Synthesis of Novel Methyl 3-(hetero)arylthieno[3,2-b]pyridine-2-carboxylates and Antitumor Activity Evaluation: Studies In Vitro and In Ovo Grafts of Chick Chorioallantoic Membrane (CAM) with a Triple Negative Breast Cancer Cell Line
Abstract
1. Introduction
2. Results
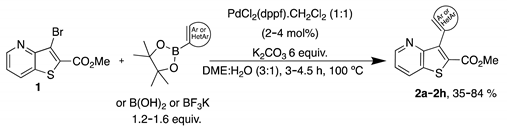
2.1. Synthesis of Methyl 3-(hetero)arylthieno[3,2-b]pyridine-2-carboxylates 2a–2h
2.2. Cell Growth Inhibitory Effect of the Synthesized Compounds on Two Different TNBC Cell Lines
2.3. Evaluation of the Cytotoxicity of the Most Promising Compounds against a Non-Tumorigenic Cell Line
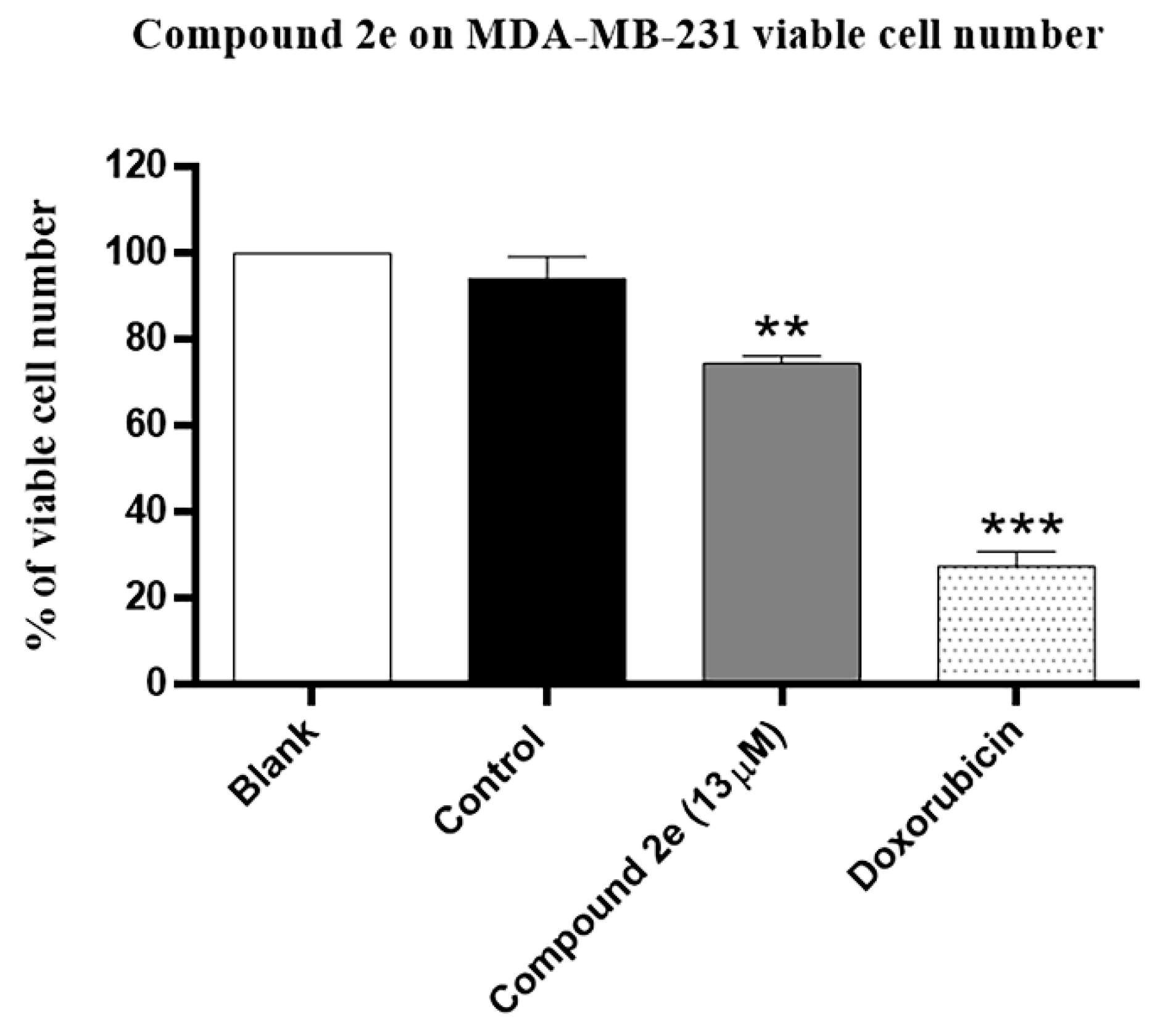
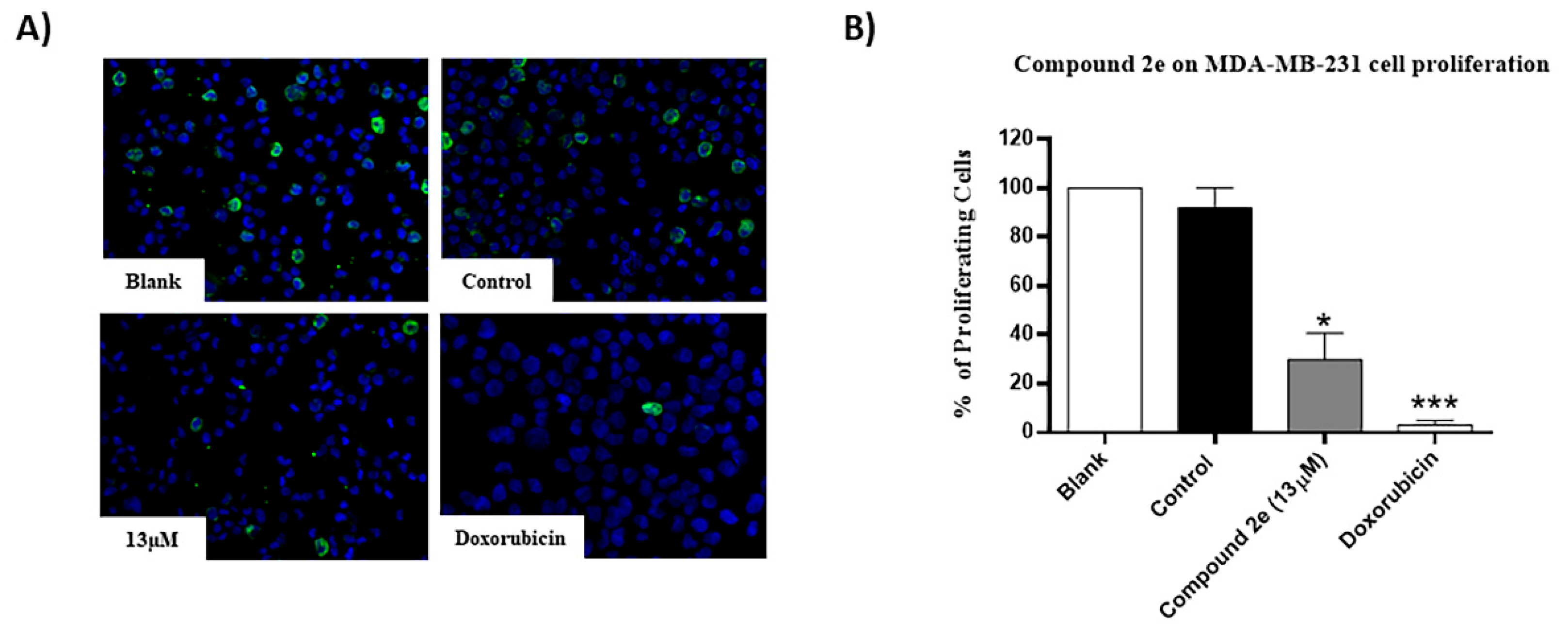
2.4. Effect of Compound 2e on Number of Viable Cells and on the Proliferation of TNBC MDA-MB-231 Cells
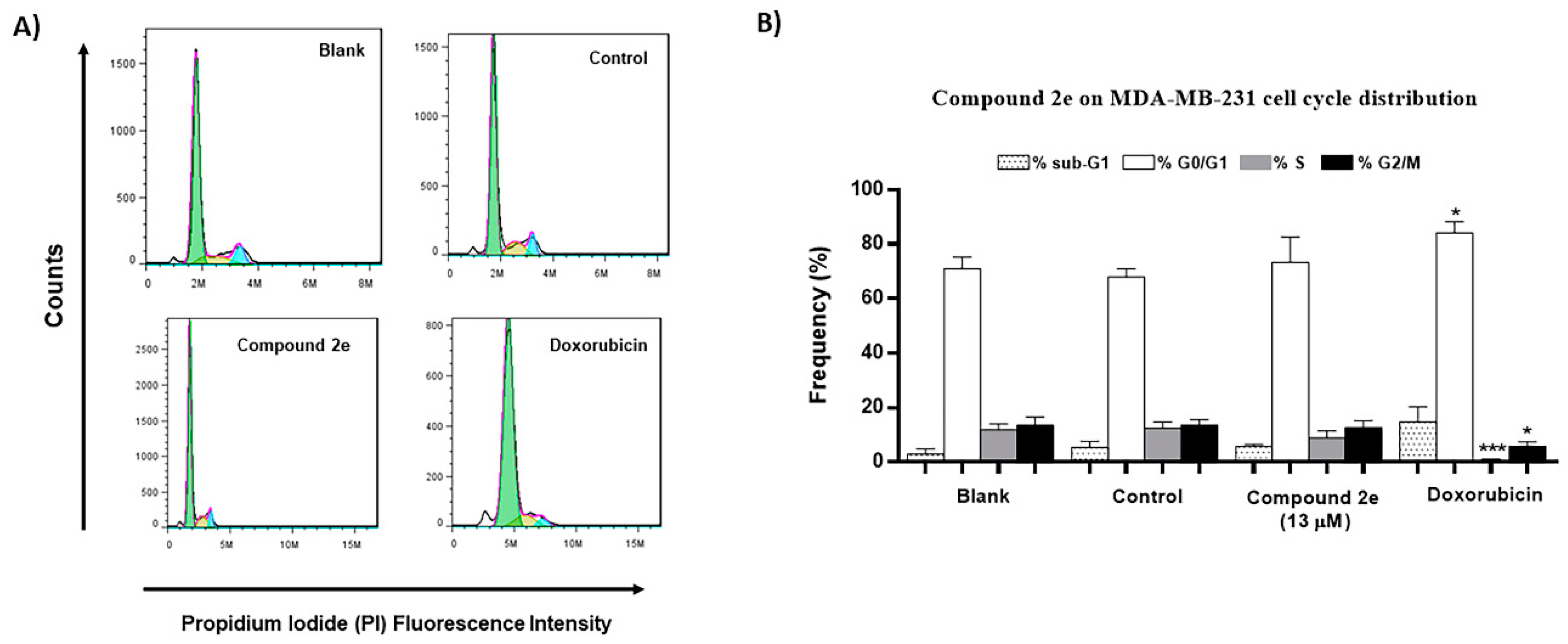
2.5. Effect of Compound 2e on Cell Cycle Profile in TNBC MDA-MB-231 Cells
2.6. Antitumor Effect of Compound 2e in Ovo Grafted with MDA-MB-231 Cells Using the Chick Chorioallantoic Membrane (CAM) Assay
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.1.1. General Procedure for the Suzuki-Miyaura Cross-coupling Products 2a–h
Methyl 3-phenylthieno[3,2-b]pyridine-2-carboxylate (2a)
Methyl 3-(p-tolyl)thieno[3,2-b]pyridine-2-carboxylate (2b)
Methyl 3-(4-methoxyphenyl)thieno[3,2-b]pyridine-2-carboxylate (2c)
Methyl 3-[4-(trifluoromethyl)phenyl]thieno[3,2-b]pyridine-2-carboxylate (2d)
Methyl 3-(4-chlorophenyl)thieno[3,2-b]pyridine-2-carboxylate (2e)
Methyl 3-(4-cyanophenyl)thieno[3,2-b]pyridine-2-carboxylate (2f)
Methyl 3-(pyridin-4-yl)thieno[3,2-b]pyridine-2-carboxylate (2g)
Methyl 3-(furan-3-yl)thieno[3,2-b]pyridine-2-carboxylate (2h)
4.2. In Vitro Antitumor Evaluation
4.2.1. Reagents
4.2.2. Solutions of the Compounds
4.2.3. Cell Cultures
4.2.4. Trypan Blue Exclusion Assay
4.2.5. Cell Growth Inhibition Assay (SRB Assay)
