), 32.1 (C-10″), 34.4 (C-2″), 70.8 (7-OCH2), 74.3 (3-OCH2), 99.6 (C-8), 108.3 (C-7′), 108.9 (C-6), 109.1 (C-4′), 111.9 (C-4a), 117.8 (C-2′), 123.9 (C-6′), 124.6 (C-5′), 126.4 (C-2‴), 127.7, 128.1, 128.1, 128.5, 128.6, 128.9, 129.2, 129.5 (Ar-C), 135.6 (7-OCH2C(Ar)), 136.6 (3-OCH2C(Ar)), 139.3 (C-3), 140.0 (C-1‴), 147.3 (C-3′a), 149.0 (C-7′a), 150.9 (C-5), 155.0 (C-2), 157.9 (C-9), 162.4 (C-7), 172.6 (C-1″), 173.2 (C-4). IR: νmax/cm−1; 695, 749, 776, 818, 841, 908, 948, 980, 1018, 1042, 1079, 1105, 1151, 1182, 1209, 1234, 1255, 1292, 1316, 1333, 1366, 1397, 1444, 1494, 1625, 1765, 2853, 2923, 3032, 3065. HRMS (ESI+): Found (MNa+) 851.3527, C54H52NaO8 requires 851.3554.
2-(3′,4′-Dihydroxyphenyl)-3,7-dihydroxy-4-oxo-4H-chromen-5-yl dodecanoate (5e): The reaction was carried out according to general procedure B with 4e with (0.22 g, 0.26 mmol) and 20% Pd(OH)2/C (37 mg, 0.05 mmol). The reaction was stirred for 4 d. The product was purified by flash chromatography (2:1 Petroleum ether:EtOAc) to give the title compound 5e (90 mg, 70%) as a yellow solid. Rf: 0.39 (1:1 Petroleum ether:EtOAc). M.P.: 165–168 °C. δH (400 MHz; d6-DMSO): 0.85 (3H, t, J = 7.0 Hz, 12″-H), 1.20–1.33 (14H, broad m, 5″-H, 6″-H, 7″-H, 8″-H, 9″-H, 10″-H and 11″-H), 1.34–1.38 (2H, m, 4″-H), 1.67 (2H, p, J = 7.6 Hz, 3″-H), 2.66 (2H, t, J = 7.6 Hz, 2″-H), 6.52 (1H, d, J = 2.1 Hz, 6-H), 6.82 (1H, d, J = 2.1 Hz, 8-H), 6.87 (1H, d, J = 8.5 Hz, 5′-H), 7.51 (1H, dd, J = 8.5, 2.3 Hz, 6′-H), 7.66 (1H, d, J = 2.3 Hz, 2′-H), 8.96 (1H, s, 3-OH), 9.25 (1H, s, 4′-OH), 9.50 (1H, s, 3′-OH), 11.01 (1H, s, 7-OH). δC (100 MHz; d6-DMSO): 13.9 (C-12″), 22.1 (C-11″), 24.0 (C-3″), 28.5, 28.7, 28.8, 28.9, 29.0 (C-4″, C-5″, C-6″, C-7″, C-8″ and C-9″), 31.3 (C-10″), 33.5 (C-2″), 100.1 (C-8), 107.7 (C-4a), 108.2 (C-6), 114.8 (C-2′), 115.6 (C-5′), 119.5 (C-6′), 122.0 (C-1′), 137.2 (C-3), 144.0 (C-2), 145.0 (C-3′), 147.3 (C-4′), 149.9 (C-5), 156.9 (C-8a), 161.7 (C-7), 170.6 (C-1″), 171.3 (C-4). IR: νmax/cm−1; 692, 715, 750, 791, 813, 844, 883, 928, 994, 1103, 1131, 1156, 1205, 1246, 1317, 1376, 1412, 1448, 1523, 1598, 1723, 2852, 2923, 3323. HRMS (ESI+): Found (MNa+) 507.1979, C27H32NaO8 requires 507.1989.