4.2.6. Cell Proliferation Analysis (BrdU Assay)
4.2.7. Cell Cycle Analysis
4.3. In Ovo Evaluation
In Vivo Chick Egg Chorioallantoic Membrane (CAM) Model
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef]
- Prat, A.; Parker, J.S.; Karginova, O.; Fan, C.; Livasy, C.; Herschkowitz, J.I.; He, X.; Perou, C.M. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010, 12, R68. [Google Scholar] [CrossRef]
- Sporikova, Z.; Koudelakova, V.; Trojanec, R.; Hajduch, M. Genetic markers in triple-negative breast cancer. Clin. Breast Cancer 2018, 18, e841–e850. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Munchhof, M.J.; Beebe, J.S.; Casavant, J.M.; Cooper, B.A.; Doty, J.L.; Higdon, R.C.; Hillerman, S.M.; Soderstrom, C.I.; Knauth, E.A.; Marx, M.A.; et al. Design and SAR of thienopyrimidine and thienopyridine inhibitors of VEGFR-2 kinase activity. Bioorg. Med. Chem. Lett. 2004, 14, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Boschelli, D.H.; Wu, B.; Barrios Sosa, A.C.; Chen, J.J.; Golas, J.M.; Boschelli, F. Inhibition of Src kinase activity by 7-[(2,4-dichloro-5-methoxyphenyl)amino]-2-heteroaryl-thieno[3,2-b]pyridine-6-carbonitriles. Bioorg. Med. Chem. Lett. 2005, 15, 4681–4684. [Google Scholar] [CrossRef]
- Claridge, S.; Raeppel, F.; Granger, M.C.; Bernstein, N.; Saavedra, O.; Zhan, L.; Llewellyn, D.; Wahhab, A.; Deziel, R.; Rahil, J.; et al. A discovery of a novel and potent series of thieno[3,2-b]pyridine-based inhibitors of c-Met and VEGFR2 tyrosine kinases. Bioorg. Med. Chem. Lett. 2008, 18, 2793–2798. [Google Scholar] [CrossRef]