3,7-Bis(benzyloxy)-2-(2′,2′-diphenylbenzo[d][1′,3′]dioxol-5-yl)-4-oxo-4H-chromen-5-yl palmitate (4f): The reaction was carried out according to general procedure A with 3 (0.5 g, 0.77 mmol), Et3N (0.32 mL, 2.32 mmol) and palmitoyl chloride (0.47 mL, 1.55 mmol). The crude product was purified by flash chromatography (15:1 Petroleum ether:EtOAc) give the title compound 4f (0.60 g, 88%) as a colourless oil. Rf: 0.62 (15:1 Petroleum ether:EtOAc). δH (400 MHz; CDCl3): 0.88 (3H, t, J = 6.9 Hz, 16″-H), 1.24–1.42 (22H, broad m, 5″-H, 6″-H, 7″-H, 8″-H, 9″-H, 10″-H, 11″-H, 12″-H, 13″-H, 14″-H and 15″-H), 1.43–1.49 (2H, m, 4″-H), 1.84 (2H, p, J = 7.6 Hz, 3″-H), 2.79 (2H, t, J = 7.6 Hz, 2″-H), 4.99 (2H, s, 3-OCH2), 5.13 (2H, s, 7-OCH2), 6.67 (1H, d, J = 2.4 Hz, 6-H), 6.84 (1H, d, J = 2.4 Hz, 8-H), 6.88 (1H, d, J = 8.3 Hz, 7′-H), 7.07–7.17 (3H, m Ar-H), 7.21–7.23 (2H, m, Ar-H), 7.34–7.45 (12H, m, 4′-H and Ar-H), 7.48 (1H, dd, J = 8.3, 2.0 Hz, 6′-H), 7.58–7.61 (4H, m, 2‴-H). δC (100 MHz; CDCl3): 14.3 (C-16″), 22.8 (C-15″), 24.7 (C-3″), 29.4, 29.5, 29.7, 29.8, 29.8 (C-4″, C-5″, C-6″, C-7″, C-8″, C-9″, C-10″, C-11″, C-12″ and C-13″), 32.1 (C-14″), 34.4 (C-2″), 70.8 (7-OCH2), 74.3 (3-OCH2), 99.6 (C-8), 108.3 (C-7′), 108.9 (C-6), 109.1 (C-4′), 111.9 (C-4a), 117.8 (C-2′), 123.9 (C-6′), 124.6 (C-5′), 126.4 (C-2‴), 127.7, 128.1, 128.1, 128.5, 128.6, 128.9, 129.2, 129.5 (Ar-C), 135.6 (7-OCH2C(Ar)), 136.6 (3-OCH2C(Ar)), 139.3 (C-3), 140.0 (C-1‴), 147.3 (C-3′a), 149.0 (C-7′a), 150.9 (C-5), 155.0 (C-2), 157.9 (C-8a), 162.4 (C-7), 172.6 (C-1″), 173.2 (C-4). IR: νmax/cm−1; 695, 750, 777, 818, 841, 909, 948, 980, 1018, 1043, 1107, 1151, 1183, 1209, 1234, 1255, 1292, 1316, 1333, 1366, 1398, 1444, 1494, 1625, 1766, 2852, 2922, 3034, 3611. HRMS (ESI+): Found (MNa+) 907.4189, C58H60NaO8 requires 907.4180.
2-(3′,4′-Dihydroxyphenyl)-3,7-dihydroxy-4-oxo-4H-chromen-5-yl palmitate (5f): The reaction was carried out according to general procedure B with 4f (0.26 g, 0.29 mmol) and 10% Pd/C (31 mg, 0.06 mmol). The reaction was stirred for 4 d. The crude product was purified by flash chromatography (9:1 Petroleum ether:EtOAc) to give the title compound 5f (87 mg, 55%) as a yellow solid. Rf: 0.43 (1:1 Petroleum ether:EtOAc). M.P.: 165–168 °C. δH (400 MHz; d6-DMSO): 0.84 (3H, t, J = 6.4 Hz, 16″-H), 1.17–1.34 (22H, broad m, 5″-H, 6″-H, 7″-H, 8″-H, 9″-H, 10″-H, 11″-H, 12″-H, 13″-H, 14″-H and 15″-H), 1.35–1.37 (2H, m, 4″-H), 1.67 (2H, p, J = 7.6 Hz, 3″-H), 2.66 (2H, t, J = 7.6 Hz, 2″-H), 6.52 (1H, d, J = 2.2 Hz, 6-H), 6.82 (1H, d, J = 2.2 Hz, 8-H), 6.88 (1H, d, J = 8.4 Hz, 5′-H), 7.51 (1H, dd, J = 8.4, 2.0 Hz, 6′-H), 7.66 (1H, d, J = 2.0 Hz, 2′-H), 8.98 (1H, s, 3-OH), 9.28 (1H, s, 4′-OH), 9.53 (1H, s, 3′-OH), 11.02 (1H, s, 7-OH). δC (100 MHz; d6-DMSO): 14.0 (C-16″), 22.1 (C-15″), 24.0 (C-3″), 28.5, 28.8, 28.8, 29.1, 29.1 (C-4″, C-5″, C-6″, C-7″, C-8″, C-9″, C-10″, C-11″, C-12″ and C-13″), 31.3 (C-14″), 33.5 (C-2″), 100.1 (C-8), 107.7 (C-4a), 108.2 (C-6), 114.9 (C-2′), 115.6 (C-5′), 119.6 (C-6′), 122.0 (C-1′), 137.2 (C-3), 144.0 (C-2), 145.0 (C-3′), 147.3 (C-4′), 149.9 (C-5), 156.9 (C-8a), 161.7 (C-7), 170.7 (C-1″), 171.3 (C-4). IR: νmax/cm−1; 658, 697, 720, 749, 788, 819, 844, 876, 921, 935, 992, 1080, 1113, 1149, 1196, 1218, 1244, 1281, 1334, 1365, 1379, 1421, 1456, 1469, 1501, 1520, 1555, 1587, 1616, 1741, 2851, 2922, 3122, 3316, 3548. HRMS (ESI+): Found (MNa+) 563.2597, C31H40NaO8 requires 563.2615.
2-(3′,4′-Dihydroxyphenyl)-3,7-dihydroxy-4-oxo-4H-chromen-5-yl methyl succinate (5g): The reaction was carried out firstly according to general procedure A with 3 (0.21 g, 0.32 mmol), Et3N (0.14 mL, 0.97 mmol) and methyl succinyl chloride (0.04 mL, 0.65 mmol) to give a crude ester which was taken to the next step without further purification. Then, according to general procedure B using the above produced ester (0.246 g, 0.32 mmol) and 20% Pd(OH)2/C (45 mg, 0.06 mmol). The reaction was stirred for 4 d. The product was purified by flash chromatography (1:1 Petroleum ether:EtOAc) to give the title compound 5g (0.12 g, 88% over 2 steps) as a yellow solid. Rf: 0.48 (2:1 Petroleum ether:EtOAc). M.P.: 186–189 °C. δH (400 MHz; d6-DMSO): 2.73 (2H, t, J = 6.9 Hz, 2″-H or 3″-H), 2.95 (2H, t, J = 6.9 Hz, 2″-H or 3″-H), 3.64 (2H, s, 6″-H), 6.54 (1H, d, J = 2.1 Hz, 6-H), 6.83 (1H, d, J = 2.1 Hz, 8-H), 6.88 (1H, d, J = 8.5 Hz, 5′-H), 7.51 (1H, dd, J = 8.5, 2.1 Hz, 6′-H), 7.66 (1H, d, J = 2.1 Hz, 2′-H), 9.01 (1H, s, 3-OH), 9.26 (1H, s, 4′-OH), 9.50 (1H, s, 3′-OH), 11.04 (1H, s, 7-OH). δC (100 MHz; d6-DMSO): 28.4, 29.0 (C-2″ and C-3″), 51.6 (C-6″), 100.3 (C-8), 107.6 (C-4a), 108.1 (C-6), 114.9 (C-2′), 115.6 (C-5′), 119.6 (C-6′), 122.0 (C-1′), 137.2 (C-3), 144.2 (C-2), 145.0 (C-3′), 147.4 (C-4′), 149.6 (C-5), 156.9 (C-8a), 161.7 (C-7), 170.4 (C-1″), 170.7 (C-4″), 171.3 (C-4). IR: νmax/cm−1; 667, 694, 722, 787, 814, 841, 881, 926, 993, 1038, 1079, 1104, 1152, 1205, 1248, 1271, 1308, 1378, 1413, 1444, 1505, 1519, 1549, 1588, 1623, 1730, 2928, 3340. HRMS (ESI+): Found (MNa+) 439.0640, C20H16NaO10 requires 439.0649.