- Queiroz, M.-J.R.P.; Calhelha, R.C.; Vale-Silva, L.A.; Pinto, E.; Lima, R.T.; Vasconcelos, M.H. Efficient synthesis of 6-(hetero)arylthieno[3,2-b]pyridines by Suzuki–Miyaura coupling. Evaluation of growth inhibition on human tumor cell lines, SARs and effects on the cell cycle. Eur. J. Med. Chem. 2010, 45, 5628–5634. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.-J.R.P.; Calhelha, R.C.; Vale-Silva, L.A.; Pinto, E.; Almeida, G.M.; Vasconcelos, M.H. Synthesis and evaluation of tumor cell growth inhibition of methyl 3-amino-6-[(hetero)arylethynyl]thieno[3,2-b]pyridine-2-carboxylates. Structure–activity relationships, effects on the cell cycle and apoptosis. Eur. J. Med. Chem. 2011, 46, 236–240. [Google Scholar] [CrossRef]
- Queiroz, M.-J.R.P.; Peixoto, D.; Calhelha, R.C.; Soares, P.; Dos Santos, T.; Lima, R.T.; Campos, J.F.; Abreu, R.M.V.; Ferreira, I.C.F.R.; Vasconcelos, M.H. New di(hetero)arylethers and di(hetero)arylamines in the thieno[3,2-b]pyridine series: Synthesis, growth inhibitory activity on human tumor cell lines and non-tumor cells, effects on cell cycle and on programmed cell death. Eur. J. Med. Chem. 2013, 69, 855–862. [Google Scholar] [CrossRef]
- Calhelha, R.C.; Ferreira, I.C.F.R.; Peixoto, D.; Abreu, R.M.V.; Vale-Silva, L.A.; Pinto, E.; Lima, R.T.; Alvelos, M.I.; Vasconcelos, M.H.; Queiroz, M.-J.R.P. Aminodi(hetero)arylamines in the Thieno[3,2-b]pyridine Series: Synthesis, effects in human tumor cells growth, cell cycle analysis, apoptosis and evaluation of toxicity using non-tumor cells. Molecules 2012, 17, 3834–3843. [Google Scholar] [CrossRef]
- Rodrigues, J.M.; Buisson, P.; Pereira, J.M.; Pinheiro, I.M.; Fernández-Marcelo, T.; Vasconcelos, M.H.; Berteina-Raboin, S.; Queiroz, M.-J.R.P. Synthesis of novel 8-(het)aryl-6H-pyrano[4′,3′:4,5]thieno[3,2-b] pyridines by 6-endo-dig cyclization of Sonogashira products and halolactonizations with Cu salts/NXS. Preliminary antitumor evaluation. Tetrahedron 2019, 75, 1387–1397. [Google Scholar] [CrossRef]