3.3. Antiproliferative Activity Procedures
The antiproliferative studies on HCT116 and MDA-MB-231 cell lines were conducted using the [3H]-thymidine incorporation assay method as detailed in the report by Leung et al. [25]. Essentially, the method was conducted by seeding 3000 cells in each well of the 96 well plates, with varying concentrations of inhibitors for 3 days. [3H]-thymidine is added to the cells and incubated for 6 h before harvest. The cell proliferation were then analysed using Trilux/Betaplate counter showing percentage of the cells with [3H]-thymidine incorporated into the DNA helix. The cell lines were treated with 20 µM of each of the compounds in DMSO and the antiproliferative activity was determined as cell growth percentages relative to the 100% growth in control. The IC50 of the derivatives against the cell lines were then determined using concentrations of 10 µM or below.
3.4. Radical Scavenging Activity Procedures
Preparation of Trolox standard and quercetin derivative solutions: Trolox standards were prepared by dissolving in DMSO to give a 2 mM stock solution which was stored in −80 °C until use. To generate the standard curve against ABTS, the stock solution was diluted with DMSO to obtain concentrations of 40, 60, 80, 100, 200 and 400 µM. For the standard curve against DPPH, the stock solution was diluted with DMSO to obtain concentrations of 80, 100, 200, 400, 600 and 800 µM. The samples were prepared by dissolving quercetin derivatives in DMSO to make 2mM stock solution. For ABTS radical scavenging assay, the sample stock solutions were further diluted to concentrations of 20, 40, 60, 80, 100 and 150 µM. For DPPH radical scavenging assay, the sample stock solutions were then further diluted to concentrations of 50, 100, 200, 300, 400 and 500 µM.
Preparation of ABTS radical cation solution and DPPH radical solution: The ABTS radical cation solution was prepared according to the reported procedure [26,27]. A 20 mM acetate buffer was first prepared by adding CH3COONa in H2O and then adjusting the pH of this buffer to 4.5 with AcOH. Neutral ABTS was dissolved in this buffer solution to make a 7 mM ABTS solution. A 2.45 mM persulfate solution was prepared by dissolving K2S2O8 in H2O. The ABTS solution and persulfate solution were then added together in a 1:1 ratio. This mixture was then stored in the absence of light at r.t. for 12–16 h. The absorbance of the ABTS radical cation solution was then adjusted with MeOH to 0.700 (±0.01) at 734 nM. The DPPH radical solution was prepared using a procedure described in literature by dissolving DPPH in MeOH to make 0.1 mM, which was used for immediately [28].
Radical scavenging assessment: The ABTS radical cation or DPPH radical solution (200 µL) were added to each well in the 96 well plate. The Trolox standard and quercetin samples (10 µL) were then added to the ABTS or DPPH solution. For control, DMSO (10 µL) was added to the well. This was then incubated in the absence of light for 1 h. The resulting absorbance (abs) was measured at 734 nm and 517 nm for ABTS and DPPH, respectively. The radical scavenging activity of the standards and samples were measured in triplicate within each experiment. The radical scavenging experiments were conducted three times using freshly prepared radical solutions and samples for each experiment. The percentage radical scavenging activity of Trolox standard against both ABTS and DPPH were calculated according to Equation (1).
% radical scavenging activity = [(abs of control − abs of sample)/abs of control] × 100
Statistical analysis to compare the radical scavenging activity of each derivative at each concentration to that of quercetin was conducted using a two-sample t-test (two tailed) with unequal variances with significance level of 0.05. A p-value greater than 0.05 was determined as not significantly different and p-value equal to or less than 0.05 was determined as significantly different.