- Machado, V.A.; Peixoto, D.; Costa, R.; Froufe, H.J.C.; Calhelha, R.C.; Abreu, R.M.V.; Ferreira, I.C.F.R.; Soares, R.; Queiroz, M.-J.R.P. Synthesis, antiangiogenesis evaluation and molecular docking studies of 1-aryl-3-[(thieno[3,2-b]pyridin-7-ylthio)phenyl]ureas: Discovery of a new substitution pattern for type II VEGFR-2 Tyr kinase inhibitors. Bioorg. Med. Chem. 2015, 23, 6497–6509. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.A.; Peixoto, D.; Queiroz, M.J.; Soares, R. Antiangiogenic 1-aryl-3-[3-(thieno[3,2-b]pyridin-7-ylthio)phenyl]ureas inhibit MCF-7 and MDA-MB-231 Human breast cancer cell lines through PI3K/Akt and MAPK/Erk pathways. J. Cell. Biochem. 2016, 9999, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Calhelha, R.C.; Queiroz, M.-J.R.P. Synthesis of new thieno[3,2-b]pyridine derivatives by palladium-catalyzed couplings and intramolecular cyclizations. Tetrahedron Lett. 2010, 51, 281–283. [Google Scholar] [CrossRef]
- Stefani, H.A.; Cella, R.; Vieira, A.S. Recent advances in organotrifluoroborates chemistry. Tetrahedron 2007, 63, 3623–3658. [Google Scholar] [CrossRef]
- Alonso, F.; Belestskaya, I.P.; Yus, M. Non-conventional methodologies for transition-metal catalysed carbon–carbon coupling: A critical overview. Part 2: The Suzuki reaction. Tetrahedron 2008, 64, 3047–3101. [Google Scholar]
- Maluenda, I.; Navarro, O. Recent developments in the Suzuki-Miyaura reaction: 2010–2014. Molecules. 2015, 20, 7528–7557. [Google Scholar] [CrossRef]
- Teixeira, A.; DaCunha, D.C.; Barros, L.; Caires, H.R.; Xavier, C.P.R.; Ferreira, I.C.F.R.; Vasconcelos, M.H. Eucalyptus globulus Labill. decoction extract inhibits the growth of NCI-H460 cells by increasing the p53 levels and altering the cell cycle profile. Food Funct. 2019, 10, 3188–3197. [Google Scholar] [CrossRef]
- Long, S.; Resende, D.; Kijjoa, A.; Silva, A.M.S.; Pina, A.; Fernandez-Marcelo, T.; Vasconcelos, M.H.; Sousa, E.; Pinto, M.M.M. Antitumor activity of quinazolinone alkaloids inspired by marine natural products. Mar. Drugs 2018, 16, 261. [Google Scholar] [CrossRef]
- Traxler, P.; Furet, P. Strategies toward the design of novel and selective protein tyrosine kinase inhibitors. Pharmacol. Ther. 1999, 82, 195–206. [Google Scholar] [CrossRef]
- Keating, G.M.; Santoro, A. Sorafenib: A review of its use in advanced hepatocellular carcinoma. Drugs 2009, 69, 223–240. [Google Scholar] [CrossRef]
- Bronte, G.; Andreis, D.; Bravaccini, S.; Maltoni, R.; Cecconetto, L.; Schirone, A.; Farolfi, A.; Fedeli, A.; Serra, P.; Donati, C.; et al. Sorafenib for the treatment of breast cancer. Expert Opin. on Pharmacother. 2017, 18, 621–630. [Google Scholar] [CrossRef]
- Zafrakas, M.; Papasozomenou, P.; Emmanouilides, C. Sorafenib in breast cancer treatment: A systematic review and overview of clinical trials. World J. Clin. Oncol. 2016, 7, 331–336. [Google Scholar] [CrossRef]
- Pinto, M.; Soares, P.; Ribatti, D. Thyroid hormone as a regulator of tumor induced angiogenesis. Cancer Lett. 2011, 301, 119–126. [Google Scholar] [CrossRef]
- Lokman, N.A.; Elder, A.S.F.; Ricciardelli, C.; Oehler, M.K. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef] [PubMed]
- Dattachoudhury, S.; Sharma, R.; Kumar, A.; Jaganathan, B.G. Sorafenib inhibits proliferation, migration and invasion of breast cancer cells. Oncology 2020, 98, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Fumarola, C.; Caffarra, C.; La Monica, S.; Galetti, M.; Alfieri, R.R.; Cavazzoni, A.; Galvani, E.; Generali, D.; Petronini, P.G.; Bonelli, M.A. Effects of sorafenib on energy metabolism in breast cancer cells: Role of AMPK-mTORC1 signaling. Breast Cancer Res. Treat. 2013, 141, 67–78. [Google Scholar] [CrossRef]