4. Conclusions
In conclusion, seven 5-O-acyl quercetin derivatives (5a–g) were synthesised and assessed on their antiproliferative activity and radical scavenging activity. In general, quercetin derivatives with longer 5-O-acyl carbon chain lengths displayed better activity against the HCT116 and MDA-MB-231 cancer cell lines compared to derivatives with shorter acyl chain lengths. Similarly, derivatives with longer 5-O-acyl carbon chain lengths had comparable radical scavenging activity against ABTS and DPPH radicals compared to quercetin, whilst derivatives with shorter acyl carbon chain lengths had slightly reduced activity. Amongst these derivatives prepared, the 5-O-octanoyl (5d), 5-O-lauroyl (5e) and 5-O-methyl succinyl (5g) quercetin derivatives displayed the best overall activity. These results show that the derivatisation of the 5-OH group can be used to modify the physical properties of quercetin whilst still maintaining, or even improving, the antiproliferative activity.
Supplementary Materials
The 1H and 13C NMR spectra of compounds 4a, 4b, 4e, 4f, 5a, 5b, 5c, 5d, 5e, 5f and 5g. Trolox standard curves (Figures S1 and S2) and the radical scavenging activity data of the quercetin derivatives (Figures S3 and S4).
Author Contributions
S.L., B.F. and D.B. contributed to the conceptualisation of the research. S.L. performed the syntheses of and radical scavenging activity studies on the compounds and E.L. performed the antiproliferative studies on the compounds. S.L. analysed the experimental data, wrote and edited the manuscript and D.B. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Marlborough Research Centre.
Acknowledgments
The authors wish to thank the Marlborough Research Centre for supporting this work and The University of Auckland for providing the scholarship.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Miean, K.H.; Mohamed, S. Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) Content of Edible Tropical Plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Sultana, B.; Anwar, F. Flavonols (Kaempeferol, Quercetin, Myricetin) Contents of Selected Fruits, Vegetables and Medicinal Plants. Food Chem. 2008, 108, 879–884. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health Effects of Quercetin: From Antioxidant to Nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Khan, I.A.; ur-Rehman, M.-; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer Potential of Quercetin: A Comprehensive Review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn Helen, M.I. Flavonoids as Prospective Compounds for Anti-Cancer Therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef]
- Ratnam, D.V.; Ankola, D.D.; Bhardwaj, V.; Sahana, D.K.; Kumar, M.N.V.R. Role of Antioxidants in Prophylaxis and Therapy: A Pharmaceutical Perspective. J. Control. Release 2006, 113, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Gali, H.U.; Perchellet, E.M.; Gao, X.M.; Laks, P.E.; Perchellet, J.P. Inhibitory Effects of Semisynthetic Flavonoid Derivatives on the Biochemical Markers of Tumor Promotion in Mouse Epidermis in Vivo. Cancer Lett. 1993, 72, 149–156. [Google Scholar] [CrossRef]
- Lambert, J.D.; Sang, S.; Hong, J.; Kwon, S.-J.; Lee, M.-J.; Ho, C.-T.; Yang, C.S. Peracetylation as a Means of Enhancing in Vitro Bioactivity and Bioavailability of Epigallocatechin-3-Gallate. Drug Metab. Dispos. 2006, 34, 2111–2116. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Lei, X.; Wang, L.; Guo, L.; Zhao, G.; Lin, G. Discovery and Synthesis of Novel Luteolin Derivatives as DAT Agonists. Bioorg. Med. Chem. 2010, 18, 7842–7848. [Google Scholar] [CrossRef]
- Nair, S.V.G.; Ziaullah, N.; Vasantha Rupasinghe, H.P. Fatty Acid Esters of Phloridzin Induce Apoptosis of Human Liver Cancer Cells through Altered Gene Expression. PLoS ONE 2014, 9, e107149. [Google Scholar] [CrossRef]