- Mo, L.; Song, J.G.; Lee, H.; Zhao, M.; Kim, H.Y.; Lee, Y.J.; Ko, H.W.; Han, H.K. PEGylated hyaluronic acid-coated liposome for enhanced in vivo efficacy of sorafenib via active tumor cell targeting and prolonged systemic exposure. Nanomedicine 2018, 14, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Resende, D.; Kijjoa, A.; Silva, A.M.S.; Fernandes, R.; Xavier, C.P.R.; Vasconcelos, M.H.; Sousa, E.; Pinto, M.M.M. Synthesis of new proteomimetic quinazolinone alkaloids and evaluation of their neuroprotective and antitumor effects. Molecules 2019, 24, 534. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]

 | |||
|---|---|---|---|
| Boronated Compounds | Suzuki-Miyaura Products, Yields and Reaction Times * | Boronated Compounds | Suzuki-Miyaura Products, Yields and Reaction Times * |
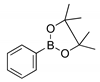 |  2a, 74%, 3 h | 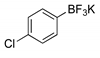 |  2e, 82%, 3 h |
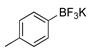 | 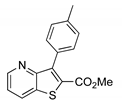 2b, 84%, 4.5 h | 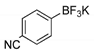 |  2f, 35%, 3 h |
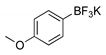 |  2c, 70%, 4 h | 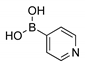 | 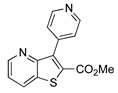 2g, 66%, 4 h |
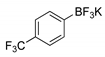 | 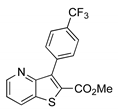 2d, 40%, 4 h | 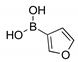 |  2h, 52%, 4.5 h |
| GI50 * Concentrations (µM) for Each Cancer Cell Line | ||
|---|---|---|
| Compound (μM) Treatment for 48 h | MDA-MB-231 | MDA-MB-468 |
| 2a | >20 | >20 |
| 2b | >10 | >10 |
| 2c | >10 | >10 |
| 2d | >10 | >30 |
| 2e | 12.56 ± 1.88 | >14 |
| 2f | 28.67 ± 1.34 | 8.73 ± 1.73 |
| 2g | >75 | >75 |
| 2h | >75 | 4.67 ± 0.68 |
| Doxorubicin | 0.068 ± 0.006 | 0.081 ± 0.009 |
| Compounds | Concentrations Tested (µM) (GI50 Concentration in the Tumor Cell Lines) | % Cell Growth in MCF-12A Cell Line (Relative to the Control) * |
|---|---|---|
| 2e | 12.56 | 88.62 ± 4.04 |
| 2f | 8.73 | 117.73 ± 3.22 |
| 2h | 4.67 | 82.13 ± 4.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, B.R.; Rebelo, R.; Rodrigues, J.M.; Xavier, C.P.R.; Vasconcelos, M.H.; Queiroz, M.-J.R.P. Synthesis of Novel Methyl 3-(hetero)arylthieno[3,2-b]pyridine-2-carboxylates and Antitumor Activity Evaluation: Studies In Vitro and In Ovo Grafts of Chick Chorioallantoic Membrane (CAM) with a Triple Negative Breast Cancer Cell Line. Molecules 2021, 26, 1594. https://doi.org/10.3390/molecules26061594
Silva BR, Rebelo R, Rodrigues JM, Xavier CPR, Vasconcelos MH, Queiroz M-JRP. Synthesis of Novel Methyl 3-(hetero)arylthieno[3,2-b]pyridine-2-carboxylates and Antitumor Activity Evaluation: Studies In Vitro and In Ovo Grafts of Chick Chorioallantoic Membrane (CAM) with a Triple Negative Breast Cancer Cell Line. Molecules. 2021; 26(6):1594. https://doi.org/10.3390/molecules26061594
Chicago/Turabian StyleSilva, Bruna R., Rita Rebelo, Juliana M. Rodrigues, Cristina P. R. Xavier, M. Helena Vasconcelos, and Maria-João R. P. Queiroz. 2021. "Synthesis of Novel Methyl 3-(hetero)arylthieno[3,2-b]pyridine-2-carboxylates and Antitumor Activity Evaluation: Studies In Vitro and In Ovo Grafts of Chick Chorioallantoic Membrane (CAM) with a Triple Negative Breast Cancer Cell Line" Molecules 26, no. 6: 1594. https://doi.org/10.3390/molecules26061594
APA StyleSilva, B. R., Rebelo, R., Rodrigues, J. M., Xavier, C. P. R., Vasconcelos, M. H., & Queiroz, M.-J. R. P. (2021). Synthesis of Novel Methyl 3-(hetero)arylthieno[3,2-b]pyridine-2-carboxylates and Antitumor Activity Evaluation: Studies In Vitro and In Ovo Grafts of Chick Chorioallantoic Membrane (CAM) with a Triple Negative Breast Cancer Cell Line. Molecules, 26(6), 1594. https://doi.org/10.3390/molecules26061594