- Mukherjee, A.; Mishra, S.; Kotla, N.K.; Manna, K.; Roy, S.; Kundu, B.; Bhattacharya, D.; Das Saha, K.; Talukdar, A. Semisynthetic Quercetin Derivatives with Potent Antitumor Activity in Colon Carcinoma. ACS Omega 2019, 4, 7285–7298. [Google Scholar] [CrossRef]
- Biasutto, L.; Marotta, E.; De Marchi, U.; Zoratti, M.; Paradisi, C. Ester-Based Precursors to Increase the Bioavailability of Quercetin. J. Med. Chem. 2007, 50, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Pereira, R.B.; Sousa, C.; Costa, A.; Andrade, P.B.; Valentão, P. Glutathione and the Antioxidant Potential of Binary Mixtures with Flavonoids: Synergisms and Antagonisms. Molecules 2013, 18, 8858–8872. [Google Scholar] [CrossRef]
- Amić, A.; Marković, Z.; Dimitrić Marković, J.M.; Stepanić, V.; Lučić, B.; Amić, D. Towards an Improved Prediction of the Free Radical Scavenging Potency of Flavonoids: The Significance of Double PCET Mechanisms. Food Chem. 2014, 152, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-H.; Li, N.-G.; Tang, Y.-P.; Wei-Li; Lian-Yin; Yang, J.-P.; Hao-Tang; Duan, J.-A. Metabolism-Based Synthesis, Biologic Evaluation and SARs Analysis of O-Methylated Analogs of Quercetin as Thrombin Inhibitors. Eur. J. Med. Chem. 2012, 54, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Hisama, M. Antimutagenic Activity of Flavonoids from Chrysanthemum Morifolium. Biosci. Biotechnol. Biochem. 2003, 67, 2091–2099. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Yang, L.; Zhou, D.; Zhang, J. Purification and Characterization of Flavonoids from the Leaves of Zanthoxylum Bungeanum and Correlation between Their Structure and Antioxidant Activity. PLoS ONE 2014, 9, e105725. [Google Scholar] [CrossRef]
- Yuldashev, M.P.; Muminova, B.A.; Drenin, A.A.; Botirov, E.K. Flavonoids from the Aerial Part of Vicia Subvillosa. Chem. Nat. Compd. 2007, 43, 34–36. [Google Scholar] [CrossRef]
- Lim, J.H.; Park, J.-W.; Min, D.S.; Chang, J.-S.; Lee, Y.H.; Park, Y.B.; Choi, K.S.; Kwon, T.K. NAG-1 up-Regulation Mediated by EGR-1 and P53 Is Critical for Quercetin-Induced Apoptosis in HCT116 Colon Carcinoma Cells. Apoptosis 2007, 12, 411–421. [Google Scholar] [CrossRef]
- Kim, H.-S.; Wannatung, T.; Lee, S.; Yang, W.K.; Chung, S.H.; Lim, J.-S.; Choe, W.; Kang, I.; Kim, S.-S.; Ha, J. Quercetin Enhances Hypoxia-Mediated Apoptosis via Direct Inhibition of AMPK Activity in HCT116 Colon Cancer. Apoptosis 2012, 17, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.-Y.; Wu, Y.-C.; Chung, J.-G.; Yang, J.-S.; Lu, H.-F.; Tsou, M.-F.; Wood, W.G.; Kuo, S.-J.; Chen, D.-R. Quercetin-Induced Apoptosis Acts through Mitochondrial- and Caspase-3-Dependent Pathways in Human Breast Cancer MDA-MB-231 Cells. Hum. Exp. Toxicol. 2009, 28, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Lee, Y.-H.; Sharma, A.R.; Park, J.-B.; Jagga, S.; Sharma, G.; Lee, S.-S.; Nam, J.-S. Quercetin Induces Apoptosis and Cell Cycle Arrest in Triple-Negative Breast Cancer Cells through Modulation of Foxo3a Activity. Korean J. Physiol. Pharmacol. 2017, 21, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Leung, E.Y.; Askarian-Amiri, M.E.; Singleton, D.C.; Ferraro-Peyret, C.; Joseph, W.R.; Finlay, G.J.; Broom, R.J.; Kakadia, P.M.; Bohlander, S.K.; Marshall, E.; et al. Derivation of Breast Cancer Cell Lines Under Physiological (5%) Oxygen Concentrations. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-Azino-Bis-3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS) Method to Measure Antioxidant Capacity of Selected Small Fruits and Comparison to Ferric Reducing Antioxidant Power (FRAP) and 2,2‘-Diphenyl-1-Picrylhydrazyl (DPPH) Methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).